Abstract
Biologic drugs are highly complex molecules produced by living cells through a multistep manufacturing process. The key characteristics of these molecules, known as critical quality attributes (CQAs), can vary based on post-translational modifications that occur in the cellular environment or during the manufacturing process. The extent of the variation in each of the CQAs must be characterized for the originator molecule and systematically matched as closely as possible by the biosimilar developer to ensure bio-similarity. The close matching of the originator fingerprint is the foundation of the biosimilarity exercise, as the analytical tools designed to measure differences at the molecular level are far more sensitive and specific than tools available to physicians during clinical trials. Biosimilar development, therefore, has a greater focus on preclinical attributes compared with the development of an original biological agent. As changes in CQAs can occur at different stages of the manufacturing process, even small modifications to the process can alter biosimilar attributes beyond the point of similarity and impact clinical effectiveness and safety. The manufacturer’s ability to provide consistent production and quality control will greatly influence the acceptance of biosimilars. To this end, preventing drift from the required specifications over time and avoiding the various implications brought by product shortage will enhance biosimilar integration into daily practice. As most prescribers are not familiar with this new drug development paradigm, educational programmes will be needed so that prescribers see biosimilars as fully equivalent, efficacious and safe medicines when compared with originator products.
Keywords: biosimilars, manufacturing, regulatory, critical quality attribute, comparability, process control
Rheumatology key messages
Variation in biologics is influenced by their manufacturing processes, which adds complexity to biosimilarity development.
Modern analytics enable the fingerprinting and replication of the critical attributes of a reference product.
A well-controlled manufacturing process ensures that the biosimilar product consistently matches the originator fingerprint.
Introduction
Biological therapies (biologics) have transformed the approach to the treatment of cancer and of several types of immune-mediated inflammatory diseases, including inflammatory rheumatic disease [1, 2] and IBD [3]. Advanced biologics, such as glycosylated mAbs and fusion proteins, have emerged as popular targets for the development of therapeutic candidates because of their high potency, their ability to bind to a wide array of molecular targets with high specificity, and their stability [4, 5]. However, unlike small-molecule drugs, which are one-dimensional and chemically defined molecular entities, biologics are much larger in size and have greater structural complexity, including primary, secondary, tertiary and, possibly, quaternary structures [6]. Their biologic activity is notably defined by their structure [7] and by the cell-based manufacturing process that is used to produce them [6].
The complexity of a biologic can best be described by the physical, chemical, biological or microbiological properties that define them [8, 9]. These properties are known as quality attributes of the biologic, and each product can have dozens of them. However, among the several quality attributes of the product, only a subset of these have a direct impact on the efficacy or safety of the product, and these are known as critical quality attributes (CQAs) [10]. Examples of the CQAs of an mAb that can impact clinical activity are shown in Table 1 [11–22]. CQAs must be routinely monitored and controlled within a specified limit or range to ensure the desired product quality is achieved during manufacturing [23]. This biological complexity makes biologics more difficult to characterize, produce and reproduce [6, 24, 25].
Table 1.
Typical critical quality attributes for a mAb, where Fc function is important (e.g. infliximab)
| Attribute | Pharmacokinetics | Efficacy | Safety/immunogenicity | |
|---|---|---|---|---|
| Structure | Sequence [11] | Variable effect (product dependent) | ||
| High-order structure [11] | Variable effect (product dependent) | Misfolding or truncation can lead to lower efficacy | Misfolding can lead to ADA formation | |
| Disulfide bonds [12] | Can impact potency | |||
| Aggregates [13,14] | Lower absorption and bioavailability in some cases; can impact FcRn binding | Variable impact on Fcγ binding | Higher aggregates can lead to ADA formation | |
| Charge heterogeneity (acidic/basic forms) [15] | Variable effect (product dependent) | Can impact potency (depending on source) | ||
| Deamidation [11,13] | Can negatively impact potency | |||
| Oxidation [11,13] | Can negatively impact potency | |||
| Content | Protein concentration [16] | Can impact dose/potency | ||
| Extractable volume [16] | Can impact dose/potency | |||
| Glysoylation profile | High mannose [13,14,17] | Higher half-life with higher mannose | Higher FCγRIII and ADCC with higher mannose | Can elicit immunogenic response |
| Sialylation (NANA or NGNA) [18] | Lower half-life with higher sialylation | Can impact ADCC | NGNA forms can cause immunogenic response | |
| Fucosylation [13,17,18] | Higher FcγRIII and ADCC with lower fucose | Can elicit immunogenic response | ||
| Bisecting GlcNAc [18] | Variable impact on half-life | Can elicit immunogenic response | ||
| Non-glycosylated forms [14,19] | Variable impact on half-life | Negative impact on efficacy | Can elicit immunogenic response | |
| Galactosylation [17–19] | Can impact C1q binding and CDC | |||
| Biological activity | Binding to Fcγ receptors [14] | Variable impact on ADCC | ||
| FcRn affinity [20] | Higher FcRn affinity associated with longer half-life | Variable impact on CDC | ||
| C1q [21] | Higher C1q affinity associated with higher CDC | |||
| ADCC [22] | Can impact mechanism of action (effector function) | |||
| CDC [22] | Can impact mechanism of action (effector function) | |||
| Process impurities | Polysorbate [16] | Can be toxic | ||
| Antifoam [16] | Can be toxic | |||
| Protein A leachate [16] | Can elicit immunogenic response | |||
| Host cell DNA [16] | Can elicit immunogenic response | |||
| Host cell protein [16] | Can elicit immunogenic response |
Data are from references [11–22]. ADA: anti-drug antibody; ADCC: antibody-dependent cell-mediated cytotoxicity; CDC: complement-dependent cytotoxicity; FcRn: neonatal Fc receptor; GlcNAc: N-acetylglucosamine; NANA: N-acetylneuraminic acid; NGNA: N-glycolylneuraminic.
A biosimilar is a biopharmaceutical that has demonstrated similar CQAs, biological function, clinical efficacy and safety to that of an already licensed biologic reference product [26–29]. Importantly, biosimilars should not be considered to be generic versions of the reference biologic, because they are not identical. Indeed, molecules of this complexity cannot be reproduced identically by the manufacturers of either the biosimilar or the originator product [6, 24, 30]. However, although not all attributes of a biologic can ever be replicated exactly, the development process for a biosimilar must focus on those quality attributes that matter most; namely, those that can have clinically relevant implications (i.e. the CQAs). Matching the originator CQAs as closely as possible is the major focus of the development of a robust biosimilar manufacturing process [6, 31–33] Biosimilarity must first be proved in an extensive analytical comparability exercise, systematically evaluating the quality and similarity of the bio-similar product and the originator product across dozens of physicochemical, biological and pharmacological CQAs, before establishing equivalence in clinical efficacy and safety [6, 27].
Given that the process defines the product, it is important that anyone intending to use a biosimilar understands the biosimilar process development exercise and how a robust manufacturing process can result in a highly similar biosimilar molecule with consistent product quality. Here, we provide a simple overview of the complex processes behind biosimilar development, production scale-up, manufacturing and quality control and highlight the direct influence that these processes have on ensuring that the clinically relevant attributes of the molecule are maintained throughout the different steps of the manufacturing process and throughout the life cycle of the product. The totality of the evidence needed to establish biosimilarity and the associated process are detailed elsewhere in this supplement (see [34]) and summarized descriptively with the associated nuances here.
