Abstract
Objective. To evaluate the effect of rituximab (RTX) in patients with RA-related interstitial lung disease (RA-ILD) and identify factors associated with outcome after treatment.
Methods. An observational study of patients with RA-ILD was conducted from a cohort of RTX-treated RA patients in a single centre for >10 years. Progression was defined by any of the following: a decrease of pre-RTX forced vital capacity (FVC) >10% or diffusion capacity of carbon monoxide (DLCO) >15% predicted, worsening of the ILD score or death from progressive ILD.
Results. Of 700 RA patients treated with RTX, 56 had RA-ILD (prevalence = 8%). After RTX, new ILD was diagnosed in 3/700 patients (incidence = 0.4%). Data for lung assessment were available for 44/56 patients. The median relative change pre- and post-RTX for FVC were −2.4% and +1.2% (P = 0.025) and for DLCO were −4.4% and −1.3% (P = 0.045). Post-RTX, 23/44 (52%) were stable and 7/44 (16%) had improved. Of the 14 (32%) with ILD that progressed, 9/56 (16%) were deaths due to progressive ILD. Factors associated with ILD progression were radiologic pattern of usual interstitial pneumonia, a previous history of lung progression and pre-RTX DLCO <46% predicted. Of those whose ILD progressed, 11/14 (79%) had severe ILD before RTX [median DLCO 42% predicted (interquartile range 41–49)].
Conclusion. In this cohort of patients where RTX was given for arthritis, most patients with ILD pre-RTX remained stable/improved after treatment over a prolonged follow-up period. Patients who deteriorated/died had the most severe ILD pre-RTX, suggesting the drug was not contributory. RTX appears to be an acceptable therapeutic choice for patients with RA-ILD and further studies are warranted.
Keywords: B cells, biologic therapies, immunosuppressant, respiratory, rheumatoid arthritis
Rheumatology key messages
-
Rituximab showed satisfactory safety in rheumatoid arthritis-related interstitial lung disease.
Lung function remained stable or improved in most patients after rituximab over a prolonged follow-up period.
Usual interstitial pneumonia, previous progression and low carbon monoxide diffusing capacity predicted lung progression post-rituximab.
Introduction
Interstitial lung disease (ILD) is a common extra-articular manifestation of RA that is reported in up to 30% of RA patients [1]. RA-ILD is the second most common cause of mortality in RA [2]. The high mortality has been attributed to uncontrolled systemic inflammatory disease, infections and complication from therapies [3, 4]. While the treatment of RA has greatly improved in recent years with the introduction of biologic therapies, the use of such agents has often been restricted in RA-ILD due to concerns over safety.
Initial concerns arose after anecdotal reports of serious respiratory adverse events following treatment with a TNF inhibitor (TNFi) in patients with pre-existing RA-ILD [5, 6], leading to preference for a non-TNFi such as rituximab (RTX). Histologically, a rationale for B cell–targeted therapy in ILD was suggested by the demonstration of follicular B cell hyperplasia and interstitial plasma cell infiltrates in (open) lung biopsy specimens of patients with RA-ILD compared with idiopathic pulmonary fibrosis (PF) [7]. Nevertheless, clinical evidence for the efficacy and safety of RTX in the context of ILD was scarce. Indeed, in the only prospective pilot study of 10 patients with progressive RA-ILD who were treated with RTX, the association between significant adverse events (included two deaths) and either RTX or underlying disease could not be determined, because of the small number of patients [8]. Furthermore, patients with RA-ILD are normally excluded from formal clinical trials due to comorbidity. Therefore, data from larger cohorts and registries are needed.
In the absence of a head-to-head trial of RTX against a standard therapy and/or other biologics, the aims of this study were to evaluate the effect of RTX in patients with RA-ILD as assessed using pulmonary function tests (PFT), imaging and mortality and to identify factors associated with outcome post-RTX.
Methods
Patients and design
All patients with moderate to severe RA who were treated with RTX in our unit between January 2004 and May 2015 were evaluated retrospectively from the Leeds Biologics Database. From this, an observational study of consecutive patients with RA-ILD was conducted. Inclusion criteria included adults (>18 years old) fulfilling the revised 1987 ACR criteria for RA [9] and detection of ILD by high-resolution CT (HRCT). The Leeds (West) Research Ethics Committee confirmed that ethical approval was not required, in accordance with the UK National Health Service Research Ethics Committee guidelines, because all treatment decisions were made prior to the evaluation of data.
Treatment protocol
All patients received a first cycle of therapy consisting of 100 mg of methylprednisolone and 1000 mg of RTX given intravenously on days 1 and 14. Further cycles consisting of the same regimen were repeated on clinical relapse. Rescue therapies with i.v. CYC and/or referral for lung transplantation could be undertaken in the event of lung progression/worsening (as defined below).
Clinical data and outcomes
Joints
Disease activity was assessed using 28-joint DAS (DAS28) at baseline and every 3 months. Response at 6 months was defined according to EULAR criteria [10].
Lung
PFT data consisting of assessment for forced vital capacity (FVC) and diffusion capacity of carbon monoxide (DLCO) were collected at 6–12 months pre-RTX, at the time of treatment with RTX, 6–12 months post-RTX and at the most recent follow-up.
