Abstract
Objective. To study disease severity and response to therapy in a large cohort of patients with anti-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR)-associated myositis.
Methods. Muscle strength, creatine kinase levels and treatments were assessed in anti-HMGCR-positive patients at each clinical visit. Univariate and multivariate analyses were used to analyse the influence of clinical characteristics on strength and the change in strength over time. Whole exome sequencing was performed in a subset of patients.
Results. Among 50 patients followed for ⩾2 years, only 22 (44%) reached full strength with immunosuppressive therapy; even among those with full strength, 55% continued to have CK levels in excess of 500 IU/l and only three could be tapered off immunosuppressive therapy. Both univariate and multivariate analysis showed that patients who were older at disease onset were stronger at all time points (P < 0.001) and improved faster (P < 0.008) than younger patients; a history of statin exposure was not independently associated with the improvement rate. Younger patients were more likely to have refractory disease (P = 0.02) than older patients. Among eight refractory patients with DNA available for testing, whole exome sequencing did not reveal pathogenic mutations in known dystrophy genes. The risk of cancer was not increased in anti-HMGCR myositis patients compared with the general population.
Conclusions. Anti-HMGCR myositis is usually a chronic disease requiring long-term immunosuppression. Although younger patients had more severe disease and a worse prognosis than older patients, they did not have evidence of a known co-existing muscular dystrophy to explain their persistent, and sometimes progressive, muscle weakness.
Keywords: polymyositis, myopathy, autoantibodies
Rheumatology key messages
Anti-hydroxy-3-methylglutaryl-coenzyme A reductase myositis is usually a chronic disease requiring long-term immunosuppression.
Younger anti-hydroxy-3-methylglutaryl-coenzyme A reductase myositis patients have more severe disease and a worse prognosis than older patients.
Anti-hydroxy-3-methylglutaryl-coenzyme A reductase myositis patients did not have an increased risk of cancer.
Introduction
Autoantibodies recognizing hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR) are specifically found in patients with autoimmune myopathy and are strongly associated with both a necrotizing myopathy on biopsy and a history of statin exposure [1–4]. In a prior analysis of 17 patients with anti-HMGCR-associated myositis followed for an average of ∼2 years (85 total clinic visits), we showed that strength improved with immunosuppressive treatment [5]. However, the relatively small number of patients included in that study did not allow us to draw definitive conclusions about the expected rate of recovery or patient characteristics that influence long-term outcomes. In the current study, we have analysed longitudinal CK levels, muscle strength, autoantibody titres and treatments in a much larger cohort of anti-HMGCR myositis patients. With data from 104 patients followed for an average of ∼3 years (739 total clinic visits), we have now defined the clinical course of those treated for anti-HMGCR myositis and identified important prognostic factors associated with the rate of recovery.
Methods
Study population and anti-HMGCR testing
All patients evaluated at the Johns Hopkins Myositis Centre with suspected myopathy were enrolled in a longitudinal study. Serum samples from each visit were screened for anti-HMGCR autoantibodies by ELISA and confirmed by immunoprecipitation of in vitro—transcribed and—translated HMGCR protein as previously described [2]. An established quantitative anti-HMGCR ELISA was used to determine anti-HMGCR levels from each patient at each visit [5].
Arm abduction and hip flexion strength were evaluated by the examining physician using the Medical Research Council scale at each visit and transformed to Kendall’s 0–10 scale as previously described [5]. Serial strength measurements for each patient were made by the same physician. Right- and left-side measurements for arm and hip strength were combined and the average was used for the calculations (possible range 0–10). Serum CK levels were included for analysis if obtained within 6 weeks of the strength testing. The presence of cancer-associated myositis (defined as the onset of cancer within 3 years of the onset of weakness), dysphagia, skin involvement (defined as presence of any DM-specific rash, that is, heliotrope rash or Gottron’s sign) and interstitial lung disease were documented.
Whole exome sequencing
When available, DNA samples from patients with a history of persistent weakness (<8 out of 10) despite at least 2 years of intensive immunosuppressant treatment were subjected to whole exome sequencing (WES) and then analysed for pathogenic mutations in genes associated with muscle disorders.