Biologics are inherently variable
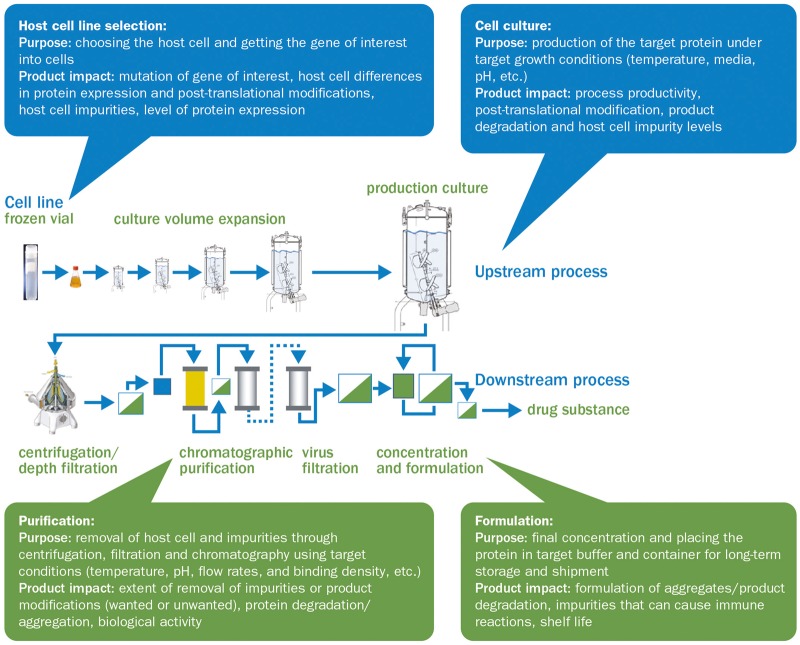
The inherent variability of biologics makes them impossible to replicate exactly. Their heterogeneity is influenced both by biological processes inside the cells that are used to express them and by the manufacturing process used to produce them (see Fig. 1) [35]. Recombinant proteins are produced by living cells, which can modify the protein structure based on their growth environment. Through several enzymatic processes, each cell expression system imprints distinct post-translational modifications (PTMs; for mAbs see Fig. 2), which may differ between cell lines, between different clones derived from the same parental cell line [6] and even between individual proteins produced by the same cell (isoform micro-heterogeneity).
Fig. 1.
The biologics manufacturing process and the manufacturing steps that affect final characteristics of biologics
Information taken from Ahmed et al. [35].
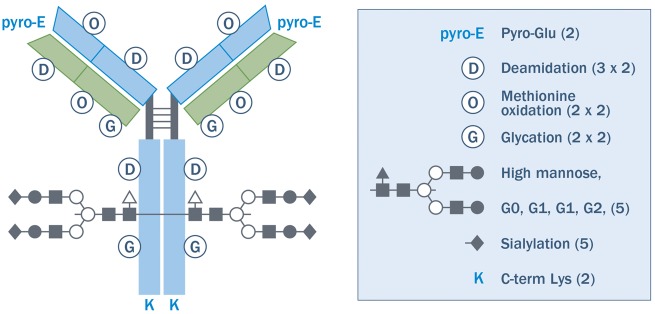
Fig. 2.
Potential mAb variants
An IgG antibody schematic is shown, with some potential structural variations resulting from post-translational modifications indicated by symbols. Each symbol is noted in the key with a list of variations. The number of variation sites in each half-antibody × the number of possible variations at each site is in parenthesis. Not all possible variants are described. For example, there are fucosylation variants in glycosylation that were not counted. If one assumes that these variants are independent and if combinations are considered, each half-antibody has 2 × 6 × 4 × 4 × 5 × 5 × 2 = 9600 possible states. If one assumes that both halves of the antibody are independent, there are 96002 ≈ 108 possible states. Reprinted from Kozlowski S, Swann P. Current and future issues in the manufacturing and development of monoclonal antibodies. Adv Drug Deliv Rev 2006;58(5–6):707–22, [37], ©2006, with permission from Elsevier.
The biochemical variability resulting from PTMs is inherent to all biological therapies and can include glycosylation, phosphorylation, deamidation, methylation and acetylation [36]. A typical mAb, for example, can have millions of molecular variants based on potential PTMs alone (Fig. 2) [37]. Several PTMs, such as glycosylation, can have a direct impact on the clinical properties of therapeutic proteins, potentially influencing their biologic activity (potency), pharmacokinetics (PK), pharmacodynamics (PD) or immunogenicity [38]. Glycosylation can be considered the most complex PTM, and its potential for clinically relevant impact and its susceptibility to change based on process conditions make it extremely challenging to control [39]. For example, the degree of fucosylation and mannosylation can have a significant impact on the effector function of a mAb [namely FcRIIIa receptor binding and antibody-dependent cell cytotoxicity (ADCC)], which plays a key role in triggering the killing of disease cells bound by the therapeutic antibody by natural killer cells [38, 40]. Likewise, the extent of terminal mannose or sialic acids can significantly alter the circulating PK half-life of an antibody or a fusion protein, and the presence of an α-Galactose epitope or N-glycolylneuraminic sialic acid can elicit an immunogenic response [38, 41].
Importantly, PTMs can result from naturally occurring processes or can be introduced by the manufacturing process used to produce biologic drugs [38]. For example, the temperature in the bioreactor or the pH of the final formulation can induce protein aggregation if not properly controlled [35], which can be associated with the immunogenicity of a biologic therapy [42, 43]. This potential for process variations to influence the immunogenicity of a compound poses some relevant clinical concerns because there are no uniform standards for the type, quantity and quality of evidence, and for guidance on experimental design for immunogenicity assays or criteria to compare the immunogenicity of biologic drugs [44]. Furthermore, the sensitivity and specificity of assays for testing immunogenic responses may still be insufficient to predict rare cases of immunogenicity [43]. Other than glycosylation and aggregation, advanced biologics can have dozens of additional CQAs, which are defined as physical, chemical or biological properties that should be within an appropriate limit, range or distribution to ensure the desired product quality (i.e. CQA ranges that ensure adequate efficacy and safety) [6, 45–47]. As these attributes are influenced not only by the cell-mediated PTMs, but also by the manufacturing process [48, 49], it is important to understand how the cell culture, purification, storage and other phases of the manufacturing process can lead to further modifications and can alter the distribution of product-related species in the final product [42]. During the cell culture phase, individual parameters, such as the temperature, pH and glucose concentration of the cell culture medium, the cell culture duration and even the type of reactor used, have the potential to alter the CQAs of the protein [37, 50]. Different steps of the purification phases can also alter the oxidation, deamidation, fragmentation and aggregation of the biological molecules [50]. As each step of the manufacturing process has multiple process parameters that can alter the quality of the product, the manufacturing process for biologics is highly challenging, with batch-to-batch variability being the norm. For example, a recent study found significant variation in the level of glycosylation in several batches of common originator biologic therapies, such as infliximab, trastuzumab and bevacizumab; a phenomenon that can also be expected in biosimilars [39]. As such, when developing a biosimilar, the variability associated with the reference product must be well understood, and the manufacturing process for the biosimilar must be carefully controlled, as even minor process alterations may have a potential irreparable impact on the qualities of the biosimilar and its comparability with the reference product [28, 30, 35, 42].
Biologic product quality changes resulting from process variation may be unintended or intended. Unintended process variation may occur owing to the impact of uncontrolled variables and can result in gradual changes over time or in a sudden shift in a quality attribute, a process called manufacturing drift [51–53]. The source of the change may not be well understood and may be an unintended result of changes outside of the manufacturer’s control, such as variability in raw material. Lack of control or understanding of the process may result in an unusable product. If the biosimilar does not meet the release or similarity criteria, it creates the potential for a supply disruption or drug recall; if several batches are affected, the potential for a drug shortage increases [54]. In addition to normal batch-to-batch variability and drift, additional changes in product quality may be the result of intentional changes made by the manufacturers of biological medicines to the manufacturing process and can range from changes in manufacturing sites to changes in suppliers or cell culture media. In addition, changes to a manufacturing process are sometimes made to introduce new technologies that can improve productivity. This type of manufacturing evolution has been observed in most, if not all, approved anti-rheumatic biologics on the market in the European Union (EU) today since their initial approval, with some having had more than 50 approved changes [35, 51, 53, 55–57]. In some cases, these changes have modified the quality of the molecule, but the majority of post-approval manufacturing changes have been considered to be non-critical because they are unlikely to have impacted the CQAs of the product [56].
