Abstract
BACKGROUND
Endometriosis is typically regarded as a premenopausal disease, resolving after natural or iatrogenic menopause due to declining oestrogen levels. Nonetheless, case reports over the years have highlighted the incidence of recurrent postmenopausal endometriosis. It is now clear that both recurrence and malignant transformation of endometriotic foci can occur in the postmenopausal period. Postmenopausal women are commonly treated with hormone replacement therapy (HRT) to treat climacteric symptoms and prevent bone loss; however, HRT may reactivate endometriosis and stimulate malignant transformation in women with a history of endometriosis. Given the uncertain risks of initiating HRT, it is difficult to determine the best menopausal management for this group of women.
OBJECTIVE AND RATIONAL
The aim of this study was to systematically review the existing literature on management of menopausal symptoms in women with a history of endometriosis. We also aimed to evaluate the published literature on the risks associated with HRT in these women, and details regarding optimal formulations and timing (i.e. initiation and duration) of HRT.
SEARCH METHODS
Four electronic databases (MEDLINE via OVID, Embase via OVID, PsycINFO via OVID and CINAHL via EbscoHost) were searched from database inception until June 2016, using a combination of relevant controlled vocabulary terms and free-text terms related to ‘menopause’ and ‘endometriosis’. Inclusion criteria were: menopausal women with a history of endometriosis and menopausal treatment including HRT or other preparations. Case reports/series, observational studies and clinical trials were included. Narrative review articles, organizational guidelines and conference abstracts were excluded, as were studies that did not report on any form of menopausal management. Articles were assessed for risk of bias and quality using GRADE criteria.
OUTCOMES
We present a synthesis of the existing case reports of endometriosis recurrence or malignant transformation in women undergoing treatment for menopausal symptoms. We highlight common presenting symptoms, potential risk factors and outcomes amongst the studies. Sparse high-quality evidence was identified, with few observational studies and only two randomized controlled trials. Given this paucity of data, no definitive conclusions can be drawn concerning risk.
WIDER IMPLICATIONS
Due to the lack of high-quality studies, it remains unclear how to advise women with a history of endometriosis regarding the management of menopausal symptoms. The absolute risk of disease recurrence and malignant transformation cannot be quantified, and the impact of HRT use on these outcomes is not known. Multicentre randomized trials or large observational studies are urgently needed to inform clinicians and patients alike.
Keywords: endometriosis, menopause, HRT, unopposed oestrogen, combined HRT, tibolone, recurrence, malignant transformation
Introduction
Endometriosis and oestrogen dependence
Endometriosis is a disease that affects an estimated 6–10% of reproductive aged women, totalling approximately 176 million women worldwide (Bulun, 2009). It is defined as the presence of endometrial-like tissue in extrauterine locations and is a chronic condition associated with debilitating pelvic pain, dyspareunia, dysuria, dysmenorrhoea and infertility. However, due to a lack of reliable diagnostic tools and the non-specific nature of the symptoms, there exists a widely recognized delay in diagnosis of 8–10 years (Ahn et al., 2017). Consequently, the economic impact is substantial, as chronic and debilitating pain from endometriosis may hinder work productivity, while infertility can cause major psychosocial and financial strain to affected women and their partners (Simoens et al., 2007, 2012).
The pathophysiology of endometriosis is complex and not completely understood. Sampson's retrograde menstruation theory, which states that endometrial cells travel backwards through the fallopian tubes during menses to reach the peritoneal cavity, has gathered the most robust support (Vercellini et al., 2014). Oestrogen dependence, progesterone resistance, inflammation and genetic predisposition represent some of the pathophysiological hallmarks of this disease (Burney and Giudice, 2012). The central feature is oestrogen-dependent growth. Endometriotic lesions pathologically overexpress oestrogen receptor beta (ERβ) (>100× higher expression compared to endometrial tissue) and have been demonstrated to express (i) high levels of steroidogenic acute regulatory protein (StAR) and P450 aromatase, and (ii) reduced levels of 17beta hydroxysteroid dehydrogenase Type 2. This expression profile results in locally elevated levels of the biologically active form of oestrogen (oestradiol) (Kitawaki et al., 2002; Bulun et al., 2012). These molecular studies are supported by clinical observations of disease regression, symptom relief and alleged ‘cures’ for endometriosis as women achieve a hypo-oestrogenic state through iatrogenic or natural menopause (Inceboz, 2015).
Transition to menopause
Understanding the altered hormonal milieu in endometriosis has enabled clinicians to exploit oestrogen dependence in their management, prescribing medications to suppress ovarian function or alter local oestrogenic effects. However, in severely symptomatic cases, first-line medical therapy (including the oral contraceptive pill or progestogens) or laparoscopic excision of endometriotic lesions may prove insufficient, and induction of menopause via GnRH analogues or oophorectomy is indicated (Dunselman et al., 2014). Surgically or medically induced menopause is associated with a swift and dramatic fall in oestrogen levels. This decline may relieve endometriosis-related symptoms, but can simultaneously trigger menopausal symptoms. These symptoms are diverse and include hot flushes, vaginal dryness, sleep and mood disturbances, night sweats and painful intercourse, among others. While these symptoms occur in many women who naturally transition into menopause, they are especially prevalent and severe in women with a sudden onset of the hypoestrogenic state (Hendrix, 2005). The gold standard for treatment of menopausal symptoms has traditionally been hormone replacement therapy (HRT). HRT has been crucial for achieving symptom relief and improving the quality of life of millions of menopausal women, although these successes have been accompanied by safety concerns regarding specific preparations and dosages (Manson et al., 2013).
Appropriateness of HRT
Many studies have explored the efficacy and safety of HRT in postmenopausal women with climacteric symptoms (Rossouw et al., 2002; Rozenberg et al., 2013); however, few studies have investigated the use of hormonal therapy in postmenopausal women with a history of endometriosis. Two specific concerns are present in this group of women. Firstly, there is the possibility that exogenous oestrogen will reactivate growth of endometriotic deposits and cause symptomatic recurrence. Secondly, there is a concern that oestrogen will promote malignant transformation of residual endometriotic tissue. Sampson first described malignant transformation of ovarian endometriosis in 1925 (Sampson, 1925) and although its pathogenesis is not fully understood, oxidative stress, inflammation and an altered hormonal milieu have been implicated as contributing factors (Nezhat et al., 2014). Malignant transformation is thought to be a multistep pathway in which normal endometriotic tissue progresses to an atypical intermediate stage, and finally to invasive carcinoma (Gadducci et al., 2014). These sequential steps towards malignancy have been associated with genetic alternations in PTEN, TP53 and ARID1A and have been demonstrated in endometriosis-associated cancers (Munksgaard and Blaakaer, 2012). In a recent animal study using a rodent model of endometriosis (adult female Sprague-Dawley rats, aged 8–12 weeks), treatment with unopposed oestrogen successfully induced malignant transformation of endometriotic foci (Wang et al., 2015). Mechanistically, oestrogens affect PTEN expression in human endometrial cells and are associated with increased proliferation, direct cell damage and increased risk of acquiring somatic mutations (Turbiner et al., 2008).
However, the impact of declining oestrogen levels should not be underestimated. Menopausal symptoms affect the lives of millions of women worldwide. The hypo-oestrogenic state can significantly impair the quality of life by making sexual intercourse uncomfortable or painful, causing sleep deprivation, or resulting in mood changes. Furthermore, declining systemic oestrogen levels are a risk factor for cardiovascular and bone disease (Gallagher, 2007; Rosano et al., 2007). The use of HRT has been shown to reduce the risk of such conditions and improve the quality of life of symptomatic women (Langer, 2017).
The decision whether or not to prescribe HRT in general, and particularly in women with a history of endometriosis, is therefore a complex clinical decision and may also take into account other risk factors, such as residual disease after surgery (Clayton et al., 1999) and obesity which causes increased aromatase activity in peripheral tissues resulting in higher systemic oestrogen levels (Zanetta et al., 2000).
Our study aimed to conduct a systematic review of the literature investigating a critical question: What is the current evidence on the management of menopausal symptoms in women with a history of endometriosis? We aimed to cover the literature on a number of sub-questions, including: What are the various treatment options to manage menopausal symptoms in these women? What are the risks associated with HRT in this cohort? Should HRT be given immediately following surgically induced menopause or be delayed? What preparations are most appropriate, and for how long should treatment be given? We aimed to synthesize the literature in a comprehensive manner, and hoped to aid the design of future research in this area. Given the prevalence of endometriosis and the inevitability of eventual menopause in these women, this is clearly an important question that warrants robust, evidence-based guidelines.
Methods
This systematic review was registered and accepted for inclusion in PROSPERO (Gemmell et al., 2016) in July 2016 (PROSPERO ID number: CRD42016042024).
Search strategy
We searched four electronic databases (MEDLINE via OVID, Embase via OVID, PsycINFO via OVID and CINAHL via EbscoHost), from database inception until 26 June 2016, using a combination of relevant controlled vocabulary terms and free-text terms searched in the title or abstract fields related to ‘menopause’ and ‘endometriosis’. No study type, language or date limits were applied to the search. An example of the search strategy used for the MEDLINE database is included in Supplementary Table S1.
Inclusion criteria
All retrieved studies were uploaded to EndNote and duplicates were deleted. One reviewer (L.G.) sifted the full library (titles/abstracts), and two reviewers (K.W., C.B.) sifted 10% of the library (randomly selected using EndNote Record Number) to assess concordance. The full text of potentially relevant articles was retrieved to assess whether the paper should be included. Inclusion criteria were that the study population included postmenopausal women with a confirmed, or clinically suspected, history of endometriosis, and the article discussed management of menopausal symptoms. All study designs were included (case reports, observational studies and clinical trials). We excluded articles that did not discuss any form of menopausal management (e.g. HRT, tibolone or other preparations). We excluded narrative review articles and organizational guidelines in an attempt to focus the review on primary literature. Conference abstracts were also excluded.
The reviewers shared their lists of included studies, and concordance was determined. When there were disparities in the list, consensus was reached through discussions between the reviewers.
Quality assessment
Quality of included studies was assessed independently by two reviewers (L.G., K.W.) using the GRADE criteria (Guyatt et al., 2011). Assigned ratings were compared and a third reviewer (C.B.) was consulted when there were disagreements.
Data extraction
Data were extracted into a standard form by one reviewer (L.G). For case reports, the following information was extracted: patient age at presentation, presenting symptoms, ureteral involvement (yes or no), type (surgical vs. natural) and timing (years previously) of menopause, stage and extent of endometriosis before menopause, reported menopausal symptoms, treatment provided, duration of follow-up, method of outcome assessment, outcome (recurrence, malignant transformation, side effects, mortality) and recommendation (if provided).
For all other study types, information on study design, study objective, sample size, participants’ characteristics, intervention, method of outcome assessment, outcome, duration of follow-up and recommendation (if provided) was recorded.
Data synthesis and analysis
The nature of the evidence retrieved by our search (predominantly case reports, and a small number of heterogeneous observational studies and clinical trials) meant that meta-analysis was not possible, thus a narrative synthesis of the data is provided.
Where possible, if not reported in the original article, risk ratios (RRs) for specific outcomes were calculated using RevMan (Review Manager (RevMan) [Computer program]. Version 5.3 2014).