HRCT scans were acquired (when clinically indicated) in patients with worsening dyspnoea and/or deterioration in lung function using a standardised method. The scans were scored independently by two radiologists (M.D., a chest radiologist with >10 years experience in reporting ILD, and G.L., a general radiologist), both blinded to lung function information and the sequence of scans. The ILD score (ILDS) was used to evaluate the presence and extent of pure ground-glass opacification (GGO), pulmonary fibrosis and honeycombing in the six lung zones, with a maximum possible total score of 24 [11]. Each paired scan (pre- and post-treatment) was then rated as 0 = worsening, 1 = same or 2 = improving. Any discrepancy was resolved by consensus. The detailed methodology for HRCT scans can be found as supplementary data, section on HRCT scans and immunoglobulin measurement, available at Rheumatology Online.
In order to account for missing PFT data of those with severe ILD who were unable to perform the test, data from the HRCT and survival status were incorporated into the overall lung response. This lung response was classified into worsening (any of either a decrease of pre-RTX FVC >10% or DLCO >15% predicted, worsening of ILDS or death from progressive lung disease) [12], improving (any of either an increase of pre-RTX FVC >10% or DLCO >15% predicted or improvement of ILDS) and stable (others that did not meet criteria for either worsening/improving).
Peripheral blood B cell subsets analysis
Peripheral blood B cell subsets were analysed using highly sensitive flow cytometry, as previously described [13], at weeks 0, 2 and 26 without knowledge of clinical status other than time since RTX. Complete B cell depletion was defined as counts <0.0001 × 109 cells/l.
Safety
Safety assessments, which included severe adverse events (SAEs) and serious infection, were recorded irrespective of a possible association with RA-ILD and/or therapy. SAEs were defined as those resulting in either hospitalization that lasted >24 h, flares requiring i.v. therapy, malignancies, life-threatening situations or death. Data for serious infections were gathered from hospital admission records using the Patient Access Centre system and were later confirmed with case notes.
Statistical analysis
Pulmonary function trends were expressed as the relative change from the start of therapy with RTX and the Wilcoxon signed-rank test was used to analyse pulmonary function changes before and after treatment. The difference in clinical characteristics between RA-ILD patients who had lung progression vs those who were stable post-RTX were analysed using the Mann–Whitney U test for continuous variables and Fisher’s exact test for categorical variables.
Progression-free survival time (measured in weeks) was calculated from the date of the first RTX infusion to either the date of progression or the date of the last updated data (May 2015). Analysis for categorically distributed variables that were relevant for ILD progression was performed using Kaplan–Meier plots and log-rank tests. Multivariate analysis was not performed due to the number of patients and nine potential predictors of ILD progression [14]. Receiver operator curves were used to measure the sensitivity and specificity of optimal thresholds for investigations predicting ILD progression. All statistical analysis was performed using SPSS 21.0 (IBM, Armonk, NY, USA) and GraphPad Prism 7.01 for Windows (GraphPad Software, La Jolla, CA, USA).
Results
Patient characteristics
Of 700 patients with RA treated with RTX, 56 patients had RA-ILD (prevalence = 8%) and were included in the analysis. Thirty-six were female and 55/56 (98%) were RF and/or anti-CCP antibody positive [median age 64 years (IQR 59–72), median RA duration 10 years (IQR 7–13), median ILD duration 5 years (IQR 3–7), median FVC 87% predicted (IQR 76–108) and median DLCO 58% predicted (IQR 43–63)] at RTX initiation. Total follow-up was 195 patient-years. Baseline characteristics are described in Table 1. Post-RTX, new ILD was diagnosed in only 3/700 patients (incidence = 0.4%).
Table 1.
Baseline characteristics of the 56 patients with RA-ILD treated with RTX
| Age at first RTX infusion, median (IQR), years | 64 (59–72) |
| Female patient, n (%) | 36 (64) |
| RF positive, n (%) | 53/56 (95) |
| ACPA positive, n (%) | 42/51 (82) |
| Anti-ENA positivea, n (%) | 6/56 (11) |
| RA disease duration at first RTX, median (IQR), years | 10 (7–13) |
| ILD disease duration at first RTX, median (IQR), years | 5 (3–7) |
| Smoking status, n (%) | |
| Never | 24 (43) |
| Ex-smoker | 25 (45) |
| Current | 7 (12) |
| Prior TNFi treatment, n (%) | 16 (29) |
| Secondary non-response for RA, n (%) | 6 (37) |
| Worsening of ILD, n (%) | 10 (63) |
| Prior CYC therapy for ILD, n (%) | 10 (18) |
| Cumulative dose of CYC, mean (s.d.), g | 6.7 (2.5) |
| Number of prior immunosuppressant failures (including TNFi and CYC but excluding steroid), median (range) | 3 (1–9) |
| Concomitant DMARDs, n (%) | |
| MTX | 28 (78) |
| AZA | 5 (14) |
| LEF | 2 (5) |
| MMF | 1 (3) |
| DAS28 at first RTX infusion, mean (s.d.) | 5.64 (1.17) |
| CRP at first RTX infusion, mean (s.d.) | 30.4 (33.2) |
| Radiographic pattern of ILD, n (%) | |
| NSIP | 33 (60) |
| UIP | 20 (36) |
| COP | 2 (3) |
| AIP | 1 (1) |
| FVC (% predicted) at first RTX infusion, median (IQR) | 87 (76–108) |
| DLCO (% predicted) at first RTX infusion, median (IQR) | 58 (43–63) |
| Expert ILDS at first RTX infusion, median (range) | 6 (2–8) |
Six patients had concurrent anti-Ro antibody positivity at RTX initiation. Of these, four had strongly positive anti-CCP antibody titres and the remaining two (without anti-CCP antibody positivity) had erosive RA.
AIP, acute interstitial pneumonia; COP, cryptogenic organizing pneumonia; ENA: extractable nuclear antigen.
