Abstract
AS is the prototypical member of the family of spondyloarthropathies, and is characterized by seronegativity, axial predominance and new bone formation, which underlie symptoms of inflammatory back pain, enthesopathy and extra-articular manifestations, including anterior uveitis, psoriasis and colitis. Patients with AS typically experience a wide variety of morbidities. These include both morbidities related to the disease itself—most prominently progressive, irreversible, structural damage to the axial or peripheral skeleton—and morbidities stemming from treatments for the disease, including toxicities from NSAID use, and increased risk of infections and immunogenicity concerns with biologics. AS is also associated with a number of comorbidities. We review the risks associated with AS, its comorbidities and its treatments, as well as strategies that can be used to mitigate these risks in patients with AS.
Keywords: ankylosing spondylitis, spondyloarthritis, morbidities, treatments, risk, treatment strategy, treatment-related risk, review
Rheumatology key messages
AS is a debilitating disease that can result in decreased mobility and quality of life.
Comprehensive care is critical in AS to mitigate morbidity and increase mobility and function.
Available therapies for AS can reduce pain and inflammation and improve quality of life.
Introduction
AS is the prototypical member of the family of spondyloarthropathies (SpAs), which also includes PsA, reactive spondyloarthritis, IBD-related or enteropathic spondyloarthritis and undifferentiated spondyloarthritis. The SpAs are a diverse group of seronegative, chronic, inflammatory, rheumatic diseases with common clinical, radiographic and genetic features [1, 2]. AS is a severe and potentially debilitating form of SpA [2]. AS affects between 0.2 and 0.5% of adults in the USA [3], and is predominantly a male disease. The male:female ratio has been reported to vary from 2:1 to 9:1 [2]. Patients with AS typically present with inflammatory back pain with varying degrees of associated enthesopathy (inflammation at sites where tendons, ligaments and joint capsule fibres attach to bone), peripheral arthritis and extra-articular manifestations [2]. Extra-articular manifestations include anterior uveitis, psoriasis, colitis and, less commonly, cardiac, renal, urogenital and cardiovascular (CV) manifestations [4].
AS, like all forms of SpA, is generally seronegative (i.e. negative for RF) and is differentiated from other forms of SpA by the axial predominance of symptoms [2]. In addition, AS is associated with new bone formation that can lead to ankyloses [5]. Although symptoms of AS typically begin before the age of 30 years, there is a delay in diagnosis of 6–8 years [6, 7]. Diagnosis of AS has long relied on evidence of structural damage to the bone via radiographic imaging [8]. Radiographic evidence of sacroiliitis is a defining feature of AS and is part of the traditional diagnostic criteria according to the internationally accepted modified New York criteria for AS [9]. The newer Assessment of SpondyloArthritis international Society (ASAS) classification criteria divide axial SpA into radiographic and non-radiographic SpA [10]. Non-radiographic SpA (nr-axSpA) refers to patients in whom sacroiliitis cannot be detected by X-ray but is visible on MRI. In patients with AS, formation of new bone and cartilage (syndesmophyte formation) in the intervertebral joints ultimately results in fusion and sclerosis of the cervical and lumbar spine, leading to loss of mobility and significant functional impairment. Finally, SpA is considered an auto-inflammatory disease controlled by innate immune cells [11].
The aetiology of SpA has a strong genetic component. The HLA-B27 gene is the predominant genetic association for the development of all forms of SpA, particularly AS, and is believed to contribute about one-third of the genetic component [12]. More than 90% of patients with AS carry the HLA-B27 gene, compared with only 6.1% in the general US population [13–15]. The prevalence of AS is closely linked to the background frequency of HLA-B27 in the population, and HLA-B27 is likely the major gene involved in AS susceptibility, but it operates in conjunction with other genes and has little impact in determining disease severity [16].
Given the multimodal complexity of AS, patients experience a wide range of morbidities related to both the disease process and its treatment, and AS patients also have a variety of associated comorbidities. Managing morbidity is an important consideration when treating patients with AS (Table 1). This review will discuss and highlight the many risks associated with AS and its treatment, with the aim of improving physician awareness.
Table 1.
Management of morbidity in AS
| AS risk framework |
|---|
| Disease-related morbidity (including functional concerns) |
| Assessment of symptoms (pain, stiffness, swelling) |
| BASDAI [17] |
| ASDAS [18] |
| Physical examination |
| Joint exam |
| Functional assessment |
| BASFI [19] |
| Imaging (X-rays, MRI) |
| Quality of life (social interaction, sexual health, body image) |
| SF-36 subscales [20] |
| EuroQoL 5 [21] |
| AS Quality-of-life Questionnaire [22] |
| Pain Disability Index [23] |
| Work ability |
| Documentation of extra-articular manifestations and/or comorbidities |
| Poor balance/risk of falls |
| Fractures |
| Track metrics over time to see if medications are making a difference |
| Treatment-related risks |
| Contraindications |
| NSAIDs in patients with IBD, CV disease |
| Adverse events |
| Poor compliance/persistence |
| Reduced efficacy of biologics |
| Routine laboratory monitoring with biologics |
| Immunogenicity with biologics |
| Psychosocial risks |
| Mental health (depression, anxiety) |
| SF-36 subscales [20] |
| DASS21 [24] |
| Alcohol abuse |
| Self-esteem issues (especially in younger patients) |
| Social participation |
ASDAS: ASAS-endorsed disease activity score; CV: cardiovascular; DASS21: Depression and Anxiety Stress Scale; SF-36: Short-Form 36.
Disease-related risks
Based on data from the National Registry of Spondyloarthritis in Spain (REGISPONSER), the most common initial symptoms of AS attributed to the disease are low back pain and sacroiliac joint syndrome, defined as alternating pain that affects the buttocks (Table 2) [25]. Comparison of the early and late cohorts (i.e. patients with disease course ⩽2 or >10 years) showed little difference in terms of the initial presenting symptoms. Progressive structural damage to the axial or peripheral skeleton resulting in irreversible physical impairment is the primary morbidity associated with AS [26]. Progressive damage associated with AS often results in a loss of function that affects the activities of daily living and substantially reduces health-related quality of life (HRQoL). Physical function directly influences patient HRQoL, work productivity, and both direct and indirect costs associated with the disease [27–30]. An inability to perform activities of daily living, such as washing and dressing, can have a significant negative effect on HRQoL [29].
Table 2.
