Abstract
Chronic pain is a major debilitating condition that is difficult to treat. Although chronic pain may appear to be a disorder of the nervous system, crucial roles for immune cells and their mediators have been identified as important contributors in various types of pain. This review focuses on how the immune system regulates pain and discusses the emerging roles of immune cells in the initiation or maintenance of chronic pain. We highlight which immune cells infiltrate damaged nerves, the dorsal root ganglia, spinal cord and tissues around free nerve endings and discuss through which mechanisms they control pain. Finally we discuss emerging roles of the immune system in resolving pain and how the immune system contributes to the transition from acute to chronic pain. We propose that targeting some of these immune processes may provide novel therapeutic opportunities for the treatment of chronic pain.
Keywords: chronic pain, immune cells, cytokines, arthritis, neuropathic, inflammatory, dorsal root ganglia, spinal cord
Rheumatology key messages
Immune cells contribute to chronic pain but have different roles in the initiation, maintenance and resolution of pain.
Modulating immune cells or immune mediators can attenuate chronic pain.
Introduction
Pain-related problems are the main reason for physician consultations [1, 2]. Chronic pain affects >20% of the population [3, 4]. Current therapies to relieve pain (e.g. NSAIDs, opioids) often fail or produce treatment-limiting side effects [5, 6]. Different causalities may be at the root of chronic pain development. Inflammation causes inflammatory pain (e.g. RA, IBD), while nerve injury as a consequence of an operation or trauma, metabolic disorders (e.g. diabetic mellitus) or auto-immune diseases (e.g. multiple sclerosis) may cause neuropathic pain. Moreover, cancer itself or its treatment (chemotherapy) may result in painful neuropathies [7].
RA and OA are common causes of chronic pain and combined account for ∼42% of the chronic pain patients in Europe [8]. Although inflammation and damage are closely linked to pain, chronic pain may not be the direct consequence of ongoing inflammation or damage because arthritis pain does not correlate well with the magnitude of inflammation or joint damage [9, 10]. Moreover, in a substantial proportion (ranging from 12 to 70%) of RA patients, pain persists even with minimal disease activity or with sustained remission [9, 11, 12]. Finally, ∼20% of OA patients with total knee replacement surgery report severe or extreme pain 3–4 years after the operation [13, 14].
Chronic pain may result from aberrant neuronal activity including ectopic discharges, peripheral sensitization of primary sensory neurons and sensitization of neurons in the CNS [15]. However, the immune system is also involved in pain regulation [16]. Microglia, the resident macrophages of the CNS, play important roles in multiple rodent models of chronic pain, including neuropathic pain, cancer-induced bone pain and chronic inflammatory pain. In these models, resident microglia switch from a quiescent inactive state to an activated phenotype that is associated with production of inflammatory mediators that increase the sensitivity of the pain system [17]. However, evidence indicates that peripheral immune cells and their mediators are also involved in regulating pain [18–20].
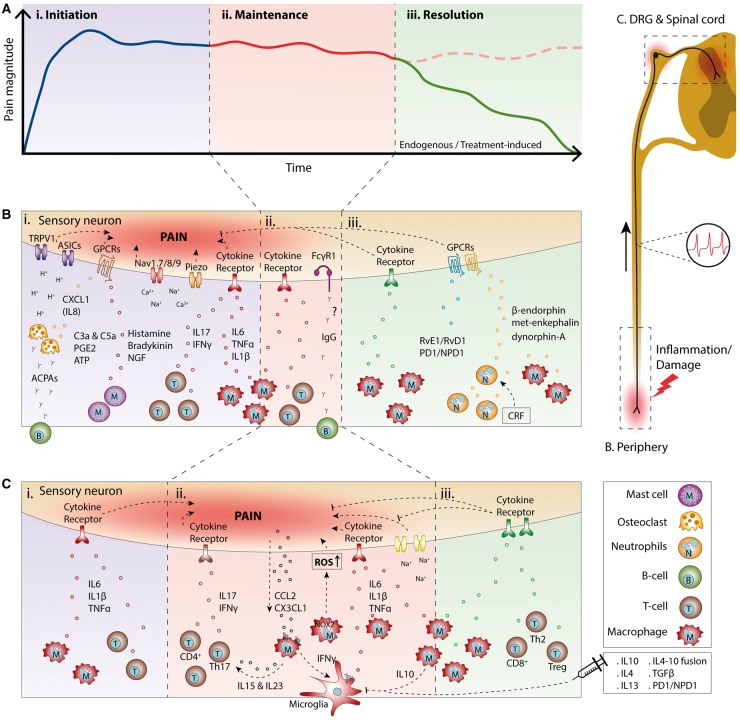
During inflammation or tissue damage, infiltrating or resident peripheral immune cells at the site of inflammation or damage produce mediators that trigger sensory neurons to produce action potentials or sensitize neurons by enhancing sensory transduction and neuronal excitability [21, 22]. However, peripheral inflammation or tissue damage also induces infiltration of immune cells into other pain-relevant sites such as peripheral nerves; dorsal root ganglia (DRG), containing the somas of the sensory neurons; or the dorsal horn of the spinal cord, which receives peripheral input to modulate pain sensitivity [19, 23, 24]. These peripheral immune cells and their mediators play different roles in the initiation and maintenance of different types of pain, and evidence exists for a role in the resolution of pain [21, 25–27]. These intricate contributions of immune cells at different stages of pain induced by inflammation (e.g. arthritis), damage (OA) or nerve damage (neuropathic) will be discussed in the following paragraphs and are summarized in Fig. 1.
Fig. 1.
Overview of the role of immune cells and their mediators at different stages of pain
(A) Time course of chronic pain induced by inflammation or damage visualizing the different stages of pain: (i) initiation, (ii)maintenance and (iii) resolution. (B and C) Schematic overview of the different types of immune cells and mediators modulating pain at different sites and during the different stages (i–iii) of pain shown in (A). (B) During inflammation or tissue damage, resident and immune cells recruited to the inflamed or damaged site secrete inflammatory mediators that act on peripheral nerves innervating the affected tissue. (C) Similarly, different immune cells migrate to the spinal cord and/or the dorsal root ganglia to modulate pain sensitivity during the different phases of pain. ACPA: anti-citrullinated protein antibody; ASIC, acid-sensing ion channel; ATP: adenosine triphosphate; CCL2: chemokine (C-C motif) ligand 2; CRF: corticotropin-releasing factor; CX3CL1: chemokine (C-X3-C motif) ligand 1; Fcγ1: Fcγ type 1 receptor; GPCR: Gprotein-coupled receptor; NGF: nerve growth factor; PD1/NPD1: protectin D1/neuroprotectin D1; ROS: reactive oxygen species.
Immune-derived mediators in pain initiation and maintenance
Several inflammatory mediators, such as bradykinin, histamine, adenosine triphosphate, neurotrophins and cytokines but also protons or damage-associated molecular patterns, activate sensory neurons to generate action potentials and/or enhance neuronal excitability and sensory transduction through neuronal receptors leading to pain and hyperalgesia [21, 22]. The contribution of cytokines in initiating pain is supported by evidence that the development of inflammatory pain is attenuated by neutralizing cytokines or blocking cytokine receptors at the site of inflammation. Neutralization of TNFα with TNFα antibodies or soluble TNF receptors attenuates the development of pain in various experimental arthritis models [28–31]. Inhibition of IL6 or IL1β signalling by an intra-articular injection of soluble gp130 or with the IL1 receptor (IL1R) antagonist anakinra, respectively, attenuates the development of pain in experimental arthritis [32, 33]. Pro-inflammatory cytokines may also maintain pain through modulating the central terminals of primary afferent neurons and/or spinal cord neurons because spinal administration of neutralizing TNFα antibodies also reduces experimental arthritis pain [31]. Some RA patients continue to experience pain after systemic anti-TNFα treatment [11], but this may be explained by systemically administered antibodies not efficiently blocking spinal TNFα. Indeed, spinal TNFα neutralization is more effective in treating arthritis pain than when administered systemically [31]. Other cytokines, such as IL15, IFNγ, IL18, IL22 and IL17, or damage associated molecules, such as high mobility group box 1 or S100, initiate or maintain pain [34, 35]. IL15 contributes to the development of neuropathic pain by promoting infiltration of macrophages and T cells into the sciatic nerve and spinal cord while IFNγ induces spontaneous neuronal firing and activates spinal microglia [36]. Nerve injury-induced allodynia is reduced after genetic or pharmacological inhibition of IL17 or IL18 and intrathecal injections of these cytokines induce pain, probably through activating spinal glial cells [37, 38]. Finally, IL22 expression is increased during the onset of experimental arthritis pain and inhibiting IL22 reduces pain [39]. Intriguingly, levels of pro-inflammatory cytokines such as IL1β, IL6 and IL18 are increased in the spinal cerebral fluid of FM, non-diabetic polyneuropathy and post-traumatic neuralgia patients [40, 41].