Results
Included studies
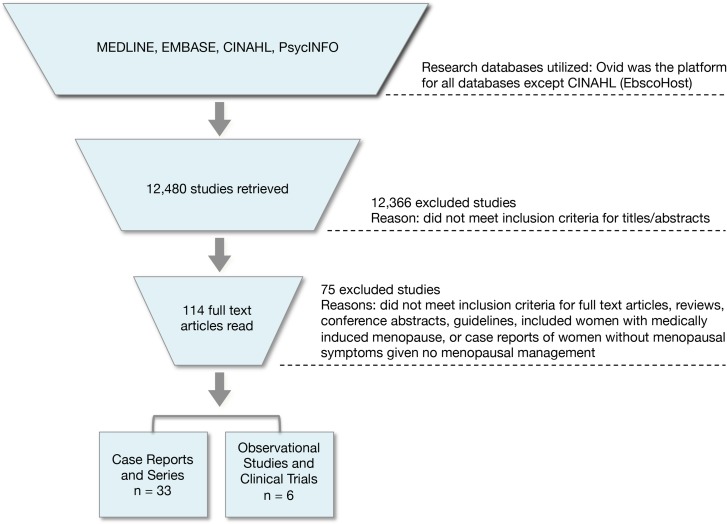
Searches across all four databases retrieved 17 488 studies. Duplicates (5008) were removed, leaving 12 480 studies (Fig. 1). After reading titles and abstracts, 12 366 failed to meet inclusion criteria. The full-text versions of the remaining 114 studies were read in their entirety. Of these 114 studies, 75 were excluded because they did not meet our inclusion criteria. This left 39 included studies: 33 case reports and 6 observational studies and clinical trials.
Figure 1.
Flow diagram depicting inclusion and exclusion decisions throughout the review process.
The majority of relevant articles identified by the search were individual case reports or case series describing the recurrence of endometriosis or malignant transformation in postmenopausal women. A small number of observational studies and clinical trials were also identified. We first present a summary of the data from case reports and case series to give context, before describing the results of the observational cohorts and trials.
Case reports and case series (33 studies; 48 patients)
There were 32 case reports/series including 42 patients identified by our search. An additional article describing endometriosis-related malignancies in six women who had taken oestrogen replacement was retrieved (Leiserowitz et al., 2003). This article is discussed separately, as insufficient data are reported for the individual women to enable inclusion in our summary statistics.
Outcome evidence provided by these reports was assessed as very low quality given their observational nature and inherent risk of publication bias. Summary characteristics of the 42 patients are presented in Table I. The age of included patients ranged between 30 and 75 years at presentation (mean age: 52 years). Of 42 patients, 40 had prior histories of endometriosis, either (i) confirmed by intraoperative visualization and/or histologically after laparoscopic excision (n = 34), (ii) suspected given the presence of symptoms (infertility, pelvic pain, menorrhagia) (n = 2), or (iii) assessed by unspecified methodology (n = 4). Two patients did not have premenopausal endometriosis diagnoses, but were speculated by the case report authors to have had such and are thus included in our analysis. There were 36 patients who went through a surgically induced menopause (procedures involving oophorectomy), and four patients went through menopause naturally (one of these four was diagnosed with premature ovarian insufficiency). An additional two patients are believed to have gone through natural menopause, and underwent oophorectomy at ages 57 and 60. HRT was given as treatment for (n = 12) or prevention of (n = 30) menopausal symptoms. The mean duration of HRT use prior to presentation was 7.8 years (range: 4 months to 20 years). Of 36 patients who had undergone hysterectomy, 31 used unopposed oestrogen therapy.
Table I.
Summary characteristics from case reports and series.
| Case reports and series (GRADE (Guyatt et al., 2011) scoring: very low quality) | |
|---|---|
| Number of patients (n) | 42 |
| Age range (years) (mean (years)) | 30–75 (52) |
| Type of menopause (n) |
|
| Mean duration of HRT (years) | 7.8 |
| Unopposed oestrogen (n) | 31 |
| Endometriosis recurrence (n) | 17 |
| Malignant transformation (n) | 25 |
| Mortality (n) | 3 |
The two main outcomes reported were endometriosis recurrence (n = 17) and malignant transformation (n = 25). For analysis, case reports and series were divided by these two main outcomes. One case series was included in both outcomes as it described three patients with endometriosis recurrence and one patient with malignant transformation (Taylor et al., 1999).
Endometriosis recurrence in women on HRT (17 patients)
Thirteen case reports and case series were identified reporting endometriosis recurrence in menopausal women given HRT for the treatment or prevention of menopausal symptoms. These included 17 patients between the ages of 30–65 (median age: 46 years) (Table II). All of the included women had undergone treatment with exogenous oestrogens in some form. Skor et al. (1977) was the earliest report retrieved by our search. This case was a 48-year-old Caucasian woman who presented with a 2-month history of painless haematuria and decreased urinary stream on voiding. She had undergone a total abdominal hysterectomy with bilateral salpingo-oophorectomy (TAH + BSO) with endometriosis found in the specimen and confirmed by histology. She had been prescribed conjugated oestrogens (Premarin 1.25 mg/day) following surgery and continued these for 6 years until her presentation. On physical examination, a 7 cm × 8 cm mass starting in the midline and extending to the left pelvic wall was palpated and the patient underwent cystoscopy. Postmenopausal bladder endometriosis was diagnosed histologically. Oestrogens were discontinued and intramuscular medroxyprogesterone acetate (1 gm per week) was administered for 2 months. Despite this, there was no significant alteration in the size of the mass. Shortly afterwards, due to symptom recurrence, the endometriotic lesion was removed surgically. The patient had no complaints 1-year post treatment. The authors commented that exogenous oestrogens play a role in the stimulation and development of postmenopausal endometriosis.
Table II.
Case reports and series reporting the postmenopausal recurrence of endometriosis after HRT in women with a history of endometriosis.
| Author, date (# patients) | Patient Age (years) | Presenting symptoms [ureter involvement] | Medical history and menopause | HRT [duration] | Diagnosis | Treatment [follow-up: patient status] |
|---|---|---|---|---|---|---|
|
48 | Painless haematuria + palpable bladder mass [No] |
|
|
Postmenopausal bladder endometriosis | Discontinued oestrogens + initiated Depo-Provera + surgery [1 year: no complaints] |
|
65 | Intermittent painless haematuria [No] |
|
|
Bladder endometriosis extending into bowel | Surgery [6 weeks: cystoscopy revealed normal appearing bladder and bimanual examination was normal] |
|
56 | Painless haematuria; two episodes of gross haematuria 10 days before admission [Yes] |
|
|
Postmenopausal ureteral endometriosis | Surgery [18 months: no complaints] |
|
64 | Painless haematuria [Yes] |
|
|
Ureteral obstruction secondary to adenomatous hyperplasia arising in endometriosis | Surgery [Not specified] |
|
|
|
|
Oestrogen-only HRT
|
|
|
|
54 | 5-month history of left iliac fossa pain, especially during her monthly withdrawal bleeding [No] |
|
|
8 cm left ovarian endometrioma adherent to sigmoid colon—uterus had extensive endometrial deposits on serosal surface | Surgery [2 years: no complaints, on Premarin to treat menopausal symptoms] |
|
30 | Recurrent haemoptysis and left-sided haemothorax [No] |
|
|
Recurrent thoracic endometriosis | Surgery [9 months: no complaints, advised to delay HRT] |
|
|
|
|
Oestrogen-only HRTAll used oestradiol implants
|
|
|
|
|
|
|
Oestrogen-only HRT and combined HRT
|
Both—endocervical endometriosis |
|
|
46 | Haematuria and rectal bleeding [No] |
|
|
5 cm endometriotic nodule in the sigmoid that was adherent to the bladder; florid endometriosis with polypoid endometrial mucosal lesions with vascular invasion involving both bowel and bladder | Surgery [Not specified] |
|
46 | 3 episodes of haemoptysis synchronous on 1st day of menstrual cycle [Unknown] |
|
|
Catamenial haemoptysis due to endobronchial endometriosis | Terlipressin + Discontinued HRT [2 years: no complaints] |
|
49 | Asymptomatic pelvic mass detected during routine examination [No] |
|
|
Intestinal endometriosis presenting as a pedunculated endometrioma | Tibolone + Surgery [Not specified] |
|
44 | Painless vaginal bleeding for 4 weeks [Yes] |
|
|
Endometriotic mass (8 × 7 × 6.5) involving the vaginal mucosa and invading into the rectal wall | Surgery [3 months: uneventful recovery and ileostomy was closed] |
TAH, total abdominal hysterectomy; SO, salpingo-oophorectomy; ERT, oestrogen-replacement therapy.
Surgical menopause refers to procedures involving bilateral oophorectomy.
Sixteen other similar accounts were retrieved from our search, with the latest report published in 2009 (Giarenis et al., 2009). The majority of cases (12 out of 17) were women with a prior hysterectomy, who took unopposed oestrogen. The remaining cases of recurrence were in women who took combined HRT.
Severity of prior endometriosis and menopause
Six patients had a history of ‘extensive’ or ‘severe’ endometriosis (Manyonda et al., 1989; Joseph et al., 1994; Taylor et al., 1999; Badawy et al., 2004). Fourteen patients underwent surgical menopause years before presentation (Skor et al., 1977; Stewart and Ireland, 1977; Kapadia et al., 1984; Ray et al., 1985; Manyonda et al., 1989; Joseph et al., 1994; Taylor et al., 1999, 2005; Badawy et al., 2004; Giarenis et al., 2009), while only two transitioned naturally to menopause (Goh and Hall, 1992; Chahine et al., 2007). One patient entered menopause as a result of premature ovarian insufficiency (Mattar et al., 2008). The median time between surgical menopause and presentation was 7.1 years (range: 4 months to 13 years).
Menopausal management
Unopposed oestrogen was implicated in numerous cases of symptom recurrence (n = 12) (Skor et al., 1977; Stewart and Ireland, 1977; Kapadia et al., 1984; Ray et al., 1985; Manyonda et al., 1989; Taylor et al., 1999, 2005; Badawy et al., 2004; Giarenis et al., 2009). Fewer studies reported recurrence in women who were using combined hormonal preparations (oestrogen and progestagen) (n = 5) (Goh and Hall, 1992; Joseph et al., 1994; Badawy et al., 2004; Chahine et al., 2007; Mattar et al., 2008). In terms of method of oestrogen administration, oral tablets (n = 5) (Skor et al., 1977; Stewart and Ireland, 1977; Kapadia et al., 1984; Ray et al., 1985; Goh and Hall, 1992), implants (n = 6) (Manyonda et al., 1989; Taylor et al., 1999, 2005) and patches (n = 3) (Badawy et al., 2004; Taylor et al., 2005) were all reported. Table III provides information on HRT dosages and regimens; however, variability in the level of detail provided by the included case reports and series limits these data.
Table III.
HRT dosages and regimens associated with recurrent endometriosis in case reports and series.
| HRT type | Dosages and regimens associated with recurrent postmenopausal endometriosis (n = 17 patients) |
|---|---|
| Oestrogen-only | 1. Premarin 1.25 mg/day (Skor et al., 1977) |
| 2. Premarin (Kapadia et al., 1984) | |
| 3. Oestrogens 2.5 mg/day (Ray et al., 1985) | |
| 4. 2.5 mg oestrogen/day, but taking twice the recommended dose (Stewart and Ireland, 1977) | |
| 5. Oestrogen patch 0.05 mg twice a week (Badawy et al., 2004) | |
| 6. Conjugated oestrogen 1.25 mg/day (Giarenis et al., 2009) | |
| 7. Oestradiol implant 100 mg 6 monthly for 5 years, subsequent 2 years of taking no hormones, then 2 weeks before admission felt run down and took three 10 μg tablets of ethinyloestradiol on consecutive days (Manyonda et al., 1989) | |
| 8. 100 mg oestradiol annual hormone implants (Manyonda et al., 1989) | |
| 9. Oestradiol implant (Taylor et al., 1999) | |
| 10. Oestradiol implant (Taylor et al., 1999) | |
| 11. Oestradiol implant (Taylor et al., 1999) | |
| 12. First oestradiol implant, then Evorel 50 patches (Taylor et al., 2005) | |
| Combined | 13. Conjugated equine oestrogens 625 mcg, norethisterone 150 mcg cyclically (Mattar et al., 2008) |
| 14. Premarin 0.625 mg daily and Provera 10 mg daily for 12 days a month (Goh and Hall, 1992) | |
| 15. Oestrogen + progesterone (Joseph et al., 1994) | |
| 16. Oestrogen patch 0.05 mg weekly followed by various regimens of oestrogen and progestogen (Badawy et al., 2004) | |
| 17. Oestradiol and cyproterone (Chahine et al., 2007) |
Presenting symptoms and sites of recurrence
As may be expected, endometriosis recurrence commonly presented with pain (n = 7): in locations typical of premenopausal endometriosis, i.e. abdomen (Taylor et al., 1999), iliac fossae (Manyonda et al., 1989; Goh and Hall, 1992), genitals (Taylor et al., 1999); and in more unusual sites such as the loin (Manyonda et al., 1989). Abnormal bleeding was also a common presentation (n = 14), including postmenopausal vaginal bleeding (Taylor et al., 1999; Badawy et al., 2004; Giarenis et al., 2009), haematuria (Skor et al., 1977; Stewart and Ireland, 1977; Kapadia et al., 1984; Ray et al., 1985; Taylor et al., 2005), rectal bleeding (Taylor et al., 2005) and also haemoptysis (Joseph et al., 1994; Chahine et al., 2007). Two women were reported to have pelvic masses (Skor et al., 1977; Mattar et al., 2008).