First signs and symptoms attributable to AS [25]
| First signs and symptoms | AS patients, n (%) | ||
|---|---|---|---|
| ≤2 years | >10 years | P-value | |
| (n = 46) | (n = 1074) | ||
| Low back pain | 33 (72) | 769 (72) | 0.98 |
| Sacroiliac syndrome | 21 (46) | 443 (41) | 0.55 |
| Neck pain | 3 (6) | 121 (11) | 0.31 |
| Dactylitis | 0 | 12 (1) | 1 |
| Arthritis, lower limbs | 9 (20) | 176 (16) | 0.57 |
| Arthritis, upper limbs | 7 (15) | 37 (3) | <0.001 |
| Enthesitis | 6 (13) | 75 (7) | 0.14 |
Significance obtained by the chi-square test for contingency tables. Comparison of REGISPONSER-Early (≤2 years) vs REGISPONSER-Late (>10 years). Adapted from: Rojas-Vargas et al. [25] First signs and symptoms of spondyloarthritis—data from an inception cohort with a disease course of two years or less (REGISPONSER-Early). Rheumatology 2009;48:404–9.
Identification of clinical and genetic factors can help predict outcomes in AS and identify patients needing more aggressive therapy. For example, Amor and colleagues [31] identified a number of poor prognostic factors in patients with SpAs, including hip involvement and early disease onset. Although there is no agreement with regard to clinical features that define severe disease, hip involvement, disease duration, ESR, CRP levels, smoking and lower socioeconomic status have all been shown to be associated with worse functional status in patients with AS [32, 33]. Additionally, older age, neck pain at disease onset and single nucleotide polymorphisms in PTPN2 and PSTPIP1 are predictors of severe functional impairment [34].
Clinicians should also be aware of the relevant extra-articular manifestations of AS (i.e. IBD, Crohn’s disease, ulcerative colitis, psoriasis and uveitis) and associated comorbidities, and understand the collective effects of these manifestations on patient management [35, 36]. Extra-articular manifestations and associated comorbidities have an overall negative effect on HRQoL [29, 35]. The prevalence of these manifestations in patients with AS is outlined in Table 3. Uveitis, psoriasis and gastrointestinal (GI) involvement are common. Anterior uveitis (inflammation of the pigmented layer of the eye) is the most frequent extra-articular presentation, with a prevalence of 18–26% [37, 38]. A strong relationship has also been noted between GI and joint inflammation in patients with SpA, including AS [35]. Colonoscopy data in patients with AS show microscopic signs of GI inflammation in up to 60% of patients [35, 39, 40], and IBD has been identified in up to 7% of patients with AS [37, 38]. The precise cause of GI inflammation in this patient group remains unknown, although it has been suggested that interaction of HLA-B27 with bacterial antigens in the gut could be a contributing factor based on studies in animals [41]. The presence of microscopic gut inflammation in patients with SpA may have prognostic and therapeutic decision-making implications; patients with chronic gut inflammation appear to have a less favourable disease course [42]. Therefore, an examination of the GI tract should be included when assessing disease-associated morbidity in patients with AS.
Table 3.
Prevalence of extra-articular immune-mediated inflammatory diseases in AS
| Inflammatory disease type, n (%) | Systematic review and meta-analysis [37]a (%) | OASIS cohort [38] (n = 216) (%) |
|---|---|---|
| IBD | 6.8 | 6.9 |
| Psoriasis | 9.3 | 4.2 |
| Uveitis | 25.8 | 18.1 |
n = 32 341 for IBD, n = 27 626 for psoriasis and n = 44 372 for uveitis. OASIS: Outcome in Ankylosing Spondylitis International Study.
Patients with AS are also at increased risk of CV disease [35, 43, 44]. There is a higher prevalence of atherosclerosis, possibly due to chronic inflammation and immune dysregulation, which may account for the elevated risk of CV disease [35]. It has been estimated that 2–10% of patients with AS have cardiac manifestations [45]. Aortic regurgitation, atrioventricular block and other less common CV disorders can occur in AS, as well as in other forms of SpA [4]. Patients with aortic root involvement are at increased risk of aortic insufficiency and cardiac conduction system disease [44, 45]. In fact, the prevalence of pacemaker use among patients with AS has been shown to be as much as 15× higher than for the general public [46].
Respiratory involvement may occur in patients with AS. Pulmonary manifestations of AS include fibrosis of the upper lobes, interstitial lung disease, and ventilatory impairment due to chest wall restriction, sleep apnoea and spontaneous pneumothorax [45]. The introduction of improved pulmonary visualization technology, such as high-resolution computed tomography, has improved the identification of lung abnormalities associated with AS [45, 47]. The natural history of lung abnormalities in AS and the potential of therapeutic interventions to halt their progression is largely unknown [45].
Osteoporosis is a well-established complication of all SpA, and can lead to spinal fractures [4]. It is believed to be caused by increased levels of inflammatory cytokines and restriction of mobility secondary to pain and reduced range of motion [4]. Chronic increases in proinflammatory cytokines such as TNF-α can inhibit the proliferation and maturation of osteocytes and stimulate osteoclastogenesis [4]. The combination of osteoporosis and a rigid ossified spine can make patients with AS more susceptible to fractures [48]. Pseudoarthroses can form when stress fractures of the thoracolumbar junction are unable to heal due to persistent motion at the stress fracture site. In patients with AS, unlike RA, bone loss is also accompanied by new bone formation [49]. The resulting phenotypical differences may thus require different approaches in therapeutic intervention [50]. Similar to RA, loss of balance is also a concern in patients with AS and may increase the risk of falls and fractures [51–53]. This affects patients with high disease activity more frequently than those with low disease activity [51, 54]. Loss of balance in AS patients is often associated with severe joint deformities, poor posture and impaired spinal mobility [53–55].
Finally, patients with AS also suffer from poor sleep quality, which is positively correlated with increased pain, poor QoL, depressed mood, higher disease activity and mobility restrictions [56]. Patients often experience fatigue, as well, which is positively correlated with disease activity and may also be related to depression, anxiety and sleep disturbance related to their disease [57].
Mitigation of disease-related risks
Early diagnosis and treatment of AS may slow the progression of the disease [58]. Increased use of MRI of the sacroiliac joints and spine has facilitated earlier diagnosis and treatment of AS because it can detect sacroiliitis in the initial stages and can predict the development of structural radiographic changes, with 60% positive predictive value, 3 years before any structural damage can be detected on plain X-ray [59]. Available therapies can reduce inflammation and improve symptoms; however, no treatments have been clearly shown to prevent structural damage to the skeleton.
