Abstract
Objective. The aim was to characterize SLE medication trends before, during and after pregnancy and to compare other commonly used medications during SLE pregnancies with non-SLE pregnancies.
Methods. Women with pregnancies ending in live birth or stillbirth were identified from the Swedish Medical Birth Register (2006–12). National registers were used to identify women with prevalent SLE during pregnancy and a sample without SLE and to identify prescription medications dispensed from 3 months pre-pregnancy until 6 months postpartum. We reported the prevalence of DMARDs, systemic CSs and NSAIDs (aspirin reported separately) in SLE pregnancies. We calculated prevalence estimates of other medications that were dispensed during pregnancy to ⩾ 5% of SLE pregnancies and for the same medications among non-SLE pregnancies.
Results. There were 483 pregnancies among women with SLE and 5723 pregnancies among women without SLE. In SLE pregnancies, 49.3% had one or more dispensing for DMARDs during pregnancy; the prevalence was 48.0% for CSs, 40.8% for aspirin and 6.0% for other NSAIDs and varied by pregnancy period. The prevalence of common medications among SLE pregnancies was 1.2- to 20-fold higher than among non-SLE pregnancies; for example, dalteparin (20.9 vs 1.0%), paracetamol (18.2 vs 2.9%) and levothyroxine (15.9 vs 4.9%).
Conclusion. In nearly half of SLE pregnancies, women were dispensed DMARDs and CSs. Commonly used medications in SLE pregnancies had far higher prevalence estimates compared with non-SLE pregnancies. Research regarding benefits and risks of commonly used medications on SLE pregnancies, breast milk and long-term outcomes for offspring is needed.
Keywords: systemic lupus erythematosus, pregnancy, medication, glucocorticoids, disease-modifying anti-rheumatic drugs
Rheumatology key messages
Pregnant women with SLE are commonly dispensed medications.
Nearly half of lupus pregnancies in Sweden used DMARDs, CSs or aspirin.
Many medications besides DMARDs are used more often in SLE pregnancies than in non-SLE pregnancies.
Introduction
The incidence of SLE is greatest among women of reproductive ages [1]. Decisions regarding medication use during pregnancy are crucial for women with this multisystem autoimmune disease. Treatment with immunosuppressants, CSs and NSAIDs during pregnancy may be indicated to treat flares or to keep disease activity under control [2]. Other medications may also be used during pregnancy to treat co-morbidities that are more common among individuals with SLE (e.g. APS, hypertension and depression) [3, 4]. The most prevalent prescription medications among pregnant women in general include antibacterials and antihistamines [5], and these may also be used commonly in SLE pregnancies.
There is limited information regarding medication use among pregnant women with SLE. Most reports from the past 15 years are based on a few hundred women or less and are often from women who attended one health-care centre, which limits generalizability [6–14]. Previous reports tended to focus on medications used to treat SLE, including HCQ, AZA and CSs, and few addressed heparin or other medications [6, 8, 11, 13, 15]. There is limited information regarding the timing and trajectory of medication use before, during and, especially, after SLE pregnancies [7, 10, 11, 14, 16]. To our knowledge, no studies have compared medication use among pregnant women with SLE vs women without SLE. Besides rheumatologists, other physicians, including obstetricians and general practitioners, prescribe medications for pregnant women with SLE. The spectrum of commonly used medications among pregnant women with SLE may not be apparent to their health-care providers. A more holistic approach to studying medication use among pregnant women with SLE is needed to gain a better understanding of the medication counselling needs of women with SLE who are pregnant or are planning pregnancy.
We used population-based health register data from Sweden to address the limited information on medication use among pregnant women with SLE. We identified the most prevalent medications among SLE pregnancies, characterized pre-pregnancy, pregnancy and postpartum medication prevalence, and compared medication prevalence among SLE pregnancies with non-SLE pregnancies.
Methods
Study population
Women with pregnancies
Nearly all deliveries in Sweden (>98%) are captured by the Medical Birth Register (MBR), which contains standardized information on maternal health during pregnancy, delivery and neonatal outcomes [5, 17]. Pregnancies with a delivery date between 5 August 2006 and 31 December 2012 were included in this study. For most of the study, the MBR captured births from 22 weeks gestation onward. However, between 2006 and 1 July 2008, stillbirths were included only if they occurred at 28 gestational weeks or later. Women could have multiple pregnancies captured during the study period.