Sites of recurrence included: the genitourinary tract (n = 14), i.e. bladder (Skor et al., 1977; Stewart and Ireland, 1977; Taylor et al., 2005), ureter (Kapadia et al., 1984; Ray et al., 1985; Manyonda et al., 1989; Taylor et al., 1999; Giarenis et al., 2009), ovary (Goh and Hall, 1992), cervix (Badawy et al., 2004), vagina (Taylor et al., 1999; Giarenis et al., 2009); gastrointestinal organs (n = 4), i.e. the bowel (Stewart and Ireland, 1977; Taylor et al., 2005; Mattar et al., 2008) and rectum (Giarenis et al., 2009); and the pulmonary system (n = 2) including the lung (Joseph et al., 1994) and bronchus (Chahine et al., 2007). Mattar et al. (2008) reported an unusual presentation involving an ovarian endometriotic cyst adherent to the small bowel with a solitary vascular pedicle (Mattar et al., 2008). The authors hypothesized spillage during previous surgery and reactivation with hormonal therapy was responsible for this presentation.
Management of postmenopausal endometriosis recurrence
Management was tailored to location and extent of recurrence. All cases, except for one (Chahine et al., 2007), required surgical excision of endometriotic tissue. Some patients required additional medical therapy involving Depo-Provera (Skor et al., 1977), Danazol (Manyonda et al., 1989) and Tibolone (Taylor et al., 1999; Mattar et al., 2008). Three patients resumed HRT after surgery (Goh and Hall, 1992; Taylor et al., 1999; Badawy et al., 2004), two of whom were prescribed oestrogen-only formulations (Goh and Hall, 1992; Badawy et al., 2004).
Outcomes were generally favourable, although reporting bias may have contributed to this finding. All patients with reported follow-up (range: immediate postoperative checks to 2 years post treatment) experienced symptom regression with no relapses in the follow-up period (Skor et al., 1977; Stewart and Ireland, 1977; Kapadia et al., 1984; Manyonda et al., 1989; Goh and Hall, 1992; Joseph et al., 1994; Taylor et al., 1999; Chahine et al., 2007; Giarenis et al., 2009).
Malignant transformation (25 patients)
Twenty case reports and series of malignant transformation of endometriotic foci in postmenopausal women with a history of endometriosis on HRT were identified. This included a total of 25 patients between the ages of 38 and 75 years old (mean: 56 years) (Table IV). An additional study by Leiserowitz and colleagues (2003) (Leiserowitz et al., 2003) detailing the malignant transformation of endometriosis in six postmenopausal women on oestrogen-only HRT (mean duration: 23.4 years) is included in Table IV, but will be discussed separately.
Table IV.
Case reports and series reporting malignant transformation of endometriotic foci after HRT in women with a history of endometriosis.
| Author, date (# patients) | Patient age (years) | Presenting symptoms [ureter involvement] | Medical history and menopause | HRT [duration] | Diagnosis | Treatment [follow-up: patient status] |
|---|---|---|---|---|---|---|
|
48 | Mild right-sided lower abdominal pain, urinary frequency, constipation for 2 months [No] |
|
|
Clear cell carcinoma arising in endometriosis of the retroperitoneum | Surgery + radiation [22 months: no evidence of disease] |
|
66 | Light vaginal bleeding [No] |
|
|
Endometrioid carcinoma arising in an endometriotic lesion of the cul-de-sac | Surgery [Unclear] |
|
|
|
|
Both oestrogen-only HRT Both conjugated oestrogen tablets
|
|
|
|
62 | Pelvic mass [No] |
|
|
Endometrioid adenocarcioma (extraluminal rectosigmoid tumour 10 cm in diameter and closely adherent to the bladder) | Surgery + radiation [6 weeks: patient deceased] |
|
38 | Intermittent vaginal bleeding of 8 weeks duration, ulcerated area over vaginal vault, polyp-like lesion on vaginal vault found 4 weeks later [No] |
|
|
Endometrial adenocarcinoma arising from an endometriotic focus | Neoadjuvant progestin therapy + surgery + radiation [Not specified] |
|
42 | Massive ascites, 6L drained [No] |
|
|
Endometroid adenocarcinoma | Surgery + chemotherapy [24 months: no evidence of disease] |
|
48 | Right flank discomfort [Yes] |
|
|
Adenosquamous endometrioid carcinoma arising from disseminated pelvic endometriosis | Surgery [Not specified] |
|
56 | Lower abdominal pain, dyspareunia, pain with bowel movements, hirsutism [Yes] |
|
|
Androgen-producing endometrioid tumour of low malignant potential (borderline tumour) arising in endometriosis in the rectovaginal septum | Surgery + chemotherapy + progestin therapy [9 months: no evidence of disease] |
|
52 | Rectal bleeding and polyp arising from sigmoid colon [Yes] |
|
|
Well-differentiated endometrial adenocarcinoma arising from endometriosis of the rectosigmoid colon | Surgery [9 months: no evidence of disease] |
|
57 | Right lower-quadrant pain, recurrent macroscopic haematuria and weight loss of 8 kg in 3 months [Yes] |
|
|
Tubulopapillary endometrioid adenocarcinoma involving blood vessels | Surgery + radiation [18 months: no evidence of disease] |
|
|
|
|
|
|
|
|
54.9 (mean) | Not reported |
|
Oestrogen-only HRT (n = 6) [23.4 years (mean), 10–32 years (range)] |
|
Surgery, chemotherapy, radiation [26 months (mean): 70% survival] |
|
|
|
|
|
|
|
|
53 | Abnormal vaginal bleeding for 2 months [Yes] |
|
|
Endometrioid adenocarcinoma affecting the vagina, bladder and rectum | Surgery + chemotherapy [6 months: no evidence of disease] |
|
62 | Abdominal mass [No] |
|
|
Endometrioid adenocarcinoma arising from endometriosis of the mesocolon | Surgery + chemotherapy [28 months: no evidence of disease] |
|
75 | Chronic abdominal pain for 2 months, left pyelonephritis [Yes] |
|
|
Ureteral malignant mullerian carcinosarcoma in a context of florid endometriosis | Surgery + aromatase inhibitor [3 months: no evidence of disease] |
|
47 | Persistent and enlarging right groin mass, right lower-quadrant tenderness [No] |
|
|
Adenosarcoma arising in endometriosis | Surgery [12 months: no evidence of disease] |
|
68 | Left-sided pelvic mass, shortness of breath [No] |
|
Oestrogen-only HRT [13 years] | Metastatic endometrioid adenocarcinoma | Chemotherapy [24 months: patient is alive and well] |
|
66 | Abdominal/pelvic pain and mass, alteration of general state, 10 kg weight loss, constipation, dysuria [Yes] |
|
|
Extragenital endometrioid carcinoma in the vesico-uterine pouch arising from endometriosis | Surgery + chemotherapy + radiation [8 months: patient deceased] |
|
59 | 8-week history of constipation, tenesmus, 7 kg weight loss [No] |
|
|
Well-demarcated, cystic, endometrioid adenocarcinoma (endometriosis-associated intestinal tumour) | Surgery [Not specified] |
|
|
|
|
|
|
|
TAH/BSO, total abdominal hysterectomy and bilateral salpingo-oophorectomy.
aArticle describes a total of 27 women with endometriosis-related malignancy; however, it is unclear how many of these women were postmenopausal. A subgroup of women with extra-ovarian disease (n = 10) included eight women with a history of hysterectomy and BSO, and one further woman who was taking unopposed oestrogens (therefore was presumably menopausal). For the purposes of this analysis, only these 10 women are described.
Severity of prior endometriosis
About 13 patients had medical histories that noted endometriosis in more than one site (Brooks and Wheeler, 1977; Duun et al., 1993; Abu et al., 1997; Powell et al., 2001; Jones et al., 2002; Petersen et al., 2002; Soliman and Evans, 2004; Milam et al., 2006; Noel et al., 2006; Efthymiou, 2009; Karanjgaokar et al., 2009), and 13 patients had histories of ovarian endometriosis (Brooks and Wheeler, 1977; Duun et al., 1993; Abu et al., 1997; Jimenez et al., 2000; Powell et al., 2001; Montamedi, 2002; Petersen et al., 2002; Soliman and Evans, 2004; Milam et al., 2006; Efthymiou, 2009; Karanjgaokar et al., 2009). Some histories specified ‘severe endometriosis’ (n = 4) (Duun et al., 1993; Abu et al., 1997; Taylor et al., 1999; Efthymiou, 2009), while others reported ‘extensive endometriosis’ (n = 4) (Reimnitz et al., 1988; Soliman and Evans, 2004; Noel et al., 2006). Some histories included comorbidities such as leiomyomas (n = 6) (Brooks and Wheeler, 1977; Reimnitz et al., 1988; Powell et al., 2001; Areia et al., 2004; Soliman and Evans, 2004; Kawate et al., 2005) and adenomyosis (n = 5) (Brooks and Wheeler, 1977; Abu et al., 1997; Powell et al., 2001; Noel et al., 2006; Karanjgaokar et al., 2009). Patients typically underwent surgical menopause (n = 22) (Brooks and Wheeler, 1977; Reimnitz et al., 1988; Duun et al., 1993; Abu et al., 1997; Taylor et al., 1999; Jimenez et al., 2000; Powell et al., 2001; Jones et al., 2002; Montamedi, 2002; Petersen et al., 2002; Areia et al., 2004; Soliman and Evans, 2004; Kawate et al., 2005; Milam et al., 2006; Noel et al., 2006; Al-Talib et al., 2008; Efthymiou, 2009; Karanjgaokar et al., 2009). Two additional patients underwent oophorectomy at ages 60 (Klug et al., 1987) and 57 (Soliman and Evans, 2004) respectively; however, it was unclear in these two cases whether the patients had already naturally transitioned to menopause.
Menopausal hormonal preparations
HRT commonly consisted of unopposed oestrogens (n = 19) (Brooks and Wheeler, 1977; Klug et al., 1987; Reimnitz et al., 1988; Duun et al., 1993; Abu et al., 1997; Taylor et al., 1999; Jimenez et al., 2000; Powell et al., 2001; Jones et al., 2002; Montamedi, 2002; Petersen et al., 2002; Areia et al., 2004; Soliman and Evans, 2004; Kawate et al., 2005; Milam et al., 2006; Noel et al., 2006; Al-Talib et al., 2008; Karanjgaokar et al., 2009) for a median duration of 6.7 years (range 3–20 years). Conjugated equine oestrogens (Premarin 1.25 mg/day or 0.625 mg/day) were frequently mentioned (n = 8) (Brooks and Wheeler, 1977; Klug et al., 1987; Reimnitz et al., 1988; Jimenez et al., 2000; Powell et al., 2001; Soliman and Evans, 2004; Kawate et al., 2005). Oestradiol implants were also implicated (n = 4) (Abu et al., 1997; Taylor et al., 1999; Jones et al., 2002; Karanjgaokar et al., 2009), as well as oestrogen injections (n = 1) (Duun et al., 1993). One interesting case of ureteral malignant mullerian carcinosarcoma in a 75-year-old woman was associated with 5 years of taking a phytoestrogen supplement (highly concentrated soy isoflavone) (Noel et al., 2006).