For WES, genomic DNA was isolated from blood with the use of a DNeasy Blood and Tissue Kit (Qiagen, Germantown, MD) and then sent to the Baylor College of Medicine Human Genome Sequencing Center for sequencing on an Illumina HiSeq 2000 (Illumina, San Diego, CA, USA) followed by Mercury pipeline analysis. The variants found in a comprehensive list of 417 genes associated with monogenic neuromuscular disorders [6] were compared with the Human gene mutation database (HGMD Professional, BIOBASE Corp., Beverly, MA, USA) to assess for possible pathogenicity.
Standard protocol approvals and patient consents
This study was approved by the Johns Hopkins Institutional Review Board and written informed consent was obtained from each participant.
Statistical analysis
Qualitative variables were expressed as percentage and absolute frequency while quantitative features were reported as mean and s.d. Creatine kinase, a highly positively skewed variable, was expressed as median, first and third quartile for descriptive purposes and transformed through a natural logarithm for regression analysis. Univariate comparisons between groups were made with the Wilcoxon rank-sum or Student’s t test for continuous variables and either a chi-square test or Fisher’s exact test for categorical variables, as appropriate. The binomial and Student’s t test were used to compare observed and expected values and Pearson’s chi-square test was used to calculate the correlation between pairs of continuous variables except when one of the variables was the creatine kinase, where Spearman’s ρ was used instead.
Indirect standardization was used to compare the number of cases of cancer that we observed in our sample during the 3 years before or after the onset of the disease with the number of cases that one would expect in the general population with the same age and sex distribution. Cancer incidence by age and sex groups was taken from the 2008 to 2012 United States Cancer Statistics registry, available at the Center for Disease Control and Prevention (https://nccd.cdc.gov/uscs/). The observed and expected numbers of cases were compared using the standardized incidence ratio (observed/expected cases of cancer) and its 95% CI. The number of years at risk for cancer was allocated to the correct age interval. Thus, if the 6 years a patient was at risk for cancer were from 41.0 to 47.0 years old, 4 years would be allocated to the 40–44 age interval and 2 years would be allocated to the 45–50 age interval. In the event that a patient had cancer, died or had <3 years of follow-up after disease onset, the number of years at risk of that patient would end at the occurrence of the event. So, if a patient died 2 years after the onset, the number of years at risk would be 5 (3 years before and 2 years after the onset).
The influence of non-modifiable risk factors (sex, race, age at disease onset and statin exposure) on initial strength was studied using linear regression models. To account for the different number of visits per patient, muscle strength and the change between visits was studied using multilevel linear regression models with random intercepts. The mean of hip flexor and arm abductor strength (range 0–10) was used as the strength outcome for regression analysis. To avoid a ceiling effect, all visits following attainment of a stable level of strength over 8 were excluded for the analysis of the change of strength between visits. Corticosteroid dose and the administration of IVIG, rituximab, MTX, AZA and mycophenolate were used as adjusting covariates. Other treatments administered to < 10% of the cohort were not included in the analysis. All statistical analyses were performed using Stata/MP 14.1 (StataCorp, College Station, TX, USA) and a two-sided P-values of 0.05 or less was considered significant for these analyses.
Results
Clinical characteristics of patients with anti-HMGCR-associated myopathy
Out of 1947 patients enrolled at the Johns Hopkins Myositis Center, 104 (5.3%) were anti-HMGCR positive. The clinical features of these patients are shown in Table 1. Of these, 100 (96%) had proximal muscle weakness, all had elevated muscle enzyme levels and 80 (77%) had a muscle biopsy showing myofibre necrosis with minimal lymphocytic inflammation. The anti-HMGCR positive patients had a mean (s.d.) of 7.1 (6.8) visits, time between visits of 8.3 (13.4) months and follow-up time of 3.4 (2.5) years. Data from a total of 739 clinic visits were available and used for analysis.
Table 1.
