Abstract
The effect of organic acids as an alternative to antibiotics on the performance of broiler chickens was evaluated by meta-analysis, identifying and quantifying the main factors that influence results. A total of 51,960 broilers from 121 articles published between 1991 and 2016 were used. Interactions of additives [non-supplemented group (control), organic acids, and growth promoter antibiotics] with microbial challenge (with or without inoculation of pathogenic microorganisms) were studied on performance variables. Moreover, the effects of organic acids, used individually or in blends, were evaluated. Relative values of average daily gain (ADG) and average daily feed intake (ADFI) were obtained in relation to control: ΔADG and ΔADFI, respectively. Analysis of variance-covariance revealed lower ADG with organic acids when compared to antibiotics (P < 0.05). There was a significant interaction between the additives and the challenge on feed conversion ratio (FCR) (P < 0.01) and on viability (P < 0.05). Without challenge, organic acids improved broilers’ FCR (P < 0.01), presenting results similar to antibiotics (P > 0.05). Under challenge, the organic acids were again effective on FCR (−5.67% in relation to control, P < 0.05), but they did not match antibiotics (−13.40% in relation to control, P < 0.01). Viability was improved only under challenge conditions, and only by antibiotics (+4.39% in relation to control, P < 0.05). ADG (P < 0.05) and FCR (P < 0.01) were increased by blends of organic acids, but not by the organic acids used alone (P > 0.05). ADFI and production factor were not influenced by the treatments (P > 0.05). ΔADFI of organic-acid supplemented group showed a linear influence on ΔADG, which increases 0.64% at every 1% increase in ΔADFI. In conclusion, organic acids can be utilized as performance enhancing, but the results are lower than those found with antibiotics, particularly under microbial challenge. The blends of organic acids provide better results than the utilization of one organic acid alone.
Keywords: additive, alternative to antibiotic, broiler, meta-analysis, organic acid
INTRODUCTION
After the European Union banned antibiotics as growth promoters in animal feed in 2006 (Castanon, 2007), the poultry industry has been facing several challenges concerning enteritis caused by the imbalance of intestinal microbiota. Some of these conditions are described as: small intestinal bacterial overgrowth, mal-absorption, and feed-passage syndrome (Huyghebaert et al., 2011). Due to these problems, there has been an international boost on research studies that search for additives to substitute the antibiotics. Among the most studied additives are organic acids, which have antimicrobial potential to control or at least minimize losses in the performance of broilers raised without chemotherapeutics.
Organic acids have been long utilized to preserve feed, and have been used as an additive to improve the performance of broilers for more than 3 decades. However, the advance of research studies and the considerable increase in the number of published articles have generated great information inconsistency, making it impossible to interpret the data under the subjective perspective of the classical approach based on qualitative literature reviews. Previous research studies show that organic acids can improve poultry growth performance (Abdel-Fattah et al., 2008; Khosravi et al., 2008; Adil et al., 2010), whereas others do not verify the same significant results (Józefiak et al., 2007; Ao et al., 2009; Isabel and Santos, 2009; Houshmand et al., 2011; Cengiz et al., 2012). Some studies has found that organic acids have a potential effect to replace the antibiotics (García et al., 2007; Chowdhury et al., 2009; Panda et al., 2009a,b; Abbas et al., 2011; Ali et al., 2014); however, several other studies showed lower performance results with organic acids when compared to the ones obtained with antibiotics (Mikkelsen et al., 2009; Fascina et al., 2012; Saki et al., 2012; Barbieri et al., 2015). The main explanation for these differences is the heterogeneity of conditions in which each experiment was carried out, differing in the chemical structure of the utilized acid and in the supplementation form (mixed or not), in the sanitary challenge conditions, in the buffering capacity of feeds, and in the feeds’ nutritional dietary value, among other factors.
Hence, the meta-analytical approach becomes a valuable option because it uses quantitative methods that allow combining different independent studies in a statistical analysis to obtain inferences with greater analytical power (St-Pierre, 2001, 2007). This tool integrates different variables to establish systematic responses adjusted to the diversity of publications (Sauvant et al., 2005, 2008), and it is also a great mechanism to identify and validate topics to be investigated in future studies. Another very reasonable justification for the use of meta-analysis refers to the transformation of research results into an applicable knowledge, because this tool considers heterogeneity among studies in a systematic way, whereas a single experiment reflects only the experimental conditions under which it was carried out (Lovatto et al., 2007).
Therefore, this study aimed to carry out a meta-analysis to evaluate the utilization of organic acids as an alternative additive to growth-promoting antibiotics on the performance of broilers, identifying and quantifying the main factors that influence the results.
MATERIALS AND METHODS
Search and Data Filtering
The digital search for studies included scientific articles published in peer-reviewed journals and was carried out in the following computer databases: Scielo, Periódicos CAPES, Scopus, Web of Science, and Google Scholar. This diversity of search engines for studies prevents potential bias toward articles found in only one database and broadens research limits. The key words used in the search were tested in English, and then in other languages: French, Portuguese, and Spanish. The search for studies lasted until the beginning of 2016.
Papers were peer-selected to ensure the quality of meta-analysis results, which depends on the eligibility of studies that compose the database. The main criteria to select the published articles were: in vivo experiments with broilers supplemented with organic acids and the presence of productive results (weight gain and feed intake) or related results (viability). Then, the studies were critically analyzed for eventual errors in the methodological structure.
A total of 191 articles evaluating the performance of broilers supplemented with organic acids were found. Forty-two articles were excluded due to the administration of antibiotics or chemotherapeutics in feed, which can interfere in the effect of organic acids [except in cases in which the antibiotics were used as a treatment (positive control)]. Sixteen articles were excluded because organic acids were combined with another additive in a single treatment (association of additives), which made impossible to evaluate only the effect of the organic acids. Eight articles were ruled out because their methodological flaws compromised the published results (examples: different start weight among birds and changes in experimental units during the evaluation period). Two articles published before 1990 were excluded due to the changes that have occurred in broilers’ genetic material and feeding practices in the present day. Two articles were not considered because they had been published in duplicate, that is, the same data were exploited in distinct publications. The lines corresponding to treatments with addition of 4% or more of organic acids were withdrawn from the database because the addition of excessive levels of organic acids changes feed intake and, consequently, broilers’ performance (Pinchasov and Jensen, 1989; Pinchasov and Elmaliah, 1994; Islam et al., 2008; Nourmohammadi et al., 2010; Esmaeilipour et al., 2011; Günal, 2013; Khosravinia et al., 2015).
