Abstract
Background
Thoracic endometriosis syndrome is a rare form of extrapelvic endometriosis characterized by the presence of functioning endometrial tissue in pleura, lung parenchyma, and airways, associated with a high rate of infertility.
Case Report
We have reported a case of successful management and treatment of thoracic endometriosis syndrome that occurred in a 37-year-old female patient. She underwent thoracoscopic resection of the lesion. During follow-up, we revealed the recurrence of a previously surgically treated thoracic endometriosis. She was initially treated with a gonadotropin-releasing hormone agonist; subsequently this was replaced by a prophylactic treatment with Dienogest.
Conclusion
The diagnosis of thoracic endometriosis is challenging. The first line of treatment is medical, whereas the surgical treatment is performed secondly. Moreover, surgical treatment can lead to a significant rate of recurrence, often reduced by a coadjutant medical treatment.
Keywords: Endometriosis, Thoracic endometriosis, Pneumothorax, Hemothorax
Introduction
Thoracic endometriosis syndrome is a rare and crippling form of extrapelvic endometriosis. It is a clinical entity, affecting women in their reproductive years, in which deposits of functional endometrial tissue are located within the pleura, the lung parenchyma, and airways [1]. Thoracic endometriosis manifests itself through various clinical presentations such as catamenial pneumothorax (PNX) (73%), catamenial hemothorax (14%), catamenial hemoptysis (7%), and lung nodules (6%) [2]. Pelvic endometriosis is associated with 30%-50% of cases. Predisposing factors are still unknown, but a systemic immune alteration probably plays a role in the ectopic presence and persistence of endometrial tissue, although this hypothesis has yet to be clearly established [3], [4], [5], [6], [7], [8], [9]. Symptoms always appear contemporaneously with the menstrual period: the typical clinical manifestation is catamenial PNX, generally observed in about 3%-6% of spontaneous PNX, most of which involving the right side. Moreover, a history of hemoptysis during the menstrual period could be a strong indicator of pulmonary endometriosis [10]. Despite its poor specificity, high-resolution computed tomography (HRCT) of the thorax during the menstrual period remains the primary imaging method to support the diagnosis. It is capable of revealing admonishing lesions as ground-glass opacities or nodules [11]. Magnetic resonance imaging (MRI) is considered more sensitive than computed tomography (CT) in blood detection during menstruation (hyperintense in T2 gradient echo), but less in spatial resolution compared to CT [12]. Bronchoscopy effectively isolates lesions located in the airways, but it cannot be used to detect the lesions located in the lung parenchyma and pleura [13]. Pulmonary endometriosis can be treated medically or surgically. Medical therapy consists of suppression of ectopic endometrium activity by progestins, danazol, or gonadotropin-releasing hormone (GnRH) analogs [10], [11], [12], [13], [14], [15]. Surgical management is performed when medical treatment fails and consists of endometrial tissue removal through video-assisted thoracoscopic surgery (VATS) or open surgery. Chemical pleurodesis could be performed in cases of catamenial PNX or hemothorax [16]. We have reported 1 case of thoracic endometriosis with recurrent episodes of catamenial PNX, associated with both pulmonary and extrapulmonary nodules and severe abdominal endometriosis.
