Abstract
Determining biosimilarity involves a comprehensive exercise with a focus on determining the comparability of the molecular characteristics and preclinical profile of the biosimilar and reference product, such that there is less need for extensive clinical testing to assure comparability of clinical outcomes. Three anti-TNF biosimilar agents are approved for patients with rheumatic diseases in the European Union. The infliximab (Remicade®) biosimilars CT-P13 (Remsima® and Inflectra®) and SB2 (Flixabi®) and the etanercept (Enbrel®) biosimilar SB4 (Benepali®) have shown close comparability to their reference medicinal products, having undergone extensive evaluations. Guidelines on the treatment of rheumatic diseases have acknowledged that biosimilars and biologic DMARDs (bDMARDs) are interchangeable in clinical practice, except when patients experience lack of efficacy or tolerability with the reference agent. Given that cost is a barrier to effective bDMARD use, the introduction of less costly biosimilars is likely to widen access and dissipate treatment inequalities. Physicians faced with prescribing decisions should be reassured by the robust and exhaustive process that is involved in assuring comparability of biosimilars with their reference agents. De novo usage of a biosimilar and switching to a biosimilar following lack of efficacy or tolerability with a different reference biologic agent are likely to be strategies most easily adopted, although switching during successful treatment should also be considered given the potential cost implications. The introduction of biosimilar bDMARDs has the potential to improve patient access to effective biologic therapy, to better accommodate restraints within healthcare budgets and to improve overall patient outcomes.
Keywords: biosimilar, CT-P13, rheumatic disease, SB2, SB4
Rheumatology key messages
CT-P13 and SB2 (infliximab biosimilars) and SB4 (etanercept biosimilar) are approved for the treatment of several rheumatic diseases.
Biosimilars and their reference agents have been shown to be interchangeable.
Introduction of biosimilars may widen access to biologic therapy and improve overall patient outcomes.
Introduction
Biologic agents are an important therapeutic option in the treatment of patients with rheumatic diseases, including RA [1], AS [2] and PsA [3]. Guidelines and recommendations for the use of biologic agents, and anti-TNF agents in particular, do not prioritize the use of any one of these agents [2–8]. Therefore, the choice of the biologic agent for a particular patient may hinge on other clinical considerations, such as dosing frequency, route and mode of administration, the presence of comorbidities and the safety/adverse-event profile of the candidate drug [1].
Despite the fact that biologic agents are highly effective in the treatment of rheumatic diseases, and are often considered to be cost-effective for patients who have not responded adequately to conventional treatment, patients are unlikely to be treated with these agents first-line, and may even encounter barriers to their use as second-line therapy [9]. This, in part, reflects the high costs of these agents and administrative restrictions [9]. Furthermore, among those patients who receive a biologic treatment, a significant proportion of patients either do not respond to initial treatment or lose responsiveness [10], and more than 1 in 10 patients typically withdraws due to side effects [11]. Access to biologic agents per se, and to a wider range of alternative biologic agents, is therefore a key consideration in improving the treatment, and therefore outcomes, for patients with rheumatic diseases. The development of biosimilar agents that are highly comparable to the reference medicinal product provides a new route to achieving this goal. This article provides an overview of the biosimilar agents that are currently in development, or available in the clinic, for the treatment of patients with rheumatic diseases, summarizes results from some key clinical trials and discusses the potential place of biosimilars in current rheumatic disease treatment algorithms.
Introducing biosimilars in the treatment of rheumatic disease
Determining biosimilarity involves a comprehensive exercise to define and compare the characteristics of the biosimilar candidate with that of the reference medicinal product [12]. Compared with novel biologic development, biosimilar development involves a much greater focus on determining comparability of the molecular characteristics and preclinical profile, with head-to-head phases I and III clinical studies conducted thereafter to demonstrate pharmacokinetic equivalence, and to assure comparability in terms of efficacy, safety, immunogenicity and tolerability [12]. Post-marketing monitoring is implemented, for example through pharmacoepidemiological studies, to ensure consistent efficacy and continual monitoring of long-term safety. This robust and comprehensive process is designed to ensure confidence in the clinical profile in terms of comparability [13]. A detailed overview of the concept of biosimilarity and the regulatory requirements that are needed to establish biosimilarity, as defined by the European Medicines Agency (EMA), is provided in the first article of this supplement by Declerck and Rezk. To date, biosimilar innovation in rheumatology has focused on the development of biosimilar versions of infliximab and etanercept.
Infliximab biosimilars
Infliximab (Remicade®) is an anti-TNF agent that is approved for use in adult patients with severe active and/or progressive RA, severe active ankylosing spondylitis, active and progressive PsA, moderate to severe plaque psoriasis, moderately to severely active Crohn's disease and fistulizing active Crohn’s disease, and in young people aged 6–17 years with severe active Crohn’s disease or ulcerative colitis [14]. To date, two infliximab (Remicade®) biosimilars, CT-P13 (Remsima® and Inflectra®) and SB2 (Flixabi®), have been approved for patients with rheumatic diseases in the European Union (EU).
CT-P13
CT-P13 (Remsima® and Inflectra®) is a biosimilar medicinal product containing infliximab that was approved in the EU in 2013 for use in the treatment of adult patients with RA, PsA or psoriasis, ulcerative colitis, Crohn’s disease and in young people with ulcerative colitis or Crohn’s disease [15–17]. All major physicochemical characteristics and biologic activities (including affinity for soluble and transmembrane TNF) for CT-P13 have been shown to be highly comparable to those of reference infliximab [18]. However, in the regulatory assessment, a small difference was noted in the amount of afucosylated glycans of CT-P13, translating into a lower binding affinity towards specific Fc receptors and a lower ex vivo antibody-dependent cellular cytotoxicity (ADCC) in the most sensitive ADCC assay [15]. This difference was not considered to be clinically meaningful as it did not affect the activity of CT-P13 in experimental models that were considered to be more relevant to the pathophysiological conditions in patients [15].
Evidence of pharmacokinetic equivalence between CT-P13 and reference infliximab was provided by a Phase I, randomized, double-blind, parallel-group study (PLANETAS) of 250 patients with AS [19]. Following administration of either agent, at a dose of 5 mg/kg, primary endpoints [area under the concentration–time curve (AUC) at steady state and observed maximum steady-state serum concentration (Cmax,ss) between weeks 22 and 30] were equivalent for CT-P13 (32 765.8 μgh/ml and 147.0 μg/ml) and infliximab (31 359.3 μgh/ml and 144.8 μg/ml). In addition, the 90% CIs of the geometric mean ratios of both AUC at steady state and Cmax,ss were contained within the predefined equivalence margin (e.g. 80–125%) [19]. The pharmacokinetic profile of multiple doses of CT-P13 was also shown to be comparable to that of reference infliximab, administered by a 2-h intravenous infusion, in a further on-going, phase I, randomized, double-blind study, which included 19 patients with active RA who were also receiving concomitant MTX (between 12.5 and 25 mg/week, oral dose) [15].
The efficacy of CT-P13 for the treatment of RA was assessed in two randomized, double-blind, multicenter studies: the phase III PLANETRA study [20] and a supportive Japanese phase I/II study [21] (Table 1). In the PLANETRA study, patients (n = 606) with active disease, who were previously unresponsive to MTX, were treated with either CT-P13 or reference infliximab at a dose of 3 mg/kg with MTX and folic acid supplementation (see Fig. 1A). This trial met its primary end point for equivalence of efficacy as the 95% CI for the difference in the ACR20 response rate at week 30 was contained within the predefined equivalence margin (e.g. ±15%) in the intention-to-treat population (CT-P13, 60.9%; reference infliximab, 58.6%; 95% CI: –6, 10). Other secondary endpoints, including ACR50 and ACR70 response rates, demonstrated similar results with CT-P13 and reference infliximab at week 30 [20] (Table 1). Likewise, CT-P13 and reference infliximab also did not significantly differ in terms of disease activity measures at week 30, including improvements in Clinical Disease Activity Index and Simplified Disease Activity Index, or the proportion of patients who achieved low disease activity or remission based on the DAS28 (Table 1) [20]. A total of 455 patients in PLANETRA were treated up to week 54 [22]. Longer term, at week 54, the proportion of patients achieving ACR20, ACR50 and ACR70 responses continued to be similar in both treatment groups, and improvements in disease activity, as measured by mean changes from baseline in DAS28, Clinical Disease Activity Index and Simplified Disease Activity Index scores, were also maintained with each regimen [22] (Table 1). Results from the PLANETRA extension study (n = 302), which assessed the efficacy and safety of switching from reference infliximab to CT-P13 or continuing CT-P13 in patients who had completed 54 weeks of treatment, reported that response rates were maintained and did not significantly differ in the switch and maintenance groups up to 102 weeks [23] (Table 1). Further support for the comparable efficacy of CT-P13 and reference infliximab in RA comes from a small phase I/II study in Japanese patients (n = 101), in which patients were treated with either agent at a dose of 3 mg/kg with MTX supplementation [21]. The proportions of patients achieving ACR20 response at weeks 14, 30 and 54 were not significantly different between CT-P13 and reference infliximab. Furthermore, there were no significant between-group differences in ACR50, ACR70 or the EULAR response rates at any time point, with the exception of the ACR70 response rate at week 54 [21] (Table 1). Results from the extension phase of this study (n = 72), which assessed the safety and efficacy of switching from reference infliximab to CT-P13 or continuing CT-P13 in patients who had completed 54 weeks of treatment, reported that ACR response rates improved in both the switch and maintenance groups up to week 134 [24] (Table 1).