Treatment characteristics
A total of 181 cycles of RTX were administered to the 56 patients studied. The median duration of response for cycles 1–3 (C1–3) was 44 weeks (IQR 33–55), 44 (37–58) and 43 (35–66), respectively. Prior to C1, 16 were treated previously with a TNFi. Of these, 10 patients (63%) were switched to RTX due to worsening ILD, while 6 (37%) had secondary non-response in terms of RA. In C1, 36 patients (64%) received concomitant therapies with conventional synthetic DMARDs (csDMARDs): MTX = 28, AZA = 5, LEF = 2 and MMF = 1.
Ten patients received CYC prior to RTX. Of these, seven had stable ILD during RTX treatment while three required further CYC due to ILD progression. Two patients who had not previously received CYC received the treatment post-RTX due to worsening ILD.
Articular response
In C1, there was a significant reduction in DAS28 [mean pre-RTX 5.69 vs 4.07 post-RTX, mean difference −1.62 (s.d. 0.29) (95% CI −2.20, −1.05); P < 0.001]. EULAR response rates of good, moderate and poor in patients with complete data at 6 months post-RTX were 12/52 (23%), 32/52 (62%) and 8/52 (15%), respectively. Articular response was not correlated with lung response [r = 0.122 (95% CI −0.206, 0.426); P = 0.452].
Of the eight patients who were C1 non-responders, 7/8 had incomplete B cell depletion post-RTX. Six of these were re-treated at 6 months, with depletion in three patients, but all responded in C2. One of the C1 non-responders had ILD progression post-RTX. The response rates (EULAR good and moderate) for C2 and C3 were 32/40 (80%) and 23/30 (78%), respectively. At the last follow-up, 7 (13%) had secondary non-response to RTX and were switched to different biologics: tocilizumab (n = 5) and abatacept (n = 2).
In C1, 23/56 (41%) were on concomitant corticosteroid at RTX initiation. At 6 months post-RTX, cessation of corticosteroid was achieved in 3/23 (13%), 4/23 (17%) had a dose reduction >50% from baseline, 14/23 (61%) had their dose unchanged and 2/23 (9%) had their dose increased by 50% from baseline.
Lung response
Data for the overall lung assessment was available for 44/56 patients. Of these, pre- and post-RTX PFT results were recorded in 37/44. The remaining 7/44 had a death outcome reported only because they were unable to undergo either a PFT or HRCT.
PFT
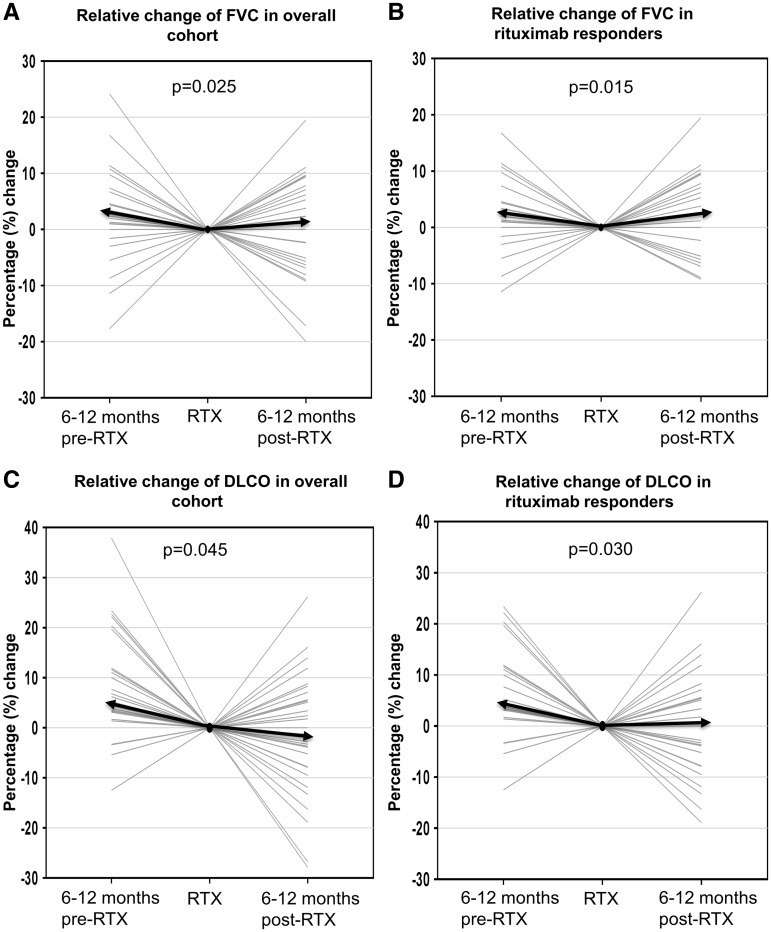
In the 6–12 months pre-RTX, there was a decline in the median relative change of FVC of −2.4% (IQR −7.1–+0.8). Ten patients had clinically significant PFT progression. In the following 6–12 months post-RTX, numerical improvement was seen in the median relative change of FVC of +1.2% (IQR −6–+8.6; median difference +4.2%; P = 0.025) (Fig. 1A). Similar numerical improvement was seen in the median relative change of DLCO [−4.4% (IQR −11.8 to −3.2) pre-RTX vs −1.3% (IQR −8.7–+6.4) post-RTX; median difference +3.7%; P = 0.045] (Fig. 1C). PFT progression was halted in 5/10 (50%) of the patients while the remaining 5 (50%) continued to progress. Post-RTX, 7/37 (19%) had improvement in PFTs, 25/37 (68%) were stable and 5/37 (13%) had worsening of their PFTs.