The functional capacity of inflammatory mediators such as cytokines to produce pain is highly dependent on the expression and composition of their receptors in sensory neurons. Indeed, a wide range of cytokine receptors are expressed on sensory neurons, allowing cytokines to act directly on there [42, 43]. During development of pain, expression of these receptors may be modulated, affecting the functional consequences of inflammatory mediators released. After peripheral nerve injury TNF receptors (TNFR1 and TNFR2) and their ligand, TNFα, are upregulated in sensory neurons [44]. However, in several models of chronic pain, TNFR1 is the main pain promoting receptor [43], yet some reports indicate involvement of TNFR2 in pain induction [45].
Sensory neurons also express IL1R, and IL1β induces activation of sensory neurons [46]. In models of neuropathic and inflammatory pain, sensory neuron IL1R1 expression is increased [43, 47, 48] and in an experimental adjuvant-induced arthritis model, the proportion of IL1R1 expressing neurons almost doubles [33]. Finally, after nerve injury IL6 and its receptor (IL6R) are upregulated. Expression of the signal-transducing component gp130 remains unchanged [49, 50]; nevertheless, sensory neuron-specific depletion of gp130 attenuates inflammatory, tumour, and arthritis pain [51, 52], indicating an important role of the receptor component in pain development.
Complement components appear to also be important contributors to pain and are increased in the affected knee of RA and OA patients [53, 54]. Moreover, C1q, factor B and C3 are increased in sciatic nerves of humans with traumatic nerve lesion-induced pain and after spinal cord injury in rodents [55, 56]. Components from the classical and non-classical pathway (e.g. C5, C3 and C1q) cause pain through triggering sensory neurons or recruiting and activating immune cells (e.g. C5a and C3a) to facilitate pain [57–59]. Intrathecal as well as intraplantar injections of C3a and C5a induce pain hypersensitivity in rodents, while blocking C5aR reduces pain in inflammatory, neuropathic and postoperative pain models [57, 60, 61].
Evidence exists that short-lived episodes of acute inflammation can induce long-lasting sensory neuron plasticity that may contribute to chronic pain development [62, 63]. This neuronal plasticity includes changes in the expression of neurotransmitters, receptors, signalling cascades and ion channels causing long-lasting altered sensory response to subsequent inflammatory mediators [64, 65]. For example, rodents challenged with a transient inflammatory stimulus such as IL6, IL1β, bradykinin or carrageenan develop short-lasting hyperalgesia. However, when signs of inflammation have resolved, even weeks later, and animals are challenged with a second inflammatory stimulus that normally induces a transient hyperalgesia (e.g. PGE2), this inflammatory mediator now induces a hyperalgesia that lasts much longer [66, 67]. Possibly, such neuronal plasticity may explain why some RA patients after arthritis flares experience pain that outlast the inflammatory response.
Immune-derived mediators resolving pain
The first evidence of immune-derived mediators that can resolve pain came from work showing that intrathecal administration of the anti-inflammatory cytokine IL10 reduces neuropathic pain [68]. Intriguingly, IL10 is important in endogenous pain resolution pathways that occur in naturally resolving transient pain conditions. For example, intrathecal injection of neutralizing IL10 antibodies prolongs transient inflammatory pain, and inhibition of IL10 signalling either genetically (IL10−/− mice) or pharmacologically (neutralizing IL10 antibodies) delays recovery from chemotherapy-induced neuropathy [25, 69]. Finally, in neuropathic pain patients cerebrospinal fluid IL10 levels are reduced compared with healthy controls [40]. The pain resolving action of IL10 may be explained by its inhibitory effects on spinal glia that maintain chronic pain [17]. IL10 inhibits glia cell activation in both inflammatory and neuropathic models of pain [69, 70]. Moreover, IL10-mediated attenuation of paclitaxel-induced mechanical allodynia is associated with decreased CD11b, TNFα and IL1β expression in the DRG, suggesting that IL10 inhibits activation and/or DRG recruitment of CD11b+ immune cells and subsequent pro-inflammatory cytokine production [71]. Nevertheless, IL10 also inhibits tetrodotoxin (TTX)-sensitive sodium channels in sensory neurons and reduces chemotherapy-induced spontaneous firing of sensory neurons in vitro, indicating IL10 modulates sensory neurons directly [69, 72]. Importantly, sensory neurons do express other anti-inflammatory cytokine receptors, such as IL4R, IL13R and TGFβR, and potentially these regulatory cytokines modulate sensory function and pain as well [42]. Indeed IL10 is not the only pain resolving cytokine since mice deficient for IL4 display mechanical allodynia and increased neuronal excitability, and patients with painful neuropathy have reduced IL4 serum levels, indicating that IL4 plays some role in controlling pain [73, 74]. Moreover, intrathecal injections of TGFβ, IL13 or sensory neuron-specific overexpression of IL4 have analgesic effects in neuropathic and inflammatory pain models [75–78]. The efficacy of these anti-inflammatory cytokines to inhibit pain is dependent on receptor expression or signalling. To our knowledge there are no data available on whether expression and/or receptor signalling are regulated in sensory neurons during chronic pain. Nevertheless, expression of IL10Rα is reduced in synovial tissue of RA patients, enabling the possibility that in such chronic inflammatory conditions sensory neuron IL10R signalling may be affected rendering these neurons less susceptible to IL10-mediated pain inhibition [79].
Despite analgesic actions of anti-inflammatory cytokines, the therapeutic potential of unmodified anti-inflammatory cytokines is limited because these cytokines work optimally in concert with each other and their relatively small size causes rapid clearance, reducing their bioavailability [80–82]. More recently these limitations have been overcome by fusion of IL4 and IL10 into one molecule that was more effective in inhibiting persistent inflammatory and neuropathic pain than the combination of the individual cytokines [83]. Moreover several viral gene therapy or non-viral transduction vectors have been employed to induce prolonged production of native cytokines to resolve chronic pain conditions [68, 78, 84, 85]. Overall these strategies show a promising perspective for the use of anti-inflammatory cytokines to treat chronic pain.
Other immune-derived mediators known to be involved in the termination programme of inflammation, such as resolvins (e.g. RvE1, RvD1) and protectins [e.g. neuroprotectin D1 (NPD1)/protectin D1 (PD1)] have strong analgesic actions. RvE1 and RvD1 suppress pain through inhibiting transient receptor potential channel activity in sensory neurons and N-methyl-D-aspartate receptors postsynaptically in the dorsal horn [86, 87]. Similarly, intrathecal NPD1/PD1 injections reduce established neuropathic pain by blocking nerve injury-induced spinal glia activation and spinal synaptic plasticity [88].
Immune cells regulating pain
Myeloid cells
Pain initiation
Monocytes/macrophages are linked to the development of pain by the production of inflammatory mediators. In neuropathic (e.g. nerve injury-induced) and inflammatory pain models [e.g. arthritis, intraplantar complete Freund’s adjuvant (CFA) injections] elevated numbers of monocytes/macrophages are observed in pain-relevant tissues such as the injured nerve, the inflamed skin or the DRG [23, 89–92] at the time pain is developing. Depletion of macrophages locally after CFA-induced paw inflammation attenuates the development of inflammatory pain, whilst depletion of macrophages at the site of inflammation during established persistent inflammatory pain does not affect pain [89, 93]. Some evidence exists for a role of myeloid cells in the initiation of pain in neuropathic pain models. Macrophages infiltrate the injured nerve after chemotherapy or sciatic nerve injury and depletion of these cells suppresses the development of neuropathic pain [23, 92].
Osteoclasts are derived from myeloid progenitors and play a role in the initiation of pain. In chronic inflammatory and degenerative diseases such as RA and OA, osteoclasts are increased in number and display increased bone resorption activities [94]. These enhanced bone resorption activities cause local acidification, activating acid-sensing ion channels and transient receptor potential channel vanilloid subfamily member 1 in sensory neurons, leading to pain [95–97]. Inhibitors of osteoclast activity reduce pain in models of OA, inflammatory pain and cancer-induced bone pain [95, 97, 98]. Similarly, in humans inhibitors of osteoclast activity reduce pain in patients with bone disorders or RA [99–101].
Although some studies have shown a role for myeloid cells or myeloid-derived osteoclasts in the initiation of pain, the majority of studies indicate roles for myeloid cells in pain maintenance.