General features of anti-hydroxy-3-methylglutaryl-coenzyme A reductase patientsa
| Patient characteristic | (95% CI) |
|---|---|
| (n = 104) | |
| Age of onset, years | 55.0 (52.4, 57.6) |
| Female sex, % | 59 (49, 68) |
| Caucasian, % | 72 (63, 80) |
| Black, % | 19 (13, 28) |
| Other races, % | 9 (5, 16) |
| Statin exposure, % | 75 (66, 82) |
| Cancer associated myositis, % | 6 (3, 12) |
| Skin involvement, % | 5 (2, 11) |
| Necrotizing muscle biopsy, % | 77 (68, 84) |
| Interstitial lung disease, % | 4 (2, 9) |
| Dysphagia, % | 27 (19, 36) |
| Corticosteroids, % | 74 (65, 81) |
| MTX, % | 50 (40, 59) |
| IVIG, % | 39 (30, 48) |
| AZA, % | 23 (16, 32) |
| Mycophenolate, % | 18 (12, 27) |
| Rituximab, % | 15 (9, 23) |
Variables are expressed as percentage (95% CI) except for age of onset, which is expressed in years (95% CI).
The mean age at disease onset was 55 years, 59% were women and 72% were Caucasian. The median peak CK during visits to the Johns Hopkins Myositis Center was 2812 IU/l (Q1–Q3: 1399–6821 IU/l). Dermatological involvement was rare (5%) and very few (4%) had evidence of interstitial lung disease. Likewise, the frequency of cancer-associated myositis was low (6%, n = 6) and very close to the 5.5 cases of cancer predicted to occur in the general population when corrected for the age and sex distribution of our patients (standardized incidence ratio 1.12, 95% CI: 0.4, 2.4).
To determine the frequency of statin exposure in patients of different ages, we divided the cohort into tertiles based on the age at onset of disease (Table 2). Statin exposure was lower in those younger than age 53 years at disease onset (40%) compared with those who developed muscle symptoms at an age of 61 years or older (97%; P < 0.001); none of the patients under 40 years of age had a prior statin exposure. Furthermore, controlling for age, sex and race, anti-HMGCR patients were more likely to have been exposed to statins than our previously described cohort of 37 anti-SRP positive patients (odds ratio = 32.9, 95% CI: 6.2, 175, P < 0.001) [7].
Table 2.
Distribution of statin exposure, sex and race across tertiles of age at onset
| Patient characteristic | 4–52 years (n = 35) | 53–61 years (n = 35) | 61–84 years (n = 34) |
|---|---|---|---|
| Statin exposure | 40 | 89 | 97 |
| Sex (female) | 63 | 66 | 47 |
| Caucasian | 60 | 71 | 85 |
| African American | 29 | 17 | 12 |
| Other races | 11 | 11 | 3 |
All values listed as %.
Associations between CK levels, autoantibody titres and strength
As previously reported [5], there was an association between CK levels and anti-HMGCR levels at both initial and subsequent visits (R2 = 0.36, P < 0.001) (Fig. 1). Furthermore, independently of the time elapsed from the disease onset, age at disease onset, sex, race, statin exposure or immunosuppressant treatment, CK levels (P < 0.001) and HMGCR titres (P < 0.001) were inversely associated with muscle strength. Indeed, in the multivariate analysis, for each 10-fold increase in CK level, strength declined by 1.7 points; a similar relation was observed between autoantibody titres and muscle strength.
Fig. 1.
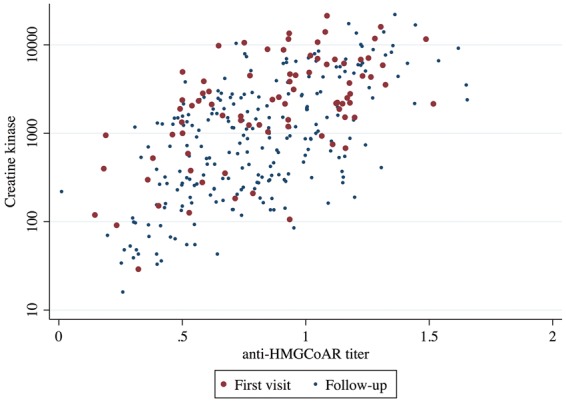
Relationship between CK levels and anti-hydroxy-3-methylglutaryl-coenzyme A reductase titres at first and follow-up visits
Treatment and clinical course of anti-HGMCR patients
Treatment regimens varied between patients based on their clinical course and individual physician preferences. The most commonly prescribed immunosuppressants were corticosteroids (74%), MTX (50%) and IVIG (39%) (Table 1). Nineteen subjects were prescribed one, 29 subjects were prescribed two, 22 subjects were prescribed three and 19 subjects were prescribed four or more different immunosuppressive treatments during the course of follow-up; these were not necessarily administered simultaneously. The mean (s.d.) number of immunosuppressive drugs per visit was 1.5 (0.9). Fifteen patients were not treated during any visit to the Johns Hopkins Myositis Center either because they were strong without treatment (four patients) or because they followed-up elsewhere before starting treatment (11 patients).
