Abstract
STUDY QUESTION
Does ambient air pollution affect fecundability?
SUMMARY ANSWER
While cycle-average air pollution exposure was not associated with fecundability, we observed some associations for acute exposure around ovulation and implantation with fecundability.
WHAT IS KNOWN ALREADY
Ambient air pollution exposure has been associated with adverse pregnancy outcomes and decrements in semen quality.
STUDY DESIGN, SIZE, DURATION
The LIFE study (2005–2009), a prospective time-to-pregnancy study, enrolled 501 couples who were followed for up to one year of attempting pregnancy.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Average air pollutant exposure was assessed for the menstrual cycle before and during the proliferative phase of each observed cycle (n = 500 couples; n = 2360 cycles) and daily acute exposure was assessed for sensitive windows of each observed cycle (n = 440 couples; n = 1897 cycles). Discrete-time survival analysis modeled the association between fecundability and an interquartile range increase in each pollutant, adjusting for co-pollutants, site, age, race/ethnicity, parity, body mass index, smoking, income and education.
MAIN RESULTS AND THE ROLE OF CHANCE
Cycle-average air pollutant exposure was not associated with fecundability. In acute models, fecundability was diminished with exposure to ozone the day before ovulation and nitrogen oxides 8 days post ovulation (fecundability odds ratio [FOR] 0.83, 95% confidence interval [CI]: 0.72, 0.96 and FOR 0.84, 95% CI: 0.71, 0.99, respectively). However, particulate matter ≤10 microns 6 days post ovulation was associated with greater fecundability (FOR 1.25, 95% CI: 1.01, 1.54).
LIMITATIONS, REASONS FOR CAUTION
Although our study was unlikely to be biased due to confounding, misclassification of air pollution exposure and the moderate study size may have limited our ability to detect an association between ambient air pollution and fecundability.
WIDER IMPLICATIONS OF THE FINDINGS
While no associations were observed for cycle-average ambient air pollution exposure, consistent with past research in the United States, exposure during critical windows of hormonal variability was associated with prospectively measured couple fecundability, warranting further investigation.
STUDY FUNDING/COMPETING INTEREST(S)
This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (Longitudinal Investigation of Fertility and the Environment study contract nos. #N01-HD-3-3355, NO1-HD-#-3356, N01-HD-3-3358 and the Air Quality and Reproductive Health Study Contract No. HHSN275200800002I, Task Order No. HHSN27500008). We declare no conflict of interest.
Keywords: air pollution, fecundability, time-to-pregnancy study, environmental risks, menstrual cycle
Introduction
Air pollution is a major environmental risk factor for poor health, and is robustly associated with cardiovascular morbidity and mortality (Langrish et al., 2012; Shah et al., 2015). Air pollution has also been associated with human reproductive and perinatal outcomes, including increased risks of stillbirth (Siddika et al., 2016), preterm birth (Stieb et al., 2012) and low birthweight (Pedersen et al., 2013). Fecundability is the probability per menstrual cycle that a couple engaging in unprotected sexual intercourse will conceive (Weinstein and Stark, 1994), and represents an underlying biological capacity for reproduction. Although environmental pollutants are ubiquitous, the effects of environmental pollutants on fecundability are little understood. Several environmental pollutants have demonstrated some suggestion of association with couple fecundability, including polychlorinated biphenyls and organochlorine pesticides (Buck Louis, 2014).
Air pollution may affect reproduction through increases in inflammation and oxidative stress (Chin, 2015), leading to disruption of the endocrine system, reduction in semen quality and changes in the uterine milieu (Checa Vizcaino et al., 2016). Animal studies have demonstrated an association between pregestational air pollution exposure and longer estrus, lower ovarian reserve and decreased fertility in mice (Veras et al., 2009). However, human studies of the association between air pollution and fecundability have been sparse and inconclusive (Frutos et al., 2015; Checa Vizcaino et al., 2016). Of four studies investigating the association between air pollution and fecundability in the general population, three suggested an adverse association between several criteria air pollutants and fecundability, although they were limited by reliance on stationary air monitoring, which does not account for local variation in air pollution, leading to exposure misclassification (Dejmek et al., 2000; Nieuwenhuijsen et al., 2014; Mahalingaiah et al., 2016; Slama et al., 2013). Furthermore, they employed either retrospective or ecological designs. Prior research by our team has shown an association between distance to roadway and fecundability, with each 200 meter greater distance from roadway associated with a 3% greater menstrual cycle-specific odds of pregnancy (95% CI 1.01, 1.06). These findings suggest traffic-related air pollution may be associated with fecundability, although other environmental and individual-level factors that may be associated with distance to roadway, such as exposure to ambient noise, need to be taken into consideration (Mendola et al., 2016).
As air pollution may influence fecundability through several mechanisms, there may be multiple windows of exposure associated with critical stages of hormonal variability. These include exposure during prior menstrual cycles in relation to hormonal changes and later growth of the endometrium, exposure during the proliferative phase in relation to ovulation (Mlynarcikova et al., 2009), and exposure during the secretory phase in relation to implantation (Gellersen et al., 2007). Prior studies have not evaluated these finer windows of exposure in relation to the menstrual cycle, and have instead evaluated either long-term chronic exposure or cycle-averaged windows of exposure based on retrospective self-report (Dejmek et al., 2000; Nieuwenhuijsen et al., 2014; Mahalingaiah et al., 2016; Slama et al., 2013).
To address the limitations of prior studies, we evaluated the association of both time-varying cycle average and acute exposure to air pollution with fecundability in a prospective time-to-pregnancy cohort study. We hypothesized that higher exposure to ambient air pollutants in the cycle prior to and the proliferative phase during an observed cycle, as well as during sensitive acute windows of an observed cycle, would be associated with diminished fecundability.
Materials and Methods
The Longitudinal Investigation of Fertility and the Environment (LIFE) Study was conducted between 2005–2009 among 501 couples in Michigan (n = 104) and Texas (n = 397) with presumed exposure to persistent organic pollutants, as fully described elsewhere (Buck Louis et al., 2011). Couples were eligible to participate if: (i) they were married or in a committed relationship, (ii) female partners were aged 18–40 and male partners 18+ years, (iii) they were able to communicate in English or Spanish, (iv) they were not off contraception for >2 menstrual cycles prior to enrollment, (v) they did not have physician diagnosed infertility and (vi) female partners had menstrual cycles between 21 and 42 days and no contraceptive hormonal injections in the previous 12 months. During the baseline visit, female partners were instructed in the use of digital home pregnancy tests (Clearblue® Easy, Clearblue, Geneva, Switzerland), with demonstrated sensitivity for detecting ≥25 mIU/mL of human chorionic gonadotropin (Cole et al., 2004), and a fertility monitor (Clearblue® Easy, Clearblue, Geneva, Switzerland), demonstrated to detect ovulation in 91% of women undergoing the gold standard of vaginal ultrasonography (Behre et al., 2000). All women had a pregnancy test at baseline to ensure they were not already pregnant. Couples were followed until pregnancy or up to one year of actively trying to become pregnant. This study was approved by the institutional review boards for all collaborating institutions, and couples provided written informed consent.