Of note, two incidences of malignancy were reported in women who had discontinued HRT several years before presentation (Karanjgaokar et al., 2009). In a case series of women over 55 years old, one woman used oestrogen implants (50–100 mg every 6 months) for 14 years post hysterectomy. She had stopped this regimen for 8 years before presenting with malignancies (adenosarcoma and endometrioid adenocarcinoma). Another patient in this series was on a regimen of 50 mg oestradiol and 50 mg testosterone implants for 12 years post surgical menopause. She was not on any form of HRT for 4 years before presenting with an endometrioid adenocarcinoma.
Presentation
Patients in these case reports/series presented with symptoms related to the site, extent, and type of malignancy. Vaginal bleeding was common (n = 7) (Klug et al., 1987; Reimnitz et al., 1988; Abu et al., 1997; Areia et al., 2004; Soliman and Evans, 2004; Karanjgaokar et al., 2009; Suraweera et al., 2012), as was pain in the abdomen/pelvis/buttock (n = 11) (Brooks and Wheeler, 1977; Powell et al., 2001; Montamedi, 2002; Petersen et al., 2002; Milam et al., 2006; Noel et al., 2006; Chung et al., 2008; Karanjgaokar et al., 2009). Masses were also frequently reported (n = 9) (Duun et al., 1993; Petersen et al., 2002; Soliman and Evans, 2004; Kawate et al., 2005; Milam et al., 2006; Al-Talib et al., 2008; Chung et al., 2008). Less frequent presentations included weight loss (n = 3) (Montamedi, 2002; Chung et al., 2008; Efthymiou, 2009), constipation (n = 3) (Brooks and Wheeler, 1977; Chung et al., 2008; Efthymiou, 2009) and flank pain (n = 3) (Reimnitz et al., 1988; Jimenez et al., 2000).
Malignant transformation and management
Malignant transformation of endometriotic foci was commonly diagnosed using Sampson's (Sampson, 1925) and Scott's (Scott, 1953) criteria. Endometrioid adenocarcinoma was by far the most commonly diagnosed HRT-associated malignancy in patients with a history of endometriosis (n = 18) (Klug et al., 1987; Reimnitz et al., 1988; Duun et al., 1993; Abu et al., 1997; Taylor et al., 1999; Jones et al., 2002; Montamedi, 2002; Petersen et al., 2002; Areia et al., 2004; Soliman and Evans, 2004; Kawate et al., 2005; Al-Talib et al., 2008; Chung et al., 2008; Efthymiou, 2009; Karanjgaokar et al., 2009). Other histological types included adenosarcoma (n = 2) (Milam et al., 2006; Karanjgaokar et al., 2009), clear cell carcinoma (n = 1) (Brooks and Wheeler, 1977), mullerian carcinosarcoma (n = 1) (Noel et al., 2006), endometrial stromal sarcoma (n = 1) (Karanjgaokar et al., 2009) and an androgen-producing endometrioid borderline tumour (n = 1) (Powell et al., 2001). One interesting study reported an adenocarcinoma followed by an adenosquamous carcinoma arising in endometriotic foci 3 months later (Reimnitz et al., 1988).
Treatments varied based on histological type, grade and stage of the tumour. In only one case it was decided to forego surgical management and treat solely with chemotherapy (Al-Talib et al., 2008). In this case, the decision to initiate chemotherapy instead of surgery was based on her previous surgical history (two ileostomies) and poor prognosis due to advanced disease.
Adjuvant or neoadjuvant treatments in the form of chemotherapy (n = 9) (Reimnitz et al., 1988; Taylor et al., 1999; Powell et al., 2001; Areia et al., 2004; Kawate et al., 2005; Chung et al., 2008; Karanjgaokar et al., 2009), radiation (n = 7) (Brooks and Wheeler, 1977; Reimnitz et al., 1988; Duun et al., 1993; Abu et al., 1997; Montamedi, 2002; Soliman and Evans, 2004; Chung et al., 2008) or progestin therapy (n = 4) (Reimnitz et al., 1988; Abu et al., 1997; Powell et al., 2001) were frequently initiated. Mean follow-up was 19.4 months (range: 6 weeks to 5 years). Outcomes were generally favourable with no evidence of disease in 13 patients at follow-up (Brooks and Wheeler, 1977; Reimnitz et al., 1988; Taylor et al., 1999; Powell et al., 2001; Jones et al., 2002; Montamedi, 2002; Petersen et al., 2002; Areia et al., 2004; Soliman and Evans, 2004; Kawate et al., 2005; Milam et al., 2006; Noel et al., 2006). The patient treated solely with chemotherapy was alive and well at 2 years after presentation (Al-Talib et al., 2008).
Mortality
Although the majority of patients responded to treatment and were cured of their malignancy, three of the 25 patients diagnosed with an endometriosis-associated malignancy died as a result of their disease (Reimnitz et al., 1988; Duun et al., 1993; Chung et al., 2008). Reimnitz et al. (1988) reported the case of a 47-year-old woman with a history of extensive pelvic endometriosis. She was on conjugated oestrogens (Premarin) 0.625 mg for 5 days every week for 4 years. She was initially diagnosed with a grade two adenocarcinoma arising from an endometriotic focus and obstructing the left ureter. Subsequently she was also diagnosed with moderately differentiated adenosquamous carcinoma arising from endometriotic foci. The patient was treated with cisplatinum and cyclophosphamide chemotherapy, but died after 11 months. In the case reported by Duun et al. (1993), the patient was a 62-year-old woman with a history of severe endometriosis involving both ovaries and the rectovaginal septum. She had received intramuscular oestrogen injections for 20 years following a hysterectomy and bilateral oophorectomy. After 3 years with no treatment, she resumed another hormone substitution regimen (not specified) for hot flushes. Within a year of commencing this hormonal substitution, the patient presented with a pelvic mass diagnosed as endometrioid adenocarcinoma. About 6 weeks after tumour excision, recurrence was diagnosed and the patient died despite radiotherapy. Chung et al. (2008) reported the case of a 66-year-old woman who presented with abdominal/pelvic pain and mass, alteration of general state, 10 kg weight loss, constipation and dysuria. She had used combined HRT (oestradiol and medroxyprogesterone acetate) for 11 years. After being diagnosed with extragenital endometrioid carcinoma in the vesico-uterine pouch arising from endometriosis, she was treated with surgery, chemotherapy and radiation. The patient was deceased 8 months later.
One unique case series by Leiserowitz et al. (2003) identified by our search reported on larger numbers of women and thus is presented separately. They describe the management of 27 women with endometriosis-related malignancy, identified during a 7-year period (by their presentation to one of the authors, and review of pathology records). The authors include all women with endometriosis-related malignancy, rather than exclusively postmenopausal women. However, it is clear from the article that a number of participants were postmenopausal. In particular, 10 women were identified with extragonadal (non-ovarian) malignancy, and 9 of these were clearly menopausal (with either a medical history of hysterectomy/BSO, or reported as using HRT). Of these 10 women, their malignancies were histologically described as endometrioid (n = 5), adenosquamous (n = 2), papillary adenocarcinoma (n = 1) or adenocarcinomas not otherwise specified (n = 2). Within this group, six women had taken unopposed oestrogen therapy for a mean duration of 23.4 years (range 10–32 years). The authors therefore suggest that unopposed oestrogen use could be a risk factor for endometriosis-associated malignancy, especially of non-ovarian location. Treatments included surgery, chemotherapy and radiation with a 70% reported survival at follow-up (mean: 26.3 months).
Observational studies and clinical trials (6 studies)
Only six observational studies and clinical trials were identified by our search, highlighting the paucity of higher-level evidence in this area. These studies aimed to cover a variety of clinical questions, and the evidence for these is summarized below and in Table V. All assessed recurrence of endometriosis as their primary outcome.
Table V.
Quality assessment of observational and clinical trials assessing risk of endometriosis recurrence after HRT.
| Quality assessment | No of patients | Effect | Evidence quality | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Intervention | Control | Relative (95% CI) | |
| Comparison of HRT with no HRT | HRT | No treatment | ||||||||
| Matorras et al. (2002) | Randomized trial | Seriousa | Not serious | Not serious | Very seriousb | None | 4/115 (3.5%) | 0/57 (0.0%) | RR 4.50 (0.25 to 82.17)c |
|
| Rattanachaiyanont et al. (2003) | Observational study | Very seriousd | Not serious | Not serious | Very seriousb | None | 4/90 (4.4%) | 0/17 (0.0%) | RR 1.78 (0.10 to 31.64)c |
|
| Acien et al. (2013) | Observational study | Seriouse | Not serious | Not serious | Not applicablef | None | 0/11 (0%) | 0/8 (0%) | Not calculableg |
|
| Comparison of delayed HRT with immediate HRT | Delayed HRT | Immediate HRT | ||||||||
| Hickman et al. (1998) | Observational study | Not serious | Not serious | Not serious | Not serious | None |
|
|
HR 5.74 (1.31 to 25.23)h |
|
| Arumugam and Damodaran (1998) | Observational study | Very seriousi | Not serious | Not serious | Not applicable | None |
|
|
Not calculableg |
|
| Comparison of HRT with tibolone | HRT | Tibolone | ||||||||
| Fedele et al. (1999) | Randomized trial | Seriousj | Not serious | Not serious | Very seriousb | None | 4/10 (40.0%) | 1/11 (9.1%) | RR 4.40 (0.59 to 33.07)c |
|
| Comparison of oestrogen-only HRT with combined HRT | Oestrogen-only HRT | Combined HRT (continuous and cyclical) | ||||||||
| Rattanachaiyanont et al. (2003) | Observational study | Very seriousd | Not serious | Not serious | Very seriousb | None | 4/50 (8.0%) | 0/40 (0.0%) | RR 7.24 (0.40 to 130.54)c |
|
CI, confidence interval; HR, hazard ratio; HRT, hormone replacement therapy; RR, risk ratio.
aHigh risk of performance bias—single blinded study, with physician unaware of treatment allocation, but with access to hormone results (which would have indicated treatment with HRT or not). High risk of detection bias, as assessment for recurrence was only carried out if the clinician felt this was warranted, which may have been influenced by the participant (who was not blind to treatment allocation).
bVery wide CI for RR.
cRR calculated by the authors using Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
dHigh risk of selection bias as unclear why women were allocated to different HRT regimens (or no HRT). High risk of detection bias, as researchers would have been aware of the woman's HRT status when assessing presence of recurrence (by reviewing medical records).
eRisk of detection bias, as criteria for designating recurrence are not clearly stated.
fNot applicable as odds ratio and CI cannot be calculated.
gNo events in either group, therefore odds ratio not calculable.
hHR adjusted for stage of endometriosis, age at time of hysterectomy and postoperative adjunct medroxyprogesterone therapy.
iHigh risk of selection bias (unclear why some women started HRT after 3 months and some after 5 months), and high risk of detection bias (recurrence was only based on CA 125 levels).
jNo description of blinding for the trial, and no scoring system is reported for pain, therefore risk of detection bias.
Should HRT be given to women with previous endometriosis?