Data Systematization and Coding
The papers were critically reviewed and the necessary information was extracted and recorded. Topics that were pertinent to meta-analysis objectives were explored. The database consisted of lines representing the treatments and columns representing the exploratory variables. Some codings were utilized to form homogenous groups with common characteristics in order to include them in statistical models as sources of variation. Main codings were used to classify the groups of additives [1: control (without additive), 2: organic acids, or 3: antibiotics] and the microbial challenge [1: absence (non-inoculated broilers, −), or 2: presence (inoculated broilers, +)]. The microorganism inocula used as the microbial challenge in experiments found in the database were: Campylobacter, Clostridium, Eimeria, Escherichia or Salmonella.
Each article was coded to make its identification in the database easier (general code). Other codings were utilized to input the variability of compiled studies (experiments) in the statistical model. Therefore, a sequential number was attributed to each experiment to codify the inter-study effect. The groups with repeatedly measured responses over time were also coded to evaluate the intra-study effects.
Database Description
The database occupied 1,149 lines and 87 columns on a spreadsheet and consisted of 121 articles published from 1991 to the beginning of 2016 (mode = 2008 and 2009). Information about the papers included in the database (references) are presented in the Supplementary Data. The studies included in the database totaled 51,960 broilers, with an average of 394 broilers per experiment (mode = 180) and 102 per treatment (mode = 60). The average duration of the evaluated periods was 25 d, the maximum time was 56 d, and the minimum was 4 d. While 48% of the experiments were carried out in floor pens, 40% were done in cages, and 12% did not describe the installation type. Only 12% of the experiments were performed with challenged broilers (inoculated). The relative frequency of utilized strains was: 44% Ross, 29% Cobb, 10% Arbor Acres, 5% Hubbard, 7% from other strains, and 5% did not provide this information. The broilers’ sex was described in 77% of the experiments (of which 68% were male, 27% mixed, and 5% female). The average initial age of the evaluated periods was 8 d (ranging from 1 to 43 d) and the final average age was 32 d (ranging from 7 to 56 d). In 66% of the experiments, the diets contained ingredients with low non-starch polysaccharides (NSPs) (98% of diets were corn- and soybean-meal-based, and 2% had sorghum in place of corn); in 25% the addition of some fiber ingredient was observed (rich in NSPs) and 9% did not present information about the diets. The average nutritional values of the diets that made up the database were: 3,028 kcal/kg of ME, 20.68% CP, 1.13% of digestible lysine, 0.48% of digestible methionine, 0.82% of digestible methionine + cystine, 0.78% of digestible threonine, 3.82% of fiber, 0.92% of calcium, 0.68% of total phosphorus and 0.41% of available phosphorus. The average level of organic acid supplementation was 0.74%, with maximum value of 3.75% and minimum of 0.025%. The organic acids that formed the database were: acetic, benzoic, butyric, caprylic, citric, ethylenediaminetetraacetic (EDTA), formic, fumaric, gallic, gluconic, lactic, malic, phenylacetic, propionic, sorbic, tannic, tartaric, and acid blends. The antibiotics utilized as positive controls were: avilamycin, bacitracin, clopidol, enramycin, flavomycin, furazolidone, oxytetracycline, salinomycin, and virginiamycin.
Data Analysis
Minitab 17 Statistical Package (Minitab Inc., State College, PA) was utilized during the first step of data analysis, which comprised graphic elaboration. The Statistical Analysis System software (SAS Institute, 2012) was used for the subsequent analyses of inference with probability level at 5%.
Graphical Analysis, Correlations, and Residual Variations.
The graphic analyses were done to evaluate data distribution, allowing a general visualization of the consistency and heterogeneity of values. Different types of scatter plots were utilized not only to establish hypotheses but also to clarify key points to choose the statistical model. Also, these graphs helped to identify experiments and isolated data (treatments) under extreme conditions (Lovatto et al., 2007). The inter- and intra-study relations were assessed as suggested by Sauvant et al. (2005, 2008). In summary, the graphic analyses were utilized to control the database quality and to observe the biological coherence of values. Based on graphical analysis, correlation hypotheses (CORR procedure) were tested to determine how the results had been affected by the relation of some variables, giving support and direction in the choice of variables to adjust statistical models of subsequent analyses of variance-covariance. The normality assumptions were verified by studentized residuals throught the UNIVARIATE procedure.
Variance-Covariance Analyses.
In all analyses of variance-covariance, the study effect was considered in the model as a random-effect class variable due to the differences among the studies that form a meta-analysis database (St-Pierre, 2001). Therefore, mixed models using MIXED procedure were utilized as proposed by St-Pierre (2007). Additives (groups: control, organic acids, or antibiotics) and the microbiological challenge (− or +) as well as the interaction among them were considered fixed effect factors. The microbiological challenge was considered in the model due to its relevance in evaluations of antimicrobial additives for poultry. Although the fundamental importance of the challenge became evident more than 50 years ago (Lillie et al., 1953; Coates et al., 1963), a lot of research studies still ignore it. Analyses of variance-covariance were also performed comparing different types of organic acids to investigate the individual efficiency of each one. When needed, Tukey-Kramer's test was applied to contrast the least square means of the treatments.
The analyzed response variables of productive performance were: ADG, ADFI, feed conversion ratio (FCR), viability (VB) and production factor [PF: (((ADG × VB)/FCR)/10)]. The data of these variables were obtained at different ages; therefore, they were corrected by the average age (average between the initial and final ages in each evaluation), which was then input in the model as a fixed effect covariable. The quadratic effect of age was used when it had significant effect. Thus, regression equations were adjusted to predict the broilers’ performance over age. The variation of ADG and ADFI obtained by the difference between the treatments with organic acids or with antibiotics compared to the control group was calculated and expressed in percentage (ΔADG and ΔADFI represent the relative variation of ADG and ADFI, respectively). A linear regression was calculated to understand how the variation of feed intake (ΔADFI) influence the responses of weight gain (ΔADG):
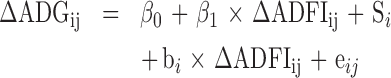 |
Description of the fixed effect part of the model: the overall (inter-study) intercept (β0) of this equation shows the gain variation that is provided by the additive (organic acids or antibiotics) when the consumption variation is zero, which may be interpreted as an indicator of maintenance requirements. The overall regression coefficient [slope (β1)] shows the extension of ΔADG change that is associated with the ΔADFI in broilers fed with organic acids or antibiotics. The significance of the fixed regression parameters was verified by t test. Random effect part of the model: Si = effect of the ith study, assumed ∼ iidN (0, 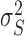 ), bi = effect of the study on the regression coefficient, assumed ∼ iidN (0,
), bi = effect of the study on the regression coefficient, assumed ∼ iidN (0, 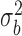 ), and the eij = residual errors, assumed ∼ iidN (0,
), and the eij = residual errors, assumed ∼ iidN (0, 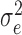 ). The Si, bi and eij were assumed to be independent random variables.