Pain maintenance
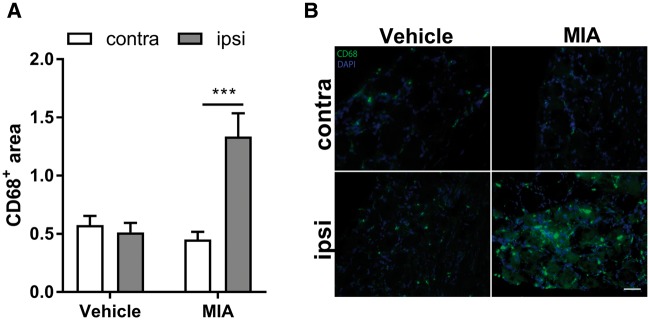
In rodent models of neuropathic pain, induced either surgically or by chemotherapy, monocytes/macrophages appear in the DRG and spinal cord at time points when pain is already established and these cells remain present for several weeks [24, 102–104]. In several chronic inflammatory pain models, including CFA-induced arthritis and experimental arthritis, macrophages are found in the DRG and spinal cord when pain is established [90, 91, 105, 106]. In a surgical model of OA, macrophages infiltrate the DRG 8 weeks after the destabilization of the medial meniscus and persist within the DRG for at least 16 weeks [107]. Similarly, 4 weeks after intra-articular administration of monosodium iodoacetate, which induces long-lasting pain by damaging the knee joints [108], the number of CD68+ macrophages in the lumbar DRG triples, suggesting a role of these cells in the maintenance of OA pain (Fig. 2). During antigen-induced arthritis, DRG-infiltrating macrophages exert a phenotype that resembles TNFα-stimulated macrophages [106]. In vitro, TNFα-stimulated macrophages promote calcitonin gene-related peptide (CGRP) release by sensory neurons, which could explain their pro-nociceptive properties [105]. Macrophage-derived IL6, TNFα and IL1β are described as important drivers of chronic pain [21]. In addition, macrophages also release reactive oxygen species that may contribute to the maintenance of pain, since Nox2+ macrophages migrate to the DRG and contribute to neuropathic pain in a reactive oxygen species-dependent mechanism [109, 110].
Fig. 2.
Macrophages infiltrate the DRG in a model of OA
(A) Unilateral intra-articular monosodium iodoacetate (MIA) injections in rats significantly increased the number of CD68+ macrophages in the lumbar DRG compared with the contralateral knee or vehicle injected rats at 4 weeks after injections (n = 6). (B) Exemplar images of the DRGs innervating the affected knee (ipsi) or the contral lateral knee (contra). Scale bar is 50 μm. Data are presented as mean and s.e.m. ***P < 0.001: statistical analyses were performed by two-way analysis of variance with the Bonferroni post hoc test.
The strongest evidence of the involvement of monocytes/macrophages in the maintenance of chronic pain comes from monocyte/macrophage depletion studies. Depletion of peripheral macrophages by i.v. injections of clodronate liposomes partially reverses established paclitaxel-induced or nerve ligation-induced mechanical hyperalgesia and reduced TNFα expression in DRG [111, 112]. Moreover, monocyte/macrophage depletion with clodronate liposomes delays the progression of diabetes-induced mechanical allodynia [113]. Systemic depletion of monocytes/macrophages after sciatic nerve ligation attenuates axonal damage and hyperalgesia, whereas depletion prior to L5 spinal nerve transection has no effect on the development of neuropathic pain, indicating that macrophages play a role in the maintenance of chronic pain [114, 115].
The presence of macrophages at pain-relevant sites raises the question why these cells migrate to these tissues that are distant from the site of actual damage or inflammation. After peripheral inflammation sensory neurons produce chemokines chemokine (C-C motif) ligand 2 (CCL2) and chemokine (C-X3-C motif) ligand 1 (CX3CL1), which may drive the attraction of macrophages [116, 117]. Similarly, after chemotherapy-induced nerve injury or after knee damage in an experimental osteoarthritis model, expression of CCL2 is increased in the DRG and spinal cord, and the increase in CCL2 production is associated with elevated numbers of macrophages in the DRG and spinal cord [107, 112]. CX3CL1 is anchored to the plasma membrane, but is liberated after cleavage by proteases (e.g. cathepsin S) produced by activated microglia [118]. After nerve injury soluble CX3CL1 levels are increased in the DRG, whilst membrane-bound CX3CL1 is decreased [119].
In mice deficient for chemokine (C-C motif) receptor 2 (CCR2) and CX3C chemokine receptor 1 (CX3CR1), receptors for CCL2 and CX3CL1, pain and the number of monocytes/macrophages in the injured nerve or DRG are markedly reduced after a peripheral inflammation, experimental OA or chemotherapy-induced neuropathy [92, 107, 120, 121]. Moreover, blocking of spinal and DRG CX3CL1 or CCL2 during established paclitaxel-induced neuropathy inhibits macrophage recruitment to the DRG and attenuates allodynia [122, 123]. In patients with lumbar disk herniation with sciatic pain, the severity of pain is correlated with increased local expression of CX3CL1 and CCL2 in the soft tissues around nerve root. Moreover, intrathecal administration of a CCR2 antagonist inhibits neuropathic pain in a rat model of lumbar disc herniation [124, 125].
Sensory neurons also produce other chemokines after nerve injury, such as CCL21, CXCL13 and CCL7 [120, 126, 127]. Whether similar chemokines are produced during chronic inflammatory pain remains to be determined. Nevertheless, all these factors may contribute to macrophage infiltration in the DRG to regulate pain. However, it should be noted that many chemokines including CCL2 also act on chemokine receptors expressed by sensory neurons to produce pain [128]).
Pain resolution
Depletion of monocytes prior to the induction of transient inflammatory pain with IL1β or carrageenan prevents the resolution of inflammatory pain, that normally last 1–2 days but now persists for >1 week. This prevention of the resolution of a transient inflammatory hyperalgesia is dependent on IL10 production by monocytes/macrophages [25]. Moreover, reduction of G protein-coupled receptor kinase 2, an ubiquitously expressed negative regulator of G protein-coupled receptors and other signalling molecules (e.g. p38) in monocytes/macrophages increases production of TNFα whilst reducing IL10 and prevents the resolution of transient inflammatory pain [25]. The existence of pain-resolving macrophages is further supported by evidence that perineural injection of IL4-skewed macrophages reduces neuropathic pain through the production of opioid peptides including Met-enkephalin, dynorphin A and β-endorphin [129].
In conclusion, myeloid cells have distinct roles in the initiation, maintenance and resolution of pain. The functional plasticity of macrophages enables these cells to mediate both pro- and anti-nociceptive effects following injury or inflammation. As such, regulating macrophage phenotype by promoting polarization into anti-nociceptive or blocking polarization into pro-nociceptive phenotype might represent interesting avenues for potential new therapeutic strategies for chronic pain.
Neutrophils and mast cells
Pain initiation and maintenance
After an inflammation/damage, neutrophils are one of the first cells recruited to the affected tissue and may act as potential initiators of pain. However, the majority of studies indicate that there is no substantial role for neutrophils in pain induction, since the development of inflammatory pain or incisional wound pain is not affected by neutrophil depletion [61, 89, 130]. Moreover, local recruitment of polymorphonuclear cells with CXCL1 and CXCL2/3 does not induce pain [131].
Given that mast cells are frequently found in close proximity to nerve endings, they are in a unique position to activate sensory neurons and induce pain. IgE-dependent activation of human mast cells induces itch. However, upon activation mast cells also rapidly release cytokines, NGF, proteases and histamine and bradykinin that induce pain [132, 133]. In patients with chronic pain, such as inflammatory bowel syndrome, RA and FM, increased mast cell numbers are found in the inflamed tissues that correlated with the severity of pain symptoms [134, 135]. In rodents, degranulation of mast cells causes immediate hyperalgesia in wild-type but not in mast-cell deficient mice [136]. Although these results point to some role of mast cells and granulocytes in the initiation of pain, potential roles in maintaining pain are thus far unknown.
Pain resolution
Neutrophils can release opioid peptides (β-endorphin, met-enkephalin and dynorphin-A) that have anti-nociceptive effects through μ, δ or κ opioid receptors expressed by sensory neurons [137]. An anti-nociceptive role of neutrophils is evoked by corticotrophin releasing factor (CRF) injections that induce opioid secretion by neutrophils [138]. CRF attenuates CFA-induced inflammatory-hyperalgesia in rats in an opioid and granulocyte-dependent manner and intra-articular injections of CRF relieve post-operative pain in patients after arthroscopic knee surgery [138, 139].