Preclinical analytical comparability as the foundation
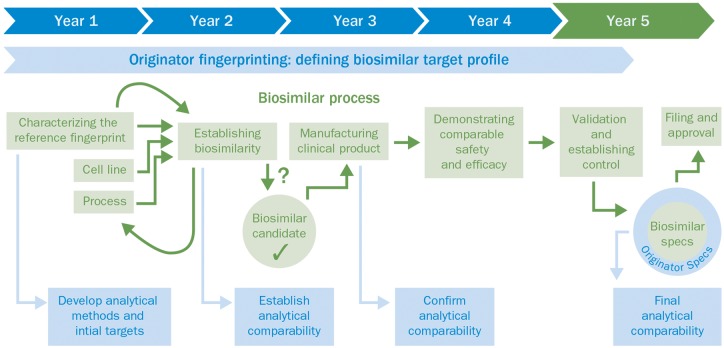
The development of new biologics emphasizes the role of clinical trials because the goal of the development process is to demonstrate de novo the risk–benefit profile of the drug candidate. However, in biosimilar development the reverse is true, because the aim of the manufacturer is to demonstrate that the biosimilar is highly similar to the reference product by demonstrating that physicochemical and biological CQAs of the biosmiliar closely match those of the originator, to be able to leverage the risk–benefit profile that has previously been established by the manufacturer of the originator product [6, 45, 46]. The European Medicines Agency (EMA) states that similarity between the biosimilar and the originator product should be established using the best possible means [27]. In much the same way that it has relied on analytical comparability studies to demonstrate that two versions of the same originator product are highly comparable after a change in the manufacturing process, the EMA has concluded that similarity is best demonstrated at the analytical level [27]. This is because, based on their high-resolution potential and their ability to assess individual molecular attributes quantitatively, analytical methods are more sensitive than clinical trials at detecting even small differences at the molecular level [58]. Physicochemical and biological analytical tools required for accurately mapping CQAs and demonstrating comparability of the biosimilar with the reference biologic (Fig. 3) have vastly improved over the past few years [45, 59]. These tools have much greater sensitivity, resolution and throughput compared with those accessible to the developers of the first generation of biologicals [42, 45, 59]. For example, the resolution potential of mass spectrometry has improved by a factor of more than 1 million in the past three decades, implying that we are now able to quantify differences in molecules at the parts-per-trillion level [60]. As a consequence, the preclinical phase, including the analytical comparability exercise, is where the most effort is concentrated because this is where most of the uncertainty regarding the similarity of two molecules is addressed and reduced (Fig. 4; also [34] in this supplement).
Fig. 3.
Comparability of the biosimilar with the originator attributes (fingerprint) during the biosimilar development process
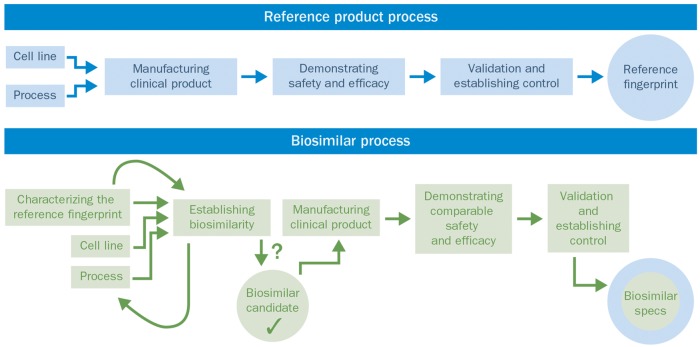
Fig. 4.
Comparison of the developmental processes for a reference (originator) product and a biosimilar
The originator fingerprint: a framework for biosimilar development
Demonstrating similarity first begins at the level of the building block, or molecular attribute. Given that advanced biologics can have many CQAs and that originator biologics have a high degree of inherent variability attributable to PTMs or to the manufacturing process, the development of a biosimilar must begin by thoroughly characterizing as many of the quality attributes of the originator as possible and establishing the range of variation for each attribute that is deemed to be critical (i.e. CQAs) [61]. The analytical characterization typically includes the assessment of physicochemical attributes (which can include primary and higher-order structure, purity and glycosylation) and functional attributes [which shed light on the molecule’s mechanism of action (MOA) and intended biological activity] [6]. The biological assays complement the structural analysis by enabling determination of the potential impact of observed structural differences between the biosimilar and reference biologic on the efficacy or safety of the product [45]. With the current status of science, these in vitro tests are particularly sensitive to detect differences between closely related molecules [62]. Put together, all of the characterized attributes and their corresponding ranges make up the fingerprint of the originator product, which provides the framework, or similarity goal posts, against which the biosimilar is developed. The aim of biosimilar process development is then to match this fingerprint, one attribute at a time, ensuring that the biosimilar is reverse engineered to similar specifications.
Defining the fingerprint of the originator biologic is an extensive exercise that involves the upfront development of highly sensitive state-of-the-art analytical methods to measure the relevant CQAs and non-CQAs of the reference product systematically. In order to establish a range that represents the expected variability of the originator biologic, several lots of the originator biologic are acquired and tested; the more lots that are analysed during the development exercise, the more confidence the biosimilar developer will have in defining the limits of similarity for each attribute. For example, during the development of SB4, a biosimilar to etanercept, the company developed 61 state-of-the-art analytical methods and tested 30 batches of EU-marketed originator and more than 30 batches of US-marketed originator during the biosimilar development process [11]. Likewise, during the development of SB2, a biosimilar to infliximab, the possible quality attributes of SB2 were compared with more than 80 lots of EU- and US-marketed originator product using more than 60 structural, physicochemical and biological analyses [17]. The results of a subset of these analyses, which demonstrated the similarity between SB2 and its originator, are shown in Table 2 [17].
Table 2.
Summarized attributes and key findings
| Category | Product quality attributes | Analytical attributes | Assessment |
|---|---|---|---|
| Physicochemical characterization | |||
| Primary structure |
|
|
|
| High-order structure | Protein secondary and tertiary structure |
|
|
| Glycosylation |
|
|
|
| Aggregation | Soluble aggregates | SEC-UV, SEC-MALLS/RI SV-AUC | Slightly higher compared with reference product in HMW analysed by SEC/UV, but SV-AUC and SEC-MALLS profiles of SB2 were similar to those of reference product |
| Fragmentation | Low molecular weight |
|
Similar to reference product |
| Charge heterogeneity |
|
CEX-HPLC and icIEF |
|
| Biological characterization | |||
| Fab-related biological activity |
|
|
|
| Fc-related biological activity |
|
|
|
2-AB: 2-aminobenzamide; ADCC: antibody-dependent cell-mediated cytotoxicity; CD: circular dichroism; CDC: complement-dependent cytotoxicity; CE-SDS: capillary electrophoresis–sodium dodecyl sulphate; CEX-HPLC: cation exchange–high-performance liquid chromatography; DSC: differential scanning calorimetry; FcRn: neonatal Fc receptors; FRET: fluorescence resonance energy transfer; Gal: galactosylated glycans; HDX-MS: hydrogen–deuterium mass spectrometry; HILIC-UPLC: hydrophilic interaction liquid chromatography–ultra-performance liquid chromatography; HMW: high molecular weight; icIEF: imaging capillary isoelectric focusing; ITF: intrinsic fluorescence spectroscopy; LC-ESIMS: liquid chromatography–electrospray ionization–mass spectrometry; LC/MS: liquid chromatography–mass spectrometry; LC-ESI-MS/MS: liquid chromatography–electrospray ionization–tandem mass spectrometry; PBMC: peripheral blood mononuclear cells; SEC: size exclusion chromatography; SEC-MALLS/RI: size exclusion chromatography–multi-angle laser light scattering/refractive index; SPR: surface plasmon resonance; SV-AUC: sedimentation velocity analytical ultracentrifugation; UV: ultraviolet; UV/VIS: ultraviolet visible. Reproduced with permission from Hong J et al. Physicochemical and biological characterization of SB2, a biosimilar of Remicade® (infliximab). MAbs 2017;9:365–38 [63], with permission from Taylor and Francis.