From our cohort of 104 patients, 50 were followed at the Johns Hopkins Myositis Center for >2 years during which time 22 (44%; mean age at onset 55.8) reached full strength. Among these, only three could be tapered off of all immunosuppressant medications. One remained clinically stable for 23 months and was then lost to follow-up; the other two have been off treatment for 10 and 49 months as of the conclusion of the study period. Despite having full strength after 2 years of follow-up, 12 patients (55%) still had CK levels over 500 IU/l.
Fifteen of the patients followed for at least 2 years had significant weakness (strength <8 out of 10) despite immunosuppressive therapy. Among these refractory patients, the mean most recent strength score was 5.8 out of 10, the median of the most recent recorded CK was 1401 IU/l (95% CI: 371, 2042 IU/l), four were African American, and four were female. The mean (s.d.) total number of medications tried by each refractory patient was 3.4 (0.3). Notably, refractory patients were younger than those who were followed for at least 2 years and had strength scores of 8 or higher (48.0 vs 57.4 years, respectively; P = 0.02). Eight refractory patients underwent WES; none of these had a known pathogenic mutation in any of the muscle dystrophy genes included in the study [6].
Univariate analysis of prognostic factors associated with strength and CK
Univariate analysis showed that black patients had higher CK levels than white patients at all clinic visits (Table 3). Although hip flexor muscle strength was no different at the first visit, black patients had weaker hip flexors than white patients at subsequent visits, including the last visit. Arm abduction strength was no different between black and white patients at the initial or subsequent visits. Although their strength was no different at the initial visit, patients with disease onset at a younger age were significantly weaker during follow-up visits than older patients (P < 0.02).
Table 3.
Association of strength and CK levels with patient characteristics in anti-hydroxy-3-methylglutaryl-coenzyme A reductase myositis: univariate analysisa
| Sex | Race | Statin exposure | Age at onset | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female | Male | P- value | Caucasian | Black | P- value | No | Yes | P- value | Correlation coefficient | P-value | ||
| Hip flexor strength at first visit | 6.2 | 5.9 | 0.5 | 5.9 | 0.5 | 6.4 | 6.3 | 0.3 | 5.6 | 0.1 | 0.4 | 6.1 (5.5, 6.6) |
| Follow-up hip flexor strength | 7.2 | 7.4 | 0.6 | 7.5 | 7.7 | 0.04 | 6.0 | 0.01 | 6.1 | 0.3 | 0.01 | 7.3 (6.7, 7.8) |
| Hip flexor strength at last visit | 7.0 | 7.3 | 0.6 | 7.5 | 5.0 | 0.004 | 0.02 | 7.5 | 5.7 | 0.2 | 0.02 | 7.1 (6.4, 7.8) |
| Arm abductor strength at first visit | 8.1 | 8.4 | 0.4 | 8.1 | 8.2 | 0.9 | 0.6 | 8.3 | 8.1 | -0.0 | 0.8 | 8.2 (7.9, 8.6) |
| Follow-up arm abductor strength | 9.0 | 9.4 | 0.1 | 9.2 | 8.7 | 0.2 | 0.002 | 9.4 | 8.4 | 0.2 | 0.09 | 9.1 (8.9, 9.4) |
| Arm abductor strength at last visit | 9.0 | 9.5 | 0.1 | 9.3 | 8.6 | 0.09 | 9.5 | 8.2 | <0.001 | 0.2 | 0.03 | 9.2 (8.9, 9.5) |
| CK at first visit | 2152 | 4493 | 0.01 | 2161 | 4146 | 0.008 | 2405 | 2181 | 0.4 | −0.2 | 0.04 | 2390 (2078, 3558) |
| Follow-up CK | 793 | 792 | 0.9 | 462 | 2846 | <0.001 | 598 | 1544 | 0.03 | −0.2 | 0.2 | 794 (450, 1232) |
| Maximum CK | 2326 | 3538 | 0.3 | 2176 | 6558 | <0.001 | 2510 | 3730 | 0.09 | −0.3 | 0.004 | 2560 (2152, 3796) |
Comparisons with statistically significant differences are in bold.