Fig. 1.
Pre- and post-treatment lung function trends
(A) Overall, the median relative change of FVC was −2.4% (IQR −7.1–+0.8) pre-RTX as compared with +1.2% (IQR −6–+8.6) post-RTX, representing a difference of + 4.2% (P = 0.025). (B) For RTX responders (improvement or stable PFT), the median difference in the change of FVC between pre-RTX vs post-RTX was +5.1% (P = 0.015). (C) Overall, the median change of DLCO was −4.4% (IQR −11.8 to − 3.2) pre-RTX compared with −1.3% (IQR −8.7 s.d.+6.4) post-RTX, representing a difference of + 3.7% (P = 0.045). (D) For RTX responders, the median difference in the change of DLCO between pre-RTX vs post-RTX was +4.4% (P = 0.030). The median change for each graph is represented by the solid black arrow.
ILDS
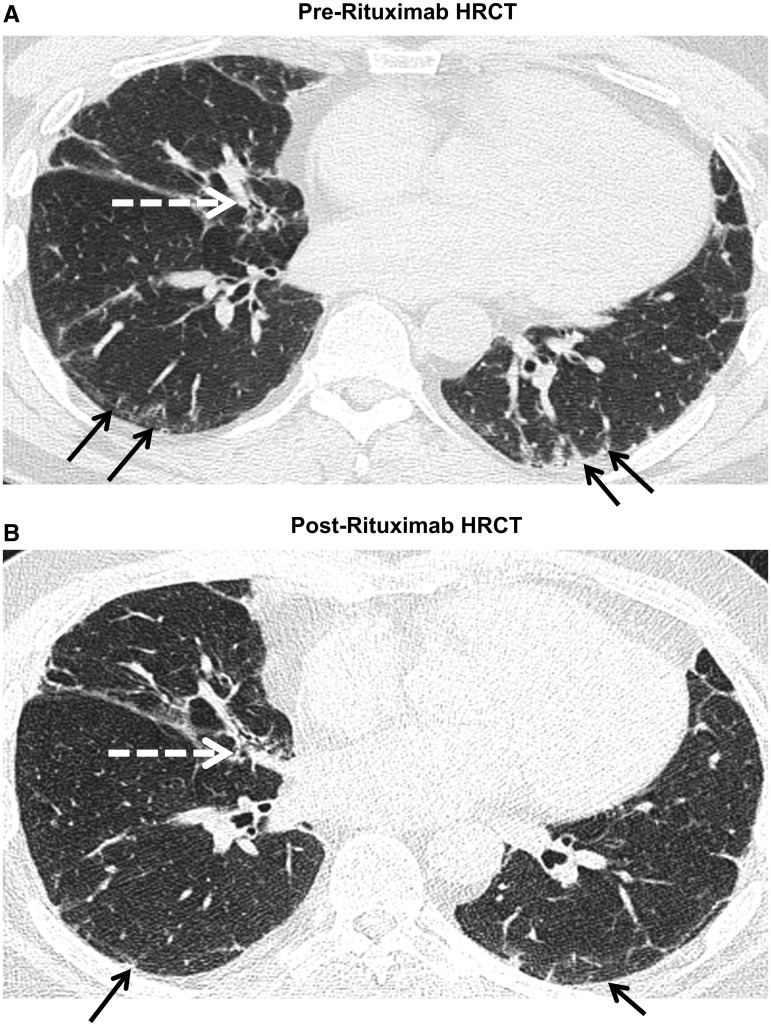
Fourteen pairs (pre- and post-RTX) of HRCTs were performed in selected patients with worsening dyspnoea and/or deterioration in lung function. Of these, 1 (7%) had improved (Fig. 2), 6 (42%) remained stable and 7 (50%) had worsening of scan imaging appearances post-RTX. There was no difference in the median pre-RTX ILDS between patients who had worsening and those who were stable (P = 0.26). The interrater agreement for the presence or absence of PF (κ = 0.63) and honeycombing (κ = 0.73) were good, whereas the interrater agreement for pure GGO was fair (κ = 0.29).
Fig. 2.
Improvement of HRCT 6 months post-RTX
The black arrow denotes intra- and interlobular thickening while the white arrow denotes traction bronchiectasis. There was an improvement of peripheral fibrosis in B compared with A following treatment with RTX.
Overall lung response
After RTX (at the latest time point with evaluable data for lung assessment), 7/44 (16%) had improved, 23/44 (52%) were stable and in 14/44 (32%) ILD had progressed. Details of individual lung response are described in supplementary Table S1, available at Rheumatology Online. Of those whose ILD progressed, 11/14 (79%) had pre-existing severe and progressive ILD (lung progression defined as above) with a median DLCO of 42% predicted (IQR 41–49) pre-RTX. Three of the 14 (21%) who had stable pre-existing ILD progressed after RTX.
Of those with severe ILD, that was, DLCO ⩽ 40% recorded at the time of RTX initiation, stabilization of ILD after therapy was seen in 4/6 (67%) of the patients. The cumulative mortality rate due to ILD progression at 3, 5 and 7 years was 13%, 16% and 16%, respectively.
Of the remaining 12 patients with incomplete data for respiratory investigations, 6 (50%) continued on RTX with no lung exacerbation, 3 (25%) switched therapy due to secondary non-response in terms of RA and 3 (25%) died of a non-ILD progression cause (Table 3).
Table 3.