Time-to-pregnancy
Menstrual cycles were first defined by applying an algorithm to distinguish menstrual bleeding from episodic bleeding, which required a bleeding duration of 2+ days with increasing intensity (Buck Louis et al., 2014). Menstrual cycle length was then calculated as beginning on the first day of menses to the last day before onset of bleeding in the next cycle. Date of ovulation for each observed cycle was determined by the fertility monitor which separated the proliferative phase of the menstrual cycle (first day of menses to ovulation) from the secretory phase (ovulation to start of menses in the next cycle). Couples could have up to two unobserved cycles of attempting pregnancy prior to enrollment, and for most couples the enrollment cycle was not fully observed and the ovulation date not assessed. Fecundability was assessed as number of self-reported and observed menstrual cycles required for a couple to achieve pregnancy or to be censored. Of those not achieving pregnancy, 53 were censored after 12 months of follow-up and 100 exited the study before 12 months. Couples contributed a total of 2360 observed cycles for the time-varying cycle-average analysis and 1897 for the acute analysis.
Ambient air pollution
Mean hourly levels of the criteria air pollutants (sulfur dioxide [SO2], nitrogen oxides [NOX], nitrogen dioxide [NO2], carbon monoxide [CO], ozone [O3], particulate matter <10 microns [PM10] and fine particulate matter <2.5 microns [PM2.5]) and five constituents of particulate matter (elemental carbon, organic compounds, sulfate, ammonium and nitrate) were estimated at a resolution of 12 × 12 km2 grid cells using modified Community Multiscale Air Quality (CMAQ) models. Air pollutant levels were estimated based on emissions inputs from the United States Environmental Protection Agency 2005 National Emissions Inventory and meteorological inputs generated by the Weather Research and Forecasting model. The raw CMAQ estimates were fused with monitor data from the United States Environmental Protection Agency Air Quality System using inverse distance weighting. The performance of this model was similar to our previously reported results (Chen et al., 2014).
To estimate residential exposure to ambient air pollution, each couple’s residential address was geocoded using ArcGIS software (Redlands, CA). Mean daily exposure to the criteria air pollutants and constituents were calculated for each couple for all days in which they were enrolled in the study and for 30–60 days prior to enrollment (for couples attempting pregnancy for 0–1 months prior to enrollment [409, 81.8%] and for 2 months prior to enrollment [91, 18.2%], respectively). One couple’s address could not be geocoded, leaving 500 couples available for analyses.
Time-varying cycle-average windows of exposure
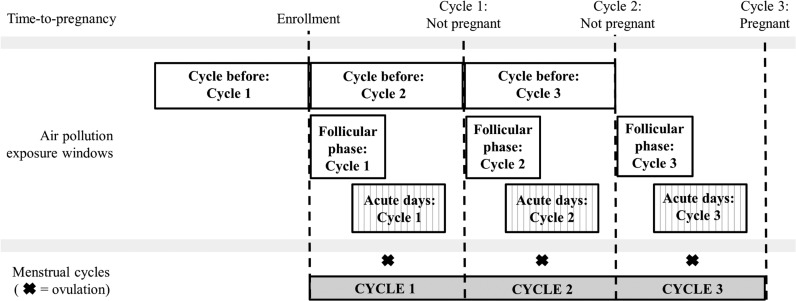
For time-varying cycle-average exposure windows, two periods of exposure were assessed: (i) mean air pollution exposure during the cycle prior to the observed cycle, and (ii) mean air pollution exposure during the proliferative phase (Days 1–10) of the observed cycle (Fig. 1). These windows are comparable to those investigated in prior research, which has focused on either chronic or cycle-average windows of exposure.
Figure 1.
Time-varying cycle average and acute air pollution exposure windows for a hypothetical couple followed for three menstrual cycles after enrollment until pregnancy in the LIFE Study (2005–2009). Time-varying cycle-average windows include the cycle before and the proliferative phase during an observed cycle. Acute days range from 5 days before to 10 days following ovulation during an observed cycle.
Acute windows of exposure
For acute windows of exposure, mean daily air pollution exposure was calculated for sensitive windows of hormonal variability of each observed cycle (range: 5 days before to 10 days following ovulation). These windows encompass potential short-term effects of air pollution on folliculogenesis, formation of the blastocyst and decidualization of the endometrium, which may be masked in longer-term averaged exposure windows (Wilcox et al., 1999).
Covariates
At the baseline visit, information on female partner age (continuous), parity conditional on gravidity (nulligravous, gravous/nulliparous and parous), race/ethnicity (Latino, non-Latino white, non-Latino black and other race/ethnicity), household income (<$40 000, $40 000–<$70 000, $70 000– <$100 000 and ≥$100 000) and education (≤high school, some college and ≥college graduate) was collected through self-report. Anthropometric measurements included measured height and weight using standardized equipment for the estimation of body mass index (BMI; <25 kg/m2, 25–<30 kg/m2 and ≥30 kg/m2). Serum cotinine concentration was used to define active cigarette smoking status (≥40.35 vs. <40.35 ng/mL) (Benowitz et al., 2009). Study site was evaluated as Michigan vs. Texas.
Statistical analysis
A total of 98 (4.2%) cycles were imputed due to implausible length of the entry or last cycle of observation with no hCG pregnancy detection given a woman’s other cycle lengths. Additionally, 46 couples were missing information on ovulation date (n = 61; 3.2% of cycles), 3 on parity, 1 on BMI, 12 on cotinine, 3 on race/ethnicity, 4 on education and 10 on household income. To impute plausible values for extreme cycle lengths and missing information on ovulation and covariates, multiple imputation using the fully conditional specification method for non-monotone missing data with predictive mean matching was implemented to generate 20 imputed datasets.
Descriptive statistics were summarized as counts and percentages for categorical variables or as means and standard deviations for continuous variables, with the exception of air pollutants, which were summarized using the median and interquartile range. Differences in distribution of variables across groups were assessed by Student’s t-test or the Wilcoxon rank sum test for continuous variables, and by Pearson’s chi-square or Fisher’s exact test for categorical variables. To assess correlations across air pollutants, Pearson product-moment correlation coefficients were calculated for mean cycle pollutant levels (first observed cycle) and mean daily pollutant levels (5 days prior to ovulation in the first observed cycle).
Discrete-time survival analysis was used to model the association between ambient air pollutant levels and fecundability. The effect estimate for the discrete-time survival model is the fecundability odds ratio (FOR). An FOR > 1 indicates a shorter time-to-pregnancy, while an FOR < 1 indicates a longer time-to-pregnancy. Survival models accounted for left truncation or time off contraception before enrollment, and censoring was assumed to be non-informative since we are unaware of empirical evidence suggesting an association with air pollution.
Air pollutant levels were standardized by modeling change in interquartile range for each pollutant and the model updated the cycle-specific exposure for each observed cycle. We used multipollutant models in our primary analyses but also evaluated single-pollutant models. For the criteria air pollutants, multipollutant models mutually adjusted for SO2, O3, NOX, CO, PM10 and PM2.5. For particulate constituents, multipollutant models adjusted for each particulate constituent and PM2.5. We a priori chose to adjust for site (Michigan vs. Texas), due to the association of site with fecundability in prior analyses (Mendola et al., 2016), as well as maternal age, race/ethnicity, BMI, parity, smoking status, household income and education, due to their association with fecundability and inclusion in prior studies (Dejmek et al., 2000; Mahalingaiah et al., 2016; Slama et al., 2013).