Case report
The patient was informed of all procedures she was to undergo, signed a consent allowing data collection for research purposes, and gave full approval for the report and publishing of the case. This case is in accordance with the Helsinki Declaration, in accordance with the Consensus-based Clinical Case Reporting Guideline Development (http://www.equator-network.org/) and the Committee on Publication Ethics (COPE) guidelines (http://publicationethics.org/), and approved by the Institutional Review Board (IRB) of the university hospital in which it was reported. A 37-year-old woman was admitted in November 2010 to our Institute with acute breathlessness, shortness of breath, chest pain, and cough typically arising during menstrual period. Initially, she had been diagnosed with serious pelvic, intestinal, bladder and abdominal wall endometriosis following a previous emergency operation that was performed because of a ruptured ovarian cyst (corpus hemorrhagicum cyst) and acute intestinal obstruction. Her menarche occurred 12 years ago, and since then she complained about severe symptoms of dysmenorrhea evaluated with visual analog scale (VAS = 9), dyspareunia (VAS = 8), dyschezia (VAS = 7), and chronic abdominal pain (VAS = 4). She reported a history of recurrent chest pain non-specifically treated and underwent multiple blood and hormone tests during this period with no results. The patient had a family history of endometriosis that occurred also in her mother, sister, aunt, cousin, and grandmother. Despite several efforts, she was unable to become pregnant. During our physical evaluation, asymmetric thorax movement and decreased breathing sound in her left hemithorax were observed. Vital parameters were stable (blood pressure 134/84 mm Hg; heart rate 95 bpm; oxygen blood saturation of 95%). A chest x-ray was performed revealing a PNX associated with areas of ground-glass haze in the anterior basal segment of the left lung, in a subpleural localization (Fig. 1). A subsequent CT scan confirmed the same lesions. A simultaneous abdominal evaluation was performed to evaluate the resumption of endometriosis disease. The patient was suffering pelvic discomfort during the gynecological evaluation, and a pelvic ultrasound (US) indicated the presence of multiple outbreaks with typical endometriosis US features of cystic ground-glass lesions involving the contralateral ovary, which had previously been operated on. Moreover, CA-125 serum was high. The patient underwent pleural and pulmonary lesion resection: mono-port VATS was performed and a macroscopically millimetric brownish and white-scattered lesion was discovered, representing small endometriosis implants into the pleural cavity. We also found emphysematous blebs and bullae matching with the anterior basal segment of the left lung where a gentle resection of lung parenchyma was performed. Histologic examination revealed the presence of endometrial tissue with glandular and stromal structures positive for estrogen receptors, which confirmed the diagnosis of pulmonary endometriosis. Consequentially, the previous catamenial episodes of PNX were linked to thoracic endometriosis. PNX was treated using chemical pleurodesis, and after surgical treatment the patient underwent medical therapy with GnRH agonists. The patient underwent a follow-up with thorax HRCT performed a month later during her menstrual cycle. Successively, an HRCT was planned 2 and 5 years later. In 2015, the patient returned to our Institute showing the same symptoms of breathlessness, cough, and pain located in the left side of the chest associated with umbilical pain. She underwent a chest MRI revealing the presence of a recurrent nodular lesion in located subpleura in the left lung, which could suggest endometriosis. A chest CT scan detected small nodules with ground-glass opacity in the left lung (Fig. 1), confirming the suspected diagnosis. Moreover, an extrapulmonary localization was detected as a ground-glass nodular lesion in the paracardiac area (Fig. 1). According to the complex thorax involvement, a fully medical treatment was performed with GnRH agonists (Enantone 3.75 mg) until the remission of symptomatology. To prevent new thorax endometriosis episodes and according to the recurrent PNX history despite surgical treatment, we decided to treat the patient prophylactically with dienogest. A 1-year follow-up was arranged, and during this period no thorax endometriosis symptoms or other recurrent episodes were reported. Furthermore, neither pelvic symptoms indicative of resumed endometriosis disease nor increased levels of CA-125 in the blood were recorded.
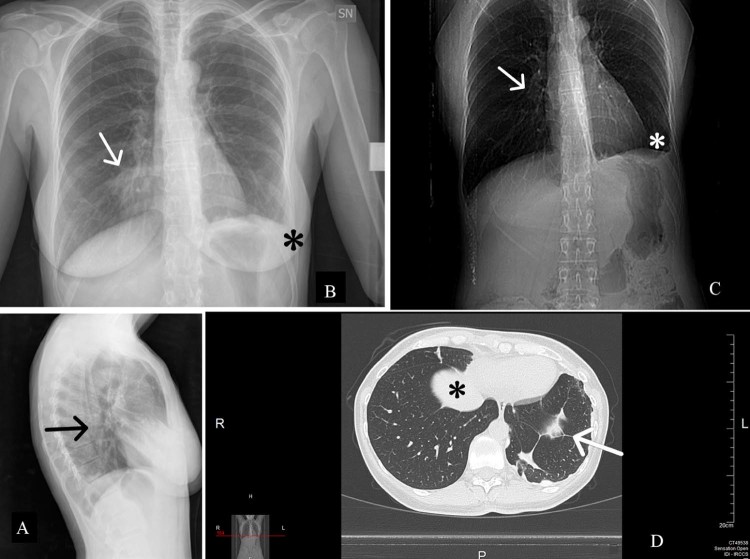
Fig. 1.