A comprehensive evaluation of disease-related morbidity should be conducted at diagnosis in all patients with AS through a careful patient history and the use of disease-specific metrics [60]. This establishes a baseline against which to assess disease progression and the benefits of treatment. Assessment of patients with AS is multidimensional and monitoring of disease progression is complex. There is no single measurement for disease activity, but rather it is the sum of many different indicators [61]. Disease activity is based on assessment of patient-reported pain, physical function, spinal stiffness, spinal mobility and global assessment of health status. In addition, levels of acute-phase reactants, either CRP or ESR, provide an objective measure of inflammation. Validated composite measures of disease activity such as BASDAI and the ASAS-endorsed disease activity score (ASDAS) and measures of function and mobility such as BASFI and spinal mobility tests (e.g. finger to floor, occiput to wall, lateral flexion and Schober’s test) are used in clinical practice to assess disease activity. For example, BASDAI consists of six questions that assess fatigue, spinal and peripheral joint pain, localized tenderness and morning stiffness (both qualitative and quantitative) [17]. Tracking these, or similar, metrics over time is important to assess whether treatment is reducing pain and improving mobility or the disease is progressing and may require more aggressive treatment. Imaging should be used for both diagnosis and to follow disease progression over time. Recent recommendations by EULAR provide detailed guidance on the use of imaging in patients with SpA, including AS [62]. These consensus guidelines recommend use of MRI to monitor disease activity in both axial and peripheral SpA. In axial SpA, conventional radiography of the sacroiliac joints and/or spine is recommended for long-term monitoring of structural damage, particularly new bone formation. Detection of syndesmophytes in the lumbar or cervical spine by conventional radiography is predictive of disease outcome and severity. The guidelines also suggest that findings of extensive inflammatory activity on MRI may be predictive of better outcomes with TNF inhibitors [62].
The primary goal of therapy in all forms of SpA is to maintain good functional status and to slow or halt disease progression defined by clinical symptoms, radiographic findings and serological markers of inflammation (e.g. CRP or ESR) [34, 43, 63, 64]. Exercise is an important component of the standard treatment approach and has been shown to improve pain, physical function, spinal mobility and patient global assessment [64]. Physical therapy and physical activity are important to help manage the symptoms of AS and physical therapy is recommended for patients with stable AS [43, 63, 65]. A Cochrane review found that an individual home-based or supervised exercise programme is better than no intervention [66]. Physical therapy helps maintain or improve spinal movement, improve fitness and decrease pain [66]. Counselling with regard to smoking cessation or abstinence is also important because smoking has been shown to be an independent risk factor for radiographic progression [67].
NSAIDs are the first choice of pharmacotherapy for AS [68]. Current guidelines state that therapy should start with an NSAID, and patients should fail at least two NSAIDs before moving to a new class of drugs [43, 63, 65]. Once patients progress on NSAIDs, they will typically receive a biologic agent. Until recently, TNF inhibitors (adalimumab, certolizumab pegol, etanercept, golimumab and infliximab) were the only biologic treatment options for patients with AS. These agents have been shown to significantly reduce pain and inflammation associated with AS and improve mobility and HRQoL [69–74]. Imaging studies have further shown that TNF inhibitors reduce spinal inflammation as measured by MRI [75, 76]. However, while treatment with TNF inhibitors is associated with a trend for decreased radiographic progression, no correlations between clinical parameters and radiographic progression were reported [75–77]. Another important outcome in the treatment of AS is management of fatigue, because fatigue is associated with both disease activity and functional ability, as well as the patient’s global sense of wellbeing and mental health [78]. Therefore, a significant reduction in fatigue may improve HRQoL. Treatment with anti-TNF mAbs (i.e. adalimumab or infliximab) is recommended for patients with AS who are also suffering from IBD as an alternative to etanercept therapy [63].
Other biologic agents have been investigated in AS and provide an additional treatment option to TNF-α inhibitors. These include secukinumab, an anti-IL-17A mAb, and ustekinumab, an anti-IL-12/23 mAb [79, 80]. Both of these agents are currently approved for treating PsA, and secukinumab has recently been approved for the treatment of AS based on evidence that it significantly reduced disease activity in active AS [79]. Treatment with ustekinumab or secukinumab has been shown to improve MRI osteitis and inflammation scores, respectively [80, 81], and regression of spinal inflammation has been observed with secukinumab [82].
Unfortunately, while biologic agents have been shown to provide significant short-term symptomatic benefits with respect to reduced pain and disease activity, improved mobility and improved function compared with placebo in patients with active AS, they have not yet been shown to prevent structural damage to the skeleton [83]. Despite aggressive treatment with biologic agents, patients with AS may still develop total spinal ankylosis, albeit patients with total spinal ankyloses may still benefit symptomatically from treatment with TNF inhibitors [74]. While pharmacotherapy effectively reduces inflammation, it is not clear that inflammation is the direct cause of syndesmophyte formation in AS. Studies have shown that progression of syndesmophytes can occur despite clinical disease remission based on inflammatory markers and clinical symptoms [64]. Therefore, treatments that can slow or prevent structural damage to the skeleton are greatly needed.
There is limited evidence that DMARDs such as MTX and SSZ provide therapeutic benefit in patients with AS [84, 85], and EULAR guidelines specifically state that these drugs are not to be used in axial disease [43]. Cochrane literature reviews of these agents found that there was not enough evidence to support any benefit from either MTX or SSZ in patients with AS [84, 85]. The use of systemic glucocorticoids is also not recommended for the treatment of AS [63].
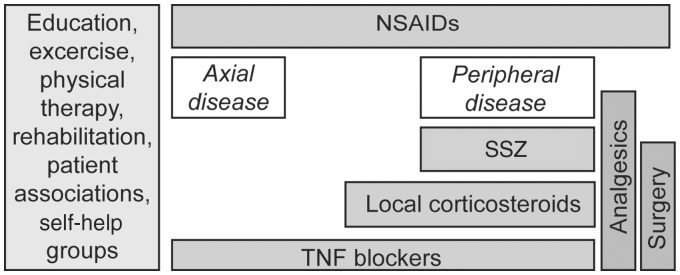
One of the major limitations in the treatment of SpAs is that treat-to-target, a therapeutic concept derived from RA and other diseases [86, 87], has not gained traction in SpA [86]. Treat-to-target implies that a clear target, such as remission or low disease activity, has been established that can be sustained over time, with an understanding of the need to treat flares and maintain tight control of disease activity [87]. Although treat-to-target has been proposed for SpA, it has not yet been adopted [60, 86, 88, 89]. This strategy requires a universal definition of the target (e.g. remission) [87]. Remission and sustained low disease activity have been suggested as possible targets in SpA [89]. Unfortunately, there is currently no consensus on the definition of remission or minimal disease activity in AS that can be used as a treatment target [90]. A composite of outcome measure may be most useful given the multifaceted nature of AS and other forms of SpA [86]. ASAS/EULAR recommendations for the management of AS are presented in Fig. 1 [91].