Strength values are expressed as means, and CK as medians. Bivariate comparisons were made using Student’s t test for the strength and the Wilcoxon rank-sum test for CK. Pearson’s r was used to calculate the correlation coefficient for strength and Spearman’s rho for the creatine kinase. Follow-up strength was defined as the mean strength of all the visits, excluding the first one.
Multivariate analysis of prognostic factors associated with strength
We performed multivariate regression models to analyse the association of sex, race, age at disease onset, time from disease onset and treatments received with strength. This confirmed that patients who were older at disease onset were stronger than those who were younger at disease onset (P < 0.001) (Table 4). Indeed, each additional 10 years at age of onset was associated with an increased strength of almost 0.5 points. Statin exposure was also significantly associated with greater strength using the same adjusting covariates (data not shown).
Table 4.
Association of patient characteristics with strength and change of strength in anti-hydroxy-3-methylglutaryl-coenzyme A reductase patients: multivariate analysis
| Strength at first visita | Strength at any time pointb | Change in strength between visitsc | |
|---|---|---|---|
| Coefficient (95% CI) | Coefficient (95% CI) | Coefficient (95% CI) | |
| Age of onset (each 10 years) | 0.43 (0.05, 0.81)* | 0.47 (0.20, 0.74)*** | 2.19 (0.89, 3.49)*** |
| Female vs Male | −0.12 (−0.96, 0.73) | −0.16 (−0.79, 0.46) | −0.11 (−2.79, 2.58) |
| Black vs Caucasian | 0.76 (−0.35, 1.87) | −0.22 (−1.03, 0.59) | 2.71 (−0.82, 6.24) |
Comparisons with statistically significant differences are in bold.
Linear regression. Multivariate analysis of the mean strength at the first visit adjusted by time from onset, age at onset, sex and race.
Multilevel regression with random intercepts. Multivariate analysis of the mean strength at any time point adjusted by time from onset, age at onset, sex, race and immunosuppressant drugs (corticosteroid dose and treatment with IVIG, rituximab, mycophenolate, MTX or AZA).
Multilevel regression with random intercepts. Multivariate analysis of the change in strength from visit to visit adjusted by time from onset, age at onset sex, race and immunosuppressant drugs (corticosteroid dose and treatment with IVIGs, rituximab, mycophenolate, MTX or AZA).
P < 0.05,
P < 0.001.
Multivariate analysis of prognostic factors associated with the rate of recovery of strength
Multivariate regression analyses confirmed that older patients had a faster rate of strength recovery than younger patients independent of sex, race, time from disease onset and treatments received (P < 0.001) (Table 4). For each additional 10 years of age at disease onset, patients recovered an additional 2.2 muscle strength points (95% CI: 0.9, 3.5 strength points/10 years). When included in the multivariate regression model, statin exposure was not independently associated with the rate of muscle strength improvement (P = 0.5). However, patient age at onset of disease did remain significantly associated with the rate of improvement in this analysis (P = 0.02).
We also studied the time to reach normal or near-normal strength (8 or above on the Kendall scale) using Kaplan–Meier estimates. This revealed that 41% of all anti-HMGCR patients reached at least near-normal strength within 2 years of disease onset while 65% reached this level of improvement within 4 years. However, ∼85% of the patients in the oldest tertile at disease onset reached near normal strength after 4 years whereas only ∼45% of the younger patients reached this level of improvement in the same time frame (Fig. 2). Cox regression confirmed that older patients at disease onset recovered strength faster than younger patients independent of sex, race, strength at first visit and time from onset to the first visit (hazard ratio = 1.3, P < 0.02).
Fig. 2.