T cells
Pain initiation and maintenance
Some evidence suggests that T cells control the initiation of neuropathic pain, because T cell infiltration into damaged nerves coincidences with the time when allodynia is developing [23, 27]. In some neuropathic pain models mechanical allodynia is reduced in T cell deficient mice at time points during the development of allodynia (day 3) [140, 141]. There is some evidence that T cells infiltrate the inflamed tissue after intraplantar CFA injections. However in T cell deficient mice the pain sensitivity after CFA is not altered, suggesting that T cells do not contribute to the initiation of inflammatory pain [89].
The majority of studies indicate that T cells infiltrate spinal cord and DRGs during the maintenance of neuropathic pain and are thus more likely to contribute to the maintenance of pain [18, 140–142]. T cells are present in spinal cord starting from 1 to 2 weeks after nerve injury in different models of neuropathic pain [20, 142]. The majority of infiltrating T cells are CD4+ and produce IL17 and IFNγ [140, 142, 143]. IL15 and IL23 produced by macrophages and dendritic cells drive T helper 17 cells to the spinal cord during the maintenance of neuropathic pain [141]. In T cell deficient mice nerve injury-induced neuropathic pain is attenuated when pain has already developed, while the initial development phase of neuropathic pain is intact [27, 142]. Depletion of CD4+ T cells with intravenous CD4 antibodies also reduces hyperalgesia and allodynia once pain has already developed [23]. Similarly, in CD4−/− mice neuropathic pain is reduced only during the maintenance of neuropathic pain and is rescued by adoptive transfer of CD4+ T cells [140].
Some recent evidence suggests that the involvement of T cells in pain maintenance is sex dependent. In female mice the expression of several T cell markers in the spinal cord is almost 2-fold higher than in males after nerve injury and nerve injury-induced neuropathic pain is reduced in female but not in male T cell deficient mice [144]. This immune system-related sex difference may be explained by sex-dependent IFNγ and IL-17A expression by CD4 T cells [143, 144]. The contribution of T cells might not only be limited by sex but also by age, because the large T cell infiltration and upregulation of IFNγ in the dorsal horn after spared nerve injury is only observed in adult and not in infant rats and mice [142]. This age-dependent involvement of T cells in neuropathic pain could explain the clinical observation that in children neuropathic pain is less often observed [145]. However, increased production of IL4 and IL10 in the spinal cord also contributes to the diminished neuropathic pain development in neonatal rats and mice [146].
Resolution of pain
Adoptive transfer of T helper 2 cells reduces established neuropathic pain in an IL10-dependent manner, indicating that this T cell subset has some anti-nociceptive roles [27]. Similarly, Tregs resolve pain. Systemic application of a CD28 superagonist, a Treg population expander, reduces the development of nerve injury-induced neuropathic pain and number of infiltrating T cells in the damaged nerve [26]. Conversely, depletion of Tregs with cytotoxic CD25 antibodies or by using transgenic mice to selectively deplete FOXP3+ T cells prolongs nerve injury-induced mechanical hypersensitivity [26, 147]. In mice deficient for T cells, transient chemotherapy-induced allodynia does not resolve and the resolution is rescued by reconstitution of CD8+ T cells but not by CD4+ T cells [69]. Importantly, this CD8+ T cell-mediated resolution of chemotherapy-induced pain required IL10 signalling not by direct secretion of IL10 but rather through upregulating IL10 receptor expression in the DRG [69]. Thus, T cells also control resolution of chemotherapy-induced neuropathic pain.
Overall, T cells clearly have roles in development of neuropathic pain. However, specific T cell subtypes and their secreted inflammatory products determine whether T cells have a pro- or anti-nociceptive role. Whether T cells also regulate inflammatory or other types of pain remains to be determined.
B cells
Pain initiation and maintenance
Evidence for the involvement of B cells in the initiation of pain mainly comes from studies that show that autoantibodies can induce pain [148]. Autoantibodies against citrullinated antigens (ACPAs; e.g. against citrullinated fibrinogen, vimentin, α-enolase, collagen type II, immunoglobulin-binding protein and histone 4) are increased in patients with RA [149]. Intravenous injection of purified ACPAs from RA patients or those from arthritic mice to healthy mice induces pain and increased heat and cold sensitivity without inducing inflammation [150]. ACPAs can be present years before RA diagnosis and may explain the pain-related problems of RA patients before the onset of clinical symptoms [151]. Mechanistically, ACPAs bind to osteoclasts to induce the release of CXCL1 (equivalent to human IL8), which activates sensory neurons and induces pain [152]. The majority of ACPAs are IgGs and the Fcγ type 1 receptor (FcγR1) is expressed by some sensory neuron subsets. Intradermal injection with IgG immune complexes produces hyperalgesia dependent on FcγRI expression, indicating that IgG immune complexes also produce pain through activating neurons directly [153]. Moreover, during experimental arthritis, the number of sensory neurons expressing FcγR1 is increased suggesting that during inflammation the sensory system becomes more sensitive for IgGs [153, 154]. Autoantibodies are also detected in other autoimmune diseases associated with pain such as multiple sclerosis, Guillian–Barre syndrome and complex regional pain syndrome (CRPS) [155, 156]. Moreover, anti-neuronal antibodies are detected in patients with CRPS and B cell depletion in a mouse model of CRPS reduced pain [157, 158]. Finally, treatment of neuroblastoma with antibodies against disialoganglioside produces severe pain as a side effect, indicating that some IgGs can induce pain [159].
Concluding remarks
Immune cells and their mediators have important but distinct roles in regulating different types of pain (Fig. 1) indicating that the immune system and nervous system are intimately intertwined. The diverse roles of myeloid cells and T cells in the initiation, maintenance and resolution of inflammatory and neuropathic pain are intriguing and invite the question of whether chronic pain conditions may be the results of defects in the immune system rather than merely nervous system defects. The intricate involvement of the immune system in pain regulation also highlights possibilities for using immune approaches for the treatment of pain. Regulating the subsets of these cells by inducing anti-nociceptive phenotypes may represent a strategy to prevent debilitating chronic pain conditions. In some clinical studies strategies have been tested to interfere with myeloid cell for treating neuropathic pain (e.g. CCR2, CSF1R antagonists), but these compounds failed to reduce pain scores [160]. Other approaches including targeting B cells to prevent the production of autoantibodies (e.g. B cell depletion strategies with anti-CD20) reduce arthritis disease onset, but this study only showed a limited improvement of pain visual analogue scores [161]. Although systemic anti-inflammatory strategies may have the risk of introducing infections, local (spinal) and/or transient administration of immunomodulatory compounds may reduce these risks. Finally, the use of anti-inflammatory cytokines for pain treatment remains a very promising strategy, but to the best of our knowledge, clinical trials are yet to be conducted.
Acknowledgements
The authors thank Sabine Versteeg, University Medical Center Utrecht, Utrecht, The Netherlands for excellent technical support for the data displayed in Fig. 2.