X-ray of the chest (A, B, C) showing basal-right focal parenchymal consolidation (arrows) and costophrenic angle obliteration (white *). HRCT (D) showing paracardiac ground-glass nodular lesion (black *) and nodular ground-glass opacity in the basal segment of the left lung (arrow).
Discussion
Endometriosis is estimated to affect 3%-10% of women of fertile age, and 2%-5% of women postmenopause [17], [18]. On the contrary, thoracic endometriosis occurs with a lower incidence. Thoracic endometriosis syndrome (TES) is a pathology whereby ectopic endometrial tissue is deposited in thoracic structures. TES consists of 4 distinct clinical entities: catamenial PNX, catamenial hemithorax, hemoptysis, and pulmonary nodules. Although endometriosis in general can affect 15% of women in their reproductive years, TES remains an exceedingly rare condition. Joseph et al. described 110 cases of TES and 50%-85% of them had concomitant pelvic endometriosis [10]. Hart in 1912 was the first who documented bronchopulmunary endometriosis [1]. Various theories have been proposed to explain the pathogenesis of extrapelvic endometriosis. The currently accepted theories are microembolization and peritoneal-pleural migration. Deposits of thoracic endometriotic tissue could be detected in several sites such as tracheobronchial tree, pulmonary tissue, pleura, or diaphragm [17]. TES is characterized by catamenial symptoms that occur with the onset of menstruation. Chest pain occurs most frequently (90%), followed by dyspnea (31%), hemoptysis (7%), and cough (rare) [14]. Joseph and Sahn reported that a large number of patients (73%) suffered from catamenial PNX, whereas others had catamenial hemothorax (14%). Only 6% of cases manifested lung nodules [10]. Our patient presented dyspnea, cough, PNX, and chest pain. TES was concomitant with severe pelvic endometriosis and infertility. In accordance with the literature, our patient had a long history of deep pelvic endometriosis with severe symptoms, including dyschezia, dysmenorrhea, dyspareunia, dysuria, and chronic abdominal pain since 18 years of age. Literature reports that infertility affects 30%-50% of women with endometriosis [6], [19]. In our case, the patient had not undergone term pregnancy and she had had 3 abortions. Previous literature reported that the establishment of the diagnosis required at least 8 months, but currently it occurs earlier, thanks to an increased awareness of the syndrome [10]. However, the diagnosis of TES remains challenging. More than 60% of patients could be submitted to thoracotomy or thoracoscopy as part of the diagnostic approach [14]. Physical examination may reveal diminished or absent breath sounds on the affected side, suggesting pleural effusions or PNX. Chest x-ray radiography may reveal PNX, pleural effusions, or pulmonary nodules. CT imaging may reveal diaphragmatic endometrial implants as hypoattenuating areas or identify single or multiple pulmonary nodules and sometimes also focal ground-glass lesion opacity or consolidation. In our patient, CT showed typical ground-glass pleural lesions. MRI can detect the presence of blood and its products as hyperintense on T2-weighted spin-echo images during menstruation with increased uptake post contrast [11].
From the radiological point of view, the diagnosis of TES is extremely challenging for several reasons: first of all PNX is usually small on the chest X-rays [10]; in addition, chest X-rays and CT are not able to identify the cause of PNX (ie, that it is caused by endometriotic implants). Nevertheless, chest CT may provide important information for differential diagnosis, since it is extremely accurate in identifying the presence of pneumoperitoneum or diaphragmatic endometriotic lesions. According to recent data [20], chest MRI is superior to CT in displaying diaphragmatic endometriosis lesions: this element is of paramount importance and could be due, at least in part, to the higher contrast resolution and better characterization of hemorrhagic lesions. In addition, chest MRI is also able to detect with great accuracy even small pleural foci as small cystic hyperintense lesions on T1-weighted images of the visceral or parietal pleura [21], [22].