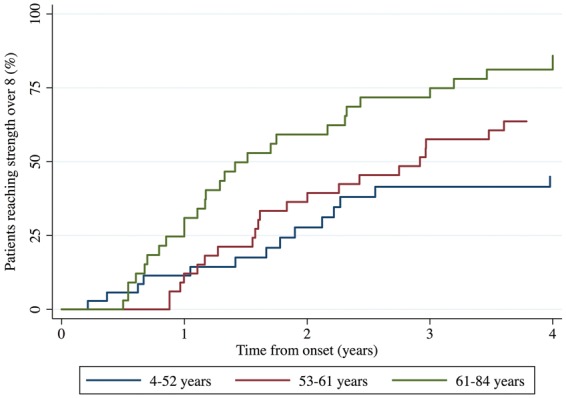
Survival analysis of strength recovery over time depending on the age at onset of weakness
Discussion
In this study we sought to identify clinical characteristics that predict disease severity and are associated with response to treatment in patients with anti-HMGCR myositis. Multivariate analysis showed that younger patients had significantly more severe weakness at the first visit compared with their older counterparts. Along with the observation that CK levels were higher in younger patients at the first clinic visit, these data indicate that younger patients present with more severe muscle disease than older anti-HMGCR myositis patients. Furthermore, multivariate linear regression analysis showed that older patients have a faster rate of strength improvement than younger patients even when adjusting for other factors such as race, gender, age at disease onset, duration of disease at first clinic visit and immunosuppressive drugs used to treat the disease. Importantly, when included in this model, statin exposure status was not independently associated with the rate of strength recovery.
Several other analyses corroborated the multivariate linear regression analysis showing that older anti-HMGCR positive patients have a better prognosis than younger patients. First, Kaplan-Meier survival estimates showed that ∼85% of patients in the oldest tertile but only ∼45% of those in the youngest tertile reached nearly full or full strength within 4 years of disease onset. Second, Cox regression demonstrated that older patients recovered strength faster than younger patients independent of sex, race, first visit strength and time from onset to the first visit. And third, refractory patients who remained weak with muscle strength scores <8 after 2 years of treatment were younger at disease onset than those who regained strength of 8 or more within 2 years of treatment.
We were surprised to find that younger age predicts more severe disease. While the reasons for this remain unclear, our analysis showed this effect was unrelated to statin exposure. One possibility could be that younger patients have greater muscle bulk and, therefore, a higher load of the HMGCR antigen. This could potentially lead to a heightened autoimmune response against the muscle and slower recovery.
Although most anti-HMGCR myositis patients were treated with immunosuppressive therapy, 3 out of 104 spontaneously improved and achieved full strength without any treatment at any point in their clinical course; two of these had normalization of CK levels. Among those patients who were treated, a significant number regained normal strength. For example, among 50 patients treated for at least 2 years, 44% reached full strength. However, the majority of these continued to have evidence of ongoing disease activity with CK levels in excess of 500 IU/l and only a handful could be weaned off immunosuppressive therapy. Notably, anti-HMGCR titres remained elevated in all patients for as long as they have been followed. These observations suggest that most, but not all, anti-HMGCR myositis patients have a chronic disease that requires long-term immunosuppressive therapy.
Importantly, we found that nearly one-third of anti-HMGCR myositis patients have refractory disease with relatively severe proximal weakness and persistently elevated CK levels despite at least 2 years of aggressive immunosuppressive therapy. In fact, some of these patients appear to develop progressive disease, with declining strength despite treatment with multiple agents. Given that autoimmune and genetic muscle disease may rarely co-exist in the same patient [8, 9] we performed WES on 8 of 16 refractory anti-HMGCR myositis patients. This analysis did not reveal pathogenic mutations in known dystrophy genes and suggests that a poorly controlled autoimmune process rather than an underlying known genetic muscle disease is responsible for the persistent weakness.
In the current study, 97% of patients over the age of 60 years had been prescribed a statin prior to developing muscle symptoms. In contrast, less than half of the general US population over the age of 60 years has been prescribed a statin (Centers for Disease Control and Prevention/National Center for Health Statistics, National Health and Nutrition Examination Survey, 2011–12). We also compared statin use in the current cohort of anti-HMGCR patients with statin exposure in our cohort of 37 anti-SRP patients. After controlling for age, sex and race, we found that anti-HMGCR patients were more likely to have been exposed to statins than anti-SRP positive patients, with an odds ratio of nearly 33 (P < 0.001). These analyses confirm a clear link between statin exposure and anti-HMGCR myositis in the Johns Hopkins Myositis Center cohort. Why a link with statins has been shown in some [1, 10–12] but not all [13–16] studies remains unclear. However, as two of the latter studies were performed in Japan and China, it could be that statins do not confer risk for anti-HMGCR myositis in all ethnic groups. Alternatively, we hypothesize that non-prescription sources of statins may be more significant in Asian countries. For example, oyster mushrooms, a common ingredient in Chinese and Japanese cuisine, can include up to 2.8% lovastatin by dry weight [17]. Future studies will be required to investigate a link between consumption of oyster mushrooms, red yeast rice and other statin-containing foods and the development of anti-HMGCR myositis.