Given the concerns of possible disease reactivation or malignant transformation of endometriotic foci, it is reasonable to consider whether treatment with HRT is justifiable in this group of women. However, in a field dominated by case reports and series, it is challenging to obtain information on risk. Our search identified a single RCT and two cohort studies that give some insight regarding the risk of HRT in this cohort of women. All three studies were assessed as very low quality by GRADE criteria (Guyatt et al., 2011).
The only RCT in this area was a single centre study from Spain, including a total of 172 women (Matorras et al., 2002). All participants had a history of endometriosis and underwent BSO. Women were randomly allocated to treatment with combined HRT (50 μg oestradiol daily administered via patches and oral micronized progesterone for 14 days out of every 30 days) or no treatment. Participants were aware of their treatment allocation, although the clinician assessing them was not. In the treatment group, HRT was started 4 weeks following surgery. All women were followed up every 6 months with a clinical review, vaginal ultrasound and hormone measurements. Recurrence of endometriosis was identified either through histological confirmation, or by clinical findings (pelvic pain and/or pelvic mass) in association with pelvic ultrasound images suggestive of endometriosis. The overall absolute recurrence rate for endometriosis in this study was low at 2.3% (4/172). However, all women who experienced recurrence of endometriosis had been assigned to the HRT treatment arm (recurrence in 3.5% (4/115) of women compared to 0% (0/57) of women in the no treatment arm). The authors also suggested that the presence of residual endometrial tissue may be a possible risk factor for disease recurrence. In this cohort, the recurrence rate was 22.2% in women who had either a subtotal hysterectomy or BSO alone (2 out of 9 women). In contrast, the rate was only 1.9% (2 out of 106 women) in those who had total hysterectomy and BSO. The authors further suggest that a greater burden of disease may increase the risk of recurrence, as shown by an increased recurrence risk for women who had peritoneal involvement of greater than 3 cm, and a non-significant trend to increased recurrence with more advanced stages of endometriosis. The authors rightly noted that their study was underpowered to detect a statistically significant change in recurrence rates between the two groups of women. However, the study raises interesting possibilities for further research into the effect of disease stage and extent on recurrence rates.
One observational study included women who took postoperative HRT (of different regimens) and those who did not (Rattanachaiyanont et al., 2003). In this retrospective, single centre cohort, the authors identified 107 women who had undergone hysterectomy and BSO for treatment of endometriosis. Women were treated with a variety of HRT regimens (total n = 90, taking unopposed oestrogen, continuous combined HRT, or cyclic HRT) or no treatment (n = 17). Recurrence was only identified in four women and all were receiving HRT, specifically unopposed oestrogen therapy. Three women had recurrent pain, and one woman had a vaginal nodule, confirmed as endometriosis on histology.
One further observational study reported on outcomes of women with deep infiltrating endometriosis and colorectal or rectovaginal disease, who underwent surgery without bowel resection (Acien et al., 2013). This retrospective comparative cohort study was conducted in Spain and included women who were operated on at one of two hospitals. Of 42 patients, 19 had a hysterectomy and BSO, whilst the remainder had conservative surgery. Of the 19 women who underwent surgical menopause, 11 were subsequently treated with HRT, comprising 1–2 years of combined oestrogen/progesterone, followed by low dose oestrogen-only HRT or tibolone, continued indefinitely. The remaining 8 women did not receive HRT. The mean follow-up was 4.3 years (standard deviation 4.5, range 1–18). During this time, no women from either group were diagnosed with recurrence of endometriosis.
Should HRT be given immediately following surgical menopause?
Further questions arise for women who undergo surgical menopause. If small deposits of endometriotic tissue remain following surgery, these may be triggered to proliferate by exogenous oestrogens given as HRT and increase the risk of recurrence or malignant transformation. Therefore, there may be a theoretical benefit in delaying the start of HRT, by allowing time for residual endometriotic tissue to regress before commencing exogenous oestrogen. Two retrieved articles, a retrospective cohort study (Hickman et al., 1998) (GRADE: low quality) and non-comparative cohort (Arumugam and Damodaran, 1998) (GRADE: very low quality), attempted to investigate this question.
The retrospective cohort study (Hickman et al., 1998) included women who underwent TAH with BSO, identified from the medical records of a single institution during a period of 12 years (1979–1991). Two groups of women were identified: those who commenced HRT within 6 weeks of their surgery (n = 60) and those who delayed starting HRT for at least 6 weeks (n = 35, mean time to starting HRT 71.1 weeks, range 7–520 weeks). Women who did not receive HRT were excluded. Information on symptom recurrence was obtained through the medical records or telephone follow-up, but a precise definition of recurrence was not reported. The mean duration of follow-up was 4.5 years. In unadjusted analyses, 4/60 (6.7%) women who began HRT immediately had recurrent pain, compared to 7/35 (20%) women who began HRT later on. Of note, in their adjusted analyses, in which endometriosis stage, age and postoperative adjunct medroxyprogesterone therapy were considered, starting oestrogen-replacement therapy (ERT) more than 6 weeks after surgical menopause had a hazard ratio of 5.7 for pain recurrence (95% CI; 1.3, 25.2). The authors therefore conclude that there is no increase in the risk of recurrence for women who commenced ERT immediately, as compared to those who delayed treatment.
A non-comparative cohort study (Arumugam and Damodaran, 1998) prospectively followed 13 women at one institution in Malaysia undergoing TAH and BSO for moderate or severe endometriosis. Patients were premenopausal at recruitment and had their endometriotic activity assessed by blood CA 125 levels taken pre-operatively and post-operatively (monthly). Eight patients received conjugated oestrogens in the form of Premarin (oral dose of 0.625 mg/day) starting 5 months post surgery, and five patients received oestrogens 3 months post surgery. Preoperative CA 125 levels were high in all 13 patients and declined to normal post surgery. Levels did not rise during the 6-month follow-up period and patients remained well and asymptomatic.
What menopausal treatments are most appropriate for women with previous endometriosis?
If a woman with a history of endometriosis does decide to opt for HRT, then the next decision must be to choose the most suitable preparation. Again, there is limited high-quality evidence on which to base this decision. Two studies, retrieved by our search, provide some insight into this question. The first was a RCT (Fedele et al., 1999) comparing HRT using transdermal oestradiol with tibolone, and the second was an observational study (Rattanachaiyanont et al., 2003) comparing oestrogen-only HRT with combined HRT. Both were assessed as very low quality using the GRADE system.
The RCT (Fedele et al., 1999) compared HRT (n = 10, transdermal oestradiol 50 mg twice weekly plus cyclic medroxyprogesterone acetate 10 mg daily for women with a uterus) and tibolone (n = 11, 2.5 mg orally once a day) in women with residual endometriosis after bilateral oophorectomy. Patients were randomized into one of the two treatment groups and followed for 1 year. Four patients in the oestradiol group experienced moderate pelvic pain during treatment compared to only one patient in the tibolone group. Furthermore, one patient in the HRT group discontinued treatment at 8 months due to the development of dyspareunia and post-coital bleeding from a vaginal mucosal endometriotic deposit. The authors concluded that tibolone may be a safer alternative for postmenopausal women with residual endometriosis, although note that their trial was very small.
The observational cohort study (Rattanachaiyanont et al., 2003) attempted to compare various HRT regimens in women who had undergone hysterectomy and BSO for endometriosis. The majority of women (n = 50) were treated with unopposed oral oestrogen. Others were prescribed either continuous combined HRT (n = 24) or cyclical HRT (n = 16). Women received either conjugated equine oestrogens or oestradiol. Finally, a small group of women received no HRT (n = 17) and were viewed as controls. Women were followed up for a mean duration of 3.5 years (range 0.5–18 years). Although there were no statistically significant differences between the groups, the only episodes of recurrence (n = 4) were found in the oestrogen-only group. One woman who suffered with recurrent symptoms whilst taking oestrogen-only HRT also had relief of her symptoms when changing to a combined preparation. The authors conclude that HRT, particularly combined oestrogen and progestin regimens, is safe for postmenopausal women with underlying endometriosis.
Discussion
In response to concerns regarding an increased risk of breast cancer raised by the Women's Health Initiative (Rossouw et al., 2002) and the Million Women Study (Collaborators, 2003), HRT usage substantially decreased (Hersh et al., 2004). Nevertheless, millions of women continue to rely on hormonal preparations for menopausal symptom relief and HRT remains the most effective treatment for menopausal vasomotor symptoms and vulvar and vaginal atrophy (Schmidt, 2012). However, amongst women with a history of endometriosis, HRT may entail additional risks, and to date there are no high-quality evidence-based guidelines to guide clinical decisions.
The articles described here provide insight into the management of menopausal symptoms amongst women with a prior history of endometriosis. The case reports and series included in our review, while limited in their usefulness in assessing prevalence, indicate that recurrence of endometriosis and malignant transformations can occur in postmenopausal women. Observational studies and clinical trials have further investigated the contingent risks of different forms and timing of HRT treatments.
Recurrence
Endometriosis is not exclusively a premenopausal disease. About 13 case reports and series found 17 cases of recurrent endometriosis in postmenopausal women taking some form of HRT. These cases included women who underwent natural and surgical menopause; however, the vast majority underwent surgical menopause, perhaps indicating more severe premenopausal disease. Similarly, Vignali and colleagues (2005) reported substantial 5-year endometriosis recurrence rates, albeit amongst premenopausal women, of 43.5% (pain) and 28% (clinical disease) for women treated with conservative surgery (preservation of the uterus and at least one ovary) (Vignali et al., 2005).
In the case reports, symptoms of postmenopausal endometriosis were similar to those reported in premenopausal endometriosis (Mounsey et al., 2006): abnormal bleeding and pain. There are few papers describing the presentation of chronic pelvic pain (CPP) in postmenopausal women specifically. From clinical experience, symptoms are not cyclical unless a cyclical HRT preparation is used; however, pain associated with bowel and bladder function is common in postmenopausal women with CPP. Dyspareunia, especially superficially, may be associated with atrophic tissues but may also present in women using HRT with well-oestrogenized tissues. Deep dyspareunia, the more pathognomonic form of painful intercourse associated with endometriosis, is rarely described. Future research should investigate the prevalence of this symptom and whether and to what extent dyspareunia (superficial or deep) may contribute to postmenopausal sexual behaviour. Perhaps the most important difference in clinical practice, however, is the need to investigate new onset pelvic pain in a postmenopausal woman, whilst guidance on the management of CPP in reproductive age women emphasizes the need to avoid over-investigation and thus over-medicalization of the symptom when no underlying cause has previously been found (Home Page. Map of Medicine Web site. http://www.mapofmedicine.com/. [Accessed January 2017]). Findings from this review would suggest that investigation is needed when a postmenopausal woman is known to have had a previous diagnosis of endometriosis due to the added risk of malignant transformation.
The genitourinary system was the most common site of presentation, with many reports involving the ureter. This may represent the bias of case reports towards documenting more severe cases. Ureter involvement is a serious complication of endometriosis, capable of causing hydronephrosis and renal failure (Choi et al., 2015). It has been suggested that most cases of ureteral endometriosis in postmenopausal women are actually a result of delayed presentation with onset prior to menopause (Yohannes, 2003). However, due to a lack of clinically relevant biomarkers and sufficiently specific imaging techniques, the onset of endometriosis remains unclear (May et al., 2010; Dunselman et al., 2014).
Prognosis was generally favourable after excision of endometriotic tissue. Randomized controlled trials corroborate these findings, reporting decreased pain and symptoms after laparoscopic surgery for endometriosis, although these trials did not specifically investigate ureteric surgery (Duffy et al., 2014).