Women with SLE
To identify women with SLE, we used the MBR and the National Patient Register, which contains information from hospitalizations since 1964, with complete nationwide coverage beginning in 1987, and from hospital-based outpatient specialist visits since 2001. The first SLE diagnosis for women included in this study occurred in 1977. Women were classified as having prevalent SLE during each pregnancy if they had the following: (i) at least two discharges from either inpatient or outpatient records with diagnosis codes indicative of SLE [International Classification of Diseases (ICD), Eighth, Ninth or Tenth Revision, ICD-8 734.1, ICD-9 710.0 or ICD-10 M32], excluding drug-induced lupus, and including at least one SLE diagnosis from a department or specialist that typically diagnoses, treats or manages SLE (rheumatology, dermatology, nephrology, internal medicine and paediatrics) and at least one SLE diagnosis before the beginning of pregnancy; or (ii) at least one SLE discharge diagnosis from a department or specialist as described above and at least one self-reported diagnosis of SLE in the MBR for the current pregnancy. Using Swedish registers, it has been shown that two inpatient or outpatient SLE diagnoses, including one from a specialist, accurately identifies women with SLE [18]. Similar case definitions yielded prevalence estimates of ∼100 SLE cases per 100 000 women of child-bearing age in the Swedish registers, which demonstrates face validity of the definition [19].
Women without SLE
Women without prevalent SLE during pregnancy were identified from individuals who were sampled from the Total Population Register as previously described [20]. Women without pregnancies or women with pregnancies ending prior to 28 weeks (2006–07) or 22 weeks (2008–12) were excluded.
Maternal and pregnancy characteristics
Maternal characteristics, including age, pre-pregnancy BMI and parity, were obtained from the MBR as were multiple gestation and gestational weeks at delivery. Maternal diagnoses before or during pregnancy, including asthma, chronic hypertension, type I or type II diabetes, mood disorders and APS, were obtained from the National Patient Register any time before delivery. We used a strict definition of APS (ICD-10 code D68.6: other thrombophilia) and a broad definition of APS (ICD-10 code O99.1: Other diseases of the blood and blood-forming organs and certain disorders involving the immune mechanism complicating pregnancy, childbirth and the puerperium). The date of the last menstrual period (LMP) was obtained from the MBR. The LMP date was most often estimated using prenatal ultrasound (89%); otherwise, maternal report of the first day of the LMP was used [21].
Medications
Prescribed drug register
The prescribed drug register, which was established in July 2005, contains information on prescription medications dispensed outside of hospitals, including Anatomical Therapeutic Chemical (ATC) classification code and date of dispensing [22]. In Sweden, prescription drugs are provided free of charge above a specified high-cost threshold (SEK 2200 in 2014) [23]. Women were linked to the prescribed drug register to identify prescription medications dispensed in the 3 months before pregnancy until 6 months after pregnancy. No information is available on i.v. infusions or medications obtained over the counter.
Timing
Women could have multiple dispensings for the same medication within a pregnancy. The timing of dispensing of medication was classified relative to the estimated date of the LMP and the delivery date. We defined medication prevalence as the proportion of pregnancies with at least one dispensing date for medications of interest during each of the following periods of interest: pregnancy, LMP date until the day before the delivery date; pre-pregnancy, 94 days before LMP date until the day before the LMP date; first trimester, LMP date until 93 days after the LMP date; second trimester, 94 days after the LMP date until 187 days after the LMP date; third trimester, 188 days after the LMP date until the day before the delivery date; first postpartum, delivery date until 93 days after the delivery date; and second postpartum, 94 days after the delivery date until 187 days after the delivery date.
SLE-related medications
We reported the prevalence of medications used to treat SLE across pregnancy periods among SLE pregnancies according to medication name and class (i.e. DMARDs, systemic CSs and NSAIDs, with aspirin reported separately). In addition to more commonly prescribed DMARDs in SLE, such as AZA, MTX and HCQ, we searched for less commonly prescribed DMARDs, such as LEF and SSZ (see supplementary material Table S1, available at Rheumatology Online, for the complete list). We stratified the four classes by term and preterm deliveries (<37 weeks gestation). The working group on anti-rheumatic drugs during pregnancy and lactation at the Fourth International Conference on Sex Hormones, Pregnancy and the Rheumatic Diseases published a recommendation in 2006 to continue HCQ during pregnancy [24]. Therefore, we conducted a secondary analysis to determine whether the prevalence of HCQ increased after the recommendation. Specifically, we stratified the prevalence of HCQ and prednisolone, separately, dispensed during pregnancy by early (2006–07) and late (2008–12) study years. Prednisolone served as a control medication, and we expected the prevalence of this medication to be similar in early and late study years. We compared prevalence estimates in early and late years using χ2 tests.