Causes of deaths in 12 patients with RA-ILD treated with RTX
| Patient no. | Pattern of ILD | FVC pre-RTX | DLCO pre-RTX | No. of cycles | Months since last RTX | Cause of death |
|---|---|---|---|---|---|---|
| 1 | UIP | 70 | 57 | 4 | 6 | Pneumonia; ILD progression despite CYC and RTX |
| 2 | UIP | 95 | 64 | 3 | 8 | Pneumonia; ILD progression; on home oxygen (LTOT) |
| 3 | UIP | N/A | N/A | 3 | 10 | ILD progression |
| 4 | UIP | 79 | 57 | 3 | 11 | ILD progression; on LTOT |
| 5 | UIP | N/A | N/A | 3 | 12 | ILD progression |
| 6 | UIP | N/A | 41 | 2 | 30 | ILD progression despite CYC was added; awaiting lung transplant |
| 7 | UIP | 72 | 41 | 1 | 36 | Pneumonia; ILD progression; on LTOT |
| 8 | UIP | N/A | N/A | 2 | 72 | Gastrointestinal bleeding secondary to colon cancer |
| 9 | NSIP | 111 | 66 | 1 | 6 | Infection after neck surgery (atlanto-axial subluxation) |
| 10 | NSIP | 53 | 41 | 1 | 9 | ILD progression; awaiting lung transplant |
| 11 | NSIP | 103 | 88 | 2 | 12 | Metastatic lung cancer |
| 12 | NSIP | N/A | 35 | 1 | 15 | Pneumonia; ILD progression despite CYC and RTX |
LTOT: Long-term oxygen therapy; N/A: Not available.
Factors associated with ILD progression
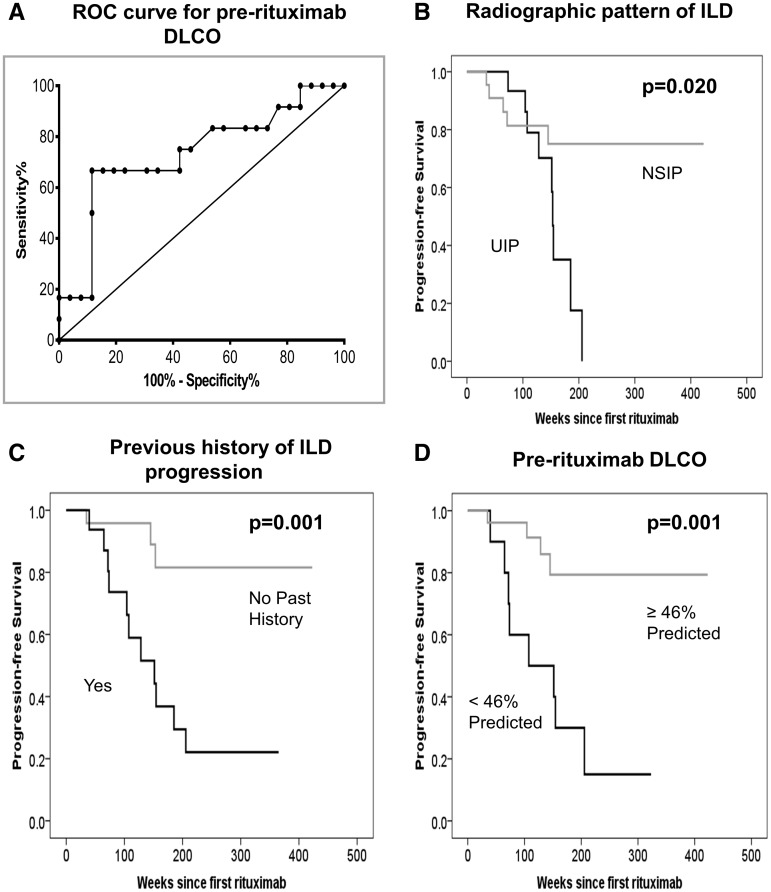
Patients whose lung function deteriorated post-RTX had a previous history of ILD progression (defined as documented radiographic or PFT progression since diagnosis), a radiographic pattern of usual interstitial pneumonia (UIP) and lower pre-RTX DLCO compared with those who were stable or improved (Table 2). The receiver operating characteristics curves indicated that a pre-RTX DLCO of 46% predicted demonstrated 67% sensitivity and 88% specificity in predicting ILD progression after therapy (Fig. 3A).
Table 2.
Baseline risk factors for ILD progression following treatment with RTX
| Characteristics | RA-ILD patients who had lung progression (n = 14) | RA-ILD patients with stable/improved lung (n = 30) | P-value |
|---|---|---|---|
| Age, median (IQR), years | 70 (61–73) | 63 (59–68) | 0.302 |
| Male, n (%) | 8 (57) | 8 (27) | 0.091 |
| ILD disease duration, median (IQR), years | 5.5 (3–9) | 6.0 (3–7) | 0.678 |
| Previous history of lung progression, n (%) | 11 (79) | 6 (20) | 0.001* |
| Ever smoking, n (%) | 8 (57) | 15 (50) | 0.752 |
| Concomitant DMARDs, n (%) | 7 (54) | 19 (66) | 0.322 |
| Corticosteroid dose, median (IQR), mg | 7.5 (1.9–10) | 0 (0–5) | 0.054 |
| Radiographic pattern of UIP, n (%) | 9 (64) | 8 (29) | 0.045* |
| CRP at first RTX infusion, median (IQR) | 24 (12–35) | 12 (1–41) | 0.348 |
| DLCO at first RTX infusion, median (IQR), % predicted | 42 (41–49) | 59 (54–64) | 0.031* |
Mann–Whitney U and Fisher’s exact tests were used appropriately to test for differences between groups. *P < 0.05, significant results.