). The Si, bi and eij were assumed to be independent random variables.
RESULTS AND DISCUSSION
Interactions of Organic Acids with Microbiological Challenge
The data adjustment for the broilers’ average age presented a significant quadratic effect in all performance variables except for the FCR, which showed better adjustment with the linear component only (Table 1). Diet-related factors were also considered during the elaboration of meta-models. Previous reviews of the literature emphasized that some variables related to diet composition might be responsible for the lack of consistency of results obtained with organic acids (Dibner and Buttin, 2002; Kim et al., 2015). Dietary inclusion of ingredients with buffering capacity, such as phosphate and bicarbonate, did not present significant adjustments (P > 0.05), possibly due to the great occurrence of empty cells corresponding to both of these variables at the end of database construction. Thus, it would be interesting to design new experiments to evaluate the interactions of organic acids with these variables. The content of crude protein in the diets was not considered due to the correlation with the broilers’ ages (−0.65, P < 0.01), in order to avoid mistakes that can be caused by multicollinearity.
Table 1.
Performance of broiler chickens supplemented with organic acids as an alternative to antibiotics, submitted or not to microbiological challenge.
| Performance2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ADG, g | ADFI, g | FCR | VB, % | PF | |||||||
| Effects1 | n | LS-Means | n | LS-Means | n | LS-Means | n | LS-Means | n | LS-Means | |
| Additive | CON | 371 | 44.7 b3 | 365 | 85.0 | 367 | 1.85 | 57 | 91.9 | 54 | 260.1 |
| OA | 688 | 45.7 b | 670 | 84.6 | 673 | 1.78 | 119 | 93.4 | 113 | 277.8 | |
| ATB | 89 | 48.5 a | 89 | 84.3 | 90 | 1.70 | 21 | 93.9 | 20 | 314.0 | |
| Challenge | – | 1063 | 47.0 | 1055 | 84.1 | 1056 | 1.73 | 173 | 97.5 | 172 | 287.3 |
| + | 85 | 45.6 | 69 | 85.1 | 74 | 1.82 | 24 | 88.7 | 15 | 280.6 | |
| Additive × Challenge | CON − | 345 | 46.0 | 343 | 83.8 | 344 | 1.76 b A | 50 | 97.3 a A | 49 | 269.0 |
| CON + | 26 | 43.4 | 22 | 86.2 | 23 | 1.94 c B | 7 | 86.6 b B | 5 | 251.2 | |
| OA − | 640 | 47.1 | 634 | 83.5 | 634 | 1.72 a A | 106 | 97.7 a A | 106 | 293.9 | |
| OA + | 48 | 44.3 | 36 | 85.7 | 39 | 1.83 b B | 13 | 89.1 ab B | 7 | 261.6 | |
| ATB − | 78 | 48.0 | 78 | 85.0 | 78 | 1.72 a A | 17 | 97.6 a A | 17 | 299.1 | |
| ATB + | 11 | 49.0 | 11 | 83.6 | 12 | 1.68 a A | 4 | 90.4 a B | 3 | 328.9 | |
| SE | 0.58 | 1.21 | 0.011 | 0.36 | 6.47 | ||||||
| Model4 | Probability of fixed effects | ||||||||||
| Additive | 0.007 | 0.918 | <0.001 | 0.001 | 0.170 | ||||||
| Challenge | 0.397 | 0.714 | 0.021 | <0.001 | 0.866 | ||||||
| Additive × Challenge | 0.241 | 0.614 | <0.001 | 0.014 | 0.539 | ||||||
| Age | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||||||
| Age × Age | <0.001 | <0.001 | - | <0.001 | <0.001 | ||||||
1CON, control (without additive); OA, organic acids; ATB, antibiotics; −, without challenge; +, with challenge.
2FCR, feed conversion ratio; VB, viability; PF, production factor = (((ADG × VB)/FCR)/10).
3LS-Means (least square means) followed by distinct letters differ by the Tukey-Kramer's test (P < 0.05). In the interactions, lower case letters represent the comparison between the additives (within the absence or the presence of challenge), and upper case letters represent the comparison between the absence and the presence of challenge (within each additive).
4Studies (experiments) entered in the model as a random-effect class variable, and the variables age (average between the initial and final age of each evaluation, expressed in d) and age × age entered in the model as fixed-effect covariates.
There was no interaction of additives with the microbiological challenge on ADG. The organic acids did not change ADG in relation to the control group, and presented lower results compared to antibiotics (−5.77%, P < 0.05). These results confirm the negative consequences caused by antibiotic withdrawal (Casewell et al., 2003). In addition, information obtained under commercial conditions identified less uniform body weight as a result of antibiotic withdrawal from feed (Engster et al., 2002). It is evident that the antibiotic ban will bring losses to broilers’ growth in the short and medium terms, which will not be fully compensated by natural additives. However, even based on the precautionary principle, the incentive to prevent the development of resistant microrganisms supports human and animal health and directs animal production to a more sustainable pathway, avoiding not only the emergence of resistant bacterial strains but also the transfer of resistant genes between animal and human bacteria (Van Den Bogaard and Stobberingh, 2000).
A significant interaction between the organic acids and the microbiological challenge was observed on FCR. Under experimental conditions carried out without microorganism inoculation, the organic acids improved broilers’ FCR in - 2.27% in relation to control (P < 0.01), and presented similar result to antibiotics. It has been suggested that the effects of organic acids go beyond modification of the intestinal microbiota, including improvements in the activity of digestive enzymes, pancreatic secretions, and gastrointestinal mucosa (Dibner and Buttin, 2002). Gains in nutrient digestibility, mainly of minerals, are also attributed to the use of organic acids (Islam et al., 2012; Emami et al., 2013). However, contradictory results found in recent studies did not confirm these benefits (Goodarzi Boroojeni et al., 2014; Ruhnke et al., 2015), suggesting more studies to clarify these points. In challenged broilers, the organic acids were again effective on FCR improvement (5.67% in relation to control, P < 0.05), but they did not compare to the effects of antibiotics (13.40% in relation to control, P < 0.01), which controlled the harmful effects of the challenge. It is clear that organic acids can be utilized to improve FCR responses, but this effect only partially suppresses deleterial effects of undesirable microbitota. Although organic acids are less efficient than antibiotics, several studies prove their efficiency against Campylobacter (Chaveerach et al., 2002; Heres et al., 2004; Skånseng et al., 2010; Guyard-Nicodéme et al., 2016), Clostridium (Timbermont et al., 2010; Mohamed et al., 2014), Eimeria (Abbas et al., 2011; Ali et al., 2014), Escherichia (Ozduven et al., 2009; Panda et al., 2009a,b; Roy et al., 2012), and Salmonella (Van Immerseel et al., 2004; Fernández-Rubio et al., 2009; Menconi et al., 2013). Moreover, Goualié et al. (2014) demonstrated the antibacterial ability of organic acids against resistant strains of Campylobacter to antibiotics that are commonly utilized in the treatment of campylobacteriosis in humans, characterizing organic acids as an alternative to minimize the problems caused by the use of antibiotics. The effect of organic acids can be explained by the lipophilic ability of these molecules to cross the cell membrane of bacteria and dissociate themselves in the more alkaline inner part, acidifying the cytoplasm and consequently impairing the cellular metabolism. The excessive exportation of protons by the bacteria to control the intracellular pH demands the consumption of adenosine triphosphate (ATP), which results in depression of the cellular energy (Ricke, 2003), retarding its growth or even causing the microorganism death. Another mechanism of the organic acid action seems to be related to the suppression of hilA, a key regulator of Salmonella capacity to invade cells in the intestinal epithelium (Van Immerseel et al., 2004, 2006).