Of note, although Allenbach and colleagues [18] recently reported that anti-HMGCR-positive myositis patients had an increased risk of cancer compared with the general population, we did not find the same association in the current study. While the reasons for this are not clear, it should be noted that the French cohort includes substantially more patients without statin exposure than does our US cohort. This raises the possibility that statin exposure might somehow be protective for cancer-associated anti-HMGCR myositis.
The current study has several limitations. For example, some patients were enrolled in the study more than a decade ago while others had only a few follow-up visits. This made it necessary to use mixed regression models to analyse the data in an unbiased manner and also prevented us from using more recently developed tools to define clinical improvement [19, 20]. Furthermore, the retrospective nature of the study design prevented us from assessing the efficacy of the different medications used to treat patients.
These limitations notwithstanding, this study provides clinically important information regarding anti-HMGCR myositis. First, we show that older anti-HMGCR patients have a more favourable prognosis than younger patients. Nonetheless, even among those patients who recovered full strength, most continued to have high CK levels and only a small minority could be tapered off immunosuppressive medications. Second, the current study demonstrates a compelling link between statin exposure and development of anti-HMGCR autoantibodies in our cohort. Third, in contrast to a recent study of French anti-HMGCR myositis patients [18], we found no increased risk of cancer in anti-HMGCR myositis patients enrolled at the Johns Hopkins Myositis Center. Finally, although some anti-HMGCR positive myositis patients develop treatment-resistant and sometimes progressive disease, we found no evidence of overlapping muscular dystrophy in these subjects. Defining effective treatment strategies for these refractory patients remains a high priority for future studies.
Acknowledgements
T.E.L. is supported by a grant (T32-AR-048522) from the National Institutes of Health and is also supported by the Rheumatology Research Foundation and the Gerome Greene Foundation. L.C.-S. is supported by the Huayi and Siuling Zhang Discovery Fund. I.P.-F is supported by a Fellowship from the Myositis Association.
Funding: This research was supported in part by the Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health and by the Huayi and Siuling Zhang Discovery Fund.
Disclosure statement: A.L.M. is listed as a patent holder for a commercially available anti-HMGCR autoantibody test licensed from Johns Hopkins to INOVA Diagnostics but relinquished all rights to receive royalties for this since moving to the NIH in June 2014. L.C.-R. and L.C.-S. have patented a diagnostic anti-HMGCR test that has been licensed by Johns Hopkins to INOVA Diagnostics; they are entitled to royalties from this patent. All other authors have declared no conflicts of interest.