Fig. 1.
ASAS/EULAR recommendations for the management of AS [91]
Flow chart summary of the recommended management of AS based on the clinical expertise and research evidence. The disease progression with time moves vertically from top to bottom. ASAS: Assessment of SpondyloArthritis international Society. Reprinted from: Zochling J et al. ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann Rheum Dis;65:442–52, Copyright 2006 [91]. With permission from the BMJ Publishing Group Ltd.
Treatment-related toxicity
In AS, the recommendation is to use the lowest dose of NSAIDs for the shortest duration, but these patients have a life-long chronic disease that may require chronic therapy. A discussion with patients regarding the adverse effects associated with NSAIDs is important because AS is the only chronic rheumatic disease where continuous treatment is justified [92]. NSAIDs have known significant risks, including dyspepsia, GI bleeding, GI obstruction, liver and renal toxicity, cardiovascular effects and hypertension [92–94]. Dependence on NSAIDs is concerning due to their toxicity. Many patients cannot take them including the elderly or those on anticoagulants due to increased risks for adverse events [94]. NSAIDs should also be avoided in patients with GI involvement [95]. The patient’s CV risk profile should be considered before prescribing NSAIDs [65]. The NSAID used should be selected according to its efficacy in a given patient and according to the patient’s risk profile [92]. Patients with AS should have their liver enzymes, creatinine levels and blood pressure monitored closely following the start of NSAID therapy.
The most common risk associated with biologic agents, including both TNF inhibitors and antibodies against interleukins, is infection. Frequently reported infectious complications include nasopharyngitis, upper respiratory tract infection, herpes simplex, influenza, Candida and pneumonia [69–74, 79]. These are generally non-serious adverse events that resolve spontaneously or with appropriate antibiotic or antifungal therapy and rarely lead to discontinuation of therapy, although serious infections have been reported. For example, in the phase III trial of infliximab, 43% of patients treated with infliximab reported ⩾1 infections compared with 36% of patients receiving placebo [73]. Rare cases of tuberculosis, granuloma of the lung and secondary malignancies have also been reported, and could potentially result from the immunosuppressive effects of these biologic agents [69, 79]. Other adverse events include infusion or injection-site reactions and elevated liver enzymes [71–74]. The availability of newer biologics with other mechanisms of action, such as IL-17A inhibition, provides an additional treatment option with a different safety profile compared with TNF inhibitors [81]. Secukinumab is the first approved agent of this class for AS and another IL-17A inhibitor, ixekizumab, is in clinical development [79, 96, 97]. Secukinumab was also associated with infections, most frequently nasopharyngitis and Candida but reactivation of latent tuberculosis was not observed with secukinumab.
Immunogenicity is another concern with all biologics that can limit their effectiveness. Approximately one-third of patients treated with TNF inhibitors will have an inadequate response or lose responsiveness to these drugs over time and, in many cases, this may be a result of antidrug antibodies (ADAs) [98]. Data on the frequency of ADAs in AS are limited, but the available data suggest rates similar to those seen in other inflammatory diseases [98]. Although ADAs are a significant problem, current clinical practice does not include routine monitoring of serum ADA or drug levels in an effort to identify the reason for poor response. Instead dose intensification and/or drug switching is routinely practiced. It has been observed that concomitant use of DMARDs with TNF inhibitors may lower the immunogenicity of TNF inhibitors in patients with SpA [99]; however, DMARDs are not recommended for the treatment of AS [43].
The combination of a DMARD and a TNF inhibitor has been found to significantly lengthen TNF inhibitor drug survival compared with TNF inhibitor monotherapy in patients with AS [100]. However, the authors of this study caution that the beneficial effect of comedication, if present, may not be large enough to justify changes in management recommendations [100]. Additionally, some patients will maintain disease remission following physician-directed dosage adjustment of TNF inhibitors [101] and this approach could be considered with care for individuals that achieve remission.
Implications of poor adherence to therapy
Treatment compliance is a concern among patients with AS. Poor adherence to treatment can undermine the potential therapeutic effect of biologic therapy, thus contributing to treatment failure, disease progression and the need for more aggressive therapy [102]. In patients treated with TNF inhibitors, older age is associated with increased adherence, whereas female sex, comorbidity and poor clinical condition at baseline are associated with decreased adherence [103]. In patients with AS, there is a high potential for poor adherence because patients may not constantly be aware of the signs and symptoms of their disease. Stopping and starting therapy may reduce efficacy; this has been demonstrated in patients with RA [104].
Psychosocial risks
Psychological factors may influence disease status and outcome, and could affect the choice of both assessment tool and treatment [105]. There is a higher risk of depression in patients with AS than in the general population [106–108]. Adherence to treatment may be more difficult in patients with comorbid depression [109]. It is important to recognize depressive behaviours such as alcoholism, non-social behaviour, drug addiction and suicidal ideation. Physicians should be attuned to psychological concerns in patients with AS, and should identify patients who may benefit from a referral for counselling.
AS can have particularly severe social and psychological effects on young patients; it usually presents in early adulthood and affects patients during their most productive years [110]. The disease can lead to self-esteem issues and social isolation. Patients may not be able to participate in social activities due to pain. Because of the early onset of AS, the psychological adverse effects of the disease occur early in life and may persist for many years.
Conclusions
Among patients with AS, and other forms of SpA, comprehensive care is critical and should aim to mitigate morbidity, increase mobility and function, and improve HRQoL. As outlined above, this may be achieved by early diagnosis and treatment with the goal of slowing or halting disease progression (defined by clinical symptoms, radiographic findings and serological markers of inflammation), treatment of SpA-associated physical morbidity through exercise, management of treatment-related toxicity and treatment of associated medical comorbidities. Effective therapies are available that can significantly reduce pain and inflammation associated with AS and improve mobility and HRQoL; however, no therapy has been shown to prevent structural damage to the skeleton. It is not clear that treating the inflammation in AS can prevent progression of syndesmophytes or avoid development of total spinal ankylosis. It is also critical to be aware of and effectively manage the many comorbidities associated with AS and to pay close attention to patients’ mental health status. In conclusion, management of patients with AS requires a multidisciplinary approach.
Acknowledgements
Technical assistance with editing and styling of the manuscript for submission was provided by Oxford PharmaGenesis Inc.