In three observational studies and one randomized clinical trial (Arumugam and Damodaran, 1998; Matorras et al., 2002; Rattanachaiyanont et al., 2003; Acien et al., 2013), there appeared to be a small association between HRT and endometriosis recurrence, but there were no statistically significant differences between treatment and control groups. The current literature assessing risks of HRT in women with a history of endometriosis are uncertain, due to paucity of sufficiently large, high-quality studies. Current guidelines, consensus statements and recommendations acknowledge this deficit, but continue to emphasize the benefits of HRT over the undefined risks for severely symptomatic women (Al Kadri et al., 2009; Johnson and Hummelshoj, 2013; Dunselman et al., 2014). However, many women with endometriosis who undergo surgical menopause are given hormonal replacement therapy as a prophylaxis before the development of menopausal symptoms. For these women, clinicians must balance the benefits to bone (Cauley et al., 2003) and cardiovascular health (Rossouw et al., 2007), particularly for younger patients, against the potential risks of recurrence or malignancy.
It is important to note that recurrence is possible even in the absence of HRT. There are reports of endometriosis recurrence in women not on any hormonal treatment (Fujiu et al., 2010; Bhat et al., 2014). In these women, other risk factors such as hyperestrogenemia and obesity may play larger roles in the pathogenesis (Punnonen et al., 1980). Incomplete definitive surgery and residual ovarian remnants are also considered risks factor for the development of postmenopausal endometriosis (Dmowski et al., 1988). It remains to be confirmed whether a genetic predisposition together with environmental factors, medication, or fat distribution increase the risk of endometriosis after menopause, as has been shown for premenopausal women (Rahmioglu et al., 2015a,b).
Malignant transformation
Our search retrieved 20 case reports and series (25 patients) of malignant transformation of endometriotic foci following HRT. Of 25 patients, 22 had undergone surgical menopause, which was not surprising given that many women had a history of severe disease with comorbidities such as leiomyomas and adenomyosis. Unopposed oestrogens were implicated in 19 patients, with conjugated equine oestrogens implicated in eight patients and oestradiol implants in four patients. Currently there are no data to indicate the absolute risk of malignant transformation in this group of women. It is likely that this is a rare outcome, but better data are urgently needed to enable women to make an informed decision about menopausal management. Fortunately, tumours arising from endometriosis are typically low grade and have a better prognosis (Heaps et al., 1990); only three deaths were reported in the literature identified by our search. Mortality was noted in the two case reports with patients who had histories of severe endometriosis and complicating factors, including increased age (Duun et al., 1993) or multiple malignancies (Reimnitz et al., 1988)
Considerations regarding type and timing of HRT
Adjusting the type and timing of the treatment plan may mitigate the potential risks of HRT highlighted by our case reports and series.
Type: oestrogen-only, combined or tibolone
Our review retrieved evidence on three main types of HRT: oestrogen-only, combined and tibolone.
A consistent theme among the case reports is the predominance of oestrogen-only HRT in women with recurrence or malignancy. The majority of case reports concerned women taking unopposed oestrogens, particularly conjugated equine oestrogens. This is not surprising given the strong association between unopposed oestrogens and endometrial cancer (Sjogren et al., 2016). As a result, current recommendations favour continuous combined preparations instead of unopposed oestrogens for women with a history of endometriosis, but the evidence remains sparse (Soliman and Hillard, 2006; Oxholm et al., 2007). We identified a single observational study that addressed this issue, including only 90 women (Rattanachaiyanont et al., 2003). Although the only women who developed recurrent symptoms were those taking oestrogen-only HRT, the study was retrospective and unable to demonstrate statistically significant differences between the groups. The authors suggested that combined HRT preparations might be the most appropriate for women with endometriosis who are using HRT. Large, randomized trials or observational studies with appropriate statistical power are clearly needed to clarify this question. Further research is urgently needed given the increased risk of breast cancer associated with combined HRT, although it is mostly in the older age group, which has been attributed to progestins (Chlebowski et al., 2013).
Tibolone therapy has also been associated with recurrence of endometriosis (Sundar et al., 2007). One RCT included in our review considered the use of tibolone, as compared with combined HRT, but the results should be interpreted with caution given the small sample size (n = 21). Fedele and colleagues (1999) concluded that tibolone (which typically has an oestrogenic effect on climacteric symptoms and bone, yet a progestogenic effect on tissues) might be a safer alternative to traditional HRT in patients with residual endometriotic disease, but no statistically significant difference was seen between the groups.
Notably, one case report highlights the importance of asking patients about their use of supplements or complementary/alternative medication. Five-year use of a highly concentrated isoflavone supplement was associated with florid recurrence of endometriosis and ureteral malignant mullerian carcinosarcoma (Noel et al., 2006). This report raises further concerns over the use of phytoestrogens in postmenopausal women with a history of endometriosis (Cotroneo and Lamartiniere, 2001), despite some clinical and animal literature suggesting a reduced risk of endometriosis with dietary isoflavones (Tsuchiya et al., 2007; Yavuz et al., 2007). Given the high prevalence of supplement use, it is important to further explore the relationship between phytoestrogens and endometriosis.
Timing: initiation and duration
Data are also lacking on the optimal time to commence HRT following surgical menopause. We identified a retrospective study in this area, comparing immediate (within 6 weeks of surgery) to delayed (≥6 weeks following surgery) commencement of HRT (Hickman et al., 1998). Although the crude incidence of recurrence was not different between the groups, increased recurrence was noted for women who delayed starting HRT after adjusting for confounders (AFS score at time of surgery, age at hysterectomy and postoperative adjunctive use of medroxyprogesterone). The authors themselves note the strong likelihood of bias in this observational study; it is probable that deferring the start of HRT would have been recommended to women felt to be at higher risk of recurrent symptoms. Additionally, we retrieved a non-comparative cohort study (Arumugam and Damodaran, 1998), which prospectively followed eight women who received conjugated oestrogens in the form of oral daily Premarin 5 months post surgical menopause, and five women who received oestrogens 3 months post surgical menopause. Women from both groups remained well and asymptomatic at 6-month follow-ups, yet clearly a much longer follow-up duration is necessary to be able to accurately assess risk of recurrence. The authors of this study also did not specify any symptoms or provide additional detail on patient status, and thus the evidence provided by this study was assessed as very low quality. Randomized trials are clearly needed to avoid this risk of bias, and have the potential to answer this question robustly.
Our search retrieved no studies investigating the total time for which women with histories of endometriosis should be treated. This is unfortunate given that it takes time to acquire mutations in endometriotic tissue, and thus duration of HRT therapy may have a large impact on probability of malignancy. Our systematic review also retrieved case reports of malignant transformation 4 and 8 years after stopping HRT treatment (Karanjgaokar et al., 2009), indicating that hormone replacement may either still have effects years after discontinuation or that the use of HRT is only one factor in malignant transformation of endometriosis.
Conclusion
Endometriosis is not exclusively a condition of the reproductive phase. Existing guidelines in this area emphasize the lack of evidence, but suggest that women should not be denied HRT treatment simply because of a history of endometriosis (Al Kadri et al., 2009; Dunselman et al., 2014). Our review indicates that women with a history of endometriosis should be carefully counselled about the possibility of disease recurrence after the menopause (Fig. 2). Although the absolute risk is unclear and likely to be low, women should be advised to seek help if they experience endometriosis-like symptoms, rather than suffer in silence. Furthermore, clinicians should adopt a cautious approach in cases of recurrence, keeping in mind the possibility of malignant transformation. For postmenopausal women with recurrent, treatment-resistant symptoms, consideration should be given to obtaining tissue for histology in order to exclude the possibility of malignancy, especially if other unusual or suspicious symptoms are present.
Figure 2.
Suggested clinical approach to postmenopausal patients with a history of endometriosis.
Although this review highlights potential risks of HRT, its substantial benefits should not be overlooked. In particular, the benefits may outweigh the costs of HRT for women with an early or surgical menopause. HRT has been shown to enhance cortical volumetric bone mineral density and compressive strength (Mikkola et al., 2011; Kuh et al., 2016). Additionally, studies have shown a reduced risk of coronary heart disease when hormonal therapy is administered to women with early natural or surgical menopause (Parker et al., 2009). Importantly, unilateral or bilateral oophorectomy prior to the onset of natural menopause is associated with an increased risk of dementia and cognitive impairment (Rocca et al., 2007); however, these risks may be reduced if HRT is administered up until the average age of natural menopause (Rocca et al., 2014).
As with any woman commencing HRT, a full and frank discussion should be held about the risks and benefits of this treatment. Currently, clinicians must balance the benefits and risks of HRT, with attention to individual risk factors (age and BMI), and choose appropriate therapies directed at specific menopausal symptoms. Patients must be actively involved in the decision process, and understand our limitations as providers to quantify specific risks. Women should be advised that there are no robust data to indicate whether HRT changes the risk of disease recurrence or malignant transformation. Small studies have suggested the possibility of increased recurrence in women who take HRT, in particular unopposed oestrogen. Some authors advocate the use of combined HRT for women with a history of endometriosis, to minimize the risk of recurrence (Moen et al., 2010; Dunselman et al., 2014), but there are still risks with combined oestrogen-progestin hormone therapy. These include an elevated risk of breast cancer both during and post-intervention mostly in older women (Chlebowski et al., 2015). Therefore care must be individualized, with the woman's personal and family history taken into account.
There are many promising areas for future research in this group of women. Our search retrieved no papers on the use of alternative selective oestrogen receptor modulators (SERMs) in postmenopausal patients with histories of endometriosis. We are aware of studies testing the gynaecologic safety of SERMS such as ospemifene and bazedoxifene, and combining these agents with oestrogens (especially bazedoxifene/conjugated oestrogens) (Mirkin et al., 2016). Such studies have been promising, and may represent a future alternative to conventional HRT for our cohort.
Summary
Our review highlights an important and severely under-researched area of gynaecology. The prevalence of endometriosis means that both specialists and general practitioners will inevitably encounter women with a history of this condition who are facing the dilemma of managing the menopause. Many women will have suffered years of debilitating symptoms before diagnosis, and then proceeded to undergo multiple treatments and operations in an attempt to regain some quality of life. These women deserve to have accurate, individualized and specific information about the risk of recurrence with different menopausal treatments, so that they can make an informed decision about their care.
Supplementary Material
Acknowledgements
The authors would like to thank Dr. Eve Fryer for her advice regarding histopathology.
Supplementary data
Supplementary data are available at Human Reproduction Update online.
Authors’ roles
L.C.G. was involved in all parts of the study. K.E.W. sorted articles, performed quality categorization and reviewed manuscript. S.K. performed the search and reviewed the manuscript. K.V. was involved in conceptualization of study and the drafting and review of the manuscript. K.T.Z. was involved in conceptualization of study and the drafting and review of the manuscript. C.M.B. sorted the articles and reviewed the manuscript and was responsible for overall conceptualization of study.
Funding
KTZ and CMB were funded by the European Commission Horizon 2020 research and innovation programme under grant agreement 692065 (project WIDENLIFE). This work was supported by an MRC project grant (MR/K011480/1).
Conflict of interest
No authors have any relevant conflicts of interest to this published work. Outside the submitted work, K.V. reports research grants from Bayer Healthcare and personal fees from Bayer Healthcare and Grunenthal GmbH; K.T.Z. and C.M.B. report research grants from Bayer Healthcare, Volition, Roche Diagnostics and MDNA Life Sciences; C.M.B. has received consultancy fees from ObsEva. The remaining authors report no conflicts of interest outside the submitted work.