In the interaction on VB, it was observed that the additives did not influence the results of broilers raised without inoculation of microorganisms, probably because the additives act on VB mainly through antimicrobial effect, attenuating the increase in mortality that may be caused by pathogenic microorganisms (Coullier et al., 2008). Under challenge, there was no difference between the additives (organic acids vs. antibiotics), but only antibiotics showed significant improvements compared to the control (+4.39%, P < 0.05). Although the increment of antibiotics on VB is significant, they were not enough to totally control the deleterious effect of the microbiological challenge on this variable. The ADFI and PF were not influenced by the effect of additives and microbiological challenge.
In Table 2, the equations of performance prediction in function of age are shown, which were planned (grouped) according to the results presented above. It is expected that FCR of broilers fed with organic acids (without challenge) increases in 0.0266 units per d, whereas under microbiological challenge the increase was estimated to be 0.0307, resulting in a difference (decrease) of 4.69% in FCR at 42 d. Under microbiological challenge, the VB obtained with organic acids was not changed by age, but on the other hand, antibiotics promote maximum VB at 18 d.
Table 2.
Regression equations, obtained by analysis of variance-covariance, to estimate the performance of broiler chickens supplemented with organic acids as an alternative to antibiotics, submitted or not to the microbiological challenge.
| Intercept | Age | Age × Age | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables1 | Group2 | Parameter | SE | P > |t| | Parameter | SE | P > |t| | Parameter | SE | P > |t| | |
| ADG, g | Additive | CON | −5.223 | 2.84 | 0.066 | 3.327 | 0.167 | <0.001 | −0.0347 | 0.0038 | <0.001 |
| OA | −0.821 | 2.61 | 0.754 | 3.257 | 0.120 | <0.001 | −0.0341 | 0.0026 | <0.001 | ||
| ATB | −0.123 | 7.68 | 0.987 | 3.874 | 0.604 | <0.001 | −0.0455 | 0.0143 | 0.002 | ||
| ADFI, g | – | – | −13.39 | 3.30 | <0.001 | 5.868 | 0.158 | <0.001 | −0.0345 | 0.0035 | <0.001 |
| FCR | Additive × Challenge | CON − | 1.272 | 0.030 | <0.001 | 0.0259 | 0.0010 | <0.001 | – | – | |
| CON + | 1.170 | 0.127 | <0.001 | 0.0369 | 0.0056 | <0.001 | – | – | |||
| OA − | 1.210 | 0.024 | <0.001 | 0.0266 | 0.0007 | <0.001 | – | – | |||
| OA + | 1.147 | 0.074 | <0.001 | 0.0307 | 0.0031 | <0.001 | – | – | |||
| ATB − | 1.225 | 0.063 | <0.001 | 0.0255 | 0.0026 | <0.001 | – | – | |||
| ATB + | 1.292 | 0.159 | <0.001 | 0.0181 | 0.0078 | <0.062 | – | – | |||
| VB, % | Additive × Challenge | CON − | 100.4 | 1.52 | <0.001 | −0.3674 | 0.127 | 0.006 | 0.0086 | 0.0026 | 0.002 |
| CON + | 254.4 | 132.0 | 0.126 | −20.94 | 17.44 | 0.296 | 0.6139 | 0.5305 | 0.312 | ||
| OA − | 99.91 | 0.85 | <0.001 | −0.3096 | 0.062 | <0.001 | 0.0070 | 0.0013 | <0.001 | ||
| OA + | 90.34 | 129.0 | 0.522 | 0.6579 | 17.34 | 0.971 | −0.0324 | 0.5328 | 0.954 | ||
| ATB − | 103.3 | 2.03 | <0.001 | −0.7014 | 0.165 | 0.004 | 0.0154 | 0.0035 | 0.003 | ||
| ATB + | 118.8 | 0.63 | 0.003 | −2.648 | 0.086 | 0.021 | 0.0716 | 0.0028 | 0.025 | ||
| PF | – | – | 106.5 | 46.76 | 0.026 | 13.52 | 2.797 | <0.001 | −0.2415 | 0.0580 | <0.001 |
1FCR, feed conversion ratio; VB, viability; PF, production factor = (((ADG × VB)/FCR)/10).
2CON, control (without additive); OA, organic acids; ATB, antibiotics; −, without challenge; +, with challenge.
Effects of Organic Acids Used Alone or Blended
Organic acids did not change broilers’ ADG (Table 3), confirming previously observed results. However, ADG increase was observed in a contrast prepared only between the control and the group with blends of organic acids (increase of 3.70% with blends, P = 0.027). These results demonstrated the superior effect of the association of several organic acids in relation to the acids used alone. Each acid has its own antimicrobial activity spectrum (Dibner and Buttin, 2002), and therefore the blends of several acids have a more general action, performing better in different conditions that exist among broiler breedings. Another explanation is related to the synergic effect that the blends may present (Kil et al., 2011; Kim et al., 2015), although there are few studies with broilers that compare the combination of blends with the individual use. It was demonstrated that the combination of only 2 acids (formic + propionic) was already sufficient to increment broilers’ weight gain, whereas the individual utilization did not bring benefits (Roy et al., 2012). It would be interesting if studies were conducted to better explain how the beneficial relations among acids occur, indicating better blends and proportions through the intensity of interactions (synergistic effect?).
Table 3.