Funding: This work was supported by Novartis Pharmaceuticals Corporation. The authors were fully responsible for all content and editorial decisions and received no financial support or other form of compensation related to the development of this manuscript.
Disclosure statement: M.J.B. has acted as a consultant for AbbVie, Celgene, Bristol-Myers Squibb, Genentech, Pfizer, Janssen, Novartis and Amgen, received speaker fees from Novartis, AbbVie and Celgene and is a shareholder of Pfizer. A.L. has declared no conflicts of interest.
References
- 1. Collantes E, Zarco P, Muñoz E. et al. Disease pattern of spondyloarthropathies in Spain: description of the first national registry (REGISPONSER)—extended report. Rheumatology 2007;46:1309–15. [DOI] [PubMed] [Google Scholar]
- 2. Zochling J, Smith EU.. Seronegative spondyloarthritis. Best Pract Res Clin Rheumatol 2010;24:747–56. [DOI] [PubMed] [Google Scholar]
- 3. Reveille JD. Epidemiology of spondyloarthritis in North America. Am J Med Sci 2011;341:284–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pereira IA, Neves FS, Castro GRW.. Extra-articular manifestations in spondyloarthritis are common and should be screened. Rheumatol Curr Res 2012;2:111. [Google Scholar]
- 5. Taurog JD, Chhabra A, Colbert RA.. Ankylosing spondylitis and axial spondyloarthritis. N Engl J Med 2016;374:2563–74. [DOI] [PubMed] [Google Scholar]
- 6. Reed MD, Dharmage S, Boers A. et al. Ankylosing spondylitis: an Australian experience. Intern Med J 2008;38:321–7. [DOI] [PubMed] [Google Scholar]
- 7. Dincer U, Cakar E, Kiralp MZ, Dursun H.. Diagnosis delay in patients with ankylosing spondylitis: possible reasons and proposals for new diagnostic criteria. Clin Rheumatol 2008;27:457–62. [DOI] [PubMed] [Google Scholar]
- 8. Schett G, Coates LC, Ash ZR, Finzel S, Conaghan PG.. Structural damage in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: traditional views, novel insights gained from TNF blockade, and concepts for the future. Arthritis Res Ther 2011;13(Suppl 1):S4.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van der Linden S, Valkenburg HA, Cats A.. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 1984;27:361–8. [DOI] [PubMed] [Google Scholar]
- 10. Rudwaleit M, van der Heijde D, Landewé R. et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009;68:777–83. [DOI] [PubMed] [Google Scholar]
- 11. Lories RJ, Baeten DL.. Differences in pathophysiology between rheumatoid arthritis and ankylosing spondylitis. Clin Exp Rheumatol 2009;27:S10–4. [PubMed] [Google Scholar]
- 12. Londono J, Santos AM, Peña P. et al. Analysis of HLA-B15 and HLA-B27 in spondyloarthritis with peripheral and axial clinical patterns. BMJ Open 2015;5:e009092.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brewerton DA, Hart FD, Nicholls A. et al. Ankylosing spondylitis and HL-A 27. Lancet 1973;1:904–7. [DOI] [PubMed] [Google Scholar]
- 14. Reveille JD, Hirsch R, Dillon CF, Carroll MD, Weisman MH.. The prevalence of HLA-B27 in the US: data from the US National Health and Nutrition Examination Survey, 2009. Arthritis Rheum 2012;64:1407–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schlosstein L, Terasaki PI, Bluestone R, Pearson CM.. High association of an HL-A antigen, W27, with ankylosing spondylitis. N Engl J Med 1973;288:704–6. [DOI] [PubMed] [Google Scholar]
- 16. Brown MA, Wordsworth BP, Reveille JD.. Genetics of ankylosing spondylitis. Clin Exp Rheumatol 2002;20:S43–9. [PubMed] [Google Scholar]
- 17. Garrett S, Jenkinson T, Kennedy LG. et al. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol 1994;21:2286–91. [PubMed] [Google Scholar]
- 18. Lukas C, Landewé R, Sieper J. et al. Development of an ASAS-endorsed disease activity score (ASDAS) in patients with ankylosing spondylitis. Ann Rheum Dis 2009;68:18–24. [DOI] [PubMed] [Google Scholar]
- 19. Calin A, Garrett S, Whitelock H. et al. A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Spondylitis Functional Index. J Rheumatol 1994;21:2281–5. [PubMed] [Google Scholar]
- 20. Ware JE Jr, Gandek B.. Overview of the SF-36 Health Survey and the International Quality of Life Assessment (IQOLA) Project. J Clin Epidemiol 1998;51:903–12. [DOI] [PubMed] [Google Scholar]
- 21. EuroQol Group. EuroQol—a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199–208. [DOI] [PubMed] [Google Scholar]
- 22. Doward LC, Spoorenberg A, Cook SA. et al. Development of the ASQoL: a quality of life instrument specific to ankylosing spondylitis. Ann Rheum Dis 2003;62:20–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tait RC, Pollard CA, Margolis RB, Duckro PN, Krause SJ.. The Pain Disability Index: psychometric and validity data. Arch Phys Med Rehabil 1987;68:438–41. [PubMed] [Google Scholar]
- 24. Lovibond SH, Lovibond PF.. Manual for the Depression Anxiety Stress Scales. Sydney: Psychology Foundation, 1993. [Google Scholar]
- 25. Rojas-Vargas M, Muñoz-Gomariz E, Escudero A. et al. First signs and symptoms of spondyloarthritis—data from an inception cohort with a disease course of two years or less (REGISPONSER-Early). Rheumatology 2009;48:404–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dagfinrud H, Mengshoel AM, Hagen KB, Loge JH, Kvien TK.. Health status of patients with ankylosing spondylitis: a comparison with the general population. Ann Rheum Dis 2004;63:1605–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Özdemir O. Quality of life in patients with ankylosing spondylitis: relationships with spinal mobility, disease activity and functional status. Rheumatol Int 2011;31:605–10. [DOI] [PubMed] [Google Scholar]
- 28. Rohekar S, Pope J.. Assessment of work disability in seronegative spondyloarthritis. Clin Exp Rheumatol 2010;28:35–40. [PubMed] [Google Scholar]
- 29. Singh JA, Strand V.. Spondyloarthritis is associated with poor function and physical health-related quality of life. J Rheumatol 2009;36:1012–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yilmaz O, Tutoglu A, Garip Y, Özcan E, Bodur H.. Health-related quality of life in Turkish patients with ankylosing spondylitis: impact of peripheral involvement on quality of life in terms of disease activity, functional status, severity of pain, and social and emotional functioning. Rheumatol Int 2013;33:1159–63. [DOI] [PubMed] [Google Scholar]