Funding: Part of this work has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 642720.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1. Salovey P, Seiber W, Smith A.. Reporting Chronic Pain Episodes on Health Surveys. Hyattsville, MD: National Center for Health Statistics, 1992. [Google Scholar]
- 2. Mantyselka P, Kumpusalo E, Ahonen R. et al. Pain as a reason to visit the doctor: a study in Finnish primary health care. Pain 2001;89:175–80. [DOI] [PubMed] [Google Scholar]
- 3. Breivik H, Eisenberg E, O'Brien T; OPENMinds. The individual and societal burden of chronic pain in Europe: the case for strategic prioritisation and action to improve knowledge and availability of appropriate care. BMC Public Health 2013;13:1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gerdle B, Bjork J, Coster L. et al. Prevalence of widespread pain and associations with work status: apopulation study. BMC Musculoskelet Disord 2008;9:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cherubino P, Sarzi-Puttini P, Zuccaro SM, Labianca R.. The management of chronic pain in important patient subgroups. Clin Drug Investig 2012;32(Suppl 1):35–44. [DOI] [PubMed] [Google Scholar]
- 6. Reid KJ, Harker J, Bala MM. et al. Epidemiology of chronic non-cancer pain in Europe: narrative review of prevalence, pain treatments and pain impact. Curr Med Res Opin 2011;27:449–62. [DOI] [PubMed] [Google Scholar]
- 7. Vellucci R. Heterogeneity of chronic pain. Clin Drug Investig 2012;32(Suppl 1):3–10. [DOI] [PubMed] [Google Scholar]
- 8. Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D.. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain 2006;10:287–333. [DOI] [PubMed] [Google Scholar]
- 9. Lee YC, Frits ML, Iannaccone CK. et al. Subgrouping of patients with rheumatoid arthritis based on pain, fatigue, inflammation, and psychosocial factors. Arthritis Rheumatol 2014;66:2006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hannan MT, Felson DT, Pincus T.. Analysis of the discordance between radiographic changes and knee pain inosteoarthritis of the knee. J Rheumatol 2000;27:1513–7. [PubMed] [Google Scholar]
- 11. Taylor P, Manger B, Alvaro-Gracia J. et al. Patient perceptions concerning pain management in the treatment of rheumatoid arthritis. J Int Med Res 2010;38:1213–24. [DOI] [PubMed] [Google Scholar]
- 12. Lee YC, Cui J, Lu B. et al. Pain persists in DAS28 rheumatoid arthritis remission but not in ACR/EULAR remission: a longitudinal observational study. Arthritis Res Ther 2011;13:R83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beswick AD, Wylde V, Gooberman-Hill R, Blom A, Dieppe P.. What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. BMJ Open 2012;2:e000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wylde V, Hewlett S, Learmonth ID, Dieppe P.. Persistent pain after joint replacement: prevalence, sensory qualities, and postoperative determinants. Pain 2011;152:566–72. [DOI] [PubMed] [Google Scholar]
- 15. Woolf CJ, Ma Q.. Nociceptors—noxious stimulus detectors. Neuron 2007;55:353–64. [DOI] [PubMed] [Google Scholar]
- 16. Ji RR, Chamessian A, Zhang YQ.. Pain regulation by non-neuronal cells and inflammation. Science 2016;354:572–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Graeber MB, Christie MJ.. Multiple mechanisms of microglia: a gatekeeper's contribution to pain states. Exp Neurol 2012;234:255–61. [DOI] [PubMed] [Google Scholar]
- 18. Grace PM, Hutchinson MR, Bishop A. et al. Adoptive transfer of peripheral immune cells potentiates allodynia in a graded chronic constriction injury model of neuropathic pain. Brain Behav Immun 2011;25:503–13. [DOI] [PubMed] [Google Scholar]
- 19. Hu P, Bembrick AL, Keay KA, McLachlan EM.. Immune cell involvement in dorsal root ganglia and spinal cord after chronic constriction or transection of the rat sciatic nerve. Brain Behav Immun 2007;21:599–616. [DOI] [PubMed] [Google Scholar]
- 20. Sweitzer SM, Hickey WF, Rutkowski MD, Pahl JL, DeLeo JA.. Focal peripheral nerve injury induces leukocyte trafficking into the central nervous system: potential relationship to neuropathic pain. Pain 2002;100:163–70. [DOI] [PubMed] [Google Scholar]
- 21. Zhang JM, An J.. Cytokines, inflammation, and pain. Int Anesthesiol Clin 2007;45:27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Basbaum AI, Bautista DM, Scherrer G, Julius D.. Cellular and molecular mechanisms of pain. Cell 2009;139:267–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kobayashi Y, Kiguchi N, Fukazawa Y. et al. Macrophage-T cell interactions mediate neuropathic pain through the glucocorticoid-induced tumor necrosis factor ligand system. J Biol Chem 2015;290:12603–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim D, You B, Lim H, Lee SJ.. Toll-like receptor 2 contributes to chemokine gene expression and macrophage infiltration in the dorsal root ganglia after peripheral nerve injury. Mol Pain 2011;7:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Willemen HL, Eijkelkamp N, Garza Carbajal A. et al. Monocytes/macrophages control resolution of transient inflammatory pain. J Pain 2014;15:496–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Austin PJ, Kim CF, Perera CJ, Moalem-Taylor G.. Regulatory T cells attenuate neuropathic pain following peripheral nerve injury and experimental autoimmune neuritis. Pain 2012;153:1916–31. [DOI] [PubMed] [Google Scholar]
- 27. Moalem G, Xu K, Yu L.. T lymphocytes play a role in neuropathic pain following peripheral nerve injury in rats. Neuroscience 2004;129:767–77. [DOI] [PubMed] [Google Scholar]
- 28. Boettger MK, Hensellek S, Richter F. et al. Antinociceptive effects of tumor necrosis factor alpha neutralization in a rat model of antigen-induced arthritis: evidence of a neuronal target. Arthritis Rheum 2008;58:2368–78. [DOI] [PubMed] [Google Scholar]
- 29. Christianson CA, Corr M, Firestein GS. et al. Characterization of the acute and persistent pain state present in K/BxN serum transfer arthritis. Pain 2010;151:394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Inglis JJ, Notley CA, Essex D. et al. Collagen-induced arthritis as a model of hyperalgesia: functional and cellular analysis of the analgesic actions of tumor necrosis factor blockade. Arthritis Rheum 2007;56:4015–23. [DOI] [PubMed] [Google Scholar]
- 31. Boettger MK, Weber K, Grossmann D. et al. Spinal tumor necrosis factor alpha neutralization reduces peripheral inflammation and hyperalgesia and suppresses autonomic responses in experimental arthritis: a role for spinal tumor necrosis factor alpha during induction and maintenance of peripheral inflammation. Arthritis Rheum 2010;62:1308–18. [DOI] [PubMed] [Google Scholar]
- 32. Boettger MK, Leuchtweis J, Kummel D. et al. Differential effects of locally and systemically administered soluble glycoprotein 130 on pain and inflammation in experimental arthritis. Arthritis Res Ther 2010;12:R140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ebbinghaus M, Uhlig B, Richter F. et al. The role of interleukin-1beta in arthritic pain: main involvement in thermal, but not mechanical, hyperalgesia in rat antigen-induced arthritis. Arthritis Rheum 2012;64:3897–907. [DOI] [PubMed] [Google Scholar]
- 34. Miller RE, Belmadani A, Ishihara S. et al. Damage-associated molecular patterns generated in osteoarthritis directly excite murine nociceptive neurons through Toll-like receptor 4. Arthritis Rheumatol 2015;67:2933–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Agalave NM, Svensson CI.. Extracellular high-mobility group box 1 protein (HMGB1) as a mediator of persistent pain. Mol Med 2015;20:569–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Toth C, Moulin DE.. Neuropathic Pain: Causes, Management and Understanding. Cambridge: Cambridge University Press; 2013. [Google Scholar]
- 37. Kim CF, Moalem-Taylor G.. Interleukin-17 contributes to neuroinflammation and neuropathic pain following peripheral nerve injury in mice. J Pain 2011;12:370–83. [DOI] [PubMed] [Google Scholar]
- 38. Miyoshi K, Obata K, Kondo T, Okamura H, Noguchi K.. Interleukin-18-mediated microglia/astrocyte interaction in the spinal cord enhances neuropathic pain processing after nerve injury. J Neurosci 2008;28:12775–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pinto LG, Talbot J, Peres RS. et al. Joint production of IL-22 participates in the initial phase of antigen-induced arthritis through IL-1beta production. Arthritis Res Ther 2015;17:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Backonja MM, Coe CL, Muller DA, Schell K.. Altered cytokine levels in the blood and cerebrospinal fluid of chronic pain patients. J Neuroimmunol 2008;195:157–63. [DOI] [PubMed] [Google Scholar]