Table 1.
Published clinical studies comparing biosimilar and originator agents
| References | Study design | Dosage regimen | Key efficacy outcomes | Immunogenicity | Key safety outcomes | Study conclusions |
|---|---|---|---|---|---|---|
| CT-P13 vs reference infliximab (INX): multinational efficacy and safety studies in patients with RA | ||||||
|
3 mg/kg IV CT-P13 or INX, weeks 0, 2, 6 then q8 wks + MTX 12.5–25 mg/wk + folic acid ≥5 mg/wk | ACR20 response rate
|
ADAs at week 14
|
Overall TEAEs
|
|
|
ACR50 response rate
|
ADAs at week 30
|
TEAEs related to treatment
|
||||
ACR70 response rate
|
Most frequently reported TEAEs related to treatment
|
|||||
Mean improvement in CDAI
| ||||||
Serious TEAEs
| ||||||
Mean improvement in SDAI
| ||||||
DAS28/CRP response rate
| ||||||
| No deaths | ||||||
Good/moderate EULAR response (CRP)
| ||||||
| Yoo et al. [22] PLANETRA study extension to week 54 |
|
3 mg/kg IV CT-P13 or INX, weeks 0, 2, 6 then q8wks + MTX 12.5 − 25 mg/wk + folic acid ≥5 mg/wk | ACR20 response rate
|
ADAs at week 54
|
Overall TEAEs
|
|
ACR50 response rate
|
TEAEs related to treatment
|
|||||
Most frequently reported TEAEs related to treatment
| ||||||
ACR70 response rate
| ||||||
Mean improvement in CDAI
| ||||||
Mean improvement in SDAI
| ||||||
Serious TEAEs:
| ||||||
Mean improvement in DAS28/CRP
| ||||||
Serious TEAEs related to treatment
| ||||||
Good/moderate EULAR response (CRP)
| ||||||
| One death in INX group; not considered related to treatment | ||||||
| Yoo et al. [23] PLANETRA study switching extension to week 102 |
|
3 mg/kg IV CT-P13 or INX, weeks 0, 2, 6 then q8wks + MTX 12.5–25 mg/wk + folic acid ≥5 mg/wk to week 54 then CT-P13/MTX/folic acid as above (weeks 64–102) | ACR20 response rate
|
ADAs at week 78
|
Overall TEAEs
|
Comparable efficacy and tolerability were observed in patients who switched from INX to CT-P13 for an additional year and in those who had long-term treatment with CT-P13 for 2 years |
ACR50 response rate
|
ADAs at week 102
|
TEAEs related to treatment
|
||||
ACR70 response rate
|
Most frequently reported TEAEs related to treatment
|
|||||
Mean improvement in DAS28/CRP
| ||||||
Serious TEAEs
| ||||||
Serious TEAEs related to treatment
| ||||||
| No deaths | ||||||
| CT-P13 vs reference infliximab (INX): Japanese pharmacokinetic efficacy and safety studies in patients with RA | ||||||
|
|
3 mg/kg IV CT-P13 or INX, weeks 0, 2, 6 then q8wks + MTX 6–16 mg/wk + folic acid ≥5 mg/wk | CT-P13/INX ratio (95% CI) in ADA-ve patients at week 14
|
ADAs at week 14
|
Overall TEAEs
|
CT-P13 and INX were pharmacokinetically equivalent and comparable in efficacy and safety when administered for 54 weeks in Japanese patients with RA |
ACR20 response rate
|
ADAs at week 30
|
Most frequently reported TEAEs related to treatment
|
||||
ACR50 response rate
|
||||||
ACR70 response rate
| ||||||
Mean improvement in CDAI
| ||||||
Serious TEAEs
| ||||||
Mean improvement in SDAI
| ||||||
Mean improvement in DAS28/CRP
| ||||||
Good/moderate EULAR response (CRP)
| ||||||
|
|
3 mg/kg IV CT-P13 or INX, weeks 0, 2, 6 then q8wks + MTX 6–16 mg/wk + folic acid ≥5 mg/wk to week 54 then CT-P13/MTX/folic acid as above (weeks 64–104); CT-P13 dose increase allowed to 10 mg/wk | ACR20 response rate
|
ADAs at week 110
|
Overall TEAEs
|
CT-P13 was well tolerated with persistent efficacy in Japanese patients with RA who maintained treatment after 54 weeks and in patients who switched to CT-P13 after 54 weeks of INX treatment |
ACR50 response rate
|
ADAs at week 134
|
Most frequently reported TEAEs related to treatment
|
||||
ACR70 response rate
|
||||||
Mean improvement in DAS28/ESR
| ||||||
Serious TEAEs
| ||||||
| CT-P13 vs reference infliximab (INX): pharmacokinetic, efficacy and safety studies in patients with AS | ||||||
|
5 mg/kg IV CT-P13 or INX, weeks 0, 2, 6 then q8wks + continued stable use of glucocorticoids/NSAIDs allowed | CT-P13/INX ratio (95% CI)
|
ADAs at week 14
|
Overall TEAEs
|
The PK profiles of CT-P13 and INX were equivalent in patients with AS. CT-P13 was well tolerated, with an efficacy and safety profile comparable to that of INX up to week 30 | |
ASAS20 response rate
|
ADAs at week 30
|
Most frequently reported TEAEs related to treatment
|
||||
ASAS40 response rate
|
||||||
Mean change in ASDAS-CRP
|
Serious TEAEs
|
|||||
Median change in BASDAI score
|
No deaths | |||||
Median change in BASFI score
| ||||||
Median change in BASMI score
| ||||||
Median change in chest expansion score (cm)
| ||||||
|
5 mg/kg IV CT-P13 or INX, weeks 0, 2, 6 then q8wks + continued stable use of glucocorticoids/NSAIDs allowed | ASAS20 response rate
|
ADAs at week 30
|
Overall TEAEs
|
|
|
TEAEs related to treatment
| ||||||
ASAS40 response rate
|
||||||
Most frequently reported TEAEs related to treatment
| ||||||
ASAS partial remission
| ||||||
Mean change in ASDAS-CRP score
| ||||||
Serious TEAEs
| ||||||
Median change in BASDAI score
| ||||||
Serious TEAEs related to treatment
| ||||||
| Two deaths (one in each treatment arm); both car accidents and not considered related to study treatment | ||||||
Median change in BASFI score
| ||||||
Median change in BASMI score
| ||||||
Median change in chest expansion score (cm)
| ||||||
|
|
5 mg/kg IV CT-P13 or INX, weeks 0, 2, 6 then q8wks + continued stable use of glucocorticoids/NSAIDs allowed then CT-P13/glucocorticoids/NSAIDs as above (weeks 62–102) | ASAS20 response rate
|
ADAs at week 78
|
Overall TEAEs
|
This is the first study to show that switching from INX to its biosimilar CT-P13 is possible without negative effects on safety or efficacy in patients with AS |
ASAS40 response rate
|
ADAs at week 101
|
TEAEs related to treatment
|
||||
ASAS partial remission
|
Most frequently reported TEAEs related to treatment
|
|||||
Mean ASDAS-CRP score
| ||||||
Mean change in BASDAI score
| ||||||
| Serious TEAEsCT-P13 maintenance: 4.5%CT-P13 switch: 4.7% | ||||||
Mean change in BASFI score
| ||||||
Serious TEAEs related to treatment
| ||||||
Mean change in BASMI score
| ||||||
| CT-P13 vs reference infliximab (INX): observational studies in patients with spondyloarthritis/rheumatic disease | ||||||
|
No dosage details provided (within label restrictions) | Median BASDAI
|
INX levels μg/ml
|
|
Switching from INX to CT-P13 was not associated with any statistically significant difference in efficacy, AEs or ADA levels | |
BASFI
|
Anti-INX antibody levels ng/ml
|
|||||
| ||||||
DAS28-CRP
| ||||||
MASES
| ||||||
VAS pain
| ||||||
Morning stiffness
| ||||||
|
|
Same dose and frequency as prior INX; 31 patients on concomitant MTX | Mean AUC for: | Three patients had ADAs to INX before CT-P13 was initiated and discontinued | One patient had neurofibromatosis and one patient had latent TB reactivation | The clinical effectiveness of CT-P13 in both patient-reported outcomes and disease activity was comparable to INX during the first year of switching, with no immediate safety signals |
Pain
| ||||||
Fatigue
| ||||||
PtGlob
| ||||||
PtAct
| ||||||
HAQ
| ||||||
DrGlob
| ||||||
| SB2 vs reference infliximab (INX): multinational efficacy and safety study in patients with RA | ||||||
| Choe et al. [35] Study to week 30 |
|
|
ACR20 response rate
|
ADAs at week 30
|
Overall TEAEs
|
Results demonstrate the equivalence of efficacy between SB2 and INX, as well as the comparability in safety, immunogenicity and PK profiles |
ACR50 response rate
|
TEAEs related to treatment
|
|||||
Most frequently reported TEAEs related to treatment
| ||||||
ACR70 response rate
| ||||||
Mean improvement in CDAI
| ||||||
Mean improvement in SDAI
| ||||||
Serious TEAEs
| ||||||
| One death in INX group (heart failure) | ||||||
Mean improvement in DAS28/ESR
| ||||||
% achieving LDAS (DAS28 ≤2.6 to ≤ 3.2)
| ||||||
% achieving remission (DAS28 ≤2.6)