- 31. Amor B, Santos RS, Nahal R, Listrat V, Dougados M.. Predictive factors for the longterm outcome of spondyloarthropathies. J Rheumatol 1994;21:1883–7. [PubMed] [Google Scholar]
- 32. Cansu DU, Çalişir C, Savaş Yavaş U, Kaşifoğlu T, Korkmaz C.. Predictors of radiographic severity and functional disability in Turkish patients with ankylosing spondylitis. Clin Rheumatol 2011;30:557–62. [DOI] [PubMed] [Google Scholar]
- 33. Doran MF, Brophy S, MacKay K, Taylor G, Calin A.. Predictors of longterm outcome in ankylosing spondylitis. J Rheumatol 2003;30:316–20. [PubMed] [Google Scholar]
- 34. Schiotis R, Bartolome N, Sánchez A. et al. Both baseline clinical factors and genetic polymorphisms influence the development of severe functional status in ankylosing spondylitis. PLoS One 2012;7:e43428.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Elewaut D, Matucci-Cerinic M.. Treatment of ankylosing spondylitis and extra-articular manifestations in everyday rheumatology practice. Rheumatology 2009;48:1029–35. [DOI] [PubMed] [Google Scholar]
- 36. Zarco P, González CM, Rodríguez de la Serna A. et al. Extra-articular disease in patients with spondyloarthritis. Baseline characteristics of the spondyloarthritis cohort of the AQUILES study. Rheumatol Clin 2015;11:83–9. [DOI] [PubMed] [Google Scholar]
- 37. Stolwijk C, van Tubergen A, Castillo-Ortiz JD, Boonen A.. Prevalence of extra-articular manifestations in patients with ankylosing spondylitis: a systematic review and meta-analysis. Ann Rheum Dis 2015;74:65–73. [DOI] [PubMed] [Google Scholar]
- 38. Essers I, Ramiro S, Stolwijk C. et al. Characteristics associated with the presence and development of extra-articular manifestations in ankylosing spondylitis: 12-year results from OASIS. Rheumatology 2015;54:633–40. [DOI] [PubMed] [Google Scholar]
- 39. De Vos M, Cuvelier C, Mielants H. et al. Ileocolonoscopy in seronegative spondylarthropathy. Gastroenterology 1989;96:339–44. [DOI] [PubMed] [Google Scholar]
- 40. Mielants H, Veys EM, Cuvelier C, De Vos M.. Course of gut inflammation in spondylarthropathies and therapeutic consequences. Baillieres Clin Rheumatol 1996;10:147–64. [DOI] [PubMed] [Google Scholar]
- 41. Taurog JD, Richardson JA, Croft JT. et al. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med 1994;180:2359–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cypers H, Van Praet L, Varkas G, Elewaut D.. Relevance of the gut/joint axis for the management of spondyloarthritis in daily clinical practice. Curr Opin Rheumatol 2014;26:371–6. [DOI] [PubMed] [Google Scholar]
- 43. Braun J, van den Berg R, Baraliakos X. et al. 2010 update of the ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann Rheum Dis 2011;70:896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lautermann D, Braun J.. Ankylosing spondylitis—cardiac manifestations. Clin Exp Rheumatol 2002;20:S11–5. [PubMed] [Google Scholar]
- 45. Momeni M, Taylor N, Tehrani M.. Cardiopulmonary manifestations of ankylosing spondylitis. Int J Rheumatol 2011;2011:728471.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bergfeldt L, Edhag O, Vedin L, Vallin H.. Ankylosing spondylitis: an important cause of severe disturbances of the cardiac conduction system. Prevalence among 223 pacemaker-treated men. Am J Med 1982;73:187–91. [DOI] [PubMed] [Google Scholar]
- 47. Özdemir O, Gülsün AM, İnanıcı F, Hasçelik HZ.. Pulmonary abnormalities on high-resolution computed tomography in ankylosing spondylitis: relationship to disease duration and pulmonary function testing. Rheumatol Int 2012;32:2031–6. [DOI] [PubMed] [Google Scholar]
- 48. Qian BP, Qiu Y, Wang B. et al. Pedicle subtraction osteotomy through pseudarthrosis to correct thoracolumbar kyphotic deformity in advanced ankylosing spondylitis. Eur Spine J 2012;21:711–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vosse D, de Vlam K.. Osteoporosis in rheumatoid arthritis and ankylosing spondylitis. Clin Exp Rheumatol 2009;27:S62–7. [PubMed] [Google Scholar]
- 50. Vosse D, Landewé R, van der Heijde D. et al. Ankylosing spondylitis and the risk of fracture: results from a large primary care-based nested case-control study. Ann Rheum Dis 2009;68:1839–42. [DOI] [PubMed] [Google Scholar]
- 51. Koerich J, Armanini KK, de Rosa lop R. et al. Evaluation of body balance in rheumatoid arthritis patients. Fisioter Pesqui 2013;20:336–42. [Google Scholar]
- 52. Murray HC, Elliott C, Barton SE, Murray A.. Do patients with ankylosing spondylitis have poorer balance than normal subjects? Rheumatology 2000;39:497–500. [DOI] [PubMed] [Google Scholar]
- 53. Sawacha Z, Carraro E, Del Din S. et al. Biomechanical assessment of balance and posture in subjects with ankylosing spondylitis. J Neuroeng Rehabil 2012;9:63.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Alkan H, Yildiz N, Sarsan A. et al. Fall risk in patients with ankylosing spondylitis. Turk J Rheumatol 2013;28:109–16. [Google Scholar]
- 55. Dursun N, Sarkaya S, Ozdolap S. et al. Risk of falls in patients with ankylosing spondylitis. J Clin Rheumatol 2015;21:76–80. [DOI] [PubMed] [Google Scholar]
- 56. Batmaz I, Sariyildiz MA, Dilek B. et al. Sleep quality and associated factors in ankylosing spondylitis: relationship with disease parameters, psychological status and quality of life. Rheumatol Int 2013;33:1039–45. [DOI] [PubMed] [Google Scholar]
- 57. Aissaoui N, Rostom S, Hakkou J. et al. Fatigue in patients with ankylosing spondylitis: prevalence and relationships with disease-specific variables, psychological status, and sleep disturbance. Rheumatol Int 2012;32:2117–24. [DOI] [PubMed] [Google Scholar]
- 58. Braun J, Baraliakos X, Deodhar A. et al. Effect of secukinumab on clinical and radiographic outcomes in ankylosing spondylitis: 2-year results from the randomised phase III MEASURE 1 study. Ann Rheum Dis 2016;76:1070–7. [DOI] [PubMed] [Google Scholar]