Video-assisted thoracoscopy reveals diaphragm perforations, nodular or plaque endometrial deposits <1 cm in size, thereby allowing a biopsy. The histology of a resected lesion demonstrated that hemosiderin-laden macrophages and inflammation are always present and characterized by the presence of lymphocytes, plasmocytes, and macrophages. Mesothelial cells are modified by inflammatory reactive stimuli and fibrosis is sometimes observed. Typical histologic findings consist of a hormone-dependent assembly of proliferative endometrial glands and stroma surrounded by hemosiderin-laden macrophages whose presence could be indicative of endometriosis [23]. In our case, endometrial glands and stroma of the histologic lesions were positive for estrogen receptors. In case of suspected endometriosis, a pelvic US could be useful because it involved 51% of cases. However, recent studies reject this idea, finding that concomitant pelvic endometriosis occurs in less than 18% of cases, supposing that it could arise from different abnormal clones of endometrial cells. Pelvic US of our patient showed deep endometriosis infiltration and associated fibrosis. Treatment of TES is divided into medical and surgical, being as challenging as diagnosis [13], [24]. Suppression of the ectopic endometrium by oral contraceptive pills, progestins, danazol, or GnRH analogs that interfere with estrogen secretion, has been largely performed in these years [15], [25]. Some studies reported that hormone therapy is more effective in catamenial hemoptysis rather than in catamenial PNX. In particular, it has been observed that GnRH agonist administration for a period longer than 1 year determines a better control of recurrences of catamenial PNX [14]. However, PNX and hemothorax may recur despite hormone therapy, suggesting the lack of complete endometrial implant regression or recurrent embolization from pelvic foci [26]. Surgical management is usually carried out when medical treatment fails [27]. Initial surgical procedures include supportive oxygen, observation, and rest if lung collapse is small. Medium to large collapse may require manual aspiration or a chest tube. A second occurrence may be treated more aggressively with a sclerosing agent. Chemical pleurodesis may be performed in cases of catamenial PNX or hemothorax [16] and concurrently with VATS exploration. GnRH agonist administration ameliorates the outcome [26]. Major surgical procedures consist of video-assisted thoracoscopic or open surgery, in which a wedge resection or limited lung segmentectomy dislodges the ectopic endometrial tissue. Hysterectomy with bilateral salpingo-oophorectomy removes estrogen secretion suppressing ectopic endometrial tissue. However, recurrences of thoracic endometriosis occur despite oophorectomy [28]. Our patient was treated both surgically and medically: she underwent chemical pleurodesis and pulmonary atypical resection. After surgery, she was treated with a GnRH agonist but afterward we added a prophylactic treatment with dienogest due to relapse. No recurrences during follow-up period were observed. In the literature, the recurrence of disease at 6-12 months after medical treatment in 50%-60% of cases and at postsurgical treatment in 5%-25% of cases has been reported [15].
A high index of suspicion is essential in women of reproductive age who have experienced cyclical chest pain, dyspnea, or hemoptysis. A recurrence of PNX and appearance of PNX during menses raised clinical suspicion of a catamenial PNX. To reach a correct diagnosis and successfully establish treatment of the patient, a multidisciplinary team consisting of a pulmonologist, thoracic surgeon, pathologist, gynecologist, and radiologist is required. Such a team can help diagnose and provide treatment of TES as soon as possible.
Footnotes
Acknowledgment: All authors have no proprietary, financial, professional, or other personal interest of any nature in any product, service, or company. The authors alone are responsible for the content and writing of the paper.
Competing Interests: None.