To address potential unmeasured area-level confounding, such as by neighborhood socioeconomic status, a secondary analysis simulated a confounder negatively correlated with PM10 at 6 days post ovulation (r = −0.1 to −0.9) and with a strength of association with fecundability 2-, 3- and 4-times the association of PM10 and fecundability. Each of the 27 confounding scenarios was simulated 1000 times. Additionally, to assess whether selection bias due to the exclusion of the 60 couples who became pregnant or withdrew after the entry cycle in the acute analyses may have influenced results, we conducted a secondary analysis setting ovulation date for the entry cycle to 14 days prior to either the start of the next menstrual cycle or positive pregnancy test (463 entry cycles). Analyses were completed in SAS version 9.4 (Cary, NC) and figures and simulations in R 3.3.1 (Vienna, Austria).
Results
Table I shows descriptive statistics for the analytic cohorts for time-varying cycle-average air pollution windows (n = 500) and acute daily windows (n = 440). For both samples, the mean age of female participants was 30 years. Most women were non-Hispanic white and were college graduates. Only 11% of households reported an income less than $40 000 per year, while 34% reported an income over $100 000 per year. Observed air pollution levels were low to moderate (Table II). While most criteria air pollutants were strongly positively correlated, O3 was negatively correlated with NOX, NO2, CO and PM10 (Supplementary Table SI). A total of 347 couples achieved pregnancy (69.4%). Participants who withdrew from the study did not substantially differ from those who completed the study, except they were more likely to have a lower educational attainment and lower household income (Buck Louis et al., 2011).
Table I.
Female partner characteristics by analytic cohort: the LIFE Study (2005–2009).
| Analytic cohort: time-varying cycle average (n = 500)a | Analytic cohort: acute analyses (n = 440)b | |||
|---|---|---|---|---|
| Mean (SD) | n (%) | Mean (SD) | n (%) | |
| Age (years) | 30.0 (4.1) | 30.1 (4.2) | ||
| Parity and gravidity | ||||
| Nulligravous | 209 (42.1) | 190 (43.5) | ||
| Gravous, nulliparous | 53 (10.7) | 42 (9.6) | ||
| Parous | 235 (47.3) | 205 (46.9) | ||
| Body mass index (kg/m2) | 27.6 (7.3) | 27.6 (7.4) | ||
| Race/ethnicity | ||||
| Non-Hispanic white | 392 (78.9) | 347 (79.2) | ||
| Non-Hispanic black | 24 (4.8) | 19 (4.3) | ||
| Hispanic | 50 (10.1) | 45 (10.3) | ||
| Other | 31 (6.2) | 27 (6.2) | ||
| Income | ||||
| <$40 000 | 55 (11.2) | 49 (11.4) | ||
| $40–<$70 000 | 102 (20.8) | 94 (21.8) | ||
| $70–<$100 000 | 166 (33.9) | 142 (33.0) | ||
| $100 000 + | 167 (34.1) | 146 (33.9) | ||
| Highest level education | ||||
| High school or lower | 27 (5.4) | 26 (6.0) | ||
| Some college | 93 (18.8) | 79 (18.1) | ||
| College graduate | 376 (75.8) | 332 (76.0) | ||
| Study site | ||||
| Michigan | 104 (20.8) | 88 (20.0) | ||
| Texas | 396 (79.2) | 352 (80.0) | ||
| Couple achieved pregnancy | ||||
| Yes | 347 (69.4) | 302 (68.6) | ||
| No | 153 (30.6) | 138 (31.4) | ||
| Number of cycles attempting pregnancy | 5.2 (3.7) | 5.7 (3.7) | ||
aExcludes one participant who could not be geocoded.
bAdditionally excludes 60 participants who achieved pregnancy or were censored at the end of the entry cycle (ovulation date not assessed).
cT-test (age, number of cycles attempting pregnancy) or Wilcoxon rank sum (gravidity, parity and body mass index) for continuous variables; Pearson’s chi-square (income, education and couple achieving pregnancy) or Fisher’s exact test (race/ethnicity) for categorical variables.
Table II.
Distribution of ambient air pollutant levels; the LIFE Study (2005–2009).
| Unit | IQR | Min | 25% | Median | 75% | Max | |
|---|---|---|---|---|---|---|---|
| Cycle average for first observed cycle (n = 500)a | |||||||
| Criteria pollutants | |||||||
| Sulfur dioxide (SO2) | ppb | 0.98 | 0.082 | 0.96 | 1.37 | 1.94 | 6.22 |
| Ozone (O3) | ppb | 9.14 | 9.97 | 23.54 | 27.85 | 32.68 | 40.54 |
| Nitrogen oxides (NOX) | ppb | 9.20 | 0.63 | 7.53 | 10.83 | 16.73 | 51.31 |
| Nitrogen dioxide (NO2) | ppb | 4.97 | 0.56 | 4.98 | 7.03 | 9.95 | 24.98 |
| Carbon monoxide (CO) | ppb | 123.70 | 75.8 | 184.0 | 250.0 | 307.7 | 500.6 |
| Particulate matter (PM10) | μg/m3 | 12.26 | 5.68 | 16.01 | 23.91 | 28.27 | 40.45 |
| Fine particular matter (PM2.5) | μg/m3 | 3.19 | 5.42 | 10.15 | 11.82 | 13.34 | 19.18 |
| Particulate constituents | |||||||
| Elemental carbon (AEC) | μg/m3 | 0.31 | 0.004 | 0.12 | 0.26 | 0.42 | 1.26 |
| Ammonium (ANH4) | μg/m3 | 0.47 | 0.38 | 0.95 | 1.16 | 1.42 | 2.53 |
| Nitrate (ANO3) | μg/m3 | 0.69 | 0.001 | 0.54 | 0.77 | 1.23 | 5.62 |
| Organic compounds (AOC) | μg/m3 | 1.10 | 0.38 | 1.44 | 1.86 | 2.54 | 6.18 |
| Sulfate (ASO4) | μg/m3 | 1.29 | 1.25 | 2.44 | 2.99 | 3.73 | 5.75 |
| Day average 5 days prior to ovulation in first observed cycle (n = 440)b | |||||||
| Criteria pollutants | |||||||
| Sulfur dioxide (SO2) | ppb | 1.47 | 0 | 0.50 | 1.05 | 1.97 | 11.80 |
| Ozone (O3) | ppb | 14.40 | 3.34 | 19.80 | 26.78 | 34.20 | 66.09 |
| Nitrogen oxides (NOX) | ppb | 10.49 | 0.19 | 5.12 | 9.24 | 15.61 | 171.02 |
| Nitrogen dioxide (NO2) | ppb | 6.62 | 0.11 | 3.73 | 6.64 | 10.35 | 47.50 |
| Carbon monoxide (CO) | ppb | 133.76 | 51.6 | 157.1 | 218.7 | 290.9 | 1250.3 |
| Particular matter (PM10) | μg/m3 | 16.57 | 0.29 | 12.76 | 21.43 | 29.33 | 64.53 |
| Fine particulate matter (PM2.5) | μg/m3 | 6.89 | 0.56 | 8.00 | 10.88 | 14.89 | 32.00 |
| Particulate constituents | |||||||
| Elemental carbon (AEC) | μg/m3 | 0.36 | 0 | 0.05 | 0.21 | 0.41 | 2.23 |
| Ammonium (ANH4) | μg/m3 | 0.92 | 0 | 0.64 | 0.99 | 1.56 | 5.54 |
| Nitrate (ANO3) | μg/m3 | 0.72 | 0 | 0.36 | 0.58 | 1.08 | 9.26 |
| Organic compounds (AOC) | μg/m3 | 1.63 | 0.01 | 0.98 | 1.71 | 2.61 | 6.99 |
| Sulfate (ASO4) | μg/m3 | 2.08 | 0 | 1.96 | 2.85 | 4.04 | 10.97 |
aExcludes one participant who could not be geocoded.
bAdditionally excludes 60 participants who achieved pregnancy or were censored at the end of the entry cycle (ovulation date not assessed).