Process development: a tougher challenge for biosimilars
Although the complexity of advanced biologics makes engineering them extremely challenging, reverse engineering an advanced biologic to match the originator fingerprint is perhaps more challenging. This is because the development of an originator biologic follows a linear sequence of steps, performed over an average time frame of 5–8 years [63, 64]. Importantly, the goal during originator process development is to produce sufficient product with high yield, while ensuring removal of process-related impurities to safe levels, and not necessarily to ensure that the originator molecule fits a constrained target range for all critical attributes [37]. To achieve this, the manufacturer first develops a preliminary manufacturing process that results in an initial version of the product that is not fully characterized or deeply evaluated, yet appropriate for the early non-clinical animal studies or first-in-human trials. The CQAs of the new molecular entity are not fully defined and, at that stage, also often not fully understood, as was the case with infliximab, where insights into its MOA in the treatment of IBD are only recently being described through the work done by the biosimiliar developers [65]. Consequently, the relationship between the molecular structure and the biological or clinical function of the molecule has not been elucidated with all of the required analytical tests. It is only after the initial clinical trials that the originator fully develops the necessary analytical tests to characterize the product and finalizes the manufacturing process and product quality profile of the originator product. In this case, the fingerprint of the originator is the end result and is controlled and defined by the manufacturers of the originator product.
The development of the manufacturing process for a biosimilar is much more complicated because the developer is faced with several constraints at the start of development. First, the development exercise must start with defining the originator fingerprint for dozens of quality attributes in order to set limits on the potential variability of the biosimilar. Second, as the manufacturing process for the originator molecule is unknown to the biosimilar developer, a new process must be engineered to ensure that the biosimilar matches the originator fingerprint as closely as possible. This iterative process requires the cell culture and purification process conditions to be adjusted continuously, while screening hundreds of new cell lines during development until the fingerprint of the biosimilar is guided into the range of similarity, one quality attribute at a time [6, 45, 46]. The biosimilar candidate can only be taken into confirmatory clinical trials once the molecule has been thoroughly characterized, the process has been well defined and the similarity of the two molecules has been confirmed. This front-loading of analytical characterization and process development ensures that there should be little residual uncertainty, in that the molecules will have similar clinical efficacy and safety because the molecules have been demonstrated to be highly similar at the molecular level, using the most sensitive analytical methods available.
The analytical comparability exercise does not end once the biosimilar candidate has been shown to meet the similarity criteria. On the contrary, the similarity assessment is an ongoing exercise that requires the biosimilar candidate to be assessed throughout the life cycle of the product, from process development, through scale-up and process validation and after any manufacturing process changes are introduced. This ongoing analytical comparability minimizes the risk that the product diverges in a clinically meaningful way from the approved molecular fingerprint.
The process defines the product: quality by design, and achieving similarity through target-directed process engineering
The target-directed development of biologics and biosimilars is known as quality by design (QbD) [66]. QbD is a systematic risk-based approach to the development of a product and the associated manufacturing processes that relies on properly identifying a drug’s CQAs and defining limits for each CQA based on its potential clinical impact (e.g. the originator fingerprint). QbD applies principles of quality risk management and differs from previous approaches to process development in ensuring that drug quality is built into every step of the product development exercise rather than relying on the final testing of the product as the only check for quality control [10]. QbD achieves this through the implementation of process controls and CQA limits at every step of the process, establishing a link between process parameters and their impact on the quality of the product to ensure that the end product meets the expected quality profile [67]. For a biosimiliar, the QbD approach relies on the upfront definition of the originator fingerprint; a set of CQAs whose functional and structural characteristics are most relevant for the clinical outcomes of the reference product [6, 68, 69]. Examples of the attributes that can form a biologic’s fingerprint, and their potential relationship to a clinical outcome, are shown in Table 1. The biosimilar product profile is then systematically analysed against the originator fingerprint throughout the development of the different phases of the manufacturing process, following an extensive iterative process development exercise that can involve thousands of experiments [10]. In all, the product and process knowledge base must include an understanding of the variability in raw materials, the relationship between process parameters and CQAs, and the association between CQAs and the clinical characteristics of the biosimilar [10].
Below, we provide an overview of the relevant steps in the development of a biosimilar manufacturing process.
Step 1: cell line selection and engineering
One of the most crucial process development decisions made during the development of a biosimilar is the choice of cell line. The cell line is one of the key determinants of the glycosylation patterns of biologicals, making the choice of the mammalian expression host very important in determining the final glycoform profile of the biosimilar [41, 70]. For example, Chinese hamster ovary-based cell lines are the most popular for the development of biological drugs because they generally produce similar glycosylation patterns to humans and have several advantages, including their ability to grow in suspension, their high specific yield and their stability to changes in pH and oxygen [71]. However, Chinese hamster ovary cell lines are unable to produce certain human glycans, while conversely also being able to produce certain glycans that are not typical in humans (such as α-gal and N-glycolylneuraminic), which could lead to increased immunogenicity [71]. Cell line engineering can also result in the over- or underexpression of certain enzymes that are responsible for the regulation of certain glycoforms. For example, ADCC can be improved dramatically by the overexpression of N-acetylglucosaminyltransferase III to increase the amount of bisecting N-acetylglucosamine forms, or by decreasing the fucose on antibodies [72]. The careful genetic engineering and monitoring of the cell lines is important during cell line selection, which is a process that can involve the screening of hundreds to thousands of clones to achieve the appropriate analytical fingerprint [72].
Step 2: cell culture process development
The cell culture process involves thawing a vial of frozen cells from a cell bank. The vial is inoculated into shake flasks to increase the cell density, and the cells are then grown in serial sub-cultivations until the target production scale is reached. The cells are maintained in growth media and are provided with the required nutrients and additives to ensure the viability of the cells. Slightly altering the cell culture conditions can have a significant impact on several CQAs, including glycosylation and impurity profiles, and must be carefully controlled [39]. To attain the quality target product profile, several parameters are optimized during process development, involving the performance of hundreds of small-scale experiments using sound statistical procedures [73]. Parameters investigated during the cell culture process development include the following: oxygen levels, lactate production, temperature, pH, osmolality and duration [72]. These parameters are continuously monitored during the scale-up of the process to ensure consistent performance as the cell culture volume is increased until the ultimate production scale is reached (e.g. 15 000–20 000 l).
Step 3: purification process optimization to guide CQAs into similarity range
Once the cell culture phase is completed, a purification process is used to recover the target protein, while removing unwanted impurities, including adventitious viruses, host cell proteins and DNA, aggregates and endotoxins [74]. As the target biosimilar is typically excreted into the cell culture fluid by the mammalian cell, the recovery process begins by removing the cellular debris through centrifugation and filtration. The cell metabolism that expresses the desired protein also generates several undesired product variants and impurities, so the purification process is designed to purify the target biosimilar while removing process impurities and additives that could potentially be harmful to patients (e.g. immunogenic). In addition, for biosimilars, the purification process must also fine-tune the biosimilarity profile of the molecule, by targeting the removal or enrichment of certain product-related attributes (isoforms, glycans, charged variants, etc.), in order to achieve the target originator biologic fingerprint. The purification process primarily relies on column chromatography and filtration to remove these undesired impurities, by exploiting the interaction between chemically functionalized resins or filters and the biochemical and/or physical properties of the proteins [74]. For example, cation or anion exchange chromatography can be used to separate positively (acidic) or negatively (basic) charged isoforms of the biosimilar resulting from C-terminal lysine heterogeneity [5, 75]. Likewise, hydrophobic interaction chromatography can be used to modify levels of misfolded and aggregated species, as these product variants are typically more hydrophobic than the correctly folded monomeric form of the protein [76]. As with the development of the cell culture process, several hundred sound statistical procedures are typically performed during process development to characterize how each operational parameter (e.g. pH, conductivity, binding capacity or flow rate) can impact the CQAs of the biosimilar and to define process controls that ensure a consistent product, batch after batch, following scale-up and routine manufacturing.