References
- 1.Alwadhi S., Kohli S., Chaudhary B., Gehlot K. Thoracic endometriosis—a rare cause of haemoptysis. J Clin Diagn Res. 2016;10:TD1–TD2. doi: 10.7860/JCDR/2016/16365.7530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hagnere P., Deswarte S., Leleu O. Thoracic endometriosis: a difficult diagnosis. Rev Mal Respir. 2011;28:908–912. doi: 10.1016/j.rmr.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 3.Vetvicka V., Laganà A.S., Salmeri F.M., Triolo O., Palmara V.I., Vitale S.G. Regulation of apoptotic pathways during endometriosis: from the molecular basis to the future perspectives. Arch Gynecol Obstet. 2016;294:897–904. doi: 10.1007/s00404-016-4195-6. [DOI] [PubMed] [Google Scholar]
- 4.Laganà A.S., Salmeri F.M., Vitale S.G., Triolo O., Götte M. Stem cell trafficking during endometriosis. Reprod Sci. 2017 doi: 10.1177/1933719116687661. [DOI] [PubMed] [Google Scholar]
- 5.Laganà A.S., Vitale S.G., Salmeri F.M., Triolo O., Ban Frangež H., Vrtačnik-Bokal E. Unus pro omnibus, omnes pro uno: a novel, evidence-based, unifying theory for the pathogenesis of endometriosis. Med Hypotheses. 2017;103:10–20. doi: 10.1016/j.mehy.2017.03.032. [DOI] [PubMed] [Google Scholar]
- 6.Vitale S.G., La Rosa V.L., Rapisarda A.M.C., Laganà A.S. Endometriosis and infertility: the impact on quality of life and mental health. J Endometr Pelvic Pain Disord. 2017;9:112–115. [Google Scholar]
- 7.Vitale S.G., Petrosino B., La Rosa V.L., Rapisarda A.M., Laganà A.S. A systematic review of the association between psychiatric disturbances and endometriosis. J Obstet Gynaecol Can. 2016;38:1079–1080. doi: 10.1016/j.jogc.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Laganà A.S., La Rosa V.L., Rapisarda A.M.C., Valenti G., Sapia F., Chiofalo B. Anxiety and depression in patients with endometriosis: impact and management challenges. Int J Womens Health. 2017;9:323–330. doi: 10.2147/IJWH.S119729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vitale S.G., La Rosa V.L., Rapisarda A.M., Laganà A.S. Impact of endometriosis on quality of life and psychological well-being. J Psychosom Obstet Gynaecol. 2016 doi: 10.1080/0167482X.2016.1244185. [DOI] [PubMed] [Google Scholar]
- 10.Joseph J., Sahn S.A. Thoracic endometriosis syndrome: new observations from an analysis of 110 cases. Am J Med. 1996;100:164–170. doi: 10.1016/s0002-9343(97)89454-5. [DOI] [PubMed] [Google Scholar]
- 11.Park C.M., Goo J.M., Lee H.J., Lee C.H., Chun E.J., Im J.G. Nodular ground-glass opacity at thin-section CT: histologic correlation and evaluation of change at follow-up. Radiographics. 2007;27:391–408. doi: 10.1148/rg.272065061. [DOI] [PubMed] [Google Scholar]
- 12.Rousset P., Rousset-Jablonski C., Alifano M., Mansuet-Lupo A., Buy J.N., Revel M.P. Thoracic endometriosis syndrome: CT and MRI features. Clin Radiol. 2014;69:323–330. doi: 10.1016/j.crad.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 13.Shiota Y., Umemura S., Arikita H., Horita N., Hiyama J., Tetsuya Ono T. A case of parenchymal pulmonary endometriosis, diagnosed by cytologic examination of bronchial washing. Respiration. 2001;68:439. doi: 10.1159/000050545. [DOI] [PubMed] [Google Scholar]
- 14.Bennett G.L., Slywotzky C.M., Cantera M., Hecht E.M. Unusual manifestations and complications of endometriosis—spectrum of imaging findings: pictorial review. AJR Am J Roentgenol. 2010;194:WS34–WS46. doi: 10.2214/AJR.07.7142. [DOI] [PubMed] [Google Scholar]