| ||||||
Good and moderate EULAR response (CRP)
| ||||||
| SB4 vs reference etanercept (ENT): multinational efficacy and safety study in patients with RA | ||||||
| Emery et al. [44] Study to week 24 |
|
|
ACR20 response rate
|
ADAs at week 30
|
Overall TEAEs
|
SB4 was shown to be equivalent with ENT in terms of efficacy. SB4 was well tolerated, with a lower immunogenicity profile. The safety profile of SB4 was comparable to that of ETN |
ACR50 response rate
|
Most appeared early (weeks 28) and most disappeared after week 12 | TEAEs related to treatment
|
||||
ACR70 response rate
|
Most frequently reported TEAEs related to treatment
|
|||||
Serious TEAEs
| ||||||
| One death in SB4 group (cardiorespiratory failure); not considered related to drug | ||||||
Mean improvement in DAS28-ESR
| ||||||
% achieving LDAS (DAS28 ≤3.2)
| ||||||
% achieving remission (DAS28 ≤2.6)
| ||||||
Good and moderate EULAR response (CRP)
| ||||||
| GP2015 vs reference etanercept (ENT): multinational efficacy and safety study in patients with plaque psoriasis | ||||||
|
|
50 mg twice/wk SC GP2015 or ENT to week 12 then 50 mg/wk SC GP2015 or ENT once weekly | PASI75 response rate at week 12
|
ADAs
|
Overall TEAEs at week 54
|
This study demonstrated the equivalent efficacy and comparable safety and immunogenicity of GP2015 and ENT in patients with moderate to severe chronic plaque-type psoriasis |
Mean difference in PASI score to week 12
|
TEAEs related to treatment
|
|||||
Proportion of IGA responders
| ||||||
PASI 50/75/90 response rate at week 52c
|
Serious TEAEs
|
|||||
Observed PASI score at week 52c
| ||||||
| One death in the ENT group in treatment period (cardiopulmonary failure); not considered related to drug | ||||||
% change in PASI score at week 52c
| ||||||
Patients enrolled with an inadequate response to prior treatment with MTX.
Patients enrolled had previously received or were eligible for phototherapy or systemic psoriasis therapy. Patients with a 50% improvement in PASI (PASI50) at week 12 were re-randomized to continue the same treatment on a once-weekly dosing schedule or to undergo a sequence of three treatment switches between GP2015 and ETN until week 30. Thereafter, patients continued treatment with the product that they had been assigned to last, up to week 52.
Raw data not provided. ≡: overall equivalence; ALT: alanine transaminase; ADA: antidrug antibody; AE: adverse event; ASAS: Assessment in AS; AST: aspartate transaminase; AUC: area under the curve; CDAI: Clinical Disease Activity Index; Cmax: maximum plasma drug concentration; DrGlob: Doctor Global Assessment Activity Score; ETN: etanercept; IGA: Investigator’s Global Assessment; INX: innovator infliximab; LDAS: low Disease Activity Score; MASES: Maastricht AS Enthesitis Score; PASI: Psoriasis Area and Severity Index; PK: pharmacokinetics; ptACT: patient disease activity; ptGlob: patient global estimate; q8wk: every 8 weeks; SDAI: Simple Disease Activity Index; TEAE: treatment-emergent adverse event; TB: tuberculosis; UTI: urinary tract infection; VAS: Visual Analogue Scale.
Fig. 1.
Study design of key studies on biosimilars
(A) CT-P13 PLANETRA study—RA. (B) CT-P13 PLANETAS study—AS. (C) SB2 phase III study—RA. (D) SB4 phase III study—RA and (E) GP2015 EGALITY study plaque-type psoriasis. Data taken from [19, 20, 22, 23, 25, 26, 35–37, 44–46, 51].
The efficacy of CT-P13 for the treatment of AS was investigated in the randomized, double-blind, multicenter PLANETAS trial, in which patients with active AS were treated with either CT-P13 or reference infliximab at a dose of 3 mg/kg (n = 250) (Fig. 1B) [19]. Although the primary end point of the study was to demonstrate pharmacokinetic equivalence between CT-P13 and reference infliximab, efficacy endpoints were also assessed. Improvements in the signs and symptoms of AS did not significantly differ between CT-P13 and reference infliximab, as assessed by the Assessment in AS (ASAS) 20 and ASAS40 response rates after 30 or 54 weeks of treatment [19, 25] (Table 1). There were also no marked between-group differences in improvements in other efficacy measures assessing disease activity, including ASDAS-CRP level, BASDAI, spinal mobility (BASMI) and physical function (BASFI) [19, 25] (Table 1). Results from the PLANETAS extension study (n = 174), which assessed the efficacy and safety of switching from reference infliximab to CT-P13 or continuing CT-P13 in patients who had completed 54 weeks of treatment, demonstrated that response rates were maintained and did not significantly differ in the switch and maintenance groups up to 102 weeks (Table 1) [26].
The efficacy of switching to CT-P13 from reference infliximab has also been examined in a range of rheumatic and inflammatory diseases. Results from a 6-month, real-life, observational study in patients with spondyloarthritis (n = 41) reported that switching from reference infliximab to CT-P13 was not associated with any statistically significant differences in efficacy, as assessed by the median BASDAI, BASFI, ASDAS, DAS28, Maastricht Ankylosing Spondylitis Enthesitis and Visual Analogue Scale pain scores, whereas the median duration of morning stiffness significantly decreased [27] (Table 1). Likewise, results from a prospective, observational study in 39 consecutive patients with established rheumatic diseases (RA, AS, PsA and JIA) who were switched to CT-P13 after a mean of 4.1 years on reference infliximab reported that the clinical effectiveness of CT-P13 in both patient-reported outcomes and disease-activity measures was comparable to reference infliximab during the first year of switching [28] (Table 1). Several registries and post-marketing studies are ongoing to evaluate CT-P13 in several indications and to further assess clinical outcomes following a switch from reference infliximab to CT-P13. For example, data from the DANBIO registry has provided support for the efficacy of switching from reference infliximab to CT-P13 in 802 patients with inflammatory arthritis (RA, PsA and axial spondyloarthritis). Three months after switching from reference infliximab to CT-P13, disease activity was largely unchanged in most patients [29]. Likewise, results from the NOR-SWITCH trial, a 52-week, randomized, double-blind, non-inferiority phase IV trial, which was funded by the Norwegian government, demonstrated that switching patients who were stable on reference infliximab to CT-P13 was not inferior to continued treatment with reference infliximab in adult patients (n = 481) with a diagnosis of five inflammatory diseases: RA, PsA, Crohn’s disease, ulcerative colitis and chronic plaque psoriasis [30]. In July 2015, the Dutch authorities began funding a similar trial (BIO-SWITCH) to study the effects on efficacy, safety and immunogenicity of switching treatment from reference infliximab to CT-P13 in patients with RA, spondyloarthritis or PsA. Results are expected in 2017 [31].
A proportion of patients with RA and AS tested positive for antidrug antibodies (ADAs) at week 54 after treatment with CT-P13 and reference infliximab in PLANETRA (41.1 and 36.0%, respectively) [22], and PLANETAS (19.5 and 23.0%, respectively) [25] (Table 1). At week 102, the proportion of ADA-positive patients was similar in the switch and maintenance groups of the PLANETRA extension (44.8 and 40.3%, respectively) [23] and PLANETAS extension (27.4 and 23.3%, respectively) [26] (Table 1). In both studies, the vast majority of patients with a positive ADA result were also positive for neutralizing antibodies. In support of these findings, results from real-world studies in patients with rheumatic disease have reported that switching from reference infliximab to CT-P13 was not associated with an increase in immunogenicity [27, 28].