References
- Abu MA, Sinha P, Totoe L, McCune G. Endometrial cancer thirteen years after total abdominal hysterectomy and bilateral salpingo-oophorectomy and hormone replacement therapy: a case report. Eur J Gynaecol Oncol 1997;18:482–483. [PubMed] [Google Scholar]
- Acien P, Nunez C, Quereda F, Velasco I, Valiente M, Vidal V. Is a bowel resection necessary for deep endometriosis with rectovaginal or colorectal involvement. Int J Womens Health 2013;5:449–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn SH, Singh V, Tayade C. Biomarkers in endometriosis: challenges and opportunities. Fertil Steril 2017;107:523–532. [DOI] [PubMed] [Google Scholar]
- Al Kadri H, Hassan S, Al-Fozan HM, Hajeer A. Hormone therapy for endometriosis and surgical menopause. Cochrane Database Syst Rev 2009;Cd005997 doi: 10.1002/14651858.CD005997.pub2. [DOI] [PubMed] [Google Scholar]
- Al-Talib A, Gilbert L, Arseneau J. Endometrioid adenocarcinoma 13 years after total abdominal hysterectomy and bilateral salpingo-oophorectomy. Saudi Med J 2008;29:1044–1047. [PubMed] [Google Scholar]
- Areia A, Sousa V, Frutuoso C, Dias I, Martins MI, de Oliveira CF. Endometrioid adenocarcinoma arising in endometriosis foci six years after estrogen replacement therapy: a case report. Eur J Gynaecol Oncol 2004;25:255–256. [PubMed] [Google Scholar]
- Arumugam K, Damodaran P. Endometriosis and estrogen replacement therapy. Med Sci Res 1998;26:333–334. [Google Scholar]
- Badawy SZA, Liberatore C, Farhat MA, Valente AL, Landas S. Cervical endometriosis stimulated by estrogen therapy following supracervical hysterectomy. J Gynecol Surg 2004;19:141–144. [Google Scholar]
- Bhat RA, Teo M, Bhat AK. Endometriosis after surgical menopause mimicking pelvic malignancy: surgeons’ predicament. Oman Med J 2014;29:226–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks JJ, Wheeler JE. Malignancy arising in extragonadal endometriosis: a case report and summary of the world literature. Cancer 1977;40:3065–3073. [DOI] [PubMed] [Google Scholar]
- Bulun SE. Endometriosis. N Engl J Med 2009;360:268–279. [DOI] [PubMed] [Google Scholar]
- Bulun SE, Monsavais D, Pavone ME, Dyson M, Xue Q, Attar E, Tokunaga H, Su EJ. Role of estrogen receptor-beta in endometriosis. Semin Reprod Med 2012;30:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril 2012;98:511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauley JA, Robbins J, Chen Z, Cummings SR, Jackson RD, LaCroix AZ, LeBoff M, Lewis CE, McGowan J, Neuner J et al. Effects of estrogen plus progestin on risk of fracture and bone mineral density: the Women's Health Initiative randomized trial. JAMA 2003;290:1729–1738. [DOI] [PubMed] [Google Scholar]
- Chahine B, Malbranque G, Lelong J, Ramon P, Tillie-Leblond I. [Catamenial hemoptysis during hormone replacement treatment]. Rev Mal Respir 2007;24:339–342. [DOI] [PubMed] [Google Scholar]
- Chlebowski RT, Manson JE, Anderson GL, Cauley JA, Aragaki AK, Stefanick ML, Lane DS, Johnson KC, Wactawski-Wende J, Chen C et al. Estrogen plus progestin and breast cancer incidence and mortality in the women's health initiative observational study. J Natl Cancer Inst 2013;105:526–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlebowski RT, Rohan TE, Manson JE, Aragaki AK, Kaunitz A, Stefanick ML, Simon MS, Johnson KC, Wactawski-Wende J, O'Sullivan MJ et al. Breast cancer after use of estrogen plus progestin and estrogen alone: analyses of data from 2 women's health initiative randomized clinical trials. JAMA Oncol 2015;1:296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JI, Yoo JG, Kim SJ, Lee HN, Kim MJ. Acute renal failure due to obstructive uropathy secondary to ureteral endometriosis. Case Rep Obstet Gynecol 2015;2015:761348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung FB, Terzibachian J-J, Govyadovskiy A, Grisey A. Postmenopausal malignant transformation of extragenital endometriosis. A case report. Gynecol Obstet Fertil 2008;36:51–55. [DOI] [PubMed] [Google Scholar]
- Clayton RD, Hawe JA, Love JC, Wilkinson N, Garry R. Recurrent pain after hysterectomy and bilateral salpingo-oophorectomy for endometriosis: evaluation of laparoscopic excision of residual endometriosis. Br J Obstet Gynaecol 1999;106:740–744. [DOI] [PubMed] [Google Scholar]
- Collaborators MWS Breast cancer and hormone-replacement therapy in the million women study. Lancet 2003;362:419–427. [DOI] [PubMed] [Google Scholar]
- Cotroneo MS, Lamartiniere CA. Pharmacologic, but not dietary, genistein supports endometriosis in a rat model. Toxicol Sci 2001;61:68–75. [DOI] [PubMed] [Google Scholar]
- Dmowski WP, Radwanska E, Rana N. Recurrent endometriosis following hysterectomy and oophorectomy: the role of residual ovarian fragments. Int J Gynaecol Obstet 1988;26:93–103. [DOI] [PubMed] [Google Scholar]
- Duffy JM, Arambage K, Correa FJ, Olive D, Farquhar C, Garry R, Barlow DH, Jacobson TZ. Laparoscopic surgery for endometriosis. Cochrane Database Syst Rev 2014;Cd011031 doi: 10.1002/14651858.CD011031.pub2. [DOI] [PubMed] [Google Scholar]
- Dunselman GA, Vermeulen N, Becker C, Calhaz-Jorge C, D'Hooghe T, De Bie B, Heikinheimo O, Horne AW, Kiesel L, Nap A et al. ESHRE guideline: management of women with endometriosis. Hum Reprod 2014;29:400–412. [DOI] [PubMed] [Google Scholar]
- Duun S, Roed-Petersen K, Michelsen JW. Endometrioid carcinoma arising from endometriosis of the sigmoid colon during estrogenic treatment. Acta Obstet Gynecol Scand 1993;72:676–678. [DOI] [PubMed] [Google Scholar]
- Efthymiou CA. Endometriosis-associated intestinal tumours: a consequence of long-term unopposed oestrogen. Ann R Coll Surg Engl 2009;91:259–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedele L, Bianchi S, Raffaelli R, Zanconato G. Comparison of transdermal estradiol and tibolone for the treatment of oophorectomized women with deep residual endometriosis. Maturitas 1999;32:189–193. [DOI] [PubMed] [Google Scholar]
- Fujiu K, Miyamoto H, Hashimoto S, Suzuki N, Takano Y, Teranishi Y, Sakuma H, Suzuki H. A case of diaphragmatic clear cell carcinoma in a patient with a medical history of ovarian endometriosis. Int J Clin Oncol 2010;15:489–492. [DOI] [PubMed] [Google Scholar]
- Gadducci A, Lanfredini N, Tana R. Novel insights on the malignant transformation of endometriosis into ovarian carcinoma. Gynecol Endocrinol 2014;30:612–617. [DOI] [PubMed] [Google Scholar]
- Gallagher JC. Effect of early menopause on bone mineral density and fractures. Menopause 2007;14:567–571. [DOI] [PubMed] [Google Scholar]
- Gemmell L, Webster KE, Kirtley S, and Becker CM The management of menopause in women with a past history of endometriosis: a systematic review. PROSPERO: International prospective register of systematic reviews2016.
- Giarenis I, Giamougiannis P, Speakman CT, Nieto JJ, Crocker SG. Recurrent endometriosis following total hysterectomy with oophorectomy mimicking a malignant neoplastic lesion: a diagnostic and therapeutic challenge. Arch Gynecol Obstet 2009;279:419–421. [DOI] [PubMed] [Google Scholar]
- Goh JT, Hall BA. Postmenopausal endometrioma and hormonal replacement therapy. Aust N Z J Obstet Gynaecol 1992;32:384–385. [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol 2011;64:380–382. [DOI] [PubMed] [Google Scholar]
- Heaps JM, Nieberg RK, Berek JS. Malignant neoplasms arising in endometriosis. Obstet Gynecol 1990;75:1023–1028. [PubMed] [Google Scholar]
- Hendrix SL. Bilateral oophorectomy and premature menopause. Am J Med 2005;118:131–135. [DOI] [PubMed] [Google Scholar]
- Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: annual trends and response to recent evidence. JAMA 2004;291:47–53. [DOI] [PubMed] [Google Scholar]
- Hickman TN, Namnoum AB, Hinton EL, Zacur HA, Rock JA. Timing of estrogen replacement therapy following hysterectomy with oophorectomy for endometriosis. Obstet Gynecol 1998;91:673–677. [DOI] [PubMed] [Google Scholar]
- Home Page Map of Medicine Web site. http://www.mapofmedicine.com/. (January 2017, date last accessed)
- Inceboz U. Endometriosis after menopause. Womens Health (Lond) 2015;11:711–715. [DOI] [PubMed] [Google Scholar]
- Jimenez RE, Tiguert R, Hurley P, An T, Grignon DJ, Lawrence D, Triest J. Unilateral hydronephrosis resulting from intraluminal obstruction of the ureter by adenosquamous endometrioid carcinoma arising from disseminated endometriosis. Urology 2000;56:331. [DOI] [PubMed] [Google Scholar]
- Johnson NP, Hummelshoj L, Consortium ftWESM . Consensus on current management of endometriosis. Hum Reprod 2013;28:1552–1568. [DOI] [PubMed] [Google Scholar]
- Jones KD, Owen E, Berresford A, Sutton C. Endometrial adenocarcinoma arising from endometriosis of the rectosigmoid colon. Gynecol Oncol 2002;86:220–222. [DOI] [PubMed] [Google Scholar]
- Joseph J, Reed CE, Sahn SA. Thoracic endometriosis. Recurrence following hysterectomy with bilateral salpingo-oophorectomy and successful treatment with talc pleurodesis. Chest 1994;106:1894–1896. [DOI] [PubMed] [Google Scholar]
- Kapadia SB, Russak RR, O'Donnell WF, Harris RN, Lecky JW. Postmenopausal ureteral endometriosis with atypical adenomatous hyperplasia following hysterectomy, bilateral oophorectomy, and long-term estrogen therapy. Obstet Gynecol 1984;64:60s–63s. [DOI] [PubMed] [Google Scholar]
- Karanjgaokar VC, Murphy DJ, Samra JS, Mann CH. Malignant transformation of residual endometriosis after hysterectomy: a case series. Fertil Steril 2009;92:2037.e2019–2021. [DOI] [PubMed] [Google Scholar]
- Kawate S, Takeyoshi I, Ikota H, Numaga Y, Sunose Y, Morishita Y. Endometrioid adenocarcinoma arising from endometriosis of the mesenterium of the sigmoid colon. Jpn J Clin Oncol 2005;35:154–157. [DOI] [PubMed] [Google Scholar]
- Kitawaki J, Kado N, Ishihara H, Koshiba H, Kitaoka Y, Honjo H. Endometriosis: the pathophysiology as an estrogen-dependent disease. J Steroid Biochem Mol Biol 2002;83:149–155. [DOI] [PubMed] [Google Scholar]
- Klug PW, Mayer HG, Leitner G. [Rare complications of endometriosis--2 case reports]. Geburtshilfe Frauenheilkd 1987;47:870–871. [DOI] [PubMed] [Google Scholar]
- Kuh D, Muthuri S, Cooper R, Moore A, Mackinnon K, Cooper C, Adams JE, Hardy R, Ward KA. Menopause, reproductive life, hormone replacement therapy, and bone phenotype at age 60–64 years: a British birth cohort. J Clin Endocrinol Metab 2016;101:3827–3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer RD. The evidence base for HRT: what can we believe. Climacteric 2017;20:91–96. [DOI] [PubMed] [Google Scholar]
- Leiserowitz GS, Gumbs JL, Oi R, Dalrymple JL, Smith LH, Ryu J, Scudder S, Russell AH. Endometriosis-related malignancies. Int J Gynecol Cancer 2003;13:466–471. [DOI] [PubMed] [Google Scholar]