Most commonly used medications among SLE pregnancies
We identified medications or vitamins/supplements that were dispensed during pregnancy to at least 5% of pregnancies with SLE using fifth level ATC classification codes; the fifth level identifies the chemical substance [25]. We calculated the prevalence of these treatments among SLE pregnancies and separately among pregnancies from the general population. Then we used generalized estimating equations to calculate prevalence ratios and 95% CIs accounting for dependence among women with more than one observed pregnancy [26]. In SLE pregnancies, we stratified prevalence estimates by pregnancy periods. Finally, we identified medication or vitamins/supplement groups that were dispensed during pregnancy to ⩾5% of SLE pregnancies using the fourth level of ATC codes; the fourth level identifies the chemical/pharmacological/therapeutic subgroups [25]. We reported prevalence estimates for fourth level groups that did not have complete overlap with treatments identified from the fifth level codes.
This project was approved by the Ethical Review Board of Karolinska Institute (PROTOKOLL 2011/1:7) on 20 July 2011 and declared exempt by Stanford University and the University of California, San Diego’s Institutional Review Board. Results with five or fewer individuals were suppressed.
Results
Cohort characteristics
We identified 483 pregnancies from 391 women with prevalent SLE and 5723 pregnancies from 4322 women without SLE. There were nine women diagnosed with SLE before the age of 16 years. SLE pregnancies had shorter gestational duration on average than non-SLE pregnancies (Table 1). Co-morbidities, including asthma, hypertension, diabetes and mood disorders, were more common among women with SLE than without SLE. Between 5 and 15% of SLE pregnancies also had a diagnostic code related to APS.
Table 1.
Maternal and pregnancy characteristics in women with and without SLE
| Maternal and pregnancy characteristics | With SLE, n = 483 | Without SLE, n = 5723 |
|---|---|---|
| Age, mean (s.d.), years | 31.7 (4.7) | 31.6 (4.9) |
| BMI, mean (s.d.), kg/m2 | 24.2 (4.1) | 24.7 (4.6) |
| Gestational weeks at delivery, mean (s.d.) | 37.8 (3.3) | 39.3 (1.9) |
| Parity, n (%) | ||
| 1 | 226 (46.8) | 2358 (41.2) |
| 2 | 180 (37.3) | 2178 (38.1) |
| 3 | 54 (11.2) | 834 (14.6) |
| 4 or more | 23 (4.8) | 353 (6.2) |
| Multiple gestation, n (%) | 9 (1.9) | 93 (1.6) |
| Asthma, n (%) | 25 (5.2) | 193 (3.4) |
| Hypertension, n (%) | 29 (6.0) | 15 (0.3) |
| Type I or type II diabetes, n (%) | 9 (1.9) | 37 (0.6) |
| Mood disorder, n (%) | 38 (7.9) | 325 (5.7) |
| APS, strict definition, n (%) | 25 (5.2) | 0 (0) |
| APS, broad definition, n (%) | 71 (14.7) | 36 (0.6) |
SLE-related medications
In SLE pregnancies, 49.3% had one or more dispensing for DMARDs during pregnancy, 48.0% for CSs, 40.8% for aspirin and 6.0% for other NSAIDs. The prevalence of these medications varied by pregnancy period (Table 2). The highest prevalence estimates were observed in the postpartum periods with the exception of aspirin, which was highest in the first and second trimesters. HCQ was the most prevalent DMARD during pregnancy (36.4%), followed by AZA (20.7%). The prevalence estimate for HCQ was highest in the first trimester and during the second postpartum period, whereas the prevalence estimate for AZA prevalence was highest in the first and second trimesters. There were no prescribed drug register-registered biologic DMARDs dispensed during pregnancy. MTX dispensings during pregnancy were rare and occurred in five or fewer individuals. There were no dispensings for mycophenolic acid during pregnancy in this cohort. Prednisolone was the most prevalent CS during pregnancy (46.2%). The prevalence of HCQ during pregnancy in 2008–12 was higher than in 2006–07 (39.1 vs 23.8%, P < 0.01), whereas the prevalence of prednisolone was similar in both time periods (46.4 vs 45.2%, P = 0.95).