- 41. Kadetoff D, Lampa J, Westman M, Andersson M, Kosek E.. Evidence of central inflammation in fibromyalgia-increased cerebrospinal fluid interleukin-8 levels. J Neuroimmunol 2012;242:33–8. [DOI] [PubMed] [Google Scholar]
- 42. Usoskin D, Furlan A, Islam S. et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci 2015;18:145–53. [DOI] [PubMed] [Google Scholar]
- 43. Miller RJ, Jung H, Bhangoo SK, White FA.. Cytokine and chemokine regulation of sensory neuron function. Handb Exp Pharmacol 2009;417–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. George A, Buehl A, Sommer C.. Tumor necrosis factor receptor 1 and 2 proteins are differentially regulated during Wallerian degeneration of mouse sciatic nerve. Exp Neurol 2005;192:163–6. [DOI] [PubMed] [Google Scholar]
- 45. Leung L, Cahill CM.. TNF-alpha and neuropathic pain—a review. J Neuroinflammation 2010;7:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Binshtok AM, Wang H, Zimmermann K. et al. Nociceptors are interleukin-1beta sensors. J Neurosci 2008;28:14062–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lee HL, Lee KM, Son SJ, Hwang SH, Cho HJ.. Temporal expression of cytokines and their receptors mRNAs in a neuropathic pain model. Neuroreport 2004;15:2807–11. [PubMed] [Google Scholar]
- 48. Li M, Shi J, Tang JR. et al. Effects of complete Freund's adjuvant on immunohistochemical distribution of IL-1beta and IL-1R I in neurons and glia cells of dorsal root ganglion. Acta Pharmacol Sin 2005;26:192–8. [DOI] [PubMed] [Google Scholar]
- 49. Brazda V, Klusakova I, Hradilova SI, Dubovy P.. Dynamic response to peripheral nerve injury detected by in situ hybridization of IL-6 and its receptor mRNAs in the dorsal root ganglia is not strictly correlated with signs of neuropathic pain. Mol Pain 2013;9:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Brazda V, Klusakova I, Svizenska I, Veselkova Z, Dubovy P.. Bilateral changes in IL-6 protein, but not in its receptor gp130, in rat dorsal root ganglia following sciatic nerve ligature. Cell Mol Neurobiol 2009;29:1053–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Andratsch M, Mair N, Constantin CE. et al. A key role for gp130 expressed on peripheral sensory nerves in pathological pain. J Neurosci 2009;29:13473–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ebbinghaus M, Segond von Banchet G, Massier J. et al. Interleukin-6-dependent influence of nociceptive sensory neurons on antigen-induced arthritis. Arthritis Res Ther 2015;17:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang Q, Rozelle AL, Lepus CM. et al. Identification of a central role for complement in osteoarthritis. Nat Med 2011;17:1674–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bhattacharjee M, Balakrishnan L, Renuse S. et al. Synovial fluid proteome in rheumatoid arthritis. Clin Proteomics 2016;13:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. de Jonge RR, van Schaik IN, Vreijling JP, Troost D, Baas F.. Expression of complement components in the peripheral nervous system. Hum Mol Genet 2004;13:295–302. [DOI] [PubMed] [Google Scholar]
- 56. Levin ME, Jin JG, Ji RR. et al. Complement activation in the peripheral nervous system following the spinal nerve ligation model of neuropathic pain. Pain 2008;137:182–201. [DOI] [PubMed] [Google Scholar]
- 57. Clark JD, Qiao Y, Li X. et al. Blockade of the complement C5a receptor reduces incisional allodynia, edema, and cytokine expression. Anesthesiology 2006;104:1274–82. [DOI] [PubMed] [Google Scholar]
- 58. Shutov LP, Warwick CA, Shi X. et al. The complement system component C5a produces thermal hyperalgesia via macrophage-to-nociceptor signaling that requires NGF and TRPV1. J Neurosci 2016;36:5055–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Li M, Peake PW, Charlesworth JA, Tracey DJ, Moalem-Taylor G.. Complement activation contributes to leukocyte recruitment and neuropathic pain following peripheral nerve injury in rats. Eur J Neurosci 2007;26:3486–500. [DOI] [PubMed] [Google Scholar]
- 60. Jang JH, Clark JD, Li X. et al. Nociceptive sensitization by complement C5a and C3a in mouse. Pain 2010;148:343–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sahbaie P, Li X, Shi X, Clark JD.. Roles of Gr-1+ leukocytes in postincisional nociceptive sensitization and inflammation. Anesthesiology 2012;117:602–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Reichling DB, Levine JD.. Critical role of nociceptor plasticity in chronic pain. Trends Neurosci 2009;32:611–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kandasamy R, Price TJ.. The pharmacology of nociceptor priming. Handb Exp Pharmacol 2015;227:15–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Woolf CJ, Salter MW.. Neuronal plasticity: increasing the gain in pain. Science 2000;288:1765–9. [DOI] [PubMed] [Google Scholar]
- 65. Zimmermann M. Pathobiology of neuropathic pain. Eur J Pharmacol 2001;429:23–37. [DOI] [PubMed] [Google Scholar]
- 66. Dina OA, McCarter GC, de Coupade C, Levine JD.. Role of the sensory neuron cytoskeleton in second messenger signaling for inflammatory pain. Neuron 2003;39:613–24. [DOI] [PubMed] [Google Scholar]
- 67. Wang H, Heijnen CJ, van Velthoven CT. et al. Balancing GRK2 and EPAC1 levels prevents and relieves chronic pain. J Clin Invest 2013;123:5023–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Milligan ED, Soderquist RG, Malone SM. et al. Intrathecal polymer-based interleukin-10 gene delivery for neuropathic pain. Neuron Glia Biol 2006;2:293–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Krukowski K, Eijkelkamp N, Laumet G. et al. CD8+ T cells and endogenous IL-10 are required for resolution of chemotherapy-induced neuropathic pain. J Neurosci 2016;36:11074–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhou Z, Peng X, Hao S, Fink DJ, Mata M.. HSV-mediated transfer of interleukin-10 reduces inflammatory pain through modulation of membrane tumor necrosis factor alpha in spinal cord microglia. Gene Ther 2008;15:183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ledeboer A, Jekich BM, Sloane EM. et al. Intrathecal interleukin-10 gene therapy attenuates paclitaxel-induced mechanical allodynia and proinflammatory cytokine expression in dorsal root ganglia in rats. Brain Behav Immun 2007;21:686–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Shen KF, Zhu HQ, Wei XH. et al. Interleukin-10 down-regulates voltage gated sodium channels in rat dorsal root ganglion neurons. Exp Neurol 2013;247:466–75. [DOI] [PubMed] [Google Scholar]
- 73. Lemmer S, Schiesser P, Geis C. et al. Enhanced spinal neuronal responses as a mechanism for the increased nociceptive sensitivity of interleukin-4 deficient mice. Exp Neurol 2015;271:198–204. [DOI] [PubMed] [Google Scholar]
- 74. Uceyler N, Rogausch JP, Toyka KV, Sommer C.. Differential expression of cytokines in painful and painless neuropathies. Neurology 2007;69:42–9. [DOI] [PubMed] [Google Scholar]
- 75. Echeverry S, Shi XQ, Haw A. et al. Transforming growth factor-beta1 impairs neuropathic pain through pleiotropic effects. Mol Pain 2009;5:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kiguchi N, Sakaguchi H, Kadowaki Y. et al. Peripheral administration of interleukin-13 reverses inflammatory macrophage and tactile allodynia in mice with partial sciatic nerve ligation. J Pharmacol Sci 2017;133:53–6. [DOI] [PubMed] [Google Scholar]
- 77. Lantero A, Tramullas M, Pilar-Cuellar F. et al. TGF-beta and opioid receptor signaling crosstalk results in improvement of endogenous and exogenous opioid analgesia under pathological pain conditions. J Neurosci 2014;34:5385–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hao S, Mata M, Glorioso JC, Fink DJ.. HSV-mediated expression of interleukin-4 in dorsal root ganglion neurons reduces neuropathic pain. Mol Pain 2006;2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Neidhart M, Jungel A, Ospelt C. et al. Deficient expression of interleukin-10 receptor alpha chain in rheumatoid arthritis synovium: limitation of animal models of inflammation. Arthritis Rheum 2005;52:3315–8. [DOI] [PubMed] [Google Scholar]
- 80. van Roon JA, Lafeber FP, Bijlsma JW.. Synergistic activity of interleukin-4 and interleukin-10 in suppression of inflammation and joint destruction in rheumatoid arthritis. Arthritis Rheum 2001;44:3–12. [DOI] [PubMed] [Google Scholar]