- 15.Marshall M.B., Ahmed Z., Kucharczuk J.C., Kaiser L.R., Shrager J.B. Catamenial pneumothorax: optimal hormonal and surgical management. Eur J Cardiothorac Surg. 2005;27:662–666. doi: 10.1016/j.ejcts.2004.12.047. [DOI] [PubMed] [Google Scholar]
- 16.Haruki T., Fujioka S., Adachi Y., Miwa K., Taniguchi Y., Nakamura H. Successful video-assisted thoracic surgery for pulmonary endometriosis: report of a case. Surg Today. 2007;37:141–144. doi: 10.1007/s00595-006-3360-0. [DOI] [PubMed] [Google Scholar]
- 17.Agarwal N., Subramanian A. Endometriosis—morphology, clinical presentations and molecular pathology. J Lab Physicians. 2010;2:1–9. doi: 10.4103/0974-2727.66699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vitale S.G., La Rosa V.L., Rapisarda A.M., Lagana A.S. Comment on: “Impact of endometriosis on quality of life and mental health: pelvic pain makes the difference.”. J Psychosom Obstet Gynaecol. 2017;38:81–82. doi: 10.1080/0167482X.2016.1244183. [DOI] [PubMed] [Google Scholar]
- 19.Soriano D., Schonman R., Gat I., Schiff E., Seidman D.S., Carp H. Thoracic endometriosis syndrome is strongly associated with severe pelvic endometriosis and infertility. J Minim Invasive Gynecol. 2012;19:742–748. doi: 10.1016/j.jmig.2012.08.773. [DOI] [PubMed] [Google Scholar]
- 20.Montoriol P.F., Da Ines D., Bourdel N., Garcier J.M., Canis M. Re: thoracic endometriosis syndrome: CT and MRI features. Clin Radiol. 2014;69:549–550. doi: 10.1016/j.crad.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 21.Marchiori E., Zanetti G., Rafful P.P., Hochhegger B. Pleural endometriosis and recurrent pneumothorax: the role of magnetic resonance imaging. Ann Thorac Surg. 2012;93:696–697. doi: 10.1016/j.athoracsur.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 22.Marchiori E., Zanetti G., Rodrigues R.S., Souza L.S., Souza Junior A.S., Francisco F.A. Pleural endometriosis: findings on magnetic resonance imaging. J Bras Pneumol. 2012;38:797–802. doi: 10.1590/s1806-37132012000600017. [DOI] [PubMed] [Google Scholar]
- 23.Ghigna M.R., Mercier O., Mussot S., Fabre D., Fadel E., Dorfmuller P. Thoracic endometriosis: clinicopathologic updates and issues about 18 cases from a tertiary referring center. Ann Diagn Pathol. 2015;19:320–325. doi: 10.1016/j.anndiagpath.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Laganà A.S., Vitale S.G., Trovato M.A., Palmara V.I., Rapisarda A.M., Granese R. Full-thickness excision versus shaving by laparoscopy for intestinal deep infiltrating endometriosis: rationale and potential treatment options. Biomed Res Int. 2016;2016:3617179. doi: 10.1155/2016/3617179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Augoulea A., Lambrinoudaki I., Christodoulakos G. Thoracic endometriosis syndrome. Respiration. 2008;75:113–119. doi: 10.1159/000105102. [DOI] [PubMed] [Google Scholar]
- 26.Slabbynck H., Laureys M., Impens N., De Vroey P., Schandevyl W. Recurring catamenial pneumothorax treated with a Gn-RH analogue. Chest. 1991;100:851. doi: 10.1378/chest.100.3.851. [DOI] [PubMed] [Google Scholar]
- 27.Alifano M., Roth T., Broet S.C., Schussler O., Magdeleinat P., Regnard J.F. Catamenial pneumothorax: a prospective study. Chest. 2003;124:1004–1008. doi: 10.1378/chest.124.3.1004. [DOI] [PubMed] [Google Scholar]
- 28.Joseph J., Reed C.E., Sahn S.A. Thoracic endometriosis. Recurrence following hysterectomy with bilateral salpingo-oophorectomy and successful treatment with talc pleurodesis. Chest. 1994;106:1894–1896. doi: 10.1378/chest.106.6.1894. [DOI] [PubMed] [Google Scholar]