Tables III and IV present multipollutant model findings. For time-varying cycle-specific air pollutant exposure during the cycle prior to or the follicular phase during the observed cycle, no significant associations were found between mean ambient air pollutant level and fecundability for either criteria air pollutants or particulate constituents. Results were similar in single-pollutant models (data not shown) and unadjusted models (Supplementary Tables SII and SIII). However, for the proliferative phase, we did observe a general trend of increased O3 and PM2.5 with lower fecundability (FOR 0.91, 95% CI 0.75, 1.10 and FOR 0.92, 95% CI 0.78, 1.10) and increased CO and PM10 with higher fecundability (FOR 1.19, 95% CI 0.91, 1.56 and FOR 1.13, 95% CI 0.89, 1.43), although estimates were imprecise.
Table III.
Association of an interquartile increase in criteria air pollutant level and fecundability, multipollutant model, adjusteda; the LIFE Study (2005–2009).
| SO2 | O3 | NOX | CO | PM10 | PM2.5 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FOR | 95% CI | FOR | 95% CI | FOR | 95% CI | FOR | 95% CI | FOR | 95% CI | FOR | 95% CI | |
| Time-varying cycle averages (n = 500)b | ||||||||||||
| Prior cycle | 0.95 | 0.80, 1.13 | 0.94 | 0.75, 1.19 | 1.13 | 0.89, 1.43 | 0.89 | 0.62, 1.29 | 1.05 | 0.78, 1.42 | 1.02 | 0.80, 1.28 |
| Proliferative phase | 1.00 | 0.87, 1.16 | 0.91 | 0.75, 1.10 | 0.95 | 0.78, 1.15 | 1.19 | 0.91, 1.56 | 1.13 | 0.89, 1.43 | 0.92 | 0.78, 1.10 |
| Days from ovulation (n = 440)c | ||||||||||||
| −5 | 0.97 | 0.88, 1.08 | 0.87 | 0.76, 0.99 | 0.93 | 0.81, 1.08 | 1.23 | 0.99, 1.54 | 1.04 | 0.85, 1.28 | 1.00 | 0.88, 1.13 |
| −4 | 0.97 | 0.87, 1.08 | 0.97 | 0.84, 1.11 | 0.99 | 0.84, 1.16 | 1.11 | 0.88, 1.40 | 0.99 | 0.81, 1.22 | 1.02 | 0.90, 1.16 |
| −3 | 1.02 | 0.93, 1.12 | 1.00 | 0.87, 1.14 | 0.91 | 0.78, 1.07 | 1.07 | 0.86, 1.34 | 1.06 | 0.87, 1.29 | 1.02 | 0.90, 1.16 |
| −2 | 1.02 | 0.92, 1.12 | 0.90 | 0.78, 1.04 | 0.91 | 0.78, 1.06 | 1.09 | 0.88, 1.34 | 1.06 | 0.86, 1.29 | 1.03 | 0.91, 1.17 |
| −1 | 1.02 | 0.93, 1.12 | 0.83 | 0.72, 0.96 | 0.89 | 0.77, 1.04 | 1.21 | 1.00, 1.48 | 1.11 | 0.90, 1.37 | 0.97 | 0.85, 1.11 |
| 0 | 1.09 | 0.99, 1.19 | 0.87 | 0.76, 1.00 | 1.00 | 0.87, 1.15 | 1.04 | 0.84, 1.28 | 1.15 | 0.93, 1.42 | 0.91 | 0.80, 1.04 |
| 1 | 1.03 | 0.94, 1.14 | 0.96 | 0.83, 1.10 | 0.98 | 0.85, 1.14 | 1.01 | 0.81, 1.25 | 1.20 | 0.98, 1.46 | 0.95 | 0.84, 1.08 |
| 2 | 1.00 | 0.90, 1.10 | 0.91 | 0.79, 1.04 | 1.06 | 0.90, 1.23 | 0.90 | 0.71, 1.14 | 1.11 | 0.91, 1.35 | 1.02 | 0.91, 1.15 |
| 3 | 1.00 | 0.90, 1.10 | 0.91 | 0.79, 1.04 | 1.03 | 0.88, 1.20 | 0.89 | 0.71, 1.10 | 1.09 | 0.90, 1.32 | 0.98 | 0.86, 1.10 |
| 4 | 1.06 | 0.96, 1.18 | 0.94 | 0.82, 1.08 | 1.11 | 0.96, 1.28 | 0.85 | 0.67, 1.07 | 1.16 | 0.95, 1.41 | 0.97 | 0.86, 1.10 |
| 5 | 0.95 | 0.86, 1.04 | 0.96 | 0.84, 1.10 | 1.04 | 0.90, 1.21 | 0.98 | 0.78, 1.24 | 1.17 | 0.95, 1.44 | 0.99 | 0.87, 1.13 |
| 6 | 1.04 | 0.95, 1.15 | 1.01 | 0.88, 1.15 | 1.01 | 0.88, 1.17 | 1.09 | 0.88, 1.36 | 1.25 | 1.01, 1.54 | 0.94 | 0.83, 1.08 |
| 7 | 1.05 | 0.95, 1.15 | 0.92 | 0.80, 1.05 | 0.92 | 0.79, 1.08 | 1.03 | 0.83, 1.28 | 1.20 | 0.98, 1.48 | 0.95 | 0.84, 1.08 |
| 8 | 1.02 | 0.94, 1.11 | 0.90 | 0.78, 1.03 | 0.84 | 0.71, 0.99 | 1.07 | 0.86, 1.34 | 1.18 | 0.97, 1.43 | 0.95 | 0.84, 1.08 |
| 9 | 0.96 | 0.86, 1.06 | 0.93 | 0.80, 1.06 | 0.90 | 0.77, 1.05 | 1.10 | 0.89, 1.37 | 1.09 | 0.89, 1.33 | 0.93 | 0.81, 1.06 |
| 10 | 1.01 | 0.91, 1.12 | 0.99 | 0.86, 1.14 | 0.99 | 0.84, 1.16 | 1.06 | 0.85, 1.31 | 1.13 | 0.92, 1.38 | 0.92 | 0.80, 1.04 |
SO2, sulfur dioxide; O3, ozone; NOX, nitrogen oxides; NO2, nitrogen dioxide; CO, carbon monoxide; PM10, particulate matter <10 μg/m3; PM2.5, fine particulate matter <2.5 μg/m3; FOR, fecundability odds ratio. Bold text indicates p < 0.05.
aAdjusted for multiple pollutants (SO2, O3, NOX, CO, PM10 and PM2.5), site (Michigan vs. Texas), maternal age (years), race/ethnicity (Latino, non-Latino white, non-Latino black or other race/ethnicity), body mass index (kg/m2), parity conditional on gravidity (nulligravous, gravous/nulliparous and parous), education (high school or less, some college and college graduate or greater), household income (<$40 000, $40– < $70 000, $70 000– < $100 000 and ≥$100 000) and smoking status (serum cotinine ≥40.35 vs. <40.35 ng/mL).
bMean air pollution level during the cycle prior to the observed cycle (prior cycle) and the first 10 days of the observed cycle (proliferative phase).
cMean daily air pollution level from 5 days before to 10 days following ovulation in the observed cycle. Excludes 60 participants who achieved pregnancy or were censored at the end of the entry cycle (ovulation date not assessed).