Fig. 3.
Receiver operating characteristics curve and risk factors for ILD progression
(A) The best cut-off point for DLCO was 46% predicted, which demonstrated 67% sensitivity and 88% specificity for prediction of ILD progression post-RTX. Progression-free survival according to (B) the radiographic pattern of UIP, (C) a previous history of lung progression and (D) DLCO <46% predicted pre-RTX, all of which were associated with time to ILD progression post-RTX.
By Kaplan–Meier analysis, a radiographic pattern of UIP, a previous history of ILD progression and pre-RTX DLCO <46% predicted were associated with the time to ILD progression [P = 0.020 (Fig. 3B), P = 0.001 (Fig. 3C) and P = 0.001 (Fig. 3D), respectively]. Smoking and concomitant treatment with csDMARDs were not associated with time to ILD progression [P = 0.773 (supplementary Fig. S1A) and P = 0.260 (supplementary Fig. S1B), available at Rheumatology Online, respectively]. Details of other clinical risk factors evaluated for lung progression can be found in the supplementary data, available at Rheumatology Online.
There was no significant association between incomplete B cell depletion and ILD progression in C1 (P = 0.268). However, a high rate of incomplete depletion was observed in this cohort in C1 [25/38 (67%)]. Of those whose ILD progressed, 9/11 of the patients (B cell data available) had incomplete B cell depletion post-RTX in C1. Of 6/9 patients who were re-treated with RTX, depletion occurred in 2/6.
Factors associated with stabilization or improvement of ILD
Of those whose ILD improved, 3/7 patients had a radiologic pattern of non-specific interstitial pneumonia (NSIP) pre-RTX, 3/7 had UIP and 1 had cryptogenic organizing pneumonia. Of those with a radiologic pattern on NSIP pre-RTX (n = 33), patients whose ILD improved or was stable post-RTX had a lower median relative change in DLCO pre-RTX compared with those who progressed post-RTX (−3.8% vs −17.5%; P = 0.037). Baseline DLCO, ILD duration, concomitant therapies with csDMARDs and corticosteroid and previous treatment with CYC were not associated with stabilization/improvement of ILD post-RTX in this group of patients (all P > 0.10).
Safety
Seventy-eight SAEs were recorded in 33 patients: 63 were hospitalization (median duration 8.5 days) and 3 malignancies (supplementary Table S2, available at Rheumatology Online). Of the 12 deaths, 9 were due to progressive ILD with a median DLCO of 41% predicted pre-RTX. Other deaths were one lung cancer, one colorectal carcinoma and one infection post-surgery (Table 3). The median time from the last RTX infusion to death was 11.5 months (range 6–72).
Fifteen serious infections (7.7/100 patient-years) were recorded in 12 patients, mostly due to chest infection. Twenty percent (n = 3) and 60% (n = 9) of the infections occurred within 3 and 6 months, respectively, of the last RTX infusion. Five of 12 patients (42%) who had serious infections were also on concomitant therapy with corticosteroid during the cycle when the infection occurred. Details regarding the association of secondary hypogammaglobulinaemia related to RTX with serious infection can be found in the supplementary data, section Immunoglobulin and serious infection, and supplementary Table S2, available at Rheumatology Online.
Discussion
This is the largest observational study to date of patients with RA-ILD treated with RTX. The majority of patients with ILD (as assessed by PFTs, imaging and survival) remained stable or improved after treatment with RTX over a prolonged follow-up period.
Our data are important to ameliorate reporting bias from case reports or series [5, 6, 15, 16]. Data from the British Society of Rheumatology Registry found no increase in overall mortality using TNFi compared with csDMARDs, but a larger proportion of deaths in TNFi-treated patients were attributable to ILD [17]. This study might have been affected by a reporting bias for this event of particular interest, confounding regarding the severity of ILD pre-treatment in each treatment group and channelling away of patients from TNFI, as this was already the established practice during the collection of these data [18]. The last could lead to an increase in reports of adverse events from registry data on the use of RTX, owing to the high morbidity and mortality that are associated with ILD. This present study was also affected by the last issue. However, by reviewing records of every patient who received RTX in a large cohort to capture every ILD patient with long-term follow-up data in a systematic way, our data help to avoid reporting bias and the difficulties in interpretation that affect case reports and registry data. The data show that RTX appears to be generally safe.
It is worth noting that RTX was given primarily for articular symptoms in this study. Although only 16% of the patients had improvement in ILD after therapy, post-RTX HRCT was not routinely performed in all patients (if stable), which could reduce the calculated response rate. A high rate of incomplete B cell depletion as measured using highly sensitive flow cytometry was observed in C1. Only a third of those whose ILD progressed had complete B cell depletion when re-treated within 12 months. Thus this might suggest resistance with residual inflammation involving other organs despite response in articular symptoms. Despite the fact that the serial HRCT scans were only undertaken in selected patients with worsening dyspnoea and/or deterioration in PFTs, only half of those cases showed radiographic progression. Together with the finding that only three patients with stable pre-existing ILD progressed post-RTX, even without knowledge of HRCT progression in the whole cohort, it is reasonable to infer that clinically significant progression is uncommon with therapy.
About a third of the patients had progression of ILD post-RTX in this study. This rate was similar to the 34% published by Dawson et al. [19] for patients with pre-existing RA-ILD who were treated with csDMARDs over a 2 year follow-up period. The demographics, baseline PFTs and definition of lung progression were similar between these two cohorts. However, the present study population was of patients who had failed non-biologic DMARDs, with a worse prognosis for both joint and lung disease. In comparison with the use of RTX in other CTDs, the rate of ILD progression in this study was also similar to the 15% reported by Keir et al. [20]. However, in the latter, RTX was given as a rescue therapy for severe and progressive ILD with a median DLCO of 24.5% at RTX initiation.