Step 4: achieving a stable formulation
After the product is purified, it is concentrated and formulated using ultrafiltration and diafiltration. The goal of the concentration step is to ensure that the product is delivered at a concentration that enables dose optimization given the route of administration. For example, although s.c. administration is often a preferred route of administration for certain medications, a high-concentration formulation must be used to minimize the injection volume [74]. However, advanced biologics, such as mAbs and fusion proteins, have a tendency to aggregate or degrade during the manufacturing process or during storage and transportation, which can impact the batch-to-batch variability, efficacy or immunogenicity of the product [77]. Moreover, many human mAbs display poor biophysical properties, such as low stability and a propensity to aggregate, which may trigger immune responses [77]. The product formulation is a crucial element of minimizing the propensity of a product to degrade and maximizing its shelf life. The development of a stable formulation step involves the optimization of buffer conditions, including pH, ionic strength, and the inclusion of excipients and stabilizers in varying amounts [77]. The product is typically subject to a myriad of solutions while exposing it to physical (e.g. agitation) or thermodynamic stress (e.g. temperature, freeze–thaw) over extended periods of time; the product is then assessed for chemical, colloidal and conformational stability [78]. The final presentation (liquid, frozen liquid or lyophilisate) will be dependent on the stability of the product in the form (ready to use); although a liquid presentation is typically preferred, in some cases the product may need to be frozen during storage or may be lyophilized to minimize chemical degradation [79]. Lyophilized forms have the disadvantage of additional costs associated with development, manufacture and testing and the need for diluents for reconstitution, although they may reduce the number of resupply batches that may be required owing to the superior stability of the lyophilized form [79]. In cases where freezing is required, a cryoprotectant (such as sucrose) is typically added to minimize cryoprecipitation or aggregation resulting from cryoconcentration [79].
Manufacturing controls ensure similarity after scale-up and post-approval
The manufacturing process for biologics is lengthy and complex, often involving many discrete unit operations and activities. Each step can have several input variables and, from start to finish, the manufacturing process involves simultaneously controlling dozens of input parameters while performing quality control checks throughout to ensure that the product meets precise allowable limits at each phase. Changes to the manufacturing process may also occur by regulatory request, by scaling up production and fine-tuning process efficiency to improve product quality and yield [9, 68], introducing process drift or shifting over time [53, 80] (Fig. 5). As a result, this manufacturing complexity can negatively impact batch-to-batch consistency and patient safety if each parameter is not properly monitored and controlled during large-scale manufacturing [6]. Rigorous life-cycle management and manufacturing quality control that follow the international pharmaceutical industry standards for comparability after manufacturing process alterations are fundamental for maintaining the biosimilar CQAs within acceptable ranges of variation [54, 68]. Adherence to these guidelines may also avoid concerns about immunogenicity, product recall and supply shortage after approval is granted [54, 68]. These process controls and guidelines, along with stringent pharmacovigilance programmes to track and accurately assess immunogenicity and adverse events after approval [43, 81], minimize the risk to the patient and are mandated by the EMA for any medicine, including biosimilars [28, 82].
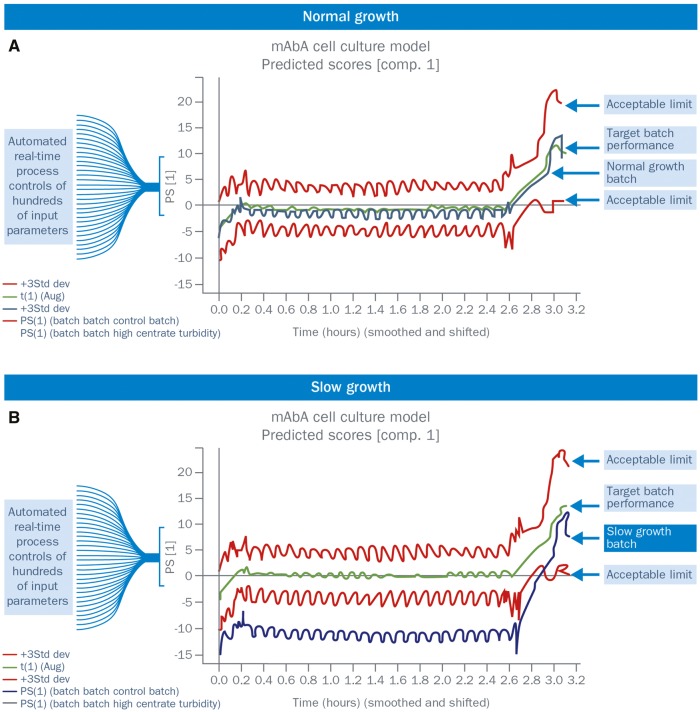
Fig. 5.
Example of an automated, real-time, quality control using multivariate batch process modelling
(A) Normal growth. (B) Slow growth. Data from several historical batches are used to correlate parameter levels with product quality, using multivariate modelling techniques, such as principal component analysis (PCA). Of the dozens of parameter inputs, those that strongly correlate with product quality are summarized by a single output (principal component), which describes a large portion of the potential variation in product quality. Historical data are also used to define acceptable limits for each parameter.
By helping to ensure consistency in product quality, these process control guidelines also help to ensure the continuity of product supply, which is a key consideration in healthcare provision [83–85]. A negative shift in product quality is frequently a consequence of low-cost manufacturing, ageing manufacturing plants, contamination and lack of good contracting practice [54]. Lack of sufficient manufacturing capacity or poor inventory practices can turn a quality issue into a disruption of supply and a potential drug shortage [54] if the manufacturer is unable to overcome a lost lot (or lots) by increasing production. These situations can lead to forced or uncontrolled switching of treatment regimens for non-medical reasons, and could increase healthcare costs by forcing a switch to an originator drug or otherwise delaying a switch to a more affordable biosimilar [54, 86–88]. Treatment switching, in particular, should instead be managed adequately through proper patient–physician education, stakeholder alignment and monitoring post-switch to minimize the potential for differences in patient-reported outcomes and prevent discontinuation for non-medical reasons (e.g. nocebo effects) [89, 90]. It is therefore important that both biosimilars and reference products are produced in state-of-the-art and specialized facilities that follow high-standard manufacturing guidelines and QbD principles [54]. Such facilities also ensure that a battery of rigorous automated in-process controls is implemented to monitor the biosimilar analytical fingerprint and batch-to-batch variability and to ensure that changes to the manufacturing process take place in real time [61], allowing for prompt assessment and troubleshooting of production drift (Fig. 4). This enables the product quality aspects to be adjusted so as to fall consistently within those of the reference product range and, as a consequence, it can prevent quality disruption, batch failure and subsequent product shortage [61]. This is an important principle, because acceptance of biosimilars into daily clinical practice can be hindered by clinicians’ reluctance to prescribe these agents in light of potential supply disruptions and product shortage, an occurrence that has been reported ever more frequently with a variety of medicines [8, 54, 88, 91]. Supply shortage reflecting manufacturing issues, as previously described, can be avoided by adopting a shortage mitigation plan [84, 85], involving effective management of drug inventory, active management of raw materials and maintenance of multi-site manufacturing capabilities, ensuring robust and secure distribution networks and instigating a rapid response to supply interruption signals (Table 3) [93]. Bearing in mind the technical challenges in developing and producing a biosimilar that matches all the CQAs of the reference product, the ultimate decision to prescribe a biosimilar instead of its reference biologic or the choice between biosimilars must take into consideration the manufacturer’s experience, record of consistent manufacturing, proven capacity and stable supply chain [93].
Table 3.
Steps to mitigate shortage of biological supply
| Key step | Rationale |
|---|---|
| Effective management of drug inventory | To minimize interruptions to drug supply |
| Active management of raw materials | To maintain continuity in manufacturing and a stable drug supply |
| Maintenance of multi-site manufacturing facilities | To create robustness in manufacturing continuity and thereby address extended supply chain interruptions |
| Implement robust and secure distribution networks | To ensure supply chain integrity to patients |
| Implement rapid response to supply interruption signals | To reduce drug shortage risk |
Data taken from [92].
Discussion
Biologic drugs are highly complex molecules that are produced by living cells through a multistep procedure. These complex molecules have dozens of CQAs that can vary based on the extent of the PTMs that occur in the cellular environment or during the manufacturing process. Each step of the manufacturing process can also impart variations to the CQAs. The extent of the variation in each of the CQAs must be characterized for the originator molecule and must be systematically matched as closely as possible by the biosimilar developer. The close matching of the originator fingerprint is the foundation of the biosimilar exercise, as the analytical tools designed to measure differences at the molecular level are far more sensitive and specific than tools available to physicians during clinical trials [58]. If two molecules are confirmed to be highly similar at the building block level, there should be little uncertainty that these molecules will have equivalent clinical efficacy and safety.