CT-P13 was generally well tolerated for up to 54 weeks of treatment in patients with RA [22] and AS [25], with a tolerability profile similar to that of reference infliximab. Overall, treatment-related adverse events (AEs) occurred in 43.7% of CT-P13 patients and 45.0% of reference infliximab patients in PLANETRA [22], and 50.0 and 51.6%, respectively, in PLANETAS [25] (Table 1). The majority of AEs were of mild to moderate intensity, with the most frequently reported treatment-related AEs being abnormal liver function tests, infusion-related reactions, latent tuberculosis and upper respiratory tract infection [22, 25]. Serious treatment-related AEs occurred in 7.6% of CT-P13 patients and 4.7% of reference infliximab patients in PLANETRA [22], and 3.1 and 4.1%, respectively, in PLANETAS [25] (Table 1). Although regulatory evaluation highlighted a numerical imbalance in serious AEs in PLANETRA, with a higher incidence of serious infections, including latent tuberculosis being noted for CT-P13, the numbers were low and the EMA considered the difference to be a chance finding [15]. According to the open-label extensions of PLANETRA and PLANETAS, CT-P13 continued to be well tolerated in the longer term, with switching from reference infliximab to CT-P13 at week 54 having no detrimental effect on safety over a further 48 weeks of treatment (Table 1) [23, 26]. Real-world studies have also reported that switching from reference infliximab to CT-P13 was generally well tolerated, with no safety signals, in patients with rheumatic diseases [24, 28] (Table 1).
SB2
SB2 (Flixabi®) is an infliximab biosimilar that has recently received marketing authorization in the EU for use in the treatment of adult patients with RA, PsA, psoriasis, ulcerative colitis or Crohn’s disease and young people with ulcerative colitis or Crohn’s disease, as for reference infliximab [32]. Overall, the physicochemical and biologic characteristics (including TNF binding and TNF-α neutralization activities and Fc-related biologic activities, such as ADCC, complement-dependent cytotoxicity, neonatal Fc receptor binding, C1q binding and Fc gamma receptor binding) for SB2 have been shown to be similar to those of reference infliximab [32]. Although a few small differences in physicochemical attributes were observed in terms of charged glycans, charge variants and high molecular mass aggregates between SB2 and reference infliximab, evidence from the related literature, structure–activity relationship studies and comparative biologic assays showed that these differences were unlikely to be clinically meaningful [33].
Evidence of pharmacokinetic equivalence between SB2 and two reference infliximab products sourced from the EU and the USA was provided by a randomized, parallel, three-arm, single-blind study of 159 healthy volunteers over 10 weeks [34]. Following administration of a single dose of 5 mg/kg, the 90% CIs for the geometric least squares mean ratios for the primary pharmacokinetic parameters [AUC from time zero to infinity (AUCinf), AUC from time zero to the last quantifiable concentration (AUClast) and Cmax] for each comparison were within the predefined equivalence margin (e.g. 80–125%) [34].
The equivalence of SB2 and reference infliximab was subsequently confirmed in a phase III, randomized, double-blind trial of 584 patients with RA, which consisted of a 54-week main study and an additional 24-week transition (switching) study [35–37] (Fig. 1C). Patients with active disease, despite prior treatment with MTX, received SB2 or reference infliximab at a dose of 3 mg/kg in conjunction with MTX for up to 30 weeks. This trial met its primary efficacy end point for equivalence of efficacy as the 95% CI for the difference in the ACR20 response rate at week 30 was contained within the predefined equivalence margin (e.g. ±15%) in the per-protocol set (SB2, 64.1%; reference infliximab, 66.0%; 95% CI: −10.26, 6.51) [35]. Other efficacy endpoints, including ACR50/70, DAS28 and EULAR response, were also similar in both treatment groups at week 30 [35] (Table 1). A total of 452 patients completed 54 weeks of treatment (available as an abstract) [36]. At week 54, patients receiving SB2 or reference infliximab demonstrated similar ACR20 response rates (50.7 vs 52.6%, respectively), ACR50 response rates (32.1 vs 29.7%, respectively) and ACR70 response rates (18.3 vs 17.7%, respectively) [36]. Likewise, other secondary efficacy parameters at week 54, such as DAS28 and EULAR response rates, were also similar between the two treatment groups. Radiographic damage, as assessed by the change in modified total sharp score from baseline to week 54, was comparable between the two treatment groups (mean change: 0.38 for SB2 vs 0.37 for reference infliximab) [36]. At week 54, patients receiving reference infliximab were randomized to either continue treatment with infliximab (n = 101) or to switch to SB2 (n = 94), whereas those previously treated with SB2 continued to receive SB2 (n = 201), but followed the randomization procedure to maintain blinding (available as an abstract) [37]. Assessments up to week 78 demonstrated that ACR response rates were sustained and comparable across treatment groups [37].
A similar proportion of patients with RA tested positive for ADAs at week 30 after treatment with SB2 and reference infliximab (55.1 and 49.7%, respectively; P = 0.212; Table 1) [35]. Likewise, at week 54, there was no significant difference in the incidence of ADAs following treatment with SB2 and reference infliximab (62.4 and 57.5%, respectively; P = 0.270) [36]. At week 78, ADAs were documented for 45.7–53.6% of patients; among patients with overall negative ADA results up to week 54, newly developed ADAs were noted in 14.6% of patients who transitioned from reference infliximab to SB2, 14.9% of patients who continued with reference infliximab and 14.1% of patients who continued with SB2 at week 78 [37]. SB2 was generally well tolerated in patients with RA, with a tolerability profile similar to that of reference infliximab [35–37]. At week 30, the incidence of treatment-emergent AEs was comparable between SB2 and reference infliximab (57.6 and 58.0%, respectively) [35]. The majority of AEs were of mild to moderate intensity, with the most frequently reported being latent tuberculosis, increased alanine aminotransferase levels and headache (Table 1). Similarly, at week 54, there was no significant difference in the incidence of treatment-emergent AEs following treatment with SB2 and reference infliximab (61.7 and 65.2%, respectively) [36]. At week 78, treatment-emergent AEs were documented for 36.2% of patients who transitioned from reference infliximab to SB2, 35.6% of patients who continued with reference infliximab and 40.3% of patients who continued with SB2 [37].
Etanercept biosimilars
Etanercept (Enbrel®) is an anti-TNF agent that is approved for use in adult patients with moderate-to-severe active and/or progressive RA, active and progressive PsA, severe active AS, severe non-radiographic axial spondyloarthritis and severe plaque psoriasis, and in young people with JIA (polyarthritis, extended oligoarthritis, PsA and enthesitis-related arthritis) and severe plaque psoriasis [38]. To date, the etanercept (Enbrel®) biosimilar SB4 (Benepali®) has been approved for patients with rheumatic diseases in the EU. As of March 2017, an estimated 30 000 patients have been treated with SB4 in Europe [39].
SB4
SB4 (Benepali®) is an etanercept biosimilar that has been approved for use in the treatment of adult patients with RA, PsA, AS, non-radiographic axial spondyloarthritis and plaque psoriasis [40, 41]. Results from a large characterization study demonstrated that SB4 is highly similar to reference etanercept in physicochemical and biologic attributes, including TNR receptor-related binding and Fc-related binding [42]. Although a few differences in quality were observed in terms of high molecular mass aggregate levels and impurity levels, these differences were sufficiently justified by the results of a structure–activity relationship study that showed that these differences did not negatively influence the key indicators of biologic activities of SB2 [42].
The comparability of the pharmacokinetics of SB4 and reference etanercept sourced from the EU (EU-E) and US (US-E) was determined in a randomized, single-blind, three-way, phase I study of 138 healthy male volunteers [43]. In each part, SB4 and reference etanercept were administered as a single 50 mg dose, and pharmacokinetics parameters were measured after 21 days; each treatment sequence (Part A: SB4 vs EU-E; Part B: SB4 vs US-E; Part C: EU-E vs US-E) was separated by a 28-day washout period. The geometric least squares mean ratios of AUCinf, AUClast and Cmax were similar between the two treatments in each part: 99.04, 98.62 and 103.71% (Part A: SB4 vs EU-E); 101.09, 100.96 and 104.36% (Part B: SB4 vs US-E); and 100.51, 101.27 and 103.29% (Part C: EU-E vs US-E), respectively, and the corresponding 90% CIs were completely contained within the pre-specified bioequivalence interval (e.g. 80–125%) [43].