With regards to mortality, 9/56 (16%) patients died from progressive ILD in this study. The survival rates at 3, 5 and 7 years (87, 84 and 84%, respectively) were similar to the data presented by the British Rheumatoid Interstitial Lung Network of patients with RA-ILD treated with RTX, with survival rates at 3, 5 and 7 years of 92, 82 and 82%, respectively [21]. Patients who deteriorated/died in this present study had severe and progressive ILD pre-RTX, limited reserve and limited treatment options, having already failed non-biologic DMARDs. Additionally, due to the length of time elapsed from the last RTX infusion before death, the drug was unlikely to be contributory.
We identified three baseline factors that were associated with ILD progression post-RTX treatment: a radiographic pattern of UIP, a previous history of lung progression and pre-RTX DLCO <46% predicted. The second concurred with other studies that patients with HRCT findings typical of UIP have a poorer prognosis than individuals with HRCT-detected features indicative of other types of interstitial pneumonia, including NSIP [22–24]. The last DLCO cut-off in this study is slightly higher than that of criterion referral for lung transplantation [25]. These added risks may prompt careful monitoring for lung function when initiating RTX. Patients with further decline in lung function post-RTX could be considered for other alternative treatments, including CYC [26, 27] and lung transplantation [28].
This study has several limitations. First, the PFTs were not undertaken in a standardized manner in all patients. As a result, the efficacy of RTX in ILD was likely to be underestimated, as the 12 patients with missing data for lung investigations (who were not observed to have clinical exacerbation of ILD during therapy) were excluded in the calculated overall lung response analysis. Next, concomitant therapy with csDMARDs was used in > 60% of patients, in line with the current licensed indication in RA, thus the effect on lung progression could not be attributed to RTX alone. Other limitations included variability in RTX re-treatment schedules and difficulty in the interpretation of HRCT due to various patterns seen in rheumatoid lungs. Although the interrater agreement for GGO was only fair, the discrepancy was resolved by consensus. Lastly, the lack of a control group made interpretation of the effect of RTX on the natural course of ILD difficult. In order to account for this, patients were used as their own controls (with pre- and post-treatment lung function trends) and provided convincing evidence of a real treatment effect attributable to RTX.
Although an efficacy signal was demonstrated in some patients with severe ILD pre-RTX, the fact that others continued to progress despite therapy argued against its therapeutic benefit, particularly those who needed treatment the most with respect to ILD. This observation suggests the importance of early diagnosis and treatment. Biomarkers for subclinical ILD are emerging [29, 30] and may help stratify patients for early therapy.
To conclude, in the absence of similar data on the effect of other non–B cell depletion therapy on the progression of RA-ILD, our findings offer reassurance that RTX appears to be an acceptable treatment choice for a group of patients with non-overlapping RA-ILD and severe arthritis who require a biologic. These data also support a definitive study of RTX for the management of RA-ILD from both an articular and a respiratory perspective.
Supplementary Material
Acknowledgements
The authors would like to thank clinicians at the Leeds Biological Monitoring Clinic, particularly Maya Buch and Sarah Bingham, and Sue Watts, Respiratory Physiologist at St James’ University Hospital, Leeds Teaching Hospitals NHS Trust for their substantial contributions to the acquisition of data. Dr Yusof is funded as a National Institute for Health Research (NIHR) Doctoral Research Fellow and Dr Vital is funded as an NIHR clinician scientist.
Funding: No specific funding was received from any funding bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article.
Disclosure statement: E.M.V. is an National Institute for Health Research clinician scientist and has received honoraria and research grant support from Roche and GSK. P.E. has received consultant fees from Bristol-Meyers Squibb, Abbott, Pfizer, MSD, Novartis, Roche and UCB and research grants paid to his employer from Abbott, Bristol-Meyers Squibb, Pfizer, MSD and Roche. S.D. has received honoraria from Roche and GSK. All other authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology Online.