Based on the superior ability of the preclinical analytical evaluation to measure molecular and physical differences between two products, it forms the foundation of the totality of the evidence required to demonstrate that a biosimilar is highly similar to the reference product. The additional sources of evidence in a biosimilar development programme are designed to address any remaining uncertainty that may not be addressed by the pre-clinical phase, and include potential non-clinical animal studies, a phase I PK equivalence study, and a phase III clinical efficacy and safety equivalence study (see [34] in this supplement). The regulations for the development of a typical biosimilar require that only a single phase III clinical trial is performed in the most sensitive clinical indication with a sensitive, reproducible end point. However, extrapolation to other indications is not taken for granted, even if the biosimilar demonstrated clinical equivalence in the phase III trial; the reduced requirement for phase III clinical data creates a potential void in evidence related to the other indications in which the biologic may be indicated. For extrapolation to be granted, the applicant must address this void by substantiating that the biosimilar shares the same MOA involved in each of the indications as the reference product [94]. The same MOA is not always shared by all of the approved indications for the reference product and, in some cases, the MOA for a given indication is not known. If the MOAs are known, they are best probed at the preclinical analytical level because highly sensitive methods can be developed to measure each CQA that could be associated with the different MOAs. For example, to allow for extrapolation of the biosimilar infliximab CT-P13 from RA (studied in the phase III trial) to the inflammatory bowel indications (not studied in pivotal trials), additional preclinical and clinical documentation was produced to show that the biosimilar did not only bind to TNF-α, but also had ADCC [95] activity, which may play an additional role in treatment of IBD [65]. In those cases where the MOA is not known, additional evidence must be provided beyond the preclinical data and the single phase III trial, such as additional PD studies or phase III studies [96]. An advantage of biosimilar mAbs is that the predominant MOA is usually known; therefore, a preclinical in vitro comparison of the biosimilar and the reference product binding to the target antigen is the primary demonstration of similar MOA [58].
Changes in CQAs can occur at different stages of the manufacturing process, requiring a deep understanding of the molecule and the manufacturing process. This reflects the complexity that characterizes each stage [50, 68, 80], be it the biosimilar molecule glycosylation pattern that is linked to the cell line used or its immunogenicity, which can be associated, for example, with the purification process and storage conditions [42]. Even small modifications to this process can alter the biosimilar attributes beyond the point of similarity and thereby impact on clinical effectiveness and safety [6, 38]. The manufacturer’s ability to provide consistent production and quality control, prevent drift from the required specifications over time and avoid the various implications brought by product shortage will greatly influence the acceptance of biosimilars and their integration into daily practice.
If well developed and demonstrated to have an equivalent quality and clinical profile to their originator counterparts, biosimilars have the potential to transform healthcare by helping to improve access to biologic therapies to underserved populations, while also creating meaningful savings in healthcare expenditures. However, as most prescribers at this moment in time are not familiar with this new drug development paradigm, educational programmes to explain the essentials, as set out in this paper, will be needed so that prescribers are able to gain confidence in the stringency involved in the development, manufacturing and approval of biosimilars as fully equivalent efficacious and safe medicines, and to provide all stakeholders (regulators, payers, prescribers and patients alike) with an objective set of considerations that should be weighed (preclinical quality of the product, clinical data and manufacturer trustworthiness) when considering the use of biosimilars. Moreover, as several biosimilars of a given product become available, understanding these concepts can help clinicians to make well-informed decisions when selecting a biosimiliar and avoid potential pitfalls related to the quality of the biosimilars and the consistency of the manufacturers producing them.
Acknowledgments
The authors would like to acknowledge the editorial support provided by inVentiv Health Medical Communications. Philip Ford and Frances Gambling from inVentiv Health Medical Communications wrote the drafts of the article based on input from both authors, and styled the article per journal requirements. The authors would also like to thank Professor Schulze-Koops for his review and detailed and constructive comments. Biogen reviewed and provided feedback on the article to the authors. The authors had full editorial control of the article, addressed all queries in the review process and take full responsibility for the whole article. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole and have given final approval of the version to be published.
Funding: This work was supported by Biogen, who provided funding for medical writing and editorial support in the development of this article.
Disclosure statement: O.A.J. is an employee of Biogen International GmbH and therefore receives salary and may own Biogen stock. A.G.V. has lectured, been on the speakers bureau and consulted for AbbVie, Amgen, Biogen, Boehringer Ingelheim, Medicines for Europe (formerly European Generic Medicines Association; EGA), Mundipharma, Pfizer/Hospira, Roche and Novartis/Sandoz; all compensation for this work was received by their hospital.
References
- 1. Isaacs JD, Cutolo M, Keystone EC, Park W, Braun J.. Biosimilars in immune-mediated inflammatory diseases: initial lessons from the first approved biosimilar anti-tumour necrosis factor monoclonal antibody. J Intern Med 2016;279:14–29. [DOI] [PubMed] [Google Scholar]
- 2. Neves H, Kwok HF.. Recent advances in the field of anti-cancer immunotherapy. BBA Clin 2015;3:280–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Côté-Daigneault J, Bouin M, Lahaie R, Colombel JF, Poitras P.. Biologics in inflammatory bowel disease: what are the data? United European Gastroenterol J 2015;3:419–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chames P, Van Regenmortel M, Weiss E, Baty D.. Therapeutic antibodies: successes, limitations and hopes for the future. Br J Pharmacol 2009;157:220–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shukla AA, Hubbard B, Tressel T, Guhan S, Low D.. Downstream processing of monoclonal antibodies – application of platform approaches. J Chromatogr B Analyt Technol Biomed Life Sci 2007;848:28–39. [DOI] [PubMed] [Google Scholar]
- 6. Bui LA, Hurst S, Finch GL. et al. Key considerations in the preclinical development of biosimilars. Drug Discov Today 2015;20(Suppl. 1):3–15. [DOI] [PubMed] [Google Scholar]
- 7. Ventola CL. Biosimilars: part 1: proposed regulatory criteria for FDA approval. P T 2013;38:270–87. [PMC free article] [PubMed] [Google Scholar]
- 8. European Medicines Agency. International Conference on Harmonization. ICH guideline Q8 (R2) on pharmaceutical development Step 5. September 2015. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002872.pdf (3 April 2017, date last accessed).
- 9. Rathore AS, Reason AJ, Weiskop A.. Defining critical quality attributes for monoclonal antibody therapeutic products. International Biopharm. July 2014. http://www.biopharminternational.com/defining-critical-quality-attributes-monoclonal-antibody-therapeutic-products?pageID=1 (3 April 2017, date last accessed).
- 10. Rathore AS, Winkle H.. Quality by design for biopharmaceuticals. Nat Biotechnol 2009;27:26–34. [DOI] [PubMed] [Google Scholar]
- 11. Cho IH, Lee N, Song D. et al. Evaluation of the structural, physicochemical, and biological characteristics of SB4, a biosimilar of etanercept. MAbs 2016;8:1136–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu H, May K.. Disulfide bond structures of IgG molecules: structural variations, chemical modifications and possible impacts to stability and biological function. MAbs 2012;4:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.A Mab: a case study in bioprocess development. CMC Biotech Working Group. Version 2.1. 30 October 2009. https://www.ispe.org/pqli/a-mab-case-study-version-2.1 (6 March 2017, date last accessed).
- 14. López-Morales CA, Miranda-Hernández MP, Juárez-Bayardo LC. et al. Physicochemical and biological characterization of a biosimilar trastuzumab. Biomed Res Int 2015;427235. doi: 10.1155/2015/427235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tebbey PW, Varga A, Naill M, Clewell J, Venema J.. Consistency of quality attributes for the glycosylated monoclonal antibody Humira® (adalimumab). MAbs 2015;7:805–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Animal Cell Biotechnology. In Biologics Production. Hauser ed. Hansjörg Hauser and Roland Wagner, Roland. De Gruyter, Berlin, Germany, 2014.