- 81. van Roon JA, van Roy JL, Gmelig-Meyling FH, Lafeber FP, Bijlsma JW.. Prevention and reversal of cartilage degradation in rheumatoid arthritis by interleukin-10 and interleukin-4. Arthritis Rheum 1996;39:829–35. [DOI] [PubMed] [Google Scholar]
- 82. Joosten LA, Lubberts E, Durez P. et al. Role of interleukin-4 and interleukin-10 in murine collagen-induced arthritis. Protective effect of interleukin-4 and interleukin-10 treatment on cartilage destruction. Arthritis Rheum 1997;40:249–60. [DOI] [PubMed] [Google Scholar]
- 83. Eijkelkamp N, Steen-Louws C, Hartgring SA. et al. IL4-10 fusion protein is a novel drug to treat persistent inflammatory pain. J Neurosci 2016;36:7353–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Cunha FQ, Poole S, Lorenzetti BB, Veiga FH, Ferreira SH.. Cytokine-mediated inflammatory hyperalgesia limited by interleukin-4. Br J Pharmacol 1999;126:45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Dengler EC, Alberti LA, Bowman BN. et al. Improvement of spinal non-viral IL-10 gene delivery by D-mannose as a transgene adjuvant to control chronic neuropathic pain. J Neuroinflammation 2014;11:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lim JY, Park CK, Hwang SW.. Biological roles of resolvins and related substances in the resolution of pain. Biomed Res Int 2015;2015:830930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ji RR, Xu ZZ, Strichartz G, Serhan CN.. Emerging roles of resolvins in the resolution of inflammation and pain. Trends Neurosci 2011;34:599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Xu ZZ, Liu XJ, Berta T. et al. Neuroprotectin/protectin D1 protects against neuropathic pain in mice after nerve trauma. Ann Neurol 2013;74:490–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ghasemlou N, Chiu IM, Julien JP, Woolf CJ.. CD11b+Ly6G− myeloid cells mediate mechanical inflammatory pain hypersensitivity. Proc Natl Acad Sci U S A 2015;112:E6808–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Inglis JJ, Nissim A, Lees DM. et al. The differential contribution of tumour necrosis factor to thermal and mechanical hyperalgesia during chronic inflammation. Arthritis Res Ther 2005;7:R807–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Segond von Banchet G, Boettger MK, Fischer N. et al. Experimental arthritis causes tumor necrosis factor-alpha-dependent infiltration of macrophages into rat dorsal root ganglia which correlates with pain-related behavior. Pain 2009;145:151–9. [DOI] [PubMed] [Google Scholar]
- 92. Old EA, Nadkarni S, Grist J. et al. Monocytes expressing CX3CR1 orchestrate the development of vincristine-induced pain. J Clin Invest 2014;124:2023–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Brack A, Labuz D, Schiltz A. et al. Tissue monocytes/macrophages in inflammation: hyperalgesia versus opioid-mediated peripheral antinociception. Anesthesiology 2004;101:204–11. [DOI] [PubMed] [Google Scholar]
- 94. Hirayama T, Danks L, Sabokbar A, Athanasou NA.. Osteoclast formation and activity in the pathogenesis of osteoporosis in rheumatoid arthritis. Rheumatology 2002;41:1232–9. [DOI] [PubMed] [Google Scholar]
- 95. Nagae M, Hiraga T, Yoneda T.. Acidic microenvironment created by osteoclasts causes bone pain associated with tumor colonization. J Bone Miner Metab 2007;25:99–104. [DOI] [PubMed] [Google Scholar]
- 96. Reeh PW, Kress M.. Molecular physiology of proton transduction in nociceptors. Curr Opin Pharmacol 2001;1:45–51. [DOI] [PubMed] [Google Scholar]
- 97. Nagae M, Hiraga T, Wakabayashi H. et al. Osteoclasts play a part in pain due to the inflammation adjacent to bone. Bone 2006;39:1107–15. [DOI] [PubMed] [Google Scholar]
- 98. Strassle BW, Mark L, Leventhal L. et al. Inhibition of osteoclasts prevents cartilage loss and pain in a rat model of degenerative joint disease. Osteoarthritis Cartilage 2010;18:1319–28. [DOI] [PubMed] [Google Scholar]
- 99. Lane JM, Khan SN, O'Connor WJ. et al. Bisphosphonate therapy in fibrous dysplasia. Clin Orthop Relat Res 2001;(382):6–12. [DOI] [PubMed] [Google Scholar]
- 100. Astrom E, Soderhall S.. Beneficial effect of long term intravenous bisphosphonate treatment of osteogenesis imperfecta. Arch Dis Child 2002;86:356–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Rovetta G, Monteforte P.. Efficacy of disodium-clodronate in the management of joint pain in rheumatoid arthritis. Six months open study. Minerva Med 2003;94:353–7. [PubMed] [Google Scholar]
- 102. Vega-Avelaira D, Geranton SM, Fitzgerald M.. Differential regulation of immune responses and macrophage/neuron interactions in the dorsal root ganglion in young and adult rats following nerve injury. Mol Pain 2009;5:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Liu CC, Lu N, Cui Y. et al. Prevention of paclitaxel-induced allodynia by minocycline: Effect on loss of peripheral nerve fibers and infiltration of macrophages in rats. Mol Pain 2010;6:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Peters CM, Jimenez-Andrade JM, Jonas BM. et al. Intravenous paclitaxel administration in the rat induces a peripheral sensory neuropathy characterized by macrophage infiltration and injury to sensory neurons and their supporting cells. Exp Neurol 2007;203:42–54. [DOI] [PubMed] [Google Scholar]
- 105. Schaible HG, von Banchet GS, Boettger MK. et al. The role of proinflammatory cytokines in the generation and maintenance of joint pain. Ann N Y Acad Sci 2010;1193:60–9. [DOI] [PubMed] [Google Scholar]
- 106. Massier J, Eitner A, Segond von Banchet G, Schaible HG.. Effects of differently activated rodent macrophages on sensory neurons: implications for arthritis pain. Arthritis Rheumatol 2015;67:2263–72. [DOI] [PubMed] [Google Scholar]
- 107. Miller RE, Tran PB, Das R. et al. CCR2 chemokine receptor signaling mediates pain in experimental osteoarthritis. Proc Natl Acad Sci U S A 2012;109:20602–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Liu P, Okun A, Ren J. et al. Ongoing pain in the MIA model of osteoarthritis. Neurosci Lett 2011;493:72–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Hackel D, Pflucke D, Neumann A. et al. The connection of monocytes and reactive oxygen species in pain. PLoS One 2013;8:e63564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Kallenborn-Gerhardt W, Hohmann SW, Syhr KM. et al. Nox2-dependent signaling between macrophages and sensory neurons contributes to neuropathic pain hypersensitivity. Pain 2014;155:2161–70. [DOI] [PubMed] [Google Scholar]
- 111. Barclay J, Clark AK, Ganju P. et al. Role of the cysteine protease cathepsin S in neuropathic hyperalgesia. Pain 2007;130:225–34. [DOI] [PubMed] [Google Scholar]
- 112. Zhang H, Li Y, de Carvalho-Barbosa M. et al. Dorsal root ganglion infiltration by macrophages contributes to paclitaxel chemotherapy-induced peripheral neuropathy. J Pain 2016;17:775–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Mert T, Gunay I, Ocal I. et al. Macrophage depletion delays progression of neuropathic pain in diabetic animals. Naunyn Schmiedebergs Arch Pharmacol 2009;379:445–52. [DOI] [PubMed] [Google Scholar]
- 114. Liu T, van Rooijen N, Tracey DJ.. Depletion of macrophages reduces axonal degeneration and hyperalgesia following nerve injury. Pain 2000;86:25–32. [DOI] [PubMed] [Google Scholar]
- 115. Rutkowski MD, Pahl JL, Sweitzer S, van Rooijen N, DeLeo JA.. Limited role of macrophages in generation of nerve injury-induced mechanical allodynia. Physiol Behav 2000;71:225–35. [DOI] [PubMed] [Google Scholar]
- 116. Jeon SM, Lee KM, Park ES, Jeon YH, Cho HJ.. Monocyte chemoattractant protein-1 immunoreactivity in sensory ganglia and hindpaw after adjuvant injection. Neuroreport 2008;19:183–6. [DOI] [PubMed] [Google Scholar]
- 117. Souza GR, Talbot J, Lotufo CM. et al. Fractalkine mediates inflammatory pain through activation of satellite glial cells. Proc Natl Acad Sci U S A 2013;110:11193–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Clark AK, Yip PK, Malcangio M.. The liberation of fractalkine in the dorsal horn requires microglial cathepsin S. J Neurosci 2009;29:6945–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Verge GM, Milligan ED, Maier SF. et al. Fractalkine (CX3CL1) and fractalkine receptor (CX3CR1) distribution in spinal cord and dorsal root ganglia under basal and neuropathic pain conditions. Eur J Neurosci 2004;20:1150–60. [DOI] [PubMed] [Google Scholar]