The equivalence of SB4 and reference etanercept was subsequently confirmed in a phase III, randomized, double-blind trial of 596 patients with RA, which consisted of a 52-week main study and an additional 48-week transition (switching) study (Fig. 1D) [44–46]. Patients with moderate to severe RA, despite prior treatment with MTX, received SB4 or reference etanercept at a dose of 50 mg every week in conjunction with MTX for up to 24 weeks. This trial met its primary efficacy end point for equivalence of efficacy as the 95% CI for the difference in the ACR20 response rate at week 24 was contained within the pre-defined equivalence margin (e.g. ±15%) in the per protocol set (SB4, 78.1%; reference etanercept, 80.3%; 95% CI: –9.41, 4.98; Table 1). Other efficacy endpoints, including ACR50/70, were also comparable in both treatment groups at week 24 [44] (Table 1). A total of 505 patients completed 52 weeks of treatment (available as an abstract) [45]. At week 52, patients receiving SB4 or reference etanercept demonstrated similar ACR20 response rates (70.2 vs 65.7%, respectively), ACR50 response rates (47.8 vs 42.1%, respectively) and ACR70 response rates (30.4 vs 24.6%, respectively) [45]. Radiographic damage, as assessed by the change in modified total sharp score from baseline to week 54, was comparable between the two treatment groups (mean change: 0.45 for SB4 vs 0.74 for reference etanercept) [45]. At week 52, patients either continued to receive SB4 (n = 126) or switched from reference etanercept to SB4 (n = 119) (available as an abstract) [46]. Assessments up to week 100 demonstrated that ACR response rates were sustained and comparable across treatment groups [46].
The incidence of ADA development up to week 24 was significantly lower with SB4 compared with reference etanercept (0.7 vs 13.1%, respectively; P < 0.001; Table 1) [44]. Likewise, at week 54, the incidence of ADAs was also significantly lower with SB4 compared with reference etanercept (1.0 vs 13.2%; P < 0.001) [45]. The overall incidence of ADAs from weeks 52 to 100 was 0.8% in patients who continued with SB4 and 0.9% in patients who switched from reference etanercept to SB4 [46].
SB4 was generally well tolerated in patients with RA, with a tolerability profile similar to that of reference etanercept [44–46]. At week 24, the incidence of treatment-emergent AEs was comparable between SB4 and reference etanercept (55.2 and 58.2%, respectively; Table 1) [44]. The majority of AEs were of mild to moderate intensity, with the most frequently reported being upper respiratory tract infection, increased alanine aminotransferase levels, injection-site erythema and nasopharyngitis (Table 1). Serious treatment-related AEs occurred in 13 patients each in the SB4 and reference etanercept groups [44]. Similarly, at week 52, there was no significant difference in the incidence of treatment-emergent AEs following treatment with SB4 and reference infliximab (58.5 and 60.3%, respectively) [45]. The incidence of treatment-emergent AEs newly occurring from weeks 52 to 100 was 47.6% in patients who continued with SB4 and 48.7% in patients who switched from reference etanercept to SB4 [46].
Other etanercept biosimilars
A further etanercept biosimilar (GP2015) is under development by Sandoz. This agent has been approved for the same indications as reference etanercept in the USA [47], and a regulatory submission for its approval in the EU was made in December 2015 [48]. Results from an analytical and non-clinical comparability exercise confirmed that GP2015 and reference etanercept are comparable in regard to functional (target binding and anti-TNF-α biologic activity), pharmacokinetics and toxicological profiles. Furthermore, the pharmacodynamic biosimilarity of GP2015 and Enbrel® was confirmed in a well-established animal model of RA [TNF-α transgenic (Tg197) mouse] [49].
Evidence of pharmacokinetic equivalence between GP2015 and reference etanercept was provided by a randomized, two-sequence, two-period, cross-over study in healthy male subjects (n = 54) [50]. Following administration of either agent, at a single dose of 50 mg/kg, the mean serum concentration–time profiles were similar between GP2015 and reference etanercept. In addition, the 90% CIs of the geometric mean ratios for the primary endpoints [Cmax, AUC from the time of the dosing and extrapolated to infinity (AUC0-inf) and AUC from the time of dosing to the last measurable concentration (AUC0-tlast)] were within the pre-defined equivalence margin (80–125%) [50].
The efficacy and safety of GP2015 were assessed in the randomized, double-blind EGALITY study, in which patients (n = 531) with moderate to severe chronic plaque-type psoriasis were treated with either GP2015 or reference etanercept [51]. The study consisted of four periods (Fig. 1E). In the first 12-week treatment period, patients received GP2015 or reference etanercept (50 mg twice weekly). In treatment period 2, patients who had achieved at least a 50% improvement in Psoriasis Area and Severity Index (PASI) 50 from baseline at week 12 were re-randomized to either continue the same treatment on a once-weekly dosing schedule or to undergo a sequence of three treatment switches between GP2015 and reference etanercept at 6-weekly intervals until week 30. During the extension phase, patients continued to receive the same treatment received during the final 6 weeks of treatment period 2. This trial met its primary end point for equivalence of efficacy as the difference in PASI75 response rates at week 12 between GP2015 and reference etanercept was –2.3%, with the 95% CI (–9.85, 5.30) being well contained within the pre-defined equivalence margin (–18, 18) [51]. The main secondary end point, mean percentage change from baseline in PASI score at week 12, was similar between GP2015 and reference etanercept (Table 1). Likewise, other endpoints, including PASI50, 75 and 90 response rates at week 52, were comparable between the continued GP2015 and reference etanercept groups and between the pooled continued and pooled switched treatment groups [51] (Table 1).
ADAs, all non-neutralizing, were limited to five patients receiving reference etanercept during treatment period 1, and one patient in the switched reference etanercept group, who had been treated with GP2015 for 12 weeks at the time of the finding [51]. The incidence of treatment-emergent AEs up to week 52 was comparable between continued GP2015 and continued reference etanercept groups and was not impacted by switching (Table 1). The incidence of serious AEs and treatment-related TEAEs was similar between the two continued treatment groups and between the two switched treatment groups [51] (Table 1).
How should biosimilars fit into current clinical practice?
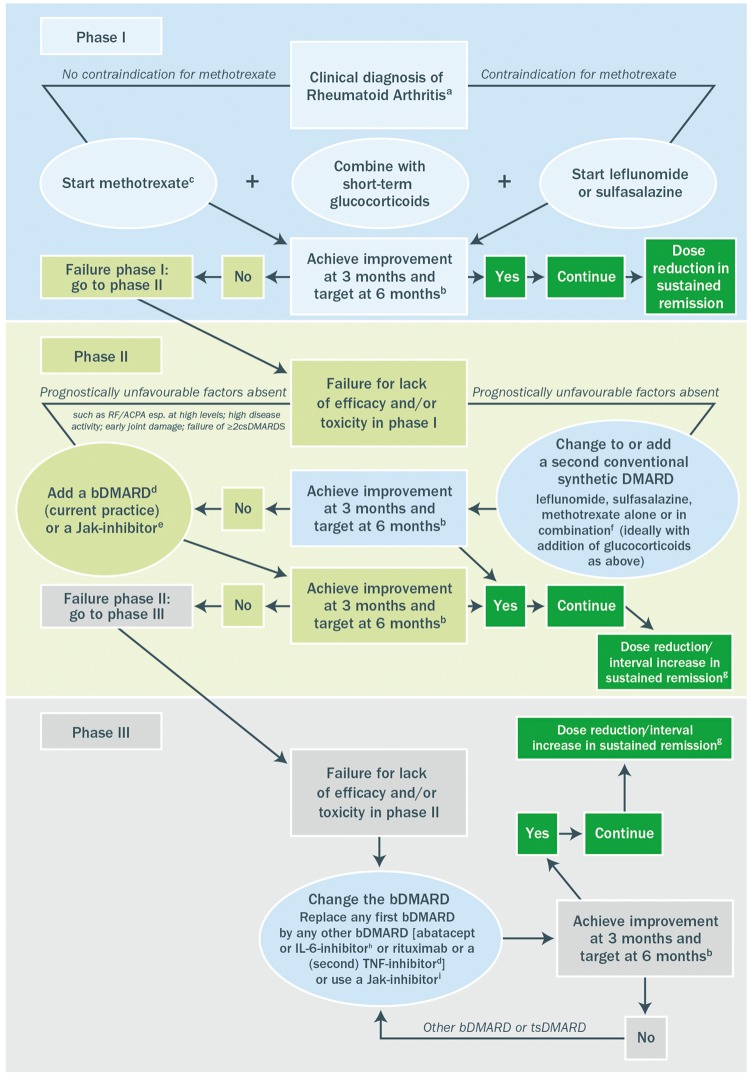
The two reference anti-TNF agents (infliximab and etanercept) and their biosimilars are indicated for use in patients with rheumatic diseases (plus psoriasis and IBD), generally following lack of response or intolerance to first-line or standard therapy, the exception being their use in patients with severe active and progressive RA, in which they are approved for first-line use [14, 16, 17, 38, 40, 52]. Guidelines on the use of biologic agents for patients with rheumatic diseases follow a similar theme [2, 3, 5–7]. For example, current European guidelines on the treatment of RA, which were updated in 2016, provide due consideration to biosimilars as part of the bDMARD treatment algorithm [6]. In those patients who do not respond adequately to first-line treatment, when alternative treatment strategies such as a treat-to-target (or tight control) approach with combination conventional synthetic DMARDs (csDMARDs) [53–55] and the use of combination csDMARDs following inadequate response to csDMARD therapy [56, 57] have failed, or those patients who experience unacceptable toxicity within 6 months of starting therapy (designated phase I), addition of firstly a bDMARD [an anti-TNF agent (adalimumab, certolizumab, etanercept, golimumab, infliximab, including EMA/FDA approved biosimilar DMARDs), abatacept, IL-6 inhibitors, or rituximab] or secondly a Jak inhibitor should be considered either following an alternative synthetic DMARD strategy or in place of an alternative synthetic DMARD strategy (designated phase II) (Fig. 2) [6]. In the scenario where this first trial of biologic therapy fails, an alternative bDMARD should be considered (an alternative anti-TNF agent, abatacept, IL-6 inhibitor, or rituximab) or a Jak inhibitor. However, it would be expected that, even when current treatment algorithms for rheumatic disease are followed, a significant proportion of patients treated with bDMARDs would be unresponsive to treatment, would lose responsiveness [10] or would experience unacceptable side effects leading to withdrawal [11].