Table IV.
Association of an interquartile increase in constituents of particulate matter and fecundability, multipollutant model, adjusteda; the LIFE Study (2005–2009).
| AEC | ANH4 | ANO3 | AOC | ASO4 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| FOR | 95% CI | FOR | 95% CI | FOR | 95% CI | FOR | 95% CI | FOR | 95% CI | |
| Time-varying cycle averages (n = 500)b | ||||||||||
| Prior cycle | 0.91 | 0.74, 1.13 | 1.07 | 0.89, 1.29 | 1.04 | 0.92, 1.17 | 0.99 | 0.86, 1.15 | 1.01 | 0.82, 1.24 |
| Proliferative phase | 1.00 | 0.83, 1.20 | 1.03 | 0.90, 1.18 | 1.00 | 0.89, 1.11 | 1.00 | 0.87, 1.14 | 1.07 | 0.92, 1.26 |
| Days from ovulation (n = 440)c | ||||||||||
| −5 | 1.02 | 0.88, 1.20 | 1.06 | 0.96, 1.17 | 1.05 | 0.97, 1.14 | 1.02 | 0.91, 1.14 | 1.05 | 0.94, 1.18 |
| −4 | 0.99 | 0.84, 1.17 | 1.06 | 0.96, 1.16 | 1.01 | 0.93, 1.10 | 0.98 | 0.88, 1.10 | 1.09 | 0.98, 1.23 |
| −3 | 0.93 | 0.78, 1.10 | 1.10 | 1.00, 1.21 | 1.02 | 0.94, 1.11 | 0.99 | 0.88, 1.11 | 1.12 | 1.01, 1.25 |
| −2 | 0.92 | 0.78, 1.08 | 1.10 | 1.00, 1.22 | 1.05 | 0.97, 1.14 | 0.96 | 0.86, 1.08 | 1.11 | 0.99, 1.23 |
| −1 | 0.96 | 0.81, 1.14 | 1.02 | 0.92, 1.12 | 1.04 | 0.96, 1.13 | 1.04 | 0.92, 1.16 | 1.01 | 0.90, 1.13 |
| 0 | 1.00 | 0.85, 1.19 | 0.95 | 0.85, 1.05 | 1.01 | 0.92, 1.10 | 1.08 | 0.97, 1.22 | 0.93 | 0.83, 1.05 |
| 1 | 0.94 | 0.79, 1.13 | 1.00 | 0.90, 1.12 | 1.06 | 0.98, 1.15 | 1.07 | 0.95, 1.20 | 0.96 | 0.86, 1.09 |
| 2 | 0.94 | 0.79, 1.12 | 1.02 | 0.92, 1.13 | 1.07 | 0.99, 1.15 | 1.00 | 0.89, 1.12 | 0.98 | 0.88, 1.10 |
| 3 | 0.95 | 0.80, 1.13 | 1.06 | 0.96, 1.18 | 1.04 | 0.96, 1.13 | 0.99 | 0.88, 1.11 | 1.05 | 0.94, 1.18 |
| 4 | 1.06 | 0.90, 1.25 | 1.00 | 0.90, 1.11 | 1.02 | 0.93, 1.11 | 1.04 | 0.92, 1.17 | 0.98 | 0.87, 1.10 |
| 5 | 1.04 | 0.90, 1.25 | 1.05 | 0.95, 1.16 | 1.06 | 0.97, 1.15 | 1.01 | 0.90, 1.14 | 1.02 | 0.90, 1.15 |
| 6 | 1.04 | 0.89, 1.21 | 1.08 | 0.98, 1.19 | 1.06 | 0.97, 1.15 | 1.00 | 0.89, 1.13 | 1.06 | 0.94, 1.19 |
| 7 | 0.93 | 0.78, 1.10 | 1.07 | 0.97, 1.19 | 0.98 | 0.90, 1.07 | 0.98 | 0.87, 1.10 | 1.12 | 0.99, 1.26 |
| 8 | 0.94 | 0.78, 1.12 | 1.01 | 0.91, 1.12 | 0.92 | 0.84, 1.02 | 1.02 | 0.91, 1.15 | 1.07 | 0.95, 1.20 |
| 9 | 0.96 | 0.81, 1.15 | 1.02 | 0.92, 1.13 | 0.97 | 0.88, 1.06 | 1.01 | 0.90, 1.14 | 1.05 | 0.93, 1.18 |
| 10 | 0.96 | 0.80, 1.14 | 1.00 | 0.90, 1.11 | 0.98 | 0.89, 1.07 | 0.98 | 0.87, 1.11 | 1.04 | 0.92, 1.17 |
AEC, elemental carbon; ANH4, ammonium; ANO3, nitrate; AOC, organic compounds; ASO4, sulfate. Bold text indicates p < 0.05.
aAdjusted for total fine particulates <2.5 microns, site (Michigan vs. Texas), maternal age (years), race/ethnicity (non-Latino white, non-Latino black, Latino or other race/ethnicity), body mass index (kg/m2), parity conditional on gravidity (nulligravous, gravous/nulliparous and parous), education (high school or less, some college and college graduate or greater), household income (<$40 000, $40– < $70 000, $70 000– < $100 000 and ≥$100 000) and smoking status (serum cotinine ≥40.35 vs. <40.35 ng/mL).
bMean air pollution level during the cycle prior to the observed cycle (prior cycle) and the first 10 days of the observed cycle (prolierative phase).
cMean daily air pollution level from 5 days before to 10 days following ovulation in the observed cycle. Excludes 60 participants who achieved pregnancy or were censored at the end of the entry cycle (ovulation date not assessed).