- 59. Oostveen J, Prevo R, den Boer J, van de Laar M.. Early detection of sacroiliitis on magnetic resonance imaging and subsequent development of sacroiliitis on plain radiography. A prospective, longitudinal study. J Rheumatol 1999;26:1953–8. [PubMed] [Google Scholar]
- 60. Rohekar S, Chan J, Tse SM. et al. 2014 Update of the Canadian Rheumatology Association/spondyloarthritis research consortium of Canada treatment recommendations for the management of spondyloarthritis. Part I: principles of the management of spondyloarthritis in Canada. J Rheumatol 2015;42:654–64. [DOI] [PubMed] [Google Scholar]
- 61. Braun J, Kiltz U, Sarholz M. et al. Monitoring ankylosing spondylitis: clinically useful markers and prediction of clinical outcomes. Expert Rev Clin Immunol 2015;11:935–46. [DOI] [PubMed] [Google Scholar]
- 62. Mandl P, Navarro-Compán V, Terslev L. et al. EULAR recommendations for the use of imaging in the diagnosis and management of spondyloarthritis in clinical practice. Ann Rheum Dis 2015;74:1327–39. [DOI] [PubMed] [Google Scholar]
- 63. Ward MM, Deodhar A, Akl EA. et al. American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network 2015 recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Rheumatol 2016;68:282–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Daikh DI, Chen PP.. Advances in managing ankylosing spondylitis. F1000Prime Rep 2014;6:78.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rohekar S, Chan J, Tse SM. et al. 2014 Update of the Canadian Rheumatology Association/Spondyloarthritis Research Consortium of Canada treatment recommendations for the management of spondyloarthritis. Part II: specific management recommendations. J Rheumatol 2015;42:665–81. [DOI] [PubMed] [Google Scholar]
- 66. Dagfinrud H, Hagen KB, Kvien TK.. Physiotherapy interventions for ankylosing spondylitis. Cochrane Database Syst Rev 2008;(1):CD002822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Poddubnyy D, Haibel H, Listing J. et al. Baseline radiographic damage, elevated acute-phase reactant levels, and cigarette smoking status predict spinal radiographic progression in early axial spondylarthritis. Arthritis Rheum 2012;64:1388–98. [DOI] [PubMed] [Google Scholar]
- 68. Singh JA, Saag KG, Bridges SL Jr. et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol 2016;68:1–26. [DOI] [PubMed] [Google Scholar]
- 69. Braun J, Brandt J, Listing J. et al. Treatment of active ankylosing spondylitis with infliximab: a randomised controlled multicentre trial. Lancet 2002;359:1187–93. [DOI] [PubMed] [Google Scholar]
- 70. Calin A, Dijkmans BA, Emery P. et al. Outcomes of a multicentre randomised clinical trial of etanercept to treat ankylosing spondylitis. Ann Rheum Dis 2004;63:1594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Davis JC Jr, Van Der Heijde D, Braun J. et al. Recombinant human tumor necrosis factor receptor (etanercept) for treating ankylosing spondylitis: a randomized, controlled trial. Arthritis Rheum 2003;48:3230–6. [DOI] [PubMed] [Google Scholar]
- 72. Haibel H, Rudwaleit M, Brandt HC. et al. Adalimumab reduces spinal symptoms in active ankylosing spondylitis: clinical and magnetic resonance imaging results of a fifty-two-week open-label trial. Arthritis Rheum 2006;54:678–81. [DOI] [PubMed] [Google Scholar]
- 73. van der Heijde D, Dijkmans B, Geusens P. et al. Efficacy and safety of infliximab in patients with ankylosing spondylitis: results of a randomized, placebo-controlled trial (ASSERT). Arthritis Rheum 2005;52:582–91. [DOI] [PubMed] [Google Scholar]
- 74. van der Heijde D, Kivitz A, Schiff MH. et al. Efficacy and safety of adalimumab in patients with ankylosing spondylitis: results of a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2006;54:2136–46. [DOI] [PubMed] [Google Scholar]
- 75. Baraliakos X, Davis J, Tsuji W, Braun J.. Magnetic resonance imaging examinations of the spine in patients with ankylosing spondylitis before and after therapy with the tumor necrosis factor α receptor fusion protein etanercept. Arthritis Rheum 2005;52:1216–23. [DOI] [PubMed] [Google Scholar]
- 76. Rudwaleit M, Baraliakos X, Listing J. et al. Magnetic resonance imaging of the spine and the sacroiliac joints in ankylosing spondylitis and undifferentiated spondyloarthritis during treatment with etanercept. Ann Rheum Dis 2005;64:1305–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Baraliakos X, Listing J, Rudwaleit M. et al. Radiographic progression in patients with ankylosing spondylitis after 2 years of treatment with the tumour necrosis factor α antibody infliximab. Ann Rheum Dis 2005;64:1462–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. van Tubergen A, Coenen J, Landewe R. et al. Assessment of fatigue in patients with ankylosing spondylitis: a psychometric analysis. Arthritis Rheum 2002;47:8–16. [DOI] [PubMed] [Google Scholar]
- 79. Baeten D, Sieper J, Braun J. et al. Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. N Engl J Med 2015;373:2534–48. [DOI] [PubMed] [Google Scholar]
- 80. Poddubnyy D, Hermann KG, Callhoff J, Listing J, Sieper J.. Ustekinumab for the treatment of patients with active ankylosing spondylitis: results of a 28-week, prospective, open-label, proof-of-concept study (TOPAS). Ann Rheum Dis 2014;73:817–23. [DOI] [PubMed] [Google Scholar]
- 81. Baeten D, Baraliakos X, Braun J. et al. Anti-interleukin-17A monoclonal antibody secukinumab in treatment of ankylosing spondylitis: a randomised, double-blind, placebo-controlled trial. Lancet 2013;382:1705–13. [DOI] [PubMed] [Google Scholar]
- 82. Baraliakos X, Borah B, Braun J. et al. Long-term effects of secukinumab on MRI findings in relation to clinical efficacy in subjects with active ankylosing spondylitis: an observational study. Ann Rheum Dis 2016;75:408–12. [DOI] [PubMed] [Google Scholar]
- 83. Braun J, Sieper J.. Ankylosing spondylitis. Lancet 2007;369:1379–90. [DOI] [PubMed] [Google Scholar]