- 17. Hong J, Lee Y, Lee C. et al. Physicochemical and biological characterization of SB2, a biosimilar of Remicade® (infliximab). MAbs 2017;9:365–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reusch D, Tejada ML.. Fc glycans of therapeutic antibodies as critical quality attributes. Glycobiology 2015;25:1325–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Raju TS, Jordan RE.. Galactosylation variations in marketed therapeutic antibodies. MAbs 2012;4:385–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shade KTC, Anthony RM.. Antibody glycosylation and inflammation. Antibodies 2013;2:392–414. [Google Scholar]
- 21. Pierri CL, Bossis F, Punzi G. et al. Molecular modeling of antibodies for the treatment of TNFα-related immunological diseases. Pharmacol Res Perspect 2016;4:e00197.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mori K, Iida S, Yamane-Ohnuki N. et al. Non-fucosylated therapeutic antibodies: the next generation of therapeutic antibodies. Cytotechnology 2007;55:109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mitchell M. Determining Criticality-Process Parameters and Quality Attributes Part I: Criticality as a Continuum. A practical roadmap in three parts that applies scientific knowledge, risk analysis, experimental data, and process monitoring throughout the three phases of the process validation lifecycle. December 2013. http://www.biopharminternational.com/determining-criticality-process-parameters-and-quality-attributes-part-i-criticality-continuum (3 April 2017, date last accessed).
- 24. Camacho LH, Frost CP, Abella E, Morrow PK, Whittaker S.. Biosimilars 101: considerations for U.S. oncologists in clinical practice. Cancer Med 2014;3:889–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee JF, Litten JB, Grampp G.. Comparability and biosimilarity: considerations for the healthcare provider. Curr Med Res Opin 2012;28:1053–8. [DOI] [PubMed] [Google Scholar]
- 26. European Medicines Agency. Questions and answers on biosimilar medicines (similar biological medicinal products). 27 September 2012. http://www.ema.europa.eu/docs/en_GB/document_library/Medicine_QA/2009/12/WC500020062.pdf (3 October 2016, date last accessed).
- 27. European Medicines Agency. Guideline on similar biological medicinal products. 22 May 2013. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2013/05/WC500142978.pdf (3 October 2016, date last accessed).
- 28. Food and Drug Administration. Guidance for Industry: Quality Considerations in Demonstrating Biosimilarity to a Reference Protein Product. April 2015. http://www.fda.gov/downloads/DrugsGuidanceComplianceRegulatoryInformation/Guidances/UCM291128.pdf (3 October 2016, date last accessed).
- 29. Weise M, Bielsky MC, De Smet K. et al. Biosimilars – why terminology matters. Nat Biotechnol 2011;29:690–3. [DOI] [PubMed] [Google Scholar]
- 30. Mellstedt H, Niederwieser D, Ludwig H.. The challenge of biosimilars. Ann Oncol 2008;19:411–9. [DOI] [PubMed] [Google Scholar]
- 31. European Medicines Agency. Guideline on the Clinical Evaluation of the Pharmacokinetics of Therapeutic Proteins. 24 January 2007. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003029.pdf (3 October 2016, date last accessed).
- 32. European Medicines Agency. Guideline on Similar Biological Medicinal Products Containing Biotechnology-derived Proteins as Active Substance: Non clinical and Clinical Issues. 18 December 2014. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/01/WC500180219.pdf (3 October 2016, date last accessed).
- 33. European Medicines Agency. Guideline on Similar Biological Medicinal Products Containing Biotechnology-derived Proteins as Active Substances: Quality Issues (revision 1). 22 May 2014. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/06/WC500167838.pdf (6 March 2016, date last accessed).
- 34. Declerck P, Rezk MF.. The road from development to approval: evaluating the body of evidence to confirm biosimilarity. Rheumatology 2017;56(Suppl. 4):iv4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ahmed I, Kaspar B, Sharma U.. Biosimilars: impact of biologic product life cycle and European experience on the regulatory trajectory in the United States. Clin Ther 2012;34:400–19. [DOI] [PubMed] [Google Scholar]
- 36. Mann M, Jensen ON.. Proteomic analysis of post-translational modifications. Nat Biotechnol 2003;21:255–61. [DOI] [PubMed] [Google Scholar]
- 37. Kozlowski S, Swann P.. Current and future issues in the manufacturing and development of monoclonal antibodies. Adv Drug Deliv Rev 2006;58:707–22. [DOI] [PubMed] [Google Scholar]
- 38. Walsh G, Jefferis R.. Post-translational modifications in the context of therapeutic proteins. Nat Biotechnol 2006;24:1241–52. [DOI] [PubMed] [Google Scholar]
- 39. Planinc A, Dejaegher B, Heyden YV. et al. Batch-to-batch N-glycosylation study of infliximab, trastuzumab and bevacizumab, and stability study of bevacizumab. Eur J Hosp Pharm 2016;1–7. doi: 10.1136/ejhpharm-2016-001022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Arora T, Padaki R, Hamburger AE. et al. Differences in binding and effector functions between classes of TNF antagonists. Cytokine 2009;45:124–31. [DOI] [PubMed] [Google Scholar]
- 41. Hossler P, Khattak SF, Li ZJ.. Optimal and consistent protein glycosylation in mammalian cell culture. Glycobiology 2009;19:936–49. [DOI] [PubMed] [Google Scholar]
- 42. Berkowitz SA, Engen JR, Mazzeo JR, Jones GB.. Analytical tools for characterizing biopharmaceuticals and the implications for biosimilars. Nat Rev Drug Discov 2012;11:527–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Locatelli F, Roger S.. Comparative testing and pharmacovigilance of biosimilars. Nephrol Dial Transplant 2006;21(Suppl. 5):v13–6. [DOI] [PubMed] [Google Scholar]
- 44. Pineda C, Castañeda Hernández G, Jacobs IA, Alvarez DF, Carini C.. Assessing the immunogenicity of biopharmaceuticals. BioDrugs 2016;30:195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Al-Sabbagh A, Olech E, McClellan JE, Kirchhoff CF.. Development of biosimilars. Semin Arthritis Rheum 2016;45:S11–8. [DOI] [PubMed] [Google Scholar]
- 46. Alten R, Cronstein BN.. Clinical trial development for biosimilars. Semin Arthritis Rheum 2015;44:S2–8. [DOI] [PubMed] [Google Scholar]
- 47. Lionberger RA, Lee SL, Lee L, Raw A, Yu LX.. Quality by design: concepts for ANDAs. AAPS J 2008;10:268–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Heinemann L, Hompesch M.. Biosimilar insulins: how similar is similar? J Diabetes Sci Technol 2011;5:741–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tsiftsoglou AS, Trouvin JH, Calvo G, Ruiz S.. Demonstration of biosimilarity, extrapolation of indications and other challenges related to biosimilars in Europe. BioDrugs 2014;28:479–86. [DOI] [PubMed] [Google Scholar]
- 50. European Medicines Agency. Guideline on Immunogenicity Assessment of monoclonoal Antibodies Intended for in vivo Clinical Use. 24 May 2012. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/06/WC500128688.pdf (3 October 2016, date last accessed).
- 51. Schiestl M, Stangler T, Torella C. et al. Acceptable changes in quality attributes of glycosylated biopharmaceuticals. Nat Biotechnol 2011;29:310–2. [DOI] [PubMed] [Google Scholar]
- 52. Munsch J. Biosimilars: new promise for reducing healthcare costs. J Biomed Res 2014;28:75–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ramanan S, Grampp G.. Drift, evolution, and divergence in biologics and biosimilars manufacturing. BioDrugs 2014;28:363–72. [DOI] [PubMed] [Google Scholar]
- 54. Woodcock J, Wosinska M.. Economic and technological drivers of generic sterile injectable drug shortages. Clin Pharmacol Ther 2013;93:170–6. [DOI] [PubMed] [Google Scholar]
- 55. Schneider CK. Biosimilars in rheumatology: the wind of change. Ann Rheum Dis 2013;72:315–8. [DOI] [PubMed] [Google Scholar]
- 56. Vezér B, Buzás Z, Sebeszta M, Zrubka Z.. Authorized manufacturing changes for therapeutic monoclonal antibodies (mAbs) in European Public Assessment Report (EPAR) documents. Curr Med Res Opin 2016;32:829–34. [DOI] [PubMed] [Google Scholar]
- 57. Kurki P, van Aerts L, Wolff-Holz E. et al. Interchangeability of biosimilars: a European perspective. BioDrugs 2017;31:83–91. [DOI] [PubMed] [Google Scholar]
- 58. Gerrard TL, Johnston G, Gaugh DR.. Biosimilars: extrapolation of clinical use to other indications. GaBI J 2015;4:118–24. [Google Scholar]
- 59. Tsuruta LR, Lopes dos Santos M, Moro AM.. Biosimilars advancements: moving on to the future. Biotechnol Prog 2015;31:1139–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Thomson B. Driving high sensitivity in biomolecular MS. Genet Engineer Biotech News 2012;32:20. [Google Scholar]
- 61. Schiel JE, Mire-Sluis A, Davis D. Monoclonal Antibody Therapeutics: The Need for Biopharmaceutical Reference Materials. ACS Symposium Series; American Chemical Society: Washington, DC. 2014. http://pubs.acs.org/doi/pdf/10.1021/bk-2014-1176.ch001 (3 April 2016, date last accessed).