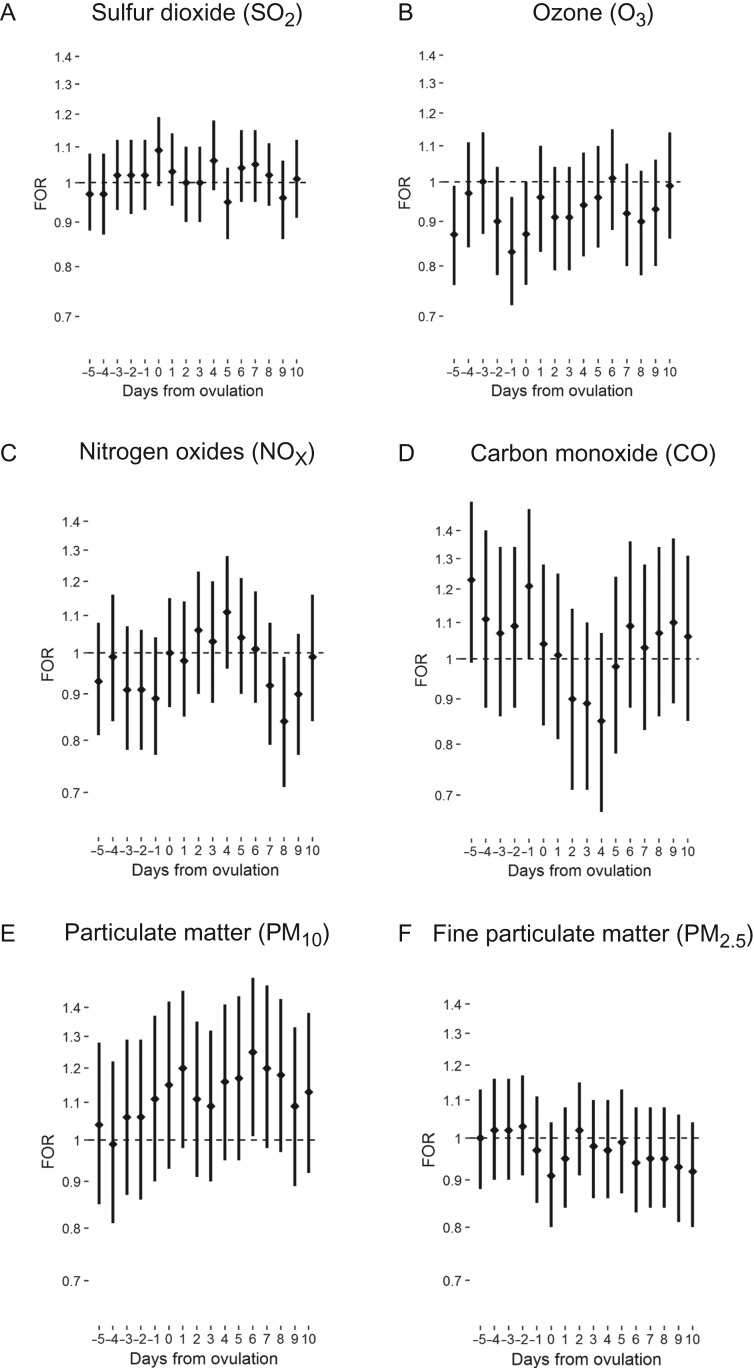
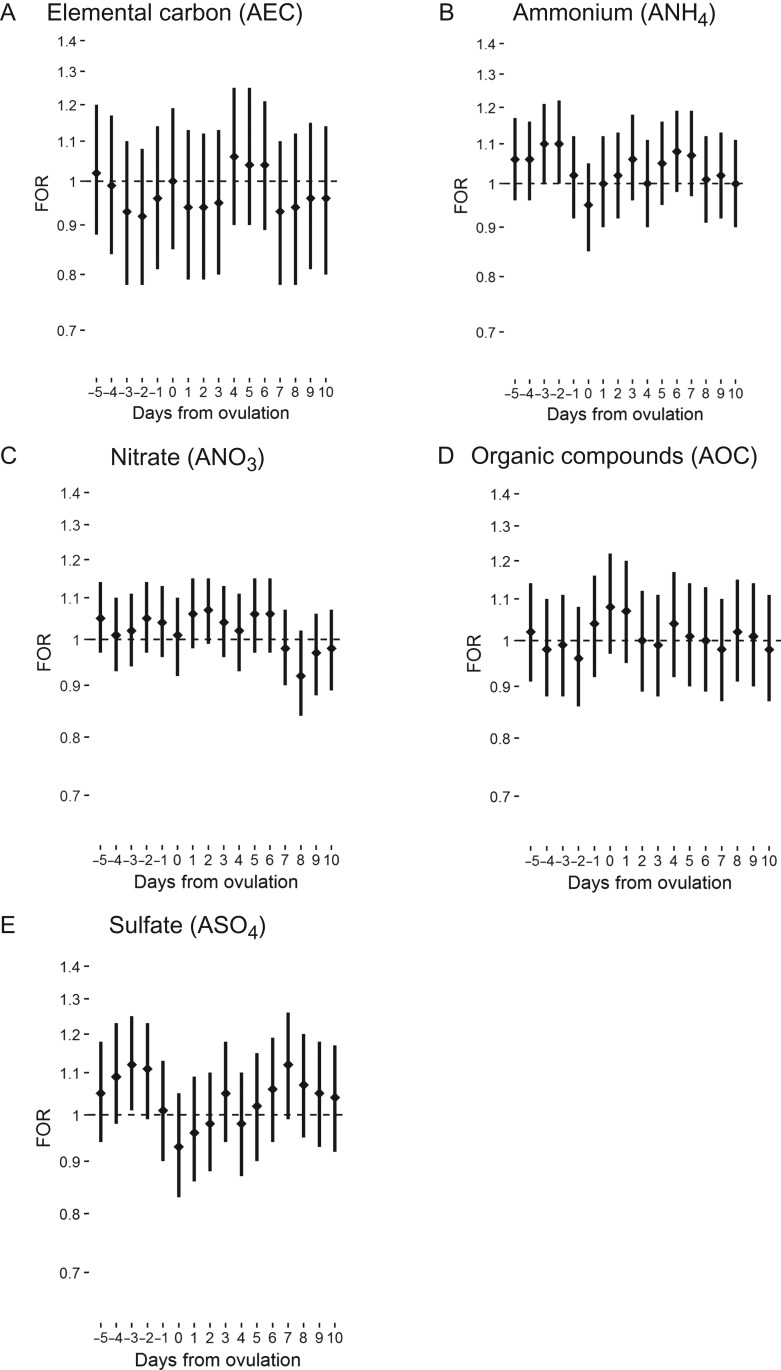
For acute average daily exposure to air pollutants and fecundability, we observed a general trend of association between greater O3, NOX and elemental carbon in the 5 days prior to ovulation and lower fecundability. An interquartile increase in O3 5 days and 1 day prior to ovulation was associated with 13% and 17% lower fecundability (FOR 0.87, 95% confidence interval (CI): 0.76, 0.99 and FOR 0.83; 95% CI: 0.72, 0.96, respectively; Fig. 2). Conversely, we observed a trend association of higher CO, ammonium and sulfate prior to ovulation with higher fecundability. An interquartile increase in sulfate 3 days prior to ovulation was associated with greater fecundability (FOR 1.12, 95% CI: 1.01, 1.25; Fig. 3). Ammonium demonstrated a similar trend, with the suggestion of an association with greater fecundability 2 and 3 days prior to ovulation. Findings were similar for single-pollutant and unadjusted models.
Figure 2.
Fecundability odds ratio (FOR) and 95% confidence interval for the association between mean daily level of criteria air pollutants and fecundability by days from ovulation; the LIFE Study (2005–2009).
Figure 3.
FOR and 95% confidence interval for the association between mean daily level of particulate constituents and fecundability by days from ovulation; the LIFE Study (2005–2009).
For mean daily exposure during the secretory phase of the menstrual cycle, a negative association was observed between O3, PM2.5, elemental carbon and nitrate concentrations around implantation (7–9 days after ovulation) and fecundability. An interquartile increase in NOX 8 days following ovulation was associated with 16% lower fecundability (FOR 0.84, 95% CI: 0.71, 0.99). Conversely, a positive association was observed for carbon monoxide, PM10, ammonium and sulfate concentrations around implantation and fecundability. An interquartile increase in PM10 6 days following ovulation was associated with 25% greater fecundability (FOR 1.25, 95% CI: 1.01, 1.54). Findings were similar in single-pollutant models and unadjusted models.
In a secondary analysis evaluating whether the results observed for PM10 6 days post ovulation and fecundability may have been due to an unmeasured confounder, we found that a confounder would need to be associated with fecundability with an odds ratio four times that of PM10 and correlated with PM10 at ρ = −0.9 for the association between PM10 and fecundability to become null (OR = 1.00) (Supplementary Table SIV).
In a secondary analysis setting ovulation date in the entry cycle to 14 days prior to the start of the next menstrual period or date of positive pregnancy test (Supplementary Tables SV and SVI), the associations between O3 1 day prior to ovulation and NOX 8 days following ovulation were moderately attenuated (FOR 0.88, 95% CI: 0.78, 1.00 and FOR 0.88, 95% CI: 0.76, 1.02, respectively). Other estimates remained similar.