References
- 1. Castelino FV, Varga J.. Interstitial lung disease in connective tissue diseases: evolving concepts of pathogenesis and management. Arthritis Res Ther 2010;12:213.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bongartz T, Nannini C, Medina-Velasquez YF. et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum 2010;62:1583–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jani M, Hirani N, Matteson EL, Dixon WG.. The safety of biologic therapies in RA-associated interstitial lung disease. Nat Rev Rheumatol 2014;10:284–94. [DOI] [PubMed] [Google Scholar]
- 4. Md Yusof MY, Vital EM, Buch MH.. B cell therapies, approved and emerging: a review of infectious risk and prevention during use. Curr Rheumatol Rep 2015;17:65. [DOI] [PubMed] [Google Scholar]
- 5. Perez-Alvarez R, Perez-de-Lis M, Diaz-Lagares C. et al. Interstitial lung disease induced or exacerbated by TNF-targeted therapies: analysis of 122 cases. Semin Arthritis Rheum 2011;41:256–64. [DOI] [PubMed] [Google Scholar]
- 6. Ostor AJ, Chilvers ER, Somerville MF. et al. Pulmonary complications of infliximab therapy in patients with rheumatoid arthritis. J Rheumatol 2006;33:622–8. [PubMed] [Google Scholar]
- 7. Atkins SR, Turesson C, Myers JL. et al. Morphologic and quantitative assessment of CD20+ B cell infiltrates in rheumatoid arthritis-associated nonspecific interstitial pneumonia and usual interstitial pneumonia. Arthritis Rheum 2006;54:635–41. [DOI] [PubMed] [Google Scholar]
- 8. Matteson EL, Bongartz T, Ryu JH. et al. Open-label, pilot study of the safety and clinical effects of rituximab in patients with rheumatoid arthritis-associated interstitial pneumonia. Open J Rheumatol Autoimmune Dis 2012;2:53. [Google Scholar]
- 9. Arnett FC, Edworthy SM, Bloch DA. et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. [DOI] [PubMed] [Google Scholar]
- 10. van Gestel AM, Prevoo ML, van’t Hof MA. et al. Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism Criteria. Arthritis Rheum 1996;39:34–40. [DOI] [PubMed] [Google Scholar]
- 11. Goldin JG, Lynch DA, Strollo DC. et al. High-resolution CT scan findings in patients with symptomatic scleroderma-related interstitial lung disease. Chest 2008;134:358–67. [DOI] [PubMed] [Google Scholar]
- 12. Raghu G, Collard HR, Egan JJ. et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dass S, Rawstron AC, Vital EM. et al. Highly sensitive B cell analysis predicts response to rituximab therapy in rheumatoid arthritis. Arthritis Rheum 2008;58:2993–9. [DOI] [PubMed] [Google Scholar]
- 14. Peduzzi P, Concato J, Feinstein AR, Holford TR.. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol 1995;48:1503–10. [DOI] [PubMed] [Google Scholar]
- 15. Bargagli E, Galeazzi M, Rottoli P.. Infliximab treatment in a patient with rheumatoid arthritis and pulmonary fibrosis. Eur Respir J 2004;24:708. [DOI] [PubMed] [Google Scholar]
- 16. Vassallo R, Matteson E, Thomas CF Jr.. Clinical response of rheumatoid arthritis-associated pulmonary fibrosis to tumor necrosis factor-alpha inhibition. Chest 2002;122:1093–6. [DOI] [PubMed] [Google Scholar]
- 17. Dixon WG, Hyrich KL, Watson KD. et al. Influence of anti-TNF therapy on mortality in patients with rheumatoid arthritis-associated interstitial lung disease: results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis 2010;69:1086–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dixon WG, Carmona L, Finckh A. et al. EULAR points to consider when establishing, analysing and reporting safety data of biologics registers in rheumatology. Ann Rheum Dis 2010;69:1596–602. [DOI] [PubMed] [Google Scholar]
- 19. Dawson JK, Fewins HE, Desmond J, Lynch MP, Graham DR.. Predictors of progression of HRCT diagnosed fibrosing alveolitis in patients with rheumatoid arthritis. Ann Rheum Dis 2002;61:517–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Keir GJ, Maher TM, Ming D. et al. Rituximab in severe, treatment-refractory interstitial lung disease. Respirology 2014;19:353–9. [DOI] [PubMed] [Google Scholar]
- 21. Iqbal K, Carty S, Dawson J, Woodhead F, Young A, Kelly C.. Survival in rheumatoid lung disease is longer in patients treated with rituximab than in those receiving anti-tumour necrosis factor therapy. Rheumatology 2016;55(Suppl 1):i86–7. [Google Scholar]
- 22. Kim EJ, Elicker BM, Maldonado F. et al. Usual interstitial pneumonia in rheumatoid arthritis-associated interstitial lung disease. Eur Respir J 2010;35:1322–8. [DOI] [PubMed] [Google Scholar]
- 23. Kelly CA, Saravanan V, Nisar M. et al. Rheumatoid arthritis-related interstitial lung disease: associations, prognostic factors and physiological and radiological characteristics—a large multicentre UK study. Rheumatology 2014;53:1676–82. [DOI] [PubMed] [Google Scholar]
- 24. Zamora-Legoff JA, Krause ML, Crowson CS, Ryu JH, Matteson EL.. Progressive decline of lung function in rheumatoid arthritis associated interstitial lung disease. Arthritis Rheumatol 2017;69:542–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Orens JB, Estenne M, Arcasoy S. et al. International guidelines for the selection of lung transplant candidates: 2006 update–a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2006;25:745–55. [DOI] [PubMed] [Google Scholar]
- 26. Hoyles RK, Ellis RW, Wellsbury J. et al. A multicenter, prospective, randomized, double-blind, placebo-controlled trial of corticosteroids and intravenous cyclophosphamide followed by oral azathioprine for the treatment of pulmonary fibrosis in scleroderma. Arthritis Rheum 2006;54:3962–70. [DOI] [PubMed] [Google Scholar]
- 27. Kelly C, Palmer E, Gordon J, Woodhead F, Nisar M, Arthanari S. et al. OP0037 pulsed cyclophosphamide in the treatment of rheumatoid arthritis-related interstitial lung disease (RA-ILD). Ann Rheum Dis 2014;73(Suppl 2):74. [Google Scholar]
- 28. Yazdani A, Singer LG, Strand V. et al. Survival and quality of life in rheumatoid arthritis-associated interstitial lung disease after lung transplantation. J Heart Lung Transplant 2014;33:514–20. [DOI] [PubMed] [Google Scholar]
- 29. Doyle TJ, Patel AS, Hatabu H. et al. Detection of rheumatoid arthritis-interstitial lung disease is enhanced by serum biomarkers. Am J Respir Crit Care Med 2015;191:1403–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen J, Doyle TJ, Liu Y. et al. Biomarkers of rheumatoid arthritis-associated interstitial lung disease. Arthritis Rheumatol 2015;67:28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.