References
- 1. Christopher-Stine L, Casciola-Rosen LA, Hong G. et al. A novel autoantibody recognizing 200-kd and 100-kd proteins is associated with an immune-mediated necrotizing myopathy. Arthritis Rheum 2010;62:2757–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mammen AL, Chung T, Christopher-Stine L. et al. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum 2011;63:713–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mammen AL. Statin-associated autoimmune myopathy. N Engl J Med 2016;374:664–9. [DOI] [PubMed] [Google Scholar]
- 4. Mammen AL, Pak K, Williams EK. et al. Anti-HMG-CoA reductase antibodies are rare in statin users, including those with self-limited musculoskeletal side-effects. Arthritis Care Res 2011;64:269–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Werner JL, Christopher-Stine L, Ghazarian SR. et al. Antibody levels correlate with creatine kinase levels and strength in anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Arthritis Rheum 2012;64:4087–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kaplan JC, Hamroun D.. The 2015 version of the gene table of monogenic neuromuscular disorders (nuclear genome). Neuromuscul Disord 2014;24:1123–53. [DOI] [PubMed] [Google Scholar]
- 7. Pinal-Fernandez I, Parks C, Werner JL. et al. Longitudinal course of disease in a large cohort of myositis patients with autoantibodies recognizing the signal recognition particle. Arthritis Care Res 2016; Advance Access published 23 April 2016, doi: 10.1002/acr.22920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mammen AL, Casciola-Rosen L, Christopher-Stine L, Lloyd TE, Wagner KR.. Myositis-specific autoantibodies are specific for myositis compared to genetic muscle disease. Neurol Neuroimmunol Neuroinflamm 2015;2:e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Claeys KG, Gorodinskaya O, Handt S. et al. Diagnostic challenge and therapeutic dilemma in necrotizing myopathy. Neurology 2013;81:932–5. [DOI] [PubMed] [Google Scholar]
- 10. Klein M, Mann H, Plestilova L. et al. Increasing incidence of immune-mediated necrotizing myopathy: single-centre experience. Rheumatology 2015;54:2010–4. [DOI] [PubMed] [Google Scholar]
- 11. Limaye V, Bundell C, Hollingsworth P. et al. Clinical and genetic associations of autoantibodies to 3-hydroxy-3-methyl-glutaryl-coenzyme a reductase in patients with immune-mediated myositis and necrotizing myopathy. Muscle Nerve 2015;52:196–203. [DOI] [PubMed] [Google Scholar]
- 12. Kassardjian CD, Lennon VA, Alfugham NB, Mahler M, Milone M.. Clinical features and treatment outcomes of necrotizing autoimmune myopathy. JAMA Neurol 2015;72:996–1003. [DOI] [PubMed] [Google Scholar]
- 13. Allenbach Y, Drouot L, Rigolet A. et al. Anti-HMGCR autoantibodies in European patients with autoimmune necrotizing myopathies: inconstant exposure to statin. Medicine 2014;93:150–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ge Y, Lu X, Peng Q, Shu X, Wang G.. Clinical characteristics of anti-3-hydroxy-3-methylglutaryl coenzyme A reductase antibodies in Chinese patients with idiopathic inflammatory myopathies. PLoS One 2015;10:e0141616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Watanabe Y, Uruha A, Suzuki S. et al. Clinical features and prognosis in anti-SRP and anti-HMGCR necrotising myopathy. J Neurol Neurosurg Psychiatry 2016;87:1038–44. [DOI] [PubMed] [Google Scholar]
- 16. Alshehri A, Choksi R, Bucelli R, Pestronk A.. Myopathy with anti-HMGCR antibodies: Perimysium and myofiber pathology. Neurol Neuroimmunol Neuroinflamm 2015;2:e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alarcon J, Aguila S, Arancibia-Avila P. et al. Production and purification of statins from Pleurotus ostreatus (Basidiomycetes) strains. Z Naturforsch C 2003;58:62–4. [DOI] [PubMed] [Google Scholar]
- 18. Allenbach Y, Keraen J, Bouvier AM. et al. High risk of cancer in autoimmune necrotizing myopathies: usefulness of myositis specific antibody. Brain 2016;139:2131–5. [DOI] [PubMed] [Google Scholar]
- 19. Rider LG, Werth VP, Huber AM. et al. Measures of adult and juvenile dermatomyositis, polymyositis, and inclusion body myositis: Physician and Patient/Parent Global Activity, Manual Muscle Testing (MMT), Health Assessment Questionnaire (HAQ)/Childhood Health Assessment Questionnaire (C-HAQ), Childhood Myositis Assessment Scale (CMAS), Myositis Disease Activity Assessment Tool (MDAAT), Disease Activity Score (DAS), Short Form 36 (SF-36), Child Health Questionnaire (CHQ), physician global damage, Myositis Damage Index (MDI), Quantitative Muscle Testing (QMT), Myositis Functional Index-2 (FI-2), Myositis Activities Profile (MAP), Inclusion Body Myositis Functional Rating Scale (IBMFRS), Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI), Cutaneous Assessment Tool (CAT), Dermatomyositis Skin Severity Index (DSSI), Skindex, and Dermatology Life Quality Index (DLQI). Arthritis Care Res 2011;63 (Suppl 11):S118–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oddis CV, Reed AM, Aggarwal R. et al. Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo-phase trial. Arthritis Rheum 2013;65:314–24. [DOI] [PMC free article] [PubMed] [Google Scholar]