Discussion
In the first prospective cohort study to assess air pollution among couples enrolled prospectively with longitudinal measurement of fecundability, we found little evidence that air pollution at moderate concentrations is associated with fecundability. We did observe associations between acute exposure to O3 prior to ovulation and NOX around implantation and a 13–17% decrement in fecundability. Conversely, sulfate prior to ovulation and PM10 around implantation were associated with 12% and 25% greater fecundability, respectively. The suggestion of a short-term effect of air pollution on fecundability is consistent with prior research on pregnancy complications (Stieb et al., 2012; Pedersen et al., 2013; Siddika et al., 2016) and myocardial infarction (Langrish et al., 2012).
Our findings are similar to those of the only other prospective study to investigate chronic exposure to air pollutants and infertility. The Nurses’ Health Study II, Mahalingaiah et al. found no association between 2- and 4-year mean exposure to coarse or fine particulates and infertility (time-to-pregnancy >12 months) among 36,294 participants of whom 2508 (7%) reported infertility (Mahalingaiah et al., 2016). The authors did, however, find an association between living <199 meters from a major roadway and a 10% greater risk of overall infertility (HR 1.1, 95% CI 1.02, 1.20), which is similar to findings in the LIFE study (Mendola et al., 2016). Although these findings are suggestive of an association between traffic-related air pollution and fecundability, the observed associations may be due to additional pathways, including exposure to noise, stress related to traffic congestion and differences in the housing and neighborhood environment.
Other previous studies have reported associations between SO2, NO2, and both fine and coarse particulate matter with couple infertility. In a retrospective cohort study in Teplice, Czech Republic among 2585 couples achieving a live birth, Dejmek et al. found that SO2 >40 μg/m3 as compared to <40 μg/m3 in the two months prior to the first unprotected menstrual cycle was associated with approximately 50% lower odds of pregnancy in the first unprotected cycle (Dejmek et al., 2000). In a similar study in Teplice among 1916 couples achieving live birth, Slama et al. found that 10 μg/m3 higher PM2.5 in the second month prior to conception and 10 μg/m3 higher NO2 in the first month prior to conception were associated with a 14% and 29% reduction in fecundability, respectively, when relying on retrospectively reported time-to-pregnancy (Slama et al., 2013). In an ecological study in Barcelona from 2001 to 2012, Nieuwenhuijsen et al. found an association between coarse particulate matter and a 12% lower population-level incidence rate ratio of fertility (Nieuwenhuijsen et al., 2014). Although suggestive, these previous studies had several limitations, including potential errors in recall and selection of couples with proven fertility in the retrospective cohort studies and confounding by area-level risk factors in the ecological study. As our mean levels of SO2 and NO2 in the first observed cycle (1.57 [standard error 0.041] and 8.09 [standard error 0.20] μg/m3, respectively) were considerably lower than those in Dejmek et al. and Slama et al. (49.9 μg/m3 and 35.9 μg/m3, respectively) (Dejmek et al., 2000; Slama et al., 2013), it is also possible that there is a threshold or non-linear association for the chronic effects of these pollutants with fecundability that we were unable to detect.
Prior work in the LIFE Study has shown that greater distance from a major roadway is associated with a greater odds of pregnancy (FOR 1.03, 95% CI 1.01, 1.06 per 200 meter greater distance) (Mendola et al., 2016). While this suggests traffic-related air pollution exposure may be associated with fecundability, there are other environmental and individual-level factors that may also be associated with distance from roadway. Although we observed an effect for NOX, a traffic-related pollutant, around the time of implantation, an association of cycle-average exposure to NOX with fecundability was not observed. Our lack of findings for cycle-averaged exposures may be due to differing routes of exposure, precision of measurement of the longer-term exposure window of interest (chronic proximity to traffic compared to shorter cycle-average exposures) or assessment of an air mixture based on traffic verses individual air pollutant species.
Our findings of a potential association between acute exposure to air pollution during sensitive windows of the observed cycle and fecundability are novel. Studies have found acute associations of O3 with premature rupture of membranes in the five hours preceding the event (Wallace et al., 2016), and CO, SO2, NO2 and PM2.5 with stillbirth in the days preceding the event (Faiz et al., 2013). Air pollution may have an acute effect on fecundability through short-term changes in inflammatory and oxidative stress pathways affecting meiotic maturation of the oocyte immediately preceding ovulation (Mlynarcikova et al., 2009) and decidualization and endometrial receptivity (Gellersen et al., 2007).
Although we observed associations between O3 preceding ovulation and NOX during implantation and diminished fecundability, we additionally found associations between several air pollutants and enhanced fecundability during critical windows of the observed cycle. These associations were counter to our hypotheses and are unlikely causal given the wealth of information on the physiological effects of air pollution. Our simulation results suggest they are unlikely to be due to confounding by a single area-level risk factor, but it is possible that these associations may be due to sampling variability or confounding by unmeasured co-pollutants. Disentangling the independent association of a given pollutant with fecundability given the interdependency of air pollutants is an important point for further study.
A major strength of this study is the use of the modified CMAQ models, which allows an estimation of both acute and time-varying cycle-average levels of a wide range of the criteria air pollutants and constituents of particulate matter, for which national coverage with ambient air monitoring varies considerably. However, some misclassification of ambient air pollution levels is likely but with no systematic direction relative to fecundability. Although the CMAQ captured a relatively precise estimate of residential exposure to ambient air pollution as compared to ambient air monitoring, estimates do not account for hours where participants were away from home as well as differences between indoor and outdoor ambient air pollutant levels.
A prospective time-to-pregnancy study is the gold standard design for estimating fecundability, and the measurement of ovulation with home fertility kits that measure luteinizing hormone allowed an investigation of acute windows of exposure around ovulation and implantation which other studies have been unable to assess (Howards et al., 2009). Despite being one of the largest couple-based prospective cohorts, the size of our cohort precluded in-depth analysis of non-linear relations and cautious interpretation of our findings is needed in light of the observational study design and potential or residual confounding including the role of other environmental exposures and the number of comparisons made without adjustment. In our analyses of acute exposure, we excluded 60 couples who became pregnant or were censored after their entry cycle, due to lack of data on ovulation. In a secondary analysis including these couples and estimating date of ovulation for the entry cycle, the associations between O3 prior to ovulation and NOX during implantation were moderately attenuated. Our study may not be broadly generalizable for two reasons: (i) our cohort included predominately non-Latino white participants of moderate to high socioeconomic status, among whom the effect of air pollution on reproductive outcomes may differ from other groups, and (ii) due to the moderate concentrations and variability of pollutants in the regions included in the study, findings may not be generalizable to regions with higher air pollution levels.
Still, this study is the first to evaluate associations of air pollution and fecundability in a time-to-pregnancy cohort. We found no association between time-varying cycle-average exposure to air pollutants and fecundability, consistent with findings from the only other prospective cohort study to evaluate air pollution and fertility in the US. While our findings are reassuring in general, they do suggest a potential role for acute exposures to several ambient air pollutants during sensitive windows of an observed cycle in relation to fecundability. Additional investigation of the potential acute effects of air pollution on critical events during the menstrual cycle merits further attention.
Supplementary data
Supplementary data are available at Human Reproduction online.