Performance of broiler chickens supplemented with individual inclusions or with blends of organic acids.
| Organic acids2 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables1 | Control | Benzoic | Butyric | Citric | Formic | Fumaric | Lactic | Blends | SE | Probability | Adjustments3 | |
| ADG, g | n | 371 | 25 | 90 | 102 | 58 | 37 | 20 | 260 | |||
| 0.62 | 0.066 | A and A × A | ||||||||||
| LS-Means | 46.0 | 45.4 | 47.2 | 46.9 | 46.2 | 45.3 | 46.4 | 47.7 | ||||
| ADFI, g | n | 365 | 25 | 82 | 102 | 58 | 37 | 20 | 256 | |||
| 1.32 | 0.874 | A and A × A | ||||||||||
| LS-Means | 84.3 | 83.5 | 83.9 | 84.5 | 83.9 | 80.9 | 83.4 | 85.0 | ||||
| FCR | n | 367 | 25 | 82 | 102 | 58 | 37 | 20 | 256 | |||
| 0.011 | <0.001 | A | ||||||||||
| LS-Means | 1.78 b4 | 1.78 ab | 1.73 ab | 1.75 ab | 1.75 ab | 1.75 ab | 1.74 ab | 1.72 a | ||||
| VB, % | n | 57 | 25 | 61 | ||||||||
| – | – | – | – | – | 0.49 | 0.375 | A and A × A | |||||
| LS-Means | 95.3 | 94.9 | 95.8 | |||||||||
| PF | n | 54 | 23 | 60 | ||||||||
| – | – | – | – | – | 7.66 | 0.205 | A and A × A | |||||
| LS-Means | 274.0 | 285.4 | 301.4 | |||||||||
1FCR, feed conversion ratio; VB, viability; PF, production factor = (((ADG × VB)/FCR)/10).
2It was considered in the analysis the organic acids with n equal to or greater than 20.
3Adjustments: studies (experiments) entered in the model as a random-effect class variable, and the variables age (A, average between the initial and final age of each evaluation, expressed in d) and age × age entered in the model as fixed-effect covariates. In all adjustments, the fixed-effect covariates presented P < 0.001.
4LS-Means (least square means) followed by distinct letters differ by the Tukey-Kramer's test (P < 0.05).
The FCR was again incremented by the action of organic acids. It can be observed that only the group with acid blends was superior to the control (P < 0.001). It was not possible to show the effect of organic acids utilized alone. Perhaps if there were a higher analytic power (more observations), some increment in FCR with some acids could be found. An option to increase the efficiency of acids is the incorporation of new technologies. The microencapsulation of butyric acid, for example, decreases the level of microbial infection in broilers (Fernández-Rubio et al., 2009). The protection provided by the microencapsulation results in gradual release of the acid throughout the gastrointestinal tract of the chicken, reaching most distal regions of the intestine (Van Den Borne et al., 2015). Van Immerseel et al. (2005) observed greater efficiency in the reduction of Salmonella colonization in the liver and spleen of broilers supplemented with a combination of a protected form and a powder form of butyric acid. It is possible that the combination of unprotected and microencapsulated acids may benefit the performance of broilers, mainly in situations with many different pathogens. The quick dissociation of unprotected acids can act on pathogens right in the first parts of the gastrointestinal tract, whereas protected organic acids act throughout the intestine, reducing the competition of endogenous nitrogen with microbiota in more distal parts. However, it was not possible to study this type of technology due to the limitations of available information in the database.
Organic acids did not influence ADFI. Although some authors have described feed intake reduction in broilers supplemented with organic acids (Pinchasov and Jensen, 1989; Pinchasov and Elmaliah, 1994; Islam et al., 2008; Nourmohammadi et al., 2010; Esmaeilipour et al., 2011; Günal, 2013; Khosravinia et al., 2015), this meta-analysis revealed that the inclusions below 4% do not harm intake significantly. VB and PF were not changed either.
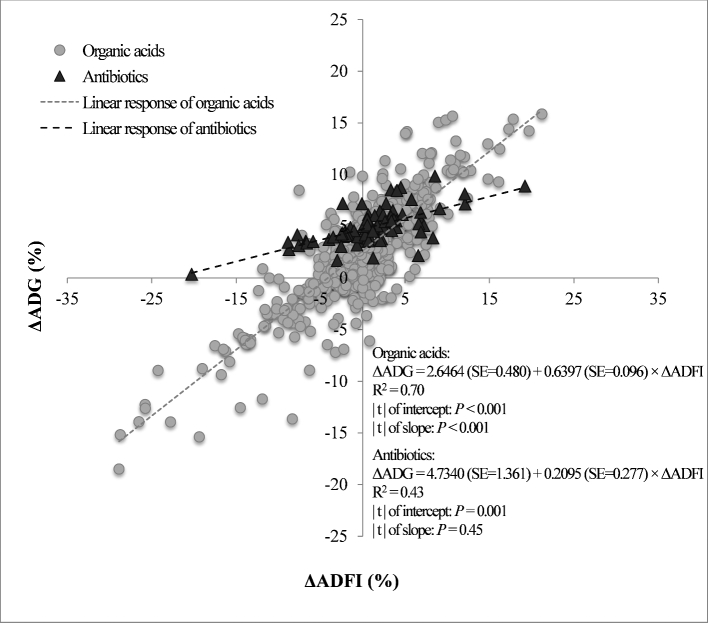
Relation Between ΔADG and ΔADFI
The relation between ΔADG and ΔADFI was studied in broilers from treatments with organic acids or with antibiotics (Figure 1). The intercepts of the equations indicated that ΔADG was 4.73% for antibiotics and 2.64% for organic acids, when ΔADFI = 0. This difference in the gain variation in relation to control group when the intake variation is zero may indicate lower metabolic loss of broilers supplemented with antibiotics or organic acids. The reduction in the microbial population due to the utilization of antibiotics promotes smaller length, weight, and thickness of the small intestine (Jukes et al., 1956; Engberg et al., 2000; Miles et al., 2006; Barbieri et al., 2015), besides a smaller cellular proliferation of the intestinal and hepatic epithelium (Krinke and Jamroz, 1996), resulting in lower energy use for body maintenance. On the other hand, organic acids can provide superior metabolic rate (Abdel-Fattah et al., 2008). In a review, Kim et al. (2015) cited that organic acids can stimulate the energetic metabolism by providing energy to cells of the intestinal epithelium, besides acting as intermediate substrates in the tricarboxylic acid cycle (Partanen and Mroz, 1999). Despite the differences in physiological responses, FCR improvement attributed to the use of both additives is mainly related to the control of pathogenic microorganisms in the gastrointestinal tract, decreasing the incidence and severity of subclinical infections, the competition for nutrients with the host and the amount of performance-depressing metabolites that are produced by bacteria (Huyghebaert et al., 2011).