- Manson JE, Chlebowski RT, Stefanick ML, Aragaki AK, Rossouw JE, Prentice RL, Anderson G, Howard BV, Thomson CA, LaCroix AZ et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women's Health Initiative randomized trials. JAMA 2013;310:1353–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manyonda IT, Neale EJ, Flynn JT, Osborn DE. Obstructive uropathy from endometriosis after hysterectomy and oophorectomy; two case reports. Eur J Obstet Gynecol Reprod Biol 1989;31:195–198. [DOI] [PubMed] [Google Scholar]
- Matorras R, Elorriaga MA, Pijoan JI, Ramon O, Rodriguez-Escudero FJ. Recurrence of endometriosis in women with bilateral adnexectomy (with or without total hysterectomy) who received hormone replacement therapy. Fertil Steril 2002;77:303–308. [DOI] [PubMed] [Google Scholar]
- Mattar CN, Pang B, Fong YF. An unexpected presentation of endometriosis—a ‘parasitic’ cyst of the bowel in a menopausal woman on hormone therapy. Ann Acad Med Singapore 2008;37:69–71. [PubMed] [Google Scholar]
- May KE, Conduit-Hulbert SA, Villar J, Kirtley S, Kennedy SH, Becker CM. Peripheral biomarkers of endometriosis: a systematic review. Hum Reprod Update 2010;16:651–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkola TM, Heinonen A, Kovanen V, Cheng S, Kujala UM, Suominen H, Alen M, Puolakka J, Ankarberg-Lindgren C, Ronkainen PH et al. Influence of long-term postmenopausal hormone-replacement therapy on estimated structural bone strength: a study in discordant monozygotic twins. J Bone Miner Res 2011;26:546–552. [DOI] [PubMed] [Google Scholar]
- Milam MR, Atkinson JB, Currie JL. Adenosarcoma arising in inguinal endometriosis. Obstet Gynecol 2006;108:753–755. [DOI] [PubMed] [Google Scholar]
- Mirkin S, Pinkerton JV, Kagan R, Thompson JR, Pan K, Pickar JH, Komm BS, Archer DF. Gynecologic safety of conjugated estrogens plus bazedoxifene: pooled analysis of five phase 3 trials. J Womens Health (Larchmt) 2016;25:431–442. [DOI] [PubMed] [Google Scholar]
- Moen MH, Rees M, Brincat M, Erel T, Gambacciani M, Lambrinoudaki I, Schenck-Gustafsson K, Tremollieres F, Vujovic S, Rozenberg S. EMAS position statement: managing the menopause in women with a past history of endometriosis. Maturitas 2010;67:94–97. [DOI] [PubMed] [Google Scholar]
- Montamedi Endometrioid adenocarcinoma in endometriosis of the ureter. A case report. Geburtshilfe Frauenheilkd 2002;62:490–494. [Google Scholar]
- Mounsey AL, Wilgus A, Slawson DC. Diagnosis and management of endometriosis. Am Fam Physician 2006;74:594–600. [PubMed] [Google Scholar]
- Munksgaard PS, Blaakaer J. The association between endometriosis and ovarian cancer: a review of histological, genetic and molecular alterations. Gynecol Oncol 2012;124:164–169. [DOI] [PubMed] [Google Scholar]
- Nezhat F, Apostol R, Mahmoud M, el Daouk M. Malignant transformation of endometriosis and its clinical significance. Fertil Steril 2014;102:342–344. [DOI] [PubMed] [Google Scholar]
- Noel JC, Anaf V, Fayt I, Wespes E. Ureteral mullerian carcinosarcoma (mixed mullerian tumor) associated with endometriosis occurring in a patient with a concentrated soy isoflavones supplementation. Arch Gynecol Obstet 2006;274:389–392. [DOI] [PubMed] [Google Scholar]
- Oxholm D, Knudsen UB, Kryger-Baggesen N, Ravn P. Postmenopausal endometriosis. Acta Obstet Gynecol Scand 2007;86:1158–1164. [DOI] [PubMed] [Google Scholar]
- Parker WH, Broder MS, Chang E, Feskanich D, Farquhar C, Liu Z, Shoupe D, Berek JS, Hankinson S, Manson JE. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the nurses’ health study. Obstet Gynecol 2009;113:1027–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen VC, Underwood JC, Wells M, Shepherd NA. Primary endometrioid adenocarcinoma of the large intestine arising in colorectal endometriosis. Histopathology 2002;40:171–176. [DOI] [PubMed] [Google Scholar]
- Powell JL, Connor GP, Henderson GS. Androgen-producing, atypically proliferating endometrioid tumor arising in endometriosis. South Med J 2001;94:450–453. [PubMed] [Google Scholar]
- Punnonen R, Klemi PJ, Nikkanen V. Postmenopausal endometriosis. Eur J Obstet Gynecol Reprod Biol 1980;11:195–200. [DOI] [PubMed] [Google Scholar]
- Rahmioglu N, Macgregor S, Drong AW, Hedman AK, Harris HR, Randall JC, Prokopenko I, Nyholt DR, Morris AP, Montgomery GW et al. Genome-wide enrichment analysis between endometriosis and obesity-related traits reveals novel susceptibility loci. Hum Mol Genet 2015. a;24:1185–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmioglu N, Montgomery GW, Zondervan KT. Genetics of endometriosis. Womens Health (Lond) 2015. b;11:577–586. [DOI] [PubMed] [Google Scholar]
- Rattanachaiyanont M, Tanmahasamut P, Angsuwatthana S, Techatraisak K, Inthawiwat S, Leerasiri P. Hormonal replacement therapy in surgical menopause with underlying endometriosis. J Med Assoc Thai 2003;86:702–707. [PubMed] [Google Scholar]
- Ray J, Conger M, Ireland K. Ureteral obstruction in postmenopausal woman with endometriosis. Urology 1985;26:577–578. [DOI] [PubMed] [Google Scholar]
- Reimnitz C, Brand E, Nieberg RK, Hacker NF. Malignancy arising in endometriosis associated with unopposed estrogen replacement. Obstet Gynecol 1988;71:444–447. [PubMed] [Google Scholar]
- Review Manager (RevMan) [Computer program]. Version 5.3 2014. The Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen.
- Rocca WA, Bower JH, Maraganore DM, Ahlskog JE, Grossardt BR, de Andrade M, Melton LJ III. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology 2007;69:1074–1083. [DOI] [PubMed] [Google Scholar]
- Rocca WA, Grossardt BR, Shuster LT. Oophorectomy, estrogen, and dementia: a 2014 update. Mol Cell Endocrinol 2014;389:7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosano GM, Vitale C, Marazzi G, Volterrani M. Menopause and cardiovascular disease: the evidence. Climacteric 2007;10:19–24. [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA 2002;288:321–333. [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM, Ko M, LaCroix AZ, Margolis KL, Stefanick ML. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 2007;297:1465–1477. [DOI] [PubMed] [Google Scholar]
- Rozenberg S, Vandromme J, Antoine C. Postmenopausal hormone therapy: risks and benefits. Nat Rev Endocrinol 2013;9:216–227. [DOI] [PubMed] [Google Scholar]
- Sampson JA. Endometrial carcinoma of the ovary, arising in endometrial tissue in that organ. Arch Surg 1925;10:1–72. [Google Scholar]
- Schmidt P. The 2012 hormone therapy position statement of: The North American Menopause Society. Menopause 2012;19:257–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott RB. Malignant changes in endometriosis. Obstet Gynecol 1953;2:283–289. [PubMed] [Google Scholar]
- Simoens S, Dunselman G, Dirksen C, Hummelshoj L, Bokor A, Brandes I, Brodszky V, Canis M, Colombo GL, DeLeire T et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod 2012;27:1292–1299. [DOI] [PubMed] [Google Scholar]
- Simoens S, Hummelshoj L, D'Hooghe T. Endometriosis: cost estimates and methodological perspective. Hum Reprod Update 2007;13:395–404. [DOI] [PubMed] [Google Scholar]
- Sjogren LL, Morch LS, Lokkegaard E. Hormone replacement therapy and the risk of endometrial cancer: a systematic review. Maturitas 2016;91:25–35. [DOI] [PubMed] [Google Scholar]
- Skor AB, Warren MM, Mueller EO Jr. Endometriosis of bladder. Urology 1977;9:689–692. [DOI] [PubMed] [Google Scholar]
- Soliman NF, Hillard TC. Hormone replacement therapy in women with past history of endometriosis. Climacteric 2006;9:325–335. [DOI] [PubMed] [Google Scholar]
- Soliman NF, Evans AJ. Malignancy arising in residual endometriosis following hysterectomy and hormone replacement therapy. J Br Menopause Soc 2004;10:123–124. [DOI] [PubMed] [Google Scholar]
- Stewart WW, Ireland GW. Vesical endometriosis in a postmenopausal woman: a case report. J Urol 1977;118:480–481. [DOI] [PubMed] [Google Scholar]
- Sundar SS, Gornall RJ, Kerr-Wilson R, Swingler GR, Kinder RB, McCarthy K. A case report of recurrent endometriosis following Tibolone hormone replacement therapy. J Obstet Gynaecol 2007;27:433–434. [DOI] [PubMed] [Google Scholar]
- Suraweera P, Gimeno-Marin C, Indusekhar R, Redman C, and Todd R Malignant transformation of endometriotic implants in a pre-and a postmenopausal woman: Two case reports and review of literature. International Journal of Gynecology and Obstetrics2012: Conference; 20th FIGO World Congress of Gynecology and Obstetrics Rome Italy. Conference Start: 20121007 Conference End: 20121012. Conference Publication: (var.pagings). 20121119 (pp S20121494–S20121495).
- Taylor AA, Kenny N, Edmonds S, Hole L, Norbrook M, English J. Postmenopausal endometriosis and malignant transformation of endometriosis: a case series. Gynecol Surg 2005;2:135–137. [Google Scholar]
- Taylor M, Bowen-Simpkins P, Barrington J. Complications of unopposed oestrogen following radical surgery for endometriosis. J Obstet Gynaecol 1999;19:647–648. [DOI] [PubMed] [Google Scholar]
- Tsuchiya M, Miura T, Hanaoka T, Iwasaki M, Sasaki H, Tanaka T, Nakao H, Katoh T, Ikenoue T, Kabuto M et al. Effect of soy isoflavones on endometriosis: interaction with estrogen receptor 2 gene polymorphism. Epidemiology 2007;18:402–408. [DOI] [PubMed] [Google Scholar]
- Turbiner J, Moreno-Bueno G, Dahiya S, Sanchez-Estevez C, Hardisson D, Prat J, Oliva E, Palacios J. Clinicopathological and molecular analysis of endometrial carcinoma associated with tamoxifen. Mod Pathol 2008;21:925–936. [DOI] [PubMed] [Google Scholar]
- Vercellini P, Vigano P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol 2014;10:261–275. [DOI] [PubMed] [Google Scholar]
- Vignali M, Bianchi S, Candiani M, Spadaccini G, Oggioni G, Busacca M. Surgical treatment of deep endometriosis and risk of recurrence. J Minim Invasive Gynecol 2005;12:508–513. [DOI] [PubMed] [Google Scholar]
- Wang CT, Wang DB, Liu KR, Li Y, Sun CX, Guo CS, Ren F. Inducing malignant transformation of endometriosis in rats by long-term sustaining hyperestrogenemia and type II diabetes. Cancer Sci 2015;106:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavuz E, Oktem M, Esinler I, Toru SA, Zeyneloglu HB. Genistein causes regression of endometriotic implants in the rat model. Fertil Steril 2007;88:1129–1134. [DOI] [PubMed] [Google Scholar]
- Yohannes P. Ureteral endometriosis. J Urol 2003;170:20–25. [DOI] [PubMed] [Google Scholar]
- Zanetta GM, Webb MJ, Li H, Keeney GL. Hyperestrogenism: a relevant risk factor for the development of cancer from endometriosis. Gynecol Oncol 2000;79:18–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.