Fig. 2.
Algorithm based on the 2016 EULAR recommendations on RA management
a2010 ACR-EULAR classification criteria can support early diagnosis. bThe treatment target is clinical remission according to ACR-EULAR definition or, if remission is unlikely to be achievable, at least low disease activity; the target should be reached after 6 months, but therapy should be adapted or changed if no sufficient improvement is seen after 3 months. cMTX should be part of the first treatment strategy; while combination therapy of csDMARDs is not preferred by the Task Force, starting with MTX does not exclude its use in combination with other csDMARDs. dTNF inhibitors (adalimumab, certolizumab, etanercept, golimumab, infliximab, including EMA/FDA approved bsDMARDs), abatacept, IL-6 inhibitors or rituximab; in patients who cannot use csDMARDs as co-medication, IL-6 inhibitors and tsDMARDs have some advantages. eCurrent practice would be to start with a bDMARD (in combination with MTX or another csDMARD) because of the long-term experience compared with tsDMARDs (Jak inhibitors). fThe most frequently used combination comprises MTX, SSZ and HCQ. gDose reduction or interval increase can be safely done with all bDMARDs with little risk of flares; stopping is associated with high flare rates; most but not all patients can recapture their good state upon re-institution of the same bDMARD. hEfficacy and safety of bDMARDs after Jak inhibitor failure is unknown; also, efficacy and safety of an IL6-pathway inhibitor after another one has failed is currently unknown. iEfficacy and safety of a Jak inhibitor after insufficient response to a previous Jak inhibitor is unknown. bDMARD: biologic DMARD; bsDMARD: biosimilar DMARD; csDMARD: conventional synthetic DMARD; EMA: European Medicines Agency; FDA, Food and Drug Administration; tsDMARD: targeted synthetic DMARD. Reproduced from EULAR recommendations for the management of RA with synthetic and biologic DMARDs: 2016 update, Smolen et al. ©2017 [6], with permission from BMJ Publishing Group Ltd.
Considerable debate has existed over whether originator and biosimilar medicines can be considered interchangeable and whether switching or substitution of biologic medicines is appropriate. Furthermore, the use of different terms, such as interchangeability, switching and substitution, has also been a source of some confusion [58] (Table 2). European guidelines consider biosimilar anti-TNF agents to be interchangeable with their reference anti-TNF products, although they should not be considered as a replacement [6] in the case of failed efficacy or unacceptable toxicity. Given the view that biosimilars are interchangeable with their reference products, when should they be used? One might consider that they may be used as any part of the treatment algorithm for certain conditions. However, this in itself brings uncertainty as to whether physicians would adopt such an approach. Certainly, biologic-naïve patients are clear candidates for use of biosimilars. Moreover, patients who are already on a reference biologic can be considered for transition to a biosimilar after appropriate discussion with a specialist. To date, experience from clinical trials, real-world studies and post-marketing experience has provided reassuring data regarding the efficacy and safety of switching patients with a range of rheumatic and inflammatory diseases from reference infliximab to the biosimilar, CT-P13 [59]. In particular, blinded studies have demonstrated that switching from reference infliximab to CT-P13 does not result in any loss of efficacy, increase in AEs or increase in immunogenicity [23, 24]. Although open-label switching can be undertaken in clinical practice, recent discontinuation rates reported in clinical trials for patients switching from reference infliximab to CT-P13 have been attributed to subjective reasons (negative expectations) and a possible nocebo effect [28, 29, 60]. Promising results have also been reported in patients switching from reference infliximab to the biosimilar SB2 and in patients switching from reference etanercept to the biosimilar SB4 [37, 46]. However, the question of switching from a reference product during successful therapy remains undetermined.
Table 2.
Definitions of interchangeability, substitution and switching
| Interchangeability | The medical practice of changing one medicine for another that is expected to achieve the same clinical effect in a given clinical setting and in any patient on the initiative or with the agreement of the prescriber |
| Substitution | Practice of dispensing one medicine instead of another equivalent and interchangeable medicine at the pharmacy level without consulting the prescriber |
| Switching | Decision by the treating physician to exchange one medicine for another medicine with the same therapeutic intent in patients who are undergoing treatment |
Information taken from [58].
The use of biosimilars in rheumatology has been a hot topic in recent years, with some physicians being cautious about using them in clinical practice [61]. This at least, in part, appears to reflect potential uncertainties among some prescribers regarding the utility of biosimilars [59]. Confidence in the clinical profile of these agents should arise from an understanding of the extensive and rigorous process undertaken to establish comparability between the biosimilar and the reference medicinal product [12]. Generally, biologic agents are associated with a high cost, which not only tests the budgets of patients and payers, but also has a detrimental effect on access to these agents, reflecting budget restrictions in many countries [62]. This has resulted in wide inequalities in their use [9]. Given that biosimilars are generally a lower cost alternative to reference medicinal products [9], this may well have an effect on uptake and prescribing behaviour. A greater number of eligible patients might be treated and inequalities in healthcare provision may begin to dissipate [9]. Indeed, budget impact analyses of the introduction of a biosimilar in several European countries have shown that switching to biosimilar therapy could result in significant cost savings and increase access to effective biologic therapy [63–65]. Significant cost savings can be made both by switching patients to a biosimilar from a reference medicinal product and offering biosimilars to patients that are treatment naïve. However, further evidence from pragmatic clinical trials that compare the most effective conventional treatment strategies with the use of biosimilars for inducing remission are required before initiation of biosimilars in treatment-naïve patients can be recommended, irrespective of the potential reduced costs.
Lower cost and increased access might also translate into greater use of biosimilars in the first-line biologic setting for certain conditions. A similar scenario could be imagined in the second and subsequent phases of biologic use. Initiating a biosimilar in treatment-naïve patients, or switching a patient to a biosimilar due to lack of efficacy or tolerability to a different reference biologic agent, is likely, however, to be considered in a different light to switching during successful treatment solely on the basis of cost. There are no specific guidelines on switching to a biosimilar and so this approach will come down to physician–patient judgement and should involve an informed decision-making process. Likewise, there is little evidence available to guide switching to a biosimilar in clinical practice, although real-world data, including the NOR-SWITCH study, are being collected. As such, it is crucial that high-quality pharmacovigilance and registry data are collected when transitioning patients to a biosimilar.
Automatic switching is one area that might cause particular concern, as this would not involve physician consultation and may impact effective pharmacovigilance, which is dependent on the transparent use of nomenclature and treatment history. In Europe, the EMA does not have the authority to designate a biosimilar to be an automatic substitute at the pharmacy [66], but such an approach could be accommodated within individual nations [52]. Notably, in Australia, the body involved with listing medicines for reimbursement—the Pharmaceutical Benefits Advisory Committee—has taken the decision to award a flag to the infliximab biosimilar InflectaTM. The awarding of this flag means that InflectaTM and the innovator Remicade® are now substitutable at the pharmacy level once the pharmacist has consulted with the patient [67].
Essentially, as more data become available, they need to be carefully scrutinized in order for an informed decision to be made as to the potential place of biosimilars in clinical practice.