Figure 1.
Relation between the variation in the average daily gain (ΔADG) due to the variation in the average daily feed intake (ΔADFI) in broilers supplemented with organic acids or antibiotics. Observations were adjusted (predicted value of ΔADG + residual) for the other variables in the model.
The inclination coefficient of antibiotic regression was not significant; therefore, it is possible to infer that antibiotic ΔADG does not depend on intake variation. Instead, the ΔADG of organic acids depend on feed intake, which increases 0.64% at every 1% increase in ΔADFI. The extension of this change can be expressed by the feed: gain ratio of 1.56 (FCR). This response makes evident that the effect of organic acids on ΔADG can be potentialized by the increase in ΔADFI. Thus, measures to increase feed intake in broilers supplemented with organic acids can be another factor to be studied to find better responses for weight gain.
We concluded that organic acids can be utilized as a performance-enhancing additive in broilers. However, the results found with organic acids are lower than the ones obtained with antibiotics, mainly in situations with microbiological challenge. In addition, the blends of organic acids present better results than the use of one single acid.
Acknowledgements
The authors thank São Paulo Research Foundation (FAPESP, Brazil) for the post-doctorate grant (Process number 2015/10144-5).
REFERENCES
- Abbas R. Z., Munawar S. H., Manzoor Z., Iqbal Z., Khan M. N., Saleemi M. K., Zia M. A., Yousaf A.. 2011. Anticoccidial effects of acetic acid on performance and pathogenic parameters in broiler chickens challenged with Eimeria tenella. Pesqui. Vet. Bras. 31:99–103. [Google Scholar]
- Abdel-Fattah S. A., El-Sanhoury M. H., N. M., El-Mednay, Abdel-Azeem F.. 2008. Thyroid activity, some blood constituents, organs morphology and performance of broiler chicks fed supplemental organic acids. Int. J. Poult. Sci. 7:215–222. [Google Scholar]
- Adil S., Banday T., Bhat G. A., Mir M. S., Rehman M.. 2010. Effect of dietary supplementation of organic acids on performance, intestinal histomorphology, and serum biochemistry of broiler chicken. Vet. Med. Int. 2010:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali A. M., Seddiek S., Khater H. F.. 2014. Effect of butyrate, clopidol and their combination on the performance of broilers infected with Eimeria máxima. Br. Poult. Sci. 55:474–482. [DOI] [PubMed] [Google Scholar]
- Ao T., Cantor A. H., Pescatore A. J., Ford M. J., Pierce J. L., Dawson K. A.. 2009. Effect of enzyme supplementation and acidification of diets on nutrient digestibility and growth performance of broiler chicks. Poult. Sci. 88:111–117. [DOI] [PubMed] [Google Scholar]
- Barbieri A., Polycarpo G. V., Cardoso R. G. A., Silva K. M., Dadalt J. C., Madeira A. M. B. N, Sousa R. L. M., Albuquerque R., Cruz-Polycarpo V. C.. 2015. Effect of probiotic and organic acids in an attempt to replace the antibiotics in diets of broiler chickens challenged with Eimeria spp. Int. J. Poult. Sci. 14:606–614. [Google Scholar]
- Casewell M., Friis C., Marco E., McMullin P., Phillips I.. 2003. The European ban on growth-promoting antibiotics and emerging consequences for human and animal health. J. Antimicrob. Chemother. 52:159–161. [DOI] [PubMed] [Google Scholar]
- Castanon J. I. R. 2007. History of the use of antibiotic as growth promoters in European poultry feeds. Poult. Sci. 86:2466–2471. [DOI] [PubMed] [Google Scholar]
- Cengiz O., Koksal B. H., Tatli O., Sevim O., Avci H., Epikmen T., Beyaz D., Buyukyoruk S., Boyacioglu M., Uner A., Onol A. G.. 2012. Influence of dietary organic acid blend supplementation and interaction with delayed feed access after hatch on broiler growth performance and intestinal health. Vet. Med. 57:515–528. [Google Scholar]
- Chaveerach P., Keuzenkamp D. A., Urlings H. A. P., Lipman L. J. A., Van Knapen F.. 2002. In vitro study on the effect of organic acids on Campylobacter jejuni/coli populations in mixtures of water and feed. Poult. Sci. 81:621–628. [DOI] [PubMed] [Google Scholar]
- Chowdhury R., Islam K. M. S., Khan M. J., Karim M. R., Haque M. N., Khatun M., Pesti G. M.. 2009. Effect of citric acid, avilamycin, and their combination on the performance, tibia ash, and immune status of broilers. Poult. Sci. 88:1616–1622. [DOI] [PubMed] [Google Scholar]
- Coates M. E., Fuller R., Harrison G. F., Lev M., Suffolk S. F.. 1963. A comparison of the growth of chicks in the Gustafsson germ-free apparatus and in a conventional environment, with and without dietary supplements of penicillin. Br. J. Nutr. 17:141–150. [DOI] [PubMed] [Google Scholar]
- Coullier C. T., Hofacre C. L., Payne A. M., Anderson D. B., Kaiser P., Mackie R. I., Gaskins H. R.. 2008. Coccidia-induced mucogenesis promotes the onset of necrotic enteritis by supporting Clostridium perfringens growth. Vet. Immunol. Immunopathol. 122:104–115. [DOI] [PubMed] [Google Scholar]
- Dibner J. J., Buttin P.. 2002. Use of organic acids as a model to study the impact of gut microflora on nutrition and metabolism. J. Appl. Poult. Res. 11:453–463. [Google Scholar]
- Emami N. K., Naeini S. Z., Ruiz-Feria C. A.. 2013. Growth performance, digestibility, immune response and intestinal morphology of male broilers fed phosphorus deficient diets supplemented with microbial phytase and organic acids. Livest. Sci. 157:506–513. [Google Scholar]
- Engberg R. M., Hedemann M. S., Leser T. D., Jensen B. B.. 2000. Effect of zinc bacitracin and salinomycin on intestinal microflora and performance of broilers. Poult. Sci. 79:1311–1319. [DOI] [PubMed] [Google Scholar]