Table 2.
SLE-related medication dispensing prevalence by pregnancy period in women with SLE
| Medication group Medication name | During pregnancya | Pre-pregnancy | Trimester 1 | Trimester 2 | Trimester 3 | Postpartum 1 | Postpartum 2 |
|---|---|---|---|---|---|---|---|
| (%) | (%) | (%) | (%) | (%) | (%) | (%) | |
| DMARDs | 49.3 | 35.4 | 38.9 | 36.7 | 28.6 | 37.1 | 40.4 |
| HCQ | 36.4 | 23.4 | 28.0 | 25.5 | 20.1 | 25.9 | 30.0 |
| AZA | 20.7 | 14.3 | 16.6 | 16.6 | 11.6 | 14.3 | 11.2 |
| Ciclosporin | 1.9 | 1.9 | 1.5 | 1.7 | 1.2 | 1.5 | 1.2 |
| Chloroquine | 1.7 | 2.1 | NA | NA | NA | 1.5 | 1.2 |
| Other DMARDb | NA | 1.7 | NA | NA | NA | 1.7 | 4.4 |
| CSs | 48.0 | 29.8 | 31.5 | 35.8 | 32.5 | 40.0 | 34.2 |
| Prednisolone | 46.2 | 28.2 | 30.4 | 34.4 | 30.6 | 38.1 | 33.1 |
| Betamethasone | 1.7 | 1.5 | NA | NA | 1.2 | 2.1 | NA |
| Other CSc | 1.5 | NA | NA | NA | NA | 1.2 | NA |
| Aspirin | 40.8 | 6.4 | 28.2 | 32.1 | 17.2 | 8.7 | 6.2 |
| Other NSAIDs | 6.0 | 8.5 | 5.0 | NA | NA | 11.0 | 8.1 |
| Diclofenac | 1.9 | 2.7 | NA | NA | NA | 6.8 | 2.5 |
| Naproxen | 1.7 | 2.1 | 1.7 | NA | 0 | 1.2 | 2.7 |
| NSAIDs excluding aspirin, diclofenac and naproxend | 2.5 | 4.4 | 2.3 | NA | 0 | 3.1 | 3.3 |
During pregnancy includes trimesters 1, 2 and 3.
Includes the following medications with n ≤ 5 during pregnancy: SSZ, mycophenolic acid, etanercept and MTX.
Includes the following medications with n ≤ 5 during pregnancy: methylprednisolone, prednisone and dexamethasone.
Includes the following medications with n ≤ 5 during pregnancy: ibuprofen, ketoprofen, dexibuprofen, celecoxib, etoricoxib and nabumetone. NA: there are five or fewer individuals.
In SLE pregnancies, 28% had no DMARD and no CS dispensings during pregnancy. When considering the three major SLE treatments, that is, HCQ, AZA and prednisolone, 14% had dispensings for HCQ only during pregnancy, 2% had dispensings for AZA only, 17% had dispensings for prednisolone only and 32% had dispensings for at least two of these treatments.