Supplementary Material
Authors’ roles
Study concept and design: P.M.; acquisition of data: G.B.L., S.S. and P.M.; statistical analysis: C.N.; interpretation and synthesis of data: C.N., E.S., S.H. and P.M.; drafting of the manuscript: C.N. and P.M.; supervision and critical revision of the manuscript for important intellectual content: C.N., E.S., S.H., G.B.L., S.S. and P.M. All authors approved of the final version to be published.
Funding
This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (Longitudinal Investigation of Fertility and the Environment study contract nos. #N01-HD-3-3355, NO1-HD-#-3356, N01-HD-3-3358 and the Air Quality and Reproductive Health Study Contract No. HHSN275200800002I, Task Order No. HHSN27500008).
Conflict of interest
None declared.
References
- Behre HM, Kuhlage J, Gassner C, Sonntag B, Schem C, Schneider HP, Nieschlag E. Prediction of ovulation by urinary hormone measurements with the home use ClearPlan Fertility Monitor: comparison with transvaginal ultrasound scans and serum hormone measurements. Hum Reprod 2000;15:2478–2482. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Bernert JT, Caraballo RS, Holiday DB, Wang J. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol 2009;169:236–248. [DOI] [PubMed] [Google Scholar]
- Buck Louis GM. Persistent environmental pollutants and couple fecundity: an overview. Reproduction 2014;147:R97–r104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck Louis GM, Schisterman EF, Sweeney AM, Wilcosky TC, Gore-Langton RE, Lynch CD, Boyd Barr D, Schrader SM, Kim S, Chen Z et al. . Designing prospective cohort studies for assessing reproductive and developmental toxicity during sensitive windows of human reproduction and development—the LIFE Study. Paediatr Perinat Epidemiol 2011;25:413–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck Louis GM, Sundaram R, Sweeney AM, Schisterman EF, Maisog J, Kannan K. Urinary bisphenol A, phthalates, and couple fecundity: the Longitudinal Investigation of Fertility and the Environment (LIFE) Study. Fertil Steril 2014;101:1359–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checa Vizcaino MA, Gonzalez-Comadran M, Jacquemin B. Outdoor air pollution and human infertility: a systematic review. Fertil Steril 2016;106:897–904. e891. [DOI] [PubMed] [Google Scholar]
- Chen G, Li J, Ying Q, Sherman S, Perkins N, Rajeshwari S, Mendola P. Evaluation of observation-fused regional air quality model results for population air pollution exposure estimation. Sci Total Environ 2014;485–486:563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin MT. Basic mechanisms for adverse cardiovascular events associated with air pollution. Heart 2015;101:253–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole LA, Khanlian SA, Sutton JM, Davies S, Rayburn WF. Accuracy of home pregnancy tests at the time of missed menses. Am J Obstet Gynecol 2004;190:100–105. [DOI] [PubMed] [Google Scholar]
- Dejmek J, Jelinek R, Solansky I, Benes I, Sram RJ. Fecundability and parental exposure to ambient sulfur dioxide. Environ Health Perspect 2000;108:647–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faiz AS, Rhoads GG, Demissie K, Lin Y, Kruse L, Rich DQ. Does ambient air pollution trigger stillbirth? Epidemiology 2013;24:538–544. [DOI] [PubMed] [Google Scholar]
- Frutos V, Gonzalez-Comadran M, Sola I, Jacquemin B, Carreras R, Checa Vizcaino MA. Impact of air pollution on fertility: a systematic review. Gynecol Endocrinol 2015;31:7–13. [DOI] [PubMed] [Google Scholar]
- Gellersen B, Brosens IA, Brosens JJ. Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives. Semin Reprod Med 2007;25:445–453. [DOI] [PubMed] [Google Scholar]
- Howards PP, Schisterman EF, Wactawski-Wende J, Reschke JE, Frazer AA, Hovey KM. Timing clinic visits to phases of the menstrual cycle by using a fertility monitor: the BioCycle Study. Am J Epidemiol 2009;169:105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langrish JP, Bosson J, Unosson J, Muala A, Newby DE, Mills NL, Blomberg A, Sandstrom T. Cardiovascular effects of particulate air pollution exposure: time course and underlying mechanisms. J Intern Med 2012;272:224–239. [DOI] [PubMed] [Google Scholar]
- Mahalingaiah S, Hart JE, Laden F, Farland LV, Hewlett MM, Chavarro J, Aschengrau A, Missmer SA. Adult air pollution exposure and risk of infertility in the Nurses’ Health Study II. Hum Reprod 2016;31:638–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendola P, Sundaram R, Louis GM, Sun L, Wallace ME, Smarr MM, Sherman S, Zhu Y, Ying Q, Liu D. Proximity to major roadways and prospectively-measured time-to-pregnancy and infertility. Sci Total Environ 2016;576:172–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlynarcikova A, Nagyova E, Fickova M, Scsukova S. Effects of selected endocrine disruptors on meiotic maturation, cumulus expansion, synthesis of hyaluronan and progesterone by porcine oocyte-cumulus complexes. Toxicol In Vitro 2009;23:371–377. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuijsen MJ, Basagana X, Dadvand P, Martinez D, Cirach M, Beelen R, Jacquemin B. Air pollution and human fertility rates. Environ Int 2014;70:9–14. [DOI] [PubMed] [Google Scholar]
- Pedersen M, Giorgis-Allemand L, Bernard C, Aguilera I, Andersen AM, Ballester F, Beelen RM, Chatzi L, Cirach M, Danileviciute A et al. . Ambient air pollution and low birthweight: a European cohort study (ESCAPE). Lancet Respir Med 2013;1:695–704. [DOI] [PubMed] [Google Scholar]
- Shah AS, Lee KK, McAllister DA, Hunter A, Nair H, Whiteley W, Langrish JP, Newby DE, Mills NL. Short term exposure to air pollution and stroke: systematic review and meta-analysis. Br Med J 2015;350:h1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddika N, Balogun HA, Amegah AK, Jaakkola JJ. Prenatal ambient air pollution exposure and the risk of stillbirth: systematic review and meta-analysis of the empirical evidence. Occup Environ Med 2016;73:573–581. [DOI] [PubMed] [Google Scholar]
- Slama R, Bottagisi S, Solansky I, Lepeule J, Giorgis-Allemand L, Sram R. Short-term impact of atmospheric pollution on fecundability. Epidemiology 2013;24:871–879. [DOI] [PubMed] [Google Scholar]
- Stieb DM, Chen L, Eshoul M, Judek S. Ambient air pollution, birth weight and preterm birth: a systematic review and meta-analysis. Environ Res 2012;117:100–111. [DOI] [PubMed] [Google Scholar]
- Veras MM, Damaceno-Rodrigues NR, Guimaraes Silva RM, Scoriza JN, Saldiva PH, Caldini EG, Dolhnikoff M. Chronic exposure to fine particulate matter emitted by traffic affects reproductive and fetal outcomes in mice. Environ Res 2009;109:536–543. [DOI] [PubMed] [Google Scholar]
- Wallace ME, Grantz KL, Liu D, Zhu Y, Kim SS, Mendola P. Exposure to ambient air pollution and premature rupture of membranes. Am J Epidemiol 2016;183:1114–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein M, Stark M. Behavioral and biological determinants of fecundability. Ann N Y Acad Sci 1994;709:128–144. [DOI] [PubMed] [Google Scholar]
- Wilcox AJ, Baird DD, Weinberg CR. Time of implantation of the conceptus and loss of pregnancy. N. Eng J Med 1999;340:1796–1799. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.