- 120. Jiang BC, Cao DL, Zhang X. et al. CXCL13 drives spinal astrocyte activation and neuropathic pain via CXCR5. J Clin Invest 2016;126:745–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Abbadie C, Lindia JA, Cumiskey AM. et al. Impaired neuropathic pain responses in mice lacking the chemokine receptor CCR2. Proc Natl Acad Sci U S A 2003;100:7947–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Zhang H, Boyette-Davis JA, Kosturakis AK. et al. Induction of monocyte chemoattractant protein-1 (MCP-1) and its receptor CCR2 in primary sensory neurons contributes to paclitaxel-induced peripheral neuropathy. J Pain 2013;14:1031–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Huang ZZ, Li D, Liu CC. et al. CX3CL1-mediated macrophage activation contributed to paclitaxel-induced DRG neuronal apoptosis and painful peripheral neuropathy. Brain Behav Immun 2014;40:155–65. [DOI] [PubMed] [Google Scholar]
- 124. Zhu X, Cao S, Zhu MD. et al. Contribution of chemokine CCL2/CCR2 signaling in the dorsal root ganglion and spinal cord to the maintenance of neuropathic pain in a rat model of lumbar disc herniation. J Pain 2014;15:516–26. [DOI] [PubMed] [Google Scholar]
- 125. Peng ZY, Chen R, Fang ZZ. et al. Increased local expressions of CX3CL1 and CCL2 are related to clinical severity in lumbar disk herniation patients with sciatic pain. J Pain Res 2017;10:157–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Imai S, Ikegami D, Yamashita A. et al. Epigenetic transcriptional activation of monocyte chemotactic protein 3 contributes to long-lasting neuropathic pain. Brain 2013;136:828–43. [DOI] [PubMed] [Google Scholar]
- 127. Biber K, Tsuda M, Tozaki-Saitoh H. et al. Neuronal CCL21 up-regulates microglia P2X4 expression and initiates neuropathic pain development. EMBO J 2011;30:1864–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Zhang ZJ, Jiang BC, Gao YJ.. Chemokines in neuron-glial cell interaction and pathogenesis of neuropathic pain. Cell Mol Life Sci 2017. (in press) doi: 10.1007/s00018-017-2513-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Pannell M, Labuz D, Celik MO. et al. Adoptive transfer of M2 macrophages reduces neuropathic pain via opioid peptides. J Neuroinflammation 2016;13:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Eijkelkamp N, Heijnen CJ, Willemen HL. et al. GRK2: a novel cell-specific regulator of severity and duration of inflammatory pain. J Neurosci 2010;30:2138–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Rittner HL, Mousa SA, Labuz D. et al. Selective local PMN recruitment by CXCL1 or CXCL2/3 injection does not cause inflammatory pain. J Leukoc Biol 2006;79:1022–32. [DOI] [PubMed] [Google Scholar]
- 132. Forsythe P, Bienenstock J.. The mast cell-nerve functional unit: a key component of physiologic and pathophysiologic responses. Chem Immunol Allergy 2012;98:196–221. [DOI] [PubMed] [Google Scholar]
- 133. Barbara G, Stanghellini V, De Giorgio R, Corinaldesi R.. Functional gastrointestinal disorders and mast cells: implications for therapy. Neurogastroenterol Motil 2006;18:6–17. [DOI] [PubMed] [Google Scholar]
- 134. Nigrovic PA, Lee DM.. Mast cells in inflammatory arthritis. Arthritis Res Ther 2005;7:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Chatterjea D, Martinov T.. Mast cells: versatile gatekeepers of pain. Mol Immunol 2015;63:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Chatterjea D, Wetzel A, Mack M. et al. Mast cell degranulation mediates compound 48/80-induced hyperalgesia in mice. Biochem Biophys Res Commun 2012;425:237–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Stein C. Opioids, sensory systems and chronic pain. Eur J Pharmacol 2013;716:179–87. [DOI] [PubMed] [Google Scholar]
- 138. Brack A, Rittner HL, Machelska H. et al. Control of inflammatory pain by chemokine-mediated recruitment of opioid-containing polymorphonuclear cells. Pain 2004;112:229–38. [DOI] [PubMed] [Google Scholar]
- 139. Likar R, Mousa SA, Steinkellner H. et al. Involvement of intra-articular corticotropin-releasing hormone in postoperative pain modulation. Clin J Pain 2007;23:136–42. [DOI] [PubMed] [Google Scholar]
- 140. Cao L, DeLeo JA.. CNS-infiltrating CD4+ T lymphocytes contribute to murine spinal nerve transection-induced neuropathic pain. Eur J Immunol 2008;38:448–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Kleinschnitz C, Hofstetter HH, Meuth SG. et al. T cell infiltration after chronic constriction injury of mouse sciatic nerve is associated with interleukin-17 expression. Exp Neurol 2006;200:480–5. [DOI] [PubMed] [Google Scholar]
- 142. Costigan M, Moss A, Latremoliere A. et al. T-cell infiltration and signaling in the adult dorsal spinal cord is a major contributor to neuropathic pain-like hypersensitivity. J Neurosci 2009;29:14415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Zhang MA, Rego D, Moshkova M. et al. Peroxisome proliferator-activated receptor (PPAR)alpha and -gamma regulate IFNgamma and IL-17A production by human T cells in a sex-specific way. Proc Natl Acad Sci U S A 2012;109:9505–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Sorge RE, Mapplebeck JC, Rosen S. et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci 2015;18:1081–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Walco GA, Dworkin RH, Krane EJ, LeBel AA, Treede RD.. Neuropathic pain in children: Special considerations. Mayo Clin Proc 2010;85(3 Suppl):S33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. McKelvey R, Berta T, Old E, Ji RR, Fitzgerald M.. Neuropathic pain is constitutively suppressed in early life by anti-inflammatory neuroimmune regulation. J Neurosci 2015;35:457–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Lees JG, Duffy SS, Perera CJ, Moalem-Taylor G.. Depletion of Foxp3+ regulatory T cells increases severity of mechanical allodynia and significantly alters systemic cytokine levels following peripheral nerve injury. Cytokine 2015;71:207–14. [DOI] [PubMed] [Google Scholar]
- 148. Mifflin KA, Kerr BJ.. Pain in autoimmune disorders. J Neurosci Res 2017;95:1282–94. [DOI] [PubMed] [Google Scholar]
- 149. Klareskog L, Catrina AI, Paget S.. Rheumatoid arthritis. Lancet 2009;373:659–72. [DOI] [PubMed] [Google Scholar]
- 150. Wigerblad G, Bas DB, Fernades-Cerqueira C. et al. Autoantibodies to citrullinated proteins induce joint pain independent of inflammation via a chemokine-dependent mechanism. Ann Rheum Dis 2016;75:730–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Bas DB, Su J, Wigerblad G, Svensson CI.. Pain in rheumatoid arthritis: models and mechanisms. Pain Manag 2016;6:265–84. [DOI] [PubMed] [Google Scholar]
- 152. Catrina AI, Svensson CI, Malmstrom V, Schett G, Klareskog L.. Mechanisms leading from systemic autoimmunity to joint-specific disease in rheumatoid arthritis. Nat Rev Rheumatol 2017;13:79–86. [DOI] [PubMed] [Google Scholar]
- 153. Jiang H, Shen X, Chen Z. et al. Nociceptive neuronal Fc-gamma receptor I is involved in IgG immune complex induced pain in the rat. Brain Behav Immun 2017;62:351–61. [DOI] [PubMed] [Google Scholar]
- 154. Qu L, Zhang P, LaMotte RH, Ma C.. Neuronal Fc-gamma receptor I mediated excitatory effects of IgG immune complex on rat dorsal root ganglion neurons. Brain Behav Immun 2011;25:1399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Dawes JM, Vincent A.. Autoantibodies and pain. Curr Opin Support Palliat Care 2016;10:137–42. [DOI] [PubMed] [Google Scholar]
- 156. Terryberry JW, Thor G, Peter JB.. Autoantibodies in neurodegenerative diseases: antigen-specific frequencies and intrathecal analysis. Neurobiol Aging 1998;19:205–16. [DOI] [PubMed] [Google Scholar]
- 157. Dirckx M, Schreurs MW, de Mos M, Stronks DL, Huygen FJ.. The prevalence of autoantibodies in complex regional pain syndrome type I. Mediators Inflamm 2015;2015:718201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Li WW, Guo TZ, Shi X. et al. Autoimmunity contributes to nociceptive sensitization in a mouse model of complex regional pain syndrome. Pain 2014;155:2377–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Yu AL, Uttenreuther-Fischer MM, Huang CS. et al. Phase I trial of a human-mouse chimeric anti-disialoganglioside monoclonal antibody ch14.18 in patients with refractory neuroblastoma and osteosarcoma. J Clin Oncol 1998;16:2169–80. [DOI] [PubMed] [Google Scholar]
- 160. Biber K, Moller T, Boddeke E, Prinz M.. Central nervous system myeloid cells as drug targets: current status and translational challenges. Nat Rev Drug Discov 2016;15:110–24. [DOI] [PubMed] [Google Scholar]
- 161. Devauchelle-Pensec V, Pennec Y, Morvan J. et al. Improvement of Sjogren's syndrome after two infusions of rituximab (anti-CD20). Arthritis Rheum 2007;57:310–7. [DOI] [PubMed] [Google Scholar]