Discussion
As of March 2017, three anti-TNF biosimilar agents have been granted approval and are available on the market for patients with rheumatic diseases in the EU. The infliximab (Remicade®) biosimilars CT-P13 (Remsima® and Inflectra®) and SB2 (Flixabi®) and the etanercept (Enbrel®) biosimilar SB4 (Benepali®) have shown close comparability to their reference medicinal products in terms of their physical, biologic and clinical characteristics, having undergone extensive evaluations. International guidelines on the treatment of RA, in particular, have acknowledged the role of biosimilars in terms of their interchangeability with reference bDMARDs, except in the case of lack of efficacy or tolerability. A similar approach can expect to be adopted in other rheumatic diseases. Given that cost is a barrier to effective bDMARD use, the introduction of less costly biosimilars is likely to widen access and dissipate treatment inequalities in several markets. Physicians faced with prescribing decisions should be reassured by the robust and exhaustive EMA process that is involved in determining the comparability of biosimilars with their reference medicinal products. De novo usage of a biosimilar and switching to a biosimilar following lack of efficacy of or lack of tolerability to a different reference biologic agent are likely to be strategies that are most easily adopted. Switching during successful treatment at the physician’s discretion should not be discounted given the potential cost implications, but more clinical experience is needed to ease current concerns. Overall, the introduction of biosimilar agents has the potential to widen patient access to effective biologic therapy, to better accommodate restraints within healthcare budgets and to improve overall patient outcomes.
Acknowledgments
The authors would like to acknowledge the editorial support provided by inVentiv Health Medical Communications. Philip Ford and Frances Gambling from inVentiv Health Medical Communications wrote the drafts of the article based on input from all authors, and styled the article per journal requirements. Biogen reviewed and provided feedback on the article to the authors. The authors had full editorial control of the article and provided their final approval of all content. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole and have given final approval to the version to be published.
Funding: This work was supported by Biogen, who provided funding for medical writing and editorial support in the development of this article.
Disclosure statement: H.S.-K. and A.S. have served as consultants for Biogen.
References
- 1. Gartlehner G, Hansen RA, Jonas BL, Thieda P, Lohr KN.. The comparative efficacy and safety of biologics for the treatment of rheumatoid arthritis: a systematic review and meta-analysis. J Rheumatol 2006;33:30–48. [PubMed] [Google Scholar]
- 2. Ward MM, Deodhar A, Akl EA. et al. American College of Rheumatology/Spondyloarthritis Research and Treatment Network 2015. Recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Rheumatol 2016;68:282–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Coates LC, Tillett W, Chandler D. et al. The 2012 BSR and BHPR guideline for the treatment of psoriatic arthritis with biologics. Rheumatology 2013;52:1754–7. [DOI] [PubMed] [Google Scholar]
- 4. Smith CH, Astey AS, Barker JN. et al. British Association of Dermatologists’ guidelines for biologic interventions for psoriasis 2009. Br J Dermatol 2009;161:987–1019. [DOI] [PubMed] [Google Scholar]
- 5. Braun J, van den Berg R, Baraliakos X. et al. 2010 update of the ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann Rheum Dis 2011;70:896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smolen JS, Landewe R, Breedveld FC. et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 2017;76:960–77. [DOI] [PubMed] [Google Scholar]
- 7. Gossec L, Smolen JS, Ramiro S. et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis 2016;75:499–510. [DOI] [PubMed] [Google Scholar]
- 8. National Institute for Health and Care Excellence. Adalimumab, etanercept, infliximab, certolizumab pegol, golimumab, tocilizumab and abatacept for rheumatoid arthritis not previously treated with DMARDs or after conventional DMARDs only have failed. August 2015. https://www.nice.org.uk/guidance/TA375/documents/rheumatoid-arthritis-adalimumab-etanercept-infliximab-certolizumab-pegol-golimumab-abatacept-and-tocilizumab-review-id537-appraisal-consultation-document2 (3 October 2016, date last accessed).
- 9. Gulacsi L, Brodszky V, Baji P. et al. Biosimilars for the management of rheumatoid arthritis. Exp Rev Clin Immunol 2015;11(Suppl 1):S43–52. [DOI] [PubMed] [Google Scholar]
- 10. Vincent FB, Morand EF, Murphy K. et al. Antidrug antibodies (ADAb) to tumour necrosis factor (TNF)-specific neutralising agents in chronic inflammatory diseases: a real issue, a clinical perspective. Ann Rheum Dis 2013;72:165–78. [DOI] [PubMed] [Google Scholar]
- 11. Singh JA, Wells GA, Christensen R. et al. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev 2011;16:CD008794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. European Medicines Agency. Guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: non clinical and clinical issues. 18 December 2014. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/01/WC500180219.pdf (3 October 2016, date last accessed).
- 13. Weiss M, Bielsky MC, De Smet K. et al. Biosimilars: what clinicians should know. Blood 2012;120:5111–7. [DOI] [PubMed] [Google Scholar]
- 14.Remicade Summary of Product Characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000240/WC500050888.pdf (3 October 2016, date last accessed).
- 15. European Medicines Agency. Assessment report. Remsima. 27 June 2013. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002576/WC500151486.pdf (3 October 2016, date last accessed).
- 16. Inflectra Summary of Product Characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002778/WC500151489.pdf (3 October 2016, date last accessed).
- 17. Remsima Summary of Product Characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002576/WC500150871.pdf (3 October 2016, date last accessed).
- 18. Jung SK, Lee KH, Jeon JW. et al. Physicochemical characterization of Remsima. MAbs 2014;6:1163–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Park W, Hrycaj P, Jeka S. et al. A randomised, double-blind, multicentre, parallel-group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT-P13 and innovator infliximab in patients with ankylosing spondylitis: the PLANETAS study. Ann Rheum Dis 2013;72:1605–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yoo DH, Hrycaj P, Miranda P. et al. A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis 2013;72:1613–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Takeuchi T, Yamanaka H, Tanaka Y. et al. Evaluation of the pharmacokinetic equivalence and 54-week efficacy and safety of CT-P13 and innovator infliximab in Japanese patients with rheumatoid arthritis. Mod Rheumatol 2015;25:817–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yoo DH, Racewicz A, Brzezicki J. et al. A Phase III randomized study to evaluate the efficacy and safety of CT-P13 compared with reference infliximab in patients with active rheumatoid arthritis: 54-week results from the PLANETRA study. Arthritis Res Ther 2016;18:82.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yoo DH, Prodanovic N, Jaworski J. et al. Efficacy and safety of CT-P13 (infliximab biosimilar) in patients with rheumatoid arthritis: comparison between switching from reference infliximab to CT-P13 and continuing CT-P13 in the PLANETRA extension study. Ann Rheum Dis 2017;76:355–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tanaka Y, Yamanaka H, Takeuchi T.. Safety and efficacy of CT-P13 in Japanese patients with rheumatoid arthritis in an extension phase or after switching from infliximab. Mod Rheumatol 2017;27:237–45. [DOI] [PubMed] [Google Scholar]
- 25. Park W, Yoo DH, Jaworski J. et al. Comparable long-term efficacy, as assessed by patient-reported outcomes, safety and pharmacokinetics, of CT-P13 and reference infliximab in patients with ankylosing spondylitis: 54-week results from the randomized, parallel-group PLANETAS study. Arthritis Res Ther 2016;18:25.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Park W, Yoo DH, Miranda P. et al. Efficacy and safety of switching from reference infliximab to CT-P13 compared with maintenance CT-P13 in ankylosing spondylitis. 102-week data from the PLANETAS extension study. Ann Rheum Dis 2017;76:346–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Benucci M, Gobbi FL, Bandinelli F. et al. Safety, efficacy and immunogenicity of switching from innovator to biosimilar infliximab in patients with spondyloarthritis: a 6-month real-life observational study. Immunol Res 2017;65:419–22. [DOI] [PubMed] [Google Scholar]
- 28. Nikiphorou E, Kautiainen H, Hannonen P. et al. Clinical effectiveness of CT-P13 (infliximab biosimilar) used as a switch from Remicade (infliximab) in patients with established rheumatic disease. Report of clinical experience based on prospective observational data. Expert Opin Biol Ther 2015;15:1677–83. [DOI] [PubMed] [Google Scholar]
- 29. Glintborg B, Sørensen IJ, Loft AG. et al. A nationwide non-medical switch from originator infliximab to biosimilar CT-P13 in 802 patients with inflammatory arthritis: 1-year clinical outcomes from the DANBIO registry. Ann Rheum Dis 2017;76:1426–31. [DOI] [PubMed] [Google Scholar]
- 30. Jørgensen KK, Olsne IC, Goll GL. et al. Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NORSWITCH): a 52-week, randomised, double-blind, noninferiority trial. Lancet 2017;389:2304–16. [DOI] [PubMed] [Google Scholar]
- 31.Sint Maartenskliniek Nijmegen, Maartenskliniek Woerden, Radboud University Medical Center Nijmegen, Rijnstate Ziekenhuis. The effect of switching treatment from innovator infliximab to infliximab biosimilar on efficacy, safety and immunogenicity in patients with rheumatoid arthritis, spondyloarthritis or psoriatic arthritis in daily clinical care [Nederlands Trial Register no. NTR5279]. Amsterdam: Dutch Cochrane Centre; 2015. http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=5279 (16 January 2017, date last accessed).