- 62. Nesbitt A, Fossati G, Bergin M. et al. Mechanism of action of certolizumab pegol (CDP870): in vitro comparison with other anti-tumor necrosis factor α agents. Inflamm Bowel Dis 2007;13:1323–32. [DOI] [PubMed] [Google Scholar]
- 63. Shankar G, Pendley C, Stein KE.. A risk-based bioanalytical strategy for the assessment of antibody immune responses against biological drugs. Nat Biotechnol 2007;25:555–61. [DOI] [PubMed] [Google Scholar]
- 64. Class JN, Langis L.. A patient-centred paradigm for the biosimilars market. GaBI J 2012;1:17–21. [Google Scholar]
- 65. Jahnsen J. Clinical experience with infliximab biosimilar Remsima (CT-P13) in inflammatory bowel disease patients. Therap Adv Gastroenterol 2016;9:322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. da Silva A, Kronthaler U, Koppenburg V. et al. Target-directed development and preclinical characterization of the proposed biosimilar rituximab GP2013. Leuk Lymphoma 2014;55:1609–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kennett RS, Kennett DA.. Quality by design applications in biosimilar pharmaceutical products. Accred Qual Assur 2008;13:681–90. [Google Scholar]
- 68. European Medicines Agency. International Conference on Harmonization. ICH topic Q 5 E: Comparability of Biotechnological/biological Products. June 2005. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002805.pdf (3 October 2016, date last accessed).
- 69. McCamish M, Woollett G.. Worldwide experience with biosimilar development. MAbs 2011;3:209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Le H, Vishwanathan N, Jacob NM, Gadgil M, Hu WS.. Cell line development for biomanufacturing processes: recent advances and an outlook. Biotechnol Lett 2015;37:1553–64. [DOI] [PubMed] [Google Scholar]
- 71. Dumont J, Euwart D, Mei B, Estes S, Kshirsagar R.. Human cell lines for biopharmaceutical manufacturing: history, status, and future perspectives. Crit Rev Biotechnol 2016;36:1110–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Li F, Vijayasankaran N, Shen AY, Kiss R, Amanullah A.. Cell culture processes for monoclonal antibody production. MAbs 2010;2:466–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Legmann R, Schreyer HB, Combs RG. et al. A predictive high-throughput scale-down model of monoclonal antibody production in CHO cells. Biotechnol Bioeng 2009;104:1107–20. [DOI] [PubMed] [Google Scholar]
- 74. Liu HF, Ma J, Winter C, Bayer R.. Recovery and purification process development for monoclonal antibody production. MAbs 2010;2:480–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yigzaw Y, Hinckley P, Hewig A, Vedantham G.. Ion exchange chromatography of proteins and clearance of aggregates. Curr Pharm Biotechnol 2009;10:421–6. [DOI] [PubMed] [Google Scholar]
- 76. Shukla AA, Thömmes J.. Recent advances in large-scale production of monoclonal antibodies and related proteins. Trends Biotechnol 2010;28:253–61. [DOI] [PubMed] [Google Scholar]
- 77. Lowe D, Dudgeon K, Rouet R. et al. Aggregation, stability, and formulation of human antibody therapeutics. Adv Protein Chem Struct Biol 2011;84:41–61. [DOI] [PubMed] [Google Scholar]
- 78. Razinkov VI, Treuheit MJ, Becker GW.. Accelerated formulation development of monoclonal antibodies (mAbs) and mAb-based modalities: review of methods and tools. J Biomol Screen 2015;20:468–83. [DOI] [PubMed] [Google Scholar]
- 79. Warne NW. Development of high concentration protein biopharmaceuticals: the use of platform approaches in formulation development. Eur J Pharm Biopharm 2011;78:208–12. [DOI] [PubMed] [Google Scholar]
- 80. Blackstone EA, Fuhr JP Jr.. Innovation and competition: will biosimilars succeed? Biotechnol Healthcare 2012;9:24–7. [PMC free article] [PubMed] [Google Scholar]
- 81. Vulto AG, Crow SA.. Risk management of biosimilars in oncology: each medicine is a work in progress. Target Oncol 2012;7 (Suppl. 1):S43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bennett CL, Chen B, Hermanson T. et al. Regulatory and clinical considerations for biosimilar oncology drugs. Lancet Oncol 2014;15:e594–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. European Medicines Agency and the European Medicines Agency Inspectors Working Group. Prevention of Drug Shortages Based on Quality and Manufacturing Issues. 23 December 2014. https://www.ispe.org/drug-shortages-initiative/report-to-ema.pdf (3 October 2016, date last accessed).
- 84. International Society of Pharmaceutical Engineering. ISPE drug shortages prevention plan 2014. http://www.ispe.org/drugshortagespreventionplan.pdf (11 October 2016, date last accessed).
- 85. Parenteral Drug Association. Drug Shortage. 2014. https://www.pda.org/scientific-and-regulatory-affairs/regulatory-resources/drug-shortage (11 October 2016, date last accessed).
- 86. Kaakeh R, Sweet BV, Reilly C. et al. Impact of drug shortages on U.S. health systems. Am J Health Syst Pharm 2011;68:1811–9. [DOI] [PubMed] [Google Scholar]
- 87. Ventola CL. The drug shortage crisis in the United States: causes, impact, and management strategies. P T 2011;36:740–57. [PMC free article] [PubMed] [Google Scholar]
- 88. Li E, Subramanian J, Anderson S. et al. Development of biosimilars in an era of oncologic drug shortages. Drug Des Devel Ther 2015;9:3247–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Razanskaite V, Bettey M, Downey L. et al. Biosimilar infliximab in inflammatory bowel disease: outcomes of a managed switching programme. J Crohns Colitis 2017;11:690–6. [DOI] [PubMed] [Google Scholar]
- 90. Wessenfield J, Stock S, Lungen M, Gerber A.. The nocebo effect: a reason for patients’ non-adherence to generic substitution? Pharmazie 2010;65:451–6. [PubMed] [Google Scholar]
- 91. Krisl JC, Fortier CR, Taber DJ.. Disruptions in the supply of medications used in transplantation: implications and management strategies for the transplant clinician. Am J Transplant 2013;13:20–30. [DOI] [PubMed] [Google Scholar]
- 92. Mica A, Mutomba M, Green L.. Steps to ensure adequate supply of biological medicines: considerations for the healthcare provider. GaBI J 2013;2:136–43. [Google Scholar]
- 93. Boone N, van der Kuy H, Scott M. et al. How to select a biosimilar. Eur J Hosp Pharm 2013;20:275–86. [Google Scholar]
- 94. Curigliano G, O’Connor DP, Rosenberg JA, Jacobs I.. Biosimilars: extrapolation for oncology. Crit Rev Oncol Hematol 2016;104:131–7. [DOI] [PubMed] [Google Scholar]
- 95. Committee for Medicinal Products for Human Use. Assessment Report: Remsima. 27 June 2013. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002576/WC500151486.pdf (3 October 2016, date last accessed).
- 96. Weise M, Kurki P, Wolff-Holz E, Bielsky MC, Schneider CK.. Biosimilars: the science of extrapolation. Blood 2014;124:3191–6. [DOI] [PubMed] [Google Scholar]