- Engster H. M., Marvil D., Stewart-Brown B.. 2002. The effect of withdrawing growth promoting antibiotics from broiler chickens: a long-term commercial industry study. J. Appl. Poult. Res. 11:431–436. [Google Scholar]
- Esmaeilipour O., Shivazad M., Moravej H., Aminzadeh S., Rezaian M., Van Krimpen M. M.. 2011. Effects of xylanase and citric acid on the performance, nutrient retention, and characteristics of gastrointestinal tract of broilers fed low-phosphorus wheat-based diets. Poult. Sci. 90:1975–1982. [DOI] [PubMed] [Google Scholar]
- Fascina V. B., Sartori J. R., Gonzales E., Carvalho F. B., Souza I. M. G. P., Polycarpo G. V., Stradiotti A. C., Pelícia V. C.. 2012. Phytogenic additives and organic acids in broiler chicken diets. Rev. Bras. Zootec. 41:2189–2197. [Google Scholar]
- Fernández-Rubio C., Ordóñez C., Abad-González J., Garcia-Gallego A., Honrubia M. P., Mallo J. J., Balaña-Fouce R.. 2009. Butyric acid-based feed additives help protect broiler chickens from Salmonella Enteritidis infection. Poult. Sci. 88:943–948. [DOI] [PubMed] [Google Scholar]
- García V., Catalá-Gregori P., Hernández F., Megías M. D., Madrid J.. 2007. Effect of formic acid and plant extracts on growth, nutrient digestibility, intestine mucosa morphology, and meat yield of broilers. J. Appl. Poult. Res. 16:555–562. [Google Scholar]
- Goodarzi Boroojeni F., Mader A., Knorr F., Ruhnke I., Röhe I., Hafeez A., Männer K., Zentek J.. 2014. The effects of different thermal treatments and organic acid levels on nutrient digestibility in broilers. Poult. Sci. 93:1159–1171. [DOI] [PubMed] [Google Scholar]
- Goualié B. G., Ouattara H. G., Akpa E. E., Quessends N. K., Bakayoko S., Niamké S. L., Dosso M.. 2014. Occurrence of multidrug resistance in Campylobacter from Ivorian poultry and analysis of bacterial response to acid shock. Food Sci. Biotechnol. 23:1185–1191. [Google Scholar]
- Günal M. 2013. The effects of sodium gluconate and microbial phytase on performance and mineral utilisation in broiler chicks. Anim. Prod. Sci. 53:316–321. [Google Scholar]
- Guyard-Nicodème M., Keita A., Quesne S., Amelot M., Poezevara T., Le Berre B., Sánchez J., Vesseur P., Martín Á., Medel P., Chemaly M.. 2016. Efficacy of feed additives against Campylobacter in live broilers during the entire rearing period. Poult. Sci. 95:298–305. [DOI] [PubMed] [Google Scholar]
- Heres L., Engel B., Urlings H. A. P., Wagenaar J. A., Van Knapen F.. 2004. Effect of acidified feed on susceptibility of broiler chickens to intestinal infection by Campylobacter and Salmonella. Vet. Microbiol. 99:259–267. [DOI] [PubMed] [Google Scholar]
- Houshmand M., Azhar K., Zulkifli I., Bejo M. H., Kamyab A.. 2011. Effects of nonantibiotic feed additives on performance, nutrient retention, gut pH, and intestinal morphology of broilers fed different levels of energy. J. Appl. Poult. Res. 20:121–128. [Google Scholar]
- Huyghebaert G., Ducatelle R., Van Immerseel F.. 2011. An update on alternatives to antimicrobial growth promoters for broilers. Vet. J. 187:182–188. [DOI] [PubMed] [Google Scholar]
- Isabel B., Santos Y.. 2009. Effects of dietary organic acids and essential oils on growth performance and carcass characteristics of broiler chickens. J. Appl. Poult. Res. 18:472–476. [Google Scholar]
- Islam K. M. S., Schuhmacher A., Aupperle H., Gropp J. M.. 2008. Fumaric acid in broiler nutrition: a dose titration study and safety aspects. Int. J. Poult. Sci. 7:903–907. [Google Scholar]
- Islam K. M. S., Schaeublin H., Wenk C., Wanner M., Liesegang A.. 2012. Effect of dietary citric acid on the performance and mineral metabolism of broiler. J. Anim. Physiol. Anim. Nutr. 96:808–817. [DOI] [PubMed] [Google Scholar]
- Józefiak D., Kaczmarek S., Bochenek M., Rutkowski A. A.. 2007. A note on effect of benzoic acid supplementation on the performance and microbiota population of broiler chickens. J. Anim. Feed Sci. 16:252–256. [Google Scholar]
- Jukes H. G., Hill D. C., Branion H. D.. 1956. Effect of feeding antibiotics on the intestinal tract of the chick. Poult. Sci. 35:716–723. [Google Scholar]
- Khosravi A., Boldaji F., Dastar B., Hasani S.. 2008. The use of some feed additives as growth promoter in broilers nutrition. Int. J. Poult. Sci. 2:1095–1099. [Google Scholar]
- Khosravinia H., Nourmohammadi R., Afzali N.. 2015. Productive performance, gut morphometry, and nutrient digestibility of broiler chicken in response to low and high dietary levels of citric acid. J. Appl. Poult. Res. 24:470–480. [Google Scholar]
- Kil D. Y., Kwon W. B., Kim B. G.. 2011. Dietary acidifiers in weanling pig diets: a review. Rev. Colom. Cienc. Pecu. 24:231–247. [Google Scholar]
- Kim J. W., Kim J. H., Kil D. Y.. 2015. Dietary organic acids for broiler chickens: a review. Rev. Colom. Cienc. Pecu. 28:109–132. [Google Scholar]
- Krinke A. L., Jamroz D.. 1996. Effects of feed antibiotic avoparcine on organ morphology in broiler chickens. Poult. Sci. 75:705–710. [DOI] [PubMed] [Google Scholar]
- Lillie R. J., Sizemore J. R., Bird H. R.. 1953. Environment and stimulation of growth of chicks by antibiotics. Poult. Sci. 32:466–475. [Google Scholar]
- Lovatto P. A., Lehnen C. R., Andretta I., Carvalho A. D., Hauschild L.. 2007. Meta-análise em pesquisas científicas - enfoque em metodologias. Rev. Bras. Zootec. 36:285–294. [Google Scholar]
- Menconi A., Reginatto A. R., Londero A., Pumford N. R., Morgan M., Hargis B. M., Tellez G.. 2013. Effect of organic acids on Salmonella Typhimurium infection in broiler chickens. Int. J. Poult. Sci. 12:72–75. [Google Scholar]
- Mikkelsen L. L., Vidanarachchi J. K., Olnood C. G., Bao Y. M., Selle P. H., Choct M.. 2009. Effect of potassium diformate on growth performance and gut microbiota in broiler chickens challenged with necrotic enteritis. Br. Poult. Sci. 50:66–75. [DOI] [PubMed] [Google Scholar]