- 32. European Medicines Agency. Summary of opinion (initial authorization). Flixabi. 1 April 2016. http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion_-_Initial_authorisation/human/004020/WC500203991.pdf (3 October 2016, date last accessed).
- 33. Hong J, Lee Y, Lee C. et al. Physicochemical and biological characterization of SB2, a biosimilar of Remicade. MAbs 2017;9:364–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shin D, Kim Y, Kim YS, Körnicke T, Fuhr R.. A randomized phase I pharmacokinetic study comparing SB2 and infliximab reference product (Remicade®) in healthy subjects. BioDrugs 2015;29:381–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Choe J-Y, Prodanovic N, Niebrzydowski. et al. A randomised, double-blind, Phase III study comparing SB2, an infliximab biosimilar, to the infliximab reference product Remicade in patients with moderate-to-severe rheumatoid arthritis despite methotrexate therapy. Ann Rheum Dis 2017;76:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Choe J-Y, Prodanovic N, Niebrzydowski J. et al. A randomized, double-blind, phase III study comparing SB2, an infliximab biosimilar, to the infliximab reference product (Remicade®) in patients with moderate to severe rheumatoid arthritis despite methotrexate therapy: 54-week results. Am Coll Rheumatol 2015;67:2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Smolen JS, Choe J-Y, Prodanovic N. et al. Comparable safety and immunogenicity and sustained efficacy after transition to SB2 (an infliximab biosimilar) vs ongoing infliximab reference product in patients with rheumatoid arthritis: results of Phase III transition study. Arthritis Rheumatol 2016;68(Suppl 10):2596. [Google Scholar]
- 38. Enbrel Summary of Product Characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000262/WC500027361.pdf (3 October 2016, date last accessed).
- 39.Biogen. Data on File. 2016.
- 40.Benepali Summary of Product Characteristics. https://www.medicines.org.uk/emc/medicine/31511 (3 October 2016, date last accessed).
- 41. European Medicines Agency. Assessment report. Benepali. 19 November 2015. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/004007/WC500200380.pdf (3 October 2016, date last accessed).
- 42. Cho IH, Lee N, Song D. et al. Evaluation of the structural, physicochemical and biological characteristics of SB4, a biosimilar of etanercept. MAbs 2016;8:1136–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lee YJ, Shin D, Kim Y. et al. A randomised Phase l pharmacokinetic study comparing SB4 and etanercept reference product (Enbrel®) in healthy subjects. Br J Pharmacol 2016;82:64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Emery P, Vencovský J, Sylwestrzak A. et al. A phase III randomised, double-blind, parallel-group study comparing SB4 with etanercept reference product in patients with active rheumatoid arthritis despite methotrexate therapy. Ann Rheum Dis 2017;76:51–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vencovsky J, Anna Sylwestrzak A, Leszczyñski P.. A Phase III, randomized, double-blind clinical study comparing SB4, an etanercept biosimilar, with etanercept reference product (Enbrel®) in patients with moderate to severe rheumatoid arthritis despite methotrexate therapy (52-week results). Arthritis Rheumatol 2015;67(Suppl 10):2055. [Google Scholar]
- 46. Emery P, Vencovský J, Sylwestrzak A. et al. Long term safety and efficacy of SB4 (etanercept biosimilar) in patients with rheumatoid arthritis: comparison between continuing SB4 and switching from etanercept reference product to SB4. Ann Rheum Dis 2016;75:236.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Novartis Press Release. Sandoz advances its biosimilars program with European Medicines Agency (EMA) acceptance of regulatory submission for biosimilar etanercept. 8 December 2015. https://www.novartis.com/news/media-releases/sandoz-advances-its-biosimilars-program-european-medicines-agency-ema-acceptance (3 October 2016, date last accessed).
- 48. Novartis Press Release. New data shows Sandoz biosimilar etanercept candidate has equivalent efficacy to originator product. 7 July 2016. https://www.novartis.com/news/media-releases/new-data-shows-sandoz-biosimilar-etanercept-candidate-has-equivalent-efficacy (3 October 2016, date last accessed).
- 49. Hofmann HP, Kronthaler U, Fritsch C. et al. Characterization and non-clinical assessment of the proposed etanercept biosimilar GP2015 with originator etanercept (Enbrel®). Expert Opin Biol Ther 2016;16:1185–95. [DOI] [PubMed] [Google Scholar]
- 50. von Richter O, Skerjanec A, Afonso M.. GP2015, a proposed etanercept biosimilar: Pharmacokinetic similarity to its reference product and comparison of its autoinjector device with prefilled syringes. Br J Clin Pharmacol 2017;83:732–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Griffiths CE, Thaci D, Gerdes S. et al. The EGALITY study: A confirmatory, randomised, double-blind study comparing the efficacy, safety and immunogenicity of GP2015, a proposed etanercept biosimilar, versus the originator product in patients with moderate to severe chronic plaque-type psoriasis. Br J Dermatol 2017;176:928–38. [DOI] [PubMed] [Google Scholar]
- 52. Flixabi Summary of Product Characteristics. 2016. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/004020/WC500208356.pdf (3 October 2016, date last accessed).
- 53. Möttönen T, Hannonen P, Leirisalo-Repo M. et al. Comparison of combination therapy with single-drug therapy in early rheumatoid arthritis: a randomised trial. FIN-RACo Trial Group. Lancet 1999;353:1568–73. [DOI] [PubMed] [Google Scholar]
- 54. Grigor C, Capell H, Stirling A. et al. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet 2004;364:263–9. [DOI] [PubMed] [Google Scholar]
- 55. Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart C. et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum 2005;52:3381–90. [DOI] [PubMed] [Google Scholar]
- 56. Scott DL, Ibrahim F, Farewell V. et al. Randomised controlled trial of Tumour necrosis factor inhibitors against combination intensive therapy with conventional disease-modifying antirheumatic drugs in established rheumatoid arthritis: the TACIT trial an associated systematic reviews. Health Technol Assess 2014;18:i–xxiv, 1–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hazlewood GS, Barnabe C, Tomlinson G. et al. Methotrexate monotherapy and methotrexate combination therapy with traditional and biologic disease modifying antirheumatic drugs for rheumatoid arthritis: abridged Cochrane systematic review and network meta analysis. BMJ 2016;353:i177.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. European Commission. What you need to know about biosimilar medicinal products. http://www.medicinesforeurope.com/wpcontent/uploads/2016/03/biosimilars_report_en.pdf (16 January 2017, date last accessed).
- 59. Braun J, Kudrin A.. Switching to biosimilar infliximab (CT-P13): evidence of clinical safety, effectiveness and impact on public health. Biologicals 2016;44:257–66. [DOI] [PubMed] [Google Scholar]
- 60. Tweehuysen L, van den Bemt BJF, van Ingen IL. et al. Clinical and immunogenicity outcomes after switching treatment from innovator infliximab to biosimilar infliximab in rheumatic diseases in daily clinical practice. Arthritis Rheumatol 2016;68(Suppl 10):627.26473409 [Google Scholar]
- 61. Dorner T, Strand V, Cornes P. et al. The changing landscape of biosimilars in rheumatology. Ann Rheum Dis 2016;75:974–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dorner T, Strand V, Cataneda-Hernandez G. et al. The role of biosimilars in the treatment of rheumatic diseases. Ann Rheum Dis 2013;72:322–8. [DOI] [PubMed] [Google Scholar]
- 63. McCarthy G, Ebel BC, Guy H.. Introduction of an infliximab biosimilar (CT-P13): A five-year budget impact analysis for the treatment of rheumatoid arthritis in Ireland. Value Health 2013;16:A558. [Google Scholar]
- 64. Kim J, Hong J, Kudrin A.. 5 year budget impact analysis of biosimilar infliximab for the treatment of rheumatoid arthritis in UK, Italy, France and Germany. Arthritis Rheumatol 2014;11(Suppl):S512. [Google Scholar]
- 65. Brodszky V, Baji P, Balogh O, Péntek M.. Budget impact analysis of biosimilar infliximab (CT-P13) for the treatment of rheumatoid arthritis in six Central and Eastern European countries. Eur J Health Econ 2014;15(Suppl 1):S65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Dranitsaris G, Amir E, Dorward K.. Biosimilars of biological drug therapies: regulatory, clinical and commercial considerations. Drugs 2011;71:1527–36. [DOI] [PubMed] [Google Scholar]
- 67. GABI. Australia's PBAC recommends substitution of biosimilars. 2015. http://www.gabionline.net/Biosimilars/General/Australia-s-PBAC-recommendssubstitution-of-biosimilars (13 March 2017, date last accessed).