- 84. Chen J, Lin S, Liu C.. Sulfasalazine for ankylosing spondylitis. Cochrane Database Syst Rev 2014;(11):CD004800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Chen J, Veras MM, Liu C, Lin J.. Methotrexate for ankylosing spondylitis. Cochrane Database Syst Rev 2013;(2):CD004524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Miedany YE. Treat to target in spondyloarthritis: the time has come. Curr Rheumatol Rev 2014;10:87–93. [DOI] [PubMed] [Google Scholar]
- 87. Wendling D. Treating to target in axial spondyloarthritis: defining the target and the arrow. Expert Rev Clin Immunol 2015;11:691–3. [DOI] [PubMed] [Google Scholar]
- 88. Schoels MM, Braun J, Dougados M. et al. Treating axial and peripheral spondyloarthritis, including psoriatic arthritis, to target: results of a systematic literature search to support an international treat-to-target recommendation in spondyloarthritis. Ann Rheum Dis 2014;73:238–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Smolen JS, Braun J, Dougados M. et al. Treating spondyloarthritis, including ankylosing spondylitis and psoriatic arthritis, to target: recommendations of an international task force. Ann Rheum Dis 2014;73:6–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sieper J. How to define remission in ankylosing spondylitis? Ann Rheum Dis 2012;71(Suppl 2):i93–5. [DOI] [PubMed] [Google Scholar]
- 91. Zochling J, van der Heijde D, Burgos-Vargas R. et al. ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann Rheum Dis 2006;65:442–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Song IH, Poddubnyy DA, Rudwaleit M, Sieper J.. Benefits and risks of ankylosing spondylitis treatment with nonsteroidal antiinflammatory drugs. Arthritis Rheum 2008;58:929–38. [DOI] [PubMed] [Google Scholar]
- 93. Kang EJ, Kavanaugh A.. Psoriatic arthritis: latest treatments and their place in therapy. Ther Adv Chronic Dis 2015;6:194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lanas A, Boers M, Nuevo J.. Gastrointestinal events in at-risk patients starting non-steroidal anti-inflammatory drugs (NSAIDs) for rheumatic diseases: the EVIDENCE study of European routine practice. Ann Rheum Dis 2015;74:675–81. [DOI] [PubMed] [Google Scholar]
- 95. Ogdie A, Schwartzman S, Husni ME.. Recognizing and managing comorbidities in psoriatic arthritis. Curr Opin Rheumatol 2015;27:118–26. [DOI] [PubMed] [Google Scholar]
- 96. ClinicalTrials.gov. A Study of Ixekizumab (LY2439821) in bDMARD-Naive Participants With Radiographic Axial Spondyloarthritis (COAST-V). https://clinicaltrials.gov/ct2/show/NCT02696785 (19 September 2016, date last accessed).
- 97.ClinicalTrials.gov. A Study of Ixekizumab (LY2439821) in TNF Inhibitor Experienced Participants With Radiographic Axial Spondyloarthritis (COAST-W). https://clinicaltrials.gov/ct2/show/NCT02696798 (19 September 2016, date last accessed).
- 98. Vincent FB, Morand EF, Murphy K. et al. Antidrug antibodies (ADAb) to tumour necrosis factor (TNF)-specific neutralising agents in chronic inflammatory diseases: a real issue, a clinical perspective. Ann Rheum Dis 2013;72:165–78. [DOI] [PubMed] [Google Scholar]
- 99. Jani M, Barton A, Warren RB, Griffiths CE, Chinoy H.. The role of DMARDs in reducing the immunogenicity of TNF inhibitors in chronic inflammatory diseases. Rheumatology 2014;53:213–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lie E, Kristensen LE, Forsblad-d'Elia H. et al. The effect of comedication with conventional synthetic disease modifying antirheumatic drugs on TNF inhibitor drug survival in patients with ankylosing spondylitis and undifferentiated spondyloarthritis: results from a nationwide prospective study. Ann Rheum Dis 2015;74:970–8. [DOI] [PubMed] [Google Scholar]
- 101. Paccou J, Baclé-Boutry MA, Solau-Gervais E, Bele-Philippe P, Flipo RM.. Dosage adjustment of anti-tumor necrosis factor-alpha inhibitor in ankylosing spondylitis is effective in maintaining remission in clinical practice. J Rheumatol 2012;39:1418–23. [DOI] [PubMed] [Google Scholar]
- 102. Blum MA, Koo D, Doshi JA.. Measurement and rates of persistence with and adherence to biologics for rheumatoid arthritis: a systematic review. Clin Ther 2011;33:901–13. [DOI] [PubMed] [Google Scholar]
- 103. López-González R, León L, Loza E. et al. Adherence to biologic therapies and associated factors in rheumatoid arthritis, spondyloarthritis and psoriatic arthritis: a systematic literature review. Clin Exp Rheumatol 2015;33:559–69. [PubMed] [Google Scholar]
- 104. Contreras-Yañez I, Ponce De León S, Cabiedes J, Rull-Gabayet M, Pascual-Ramos V.. Inadequate therapy behavior is associated to disease flares in patients with rheumatoid arthritis who have achieved remission with disease-modifying antirheumatic drugs. Am J Med Sci 2010;340:282–90. [DOI] [PubMed] [Google Scholar]
- 105. Martindale J, Smith J, Sutton CJ. et al. Disease and psychological status in ankylosing spondylitis. Rheumatology 2006;45:1288–93. [DOI] [PubMed] [Google Scholar]
- 106. Kilic G, Kilic E, Ozgocmen S.. Relationship between psychiatric status, self-reported outcome measures, and clinical parameters in axial spondyloarthritis. Medicine 2014;93:e337.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Meesters JJ, Bremander A, Bergman S. et al. The risk for depression in patients with ankylosing spondylitis: a population-based cohort study. Arthritis Res Ther 2014;16:418.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Shen B, Zhang A, Liu J. et al. Body image disturbance and quality of life in Chinese patients with ankylosing spondylitis. Psychol Psychother 2014;87:324–37. [DOI] [PubMed] [Google Scholar]
- 109. Katon W, Ciechanowski P.. Impact of major depression on chronic medical illness. J Psychosom Res 2002;53:859–63. [DOI] [PubMed] [Google Scholar]
- 110. Özgul A, Peker F, Taskaynatan MA. et al. Effect of ankylosing spondylitis on health-related quality of life and different aspects of social life in young patients. Clin Rheumatol 2006;25:168–74. [DOI] [PubMed] [Google Scholar]