- Miles R. D., Butcher G. D., Henry P. R., Littell R. C.. 2006. Effect of antibiotic growth promoters on broiler performance, intestinal growth parameters, and quantitative morphology. Poult. Sci. 85:476–485. [DOI] [PubMed] [Google Scholar]
- Mohamed M. A., El-Daly E. F., El-Azeem N. A. A., Youssef A. W., Hassan H. M. A.. 2014. Growth performance and histological changes in ileum and immune related organs of broilers fed organic acids or antibiotic growth promoter. Int. J. Poult. Sci. 13:602–610. [Google Scholar]
- Nourmohammadi R., Hosseini S. M., Farhangfar H.. 2010. Effect of dietary acidification on some blood parameters and weekly performance of broiler chickens. J. Anim. Vet. Adv. 9:3092–3097. [Google Scholar]
- Ozduven M. L., Samli H. E., Okur A. A., Koc F., Akyurek H., Senkoylu N.. 2009. Effects of mannanoligosaccharide and/or organic acid mixture on performance, blood parameters and intestinal microbiota of broiler chicks. Ital. J. Anim. Sci. 8:595–602. [Google Scholar]
- Panda A. K., Raju M. V. L. N., Rao S. V. R., Sunder G. S., Reddy M. R.. 2009a. Effect of graded levels of formic acid on gut microflora count, serum biochemical parameters, performance and carcass yield of broiler chickens. Indian J. Anim. Sci. 79:1165–1168. [Google Scholar]
- Panda A. K., Rao S. V. R., Raju M. V. L. N., Sunder G. S.. 2009b. Effect of butyric acid on performance, gastrointestinal tract health and carcass characteristics in broiler chickens. Asian-Australas. J. Anim. Sci. 22:1026–1031. [Google Scholar]
- Partanen K. H., Mroz Z.. 1999. Organic acids for performance enhancement in pig diets. Nutr. Res. Rev. 12:117–145. [DOI] [PubMed] [Google Scholar]
- Pinchasov Y., Jensen L. S.. 1989. Effect of short-chain fatty acids on voluntary feed of broiler chicks. Poult. Sci. 68:1612–1618. [Google Scholar]
- Pinchasov Y., Elmaliah S.. 1994. Broiler chick responses to anorectic agents: 1. dietary acetic and propionic acids and the digestive system. Pharmacol. Biochem. Behav. 48:371–376. [DOI] [PubMed] [Google Scholar]
- Ricke S. C. 2003. Perspectives on the use of organic acids and short chain fatty acids as antimicrobials. Poult. Sci. 82:632–639. [DOI] [PubMed] [Google Scholar]
- Roy H. S., Mukhopadhayay S. K., Niyogi D., Choudhary P. K., Ganguly S.. 2012. Organic acids as a replacer of growth promoter antibiotics in broilers: pathological and bacteriological studies on intestine. Indian J. Vet. Pathol. 36:114–116. [Google Scholar]
- Ruhnke I., Röhe I., Goodarzi Boroojeni F., Knorr F., Mader A., Hafeez A., Zentek J.. 2015. Feed supplemented with organic acids does not affect starch digestibility, nor intestinal absorptive or secretory function in broiler chickens. J. Anim. Physiol. Anim. Nutr. 99:29–35. [DOI] [PubMed] [Google Scholar]
- Saki A. A., Harcini R. N., Rahmatnejad E., Salary J.. 2012. Herbal additives and organic acids as antibiotic alternatives in broiler chickens diet for organic production. Afr. J. Biotechnol. 11:2139–2145. [Google Scholar]
- SAS Institute 2012. SAS® User Guide Version 9.3 Cary, NC SAS Institute Inc. [Google Scholar]
- Sauvant D., Schmidely P., Daudin J. J.. 2005. Les méta-analyses des données expérimentales: applications en nutrition animale. INRA Prod. Anim. 8:63–73. [Google Scholar]
- Sauvant D., Schmidely P., Dausin J. J., St-Pierre N. R.. 2008. Meta-analyses of experimental data in animal nutrition. Animal. 2:1203–1214. [DOI] [PubMed] [Google Scholar]
- Skånseng B., Kaldhusdal M., Moen B., Gjevre A. G., Johannessen G. S., Sekelja M., Trosvik P., Rudi K.. 2010. Prevention of intestinal Campylobacter jejuni colonization in broilers by combinations of in-feed organic acids. J. Appl. Microbiol. 109:1265–1273. [DOI] [PubMed] [Google Scholar]
- St-Pierre N. R. 2001. Invited review: integrating quantitative findings from multiple studies using mixed model methodology. J. Dairy Sci. 84:741–755. [DOI] [PubMed] [Google Scholar]
- St-Pierre N. R. 2007. Meta-analyses of experimental data in the animal sciences. Rev. Bras. Zootec. 36:343–358. [Google Scholar]
- Timbermont L., Lanckriet A., Dewulf J., Nollet N., Schwarzer K., Haesebrouck F., Ducatelle E., Van Immerseel F.. 2010. Control of Clostridium perfringens-induced necrotic enteritis in broilers by target-released butyric acid, fatty acids and essential oils. Avian Pathol. 39:117–121. [DOI] [PubMed] [Google Scholar]
- Van Den Bogaard A. E., Stobberingh E. E.. 2000. Epidemiology of resistance to antibiotics: links between animals and humans. Int. J. Antimicrob. Agents. 14:327–335. [DOI] [PubMed] [Google Scholar]
- Van Den Borne J. J. G. C., Heetkamp M. J. W., Buyse J., Niewold T. A.. 2015. Fat coating of Ca butyrate results in extended butyrate release in the gastrointestinal tract of broilers. Livest. Sci. 175:96–100. [Google Scholar]
- Van Immerseel F., Boyen F., Gantois I., Timbermont L., Bohez L., Pasmans F., Haesebrouck F., Ducatelle R.. 2005. Supplementation of coated butyric acid in the feed reduces colonization and shedding of Salmonella in poultry. Poult. Sci. 84:1851–1856. [DOI] [PubMed] [Google Scholar]
- Van Immerseel F., Russel J. B., Flythe M. D., Gantois I., Timbermont L., Pasmans F., Haesebrouck F., Ducatelle R.. 2006. The use of organic acids to combat Salmonella in poultry: a mechanistic explanation of the efficacy. Avian Pathol. 35:182–188. [DOI] [PubMed] [Google Scholar]
- Van Immerseel F., De Buck J., Boyen F., Bohez L., Pasmans F., Volf J., Sevcik M., Rychlik I., Haesebrouck F., Ducatelle R.. 2004. Medium-chain fatty acids decrease colonization and invasion through hilA suppression shortly after infection of chickens with Salmonella enterica serovar Enteritidis. Appl. Environ. Microbiol. 70:3582–3587. [DOI] [PMC free article] [PubMed] [Google Scholar]