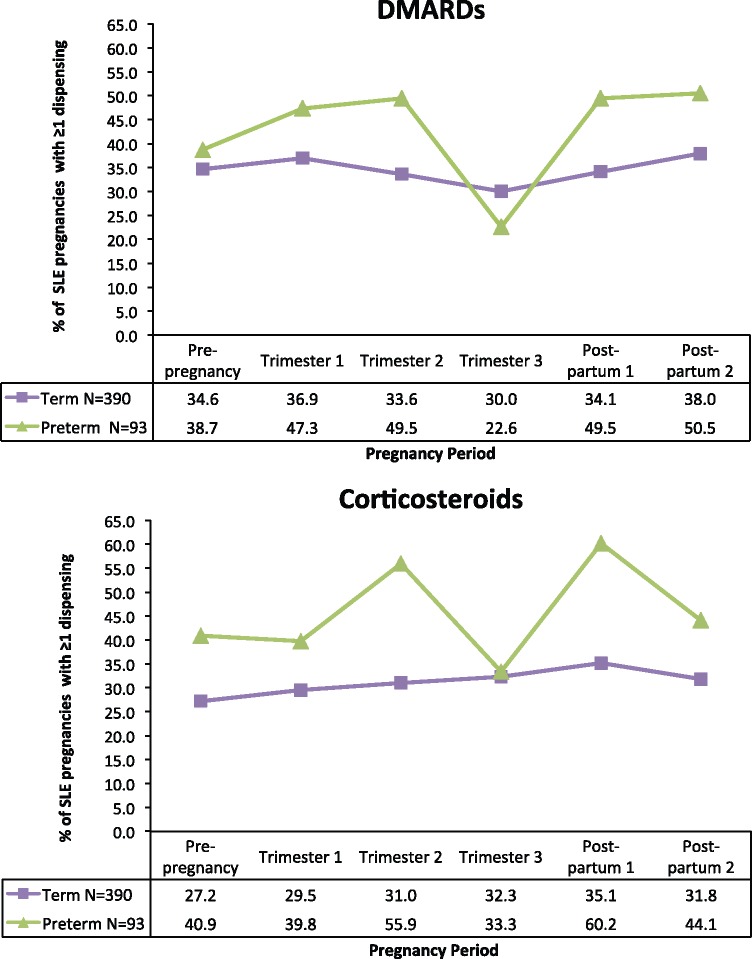
Term pregnancies had a mean gestational length of 275.8 days (s.d. 9.1) or 39 completed weeks, and preterm pregnancies had a mean gestational length of 231.5 days (s.d. 28.4) or 33 completed weeks. DMARD and CS pregnancy period-specific prevalence estimates stratified by preterm birth status are presented for SLE pregnancies in Fig. 1. Aspirin prevalence is not presented in the figure because prevalence estimates did not vary greatly between term and preterm deliveries. Other NSAID prevalence is not presented because some results had fewer than five individuals. Compared with term deliveries, DMARD prevalence was higher in the first (47.3 vs 36.9%) and second (49.5 vs 33.6%) trimesters in preterm deliveries. In the third trimester, DMARD prevalence for preterm deliveries dipped below that of term deliveries (22.6 vs 30.0%). Postpartum DMARD prevalence rebounded to ∼50% for preterm deliveries. The pattern observed for CSs was similar to that for DMARDs.
Fig. 1.
Proportion of SLE pregnancies with one or more dispensing for DMARDs or CSs, by pregnancy period
Most common medications
The prevalence of common medications among SLE pregnancies was 1.2- to 21-fold higher than among non-SLE pregnancies (Table 3); for example, dalteparin (20.9 vs 1.0%), paracetamol (18.2 vs 2.9%), levothyroxine (15.9% vs 4.9%), phenoxymethylpenicillin (also known as penicillin V; 14.3 vs 11.6%), pivmecillinam (10.8 vs 4.7%) and omeprazole (10.4 vs 2.3%). Supplements dispensed at the pharmacy, including calcium, folic acid, ferrous sulphate and cyanocabalmin, were 4- to 33-fold higher than among non-SLE pregnancies.
Table 3.
Non SLE-related medicationsa dispensed to at least 5% of SLE pregnancies, by SLE status
| Medication group | SLE, n = 483 | Non-SLE, n = 5723 | |
|---|---|---|---|
| Medication name | n (%) | n (%) | PRb (95% CIc) |
| Supplements | |||
| Calcium, combinations with vitamin D or other drugs | 106 (22.0) | 38 (0.66) | 33.05 (22.22, 49.16) |
| Folic acid | 53 (11.0) | 134 (2.3) | 4.69 (3.39, 6.48) |
| Ferrous sulphate | 34 (7.0) | 48 (0.84) | 8.39 (5.39, 13.08) |
| Cyanocabalamin | 30 (6.2) | 90 (1.6) | 3.95 (2.61, 5.97) |
| Low-molecular weight heparins | |||
| Dalteparin | 101 (20.9) | 57 (1.0) | 21.00 (14.91, 29.57) |
| Tinzaparin | 41 (8.5) | 15 (0.26) | 32.39 (16.73, 62.71) |
| Antibiotics | |||
| Phenoxymethylpenicillin (penicillin V) | 69 (14.3) | 664 (11.6) | 1.23 (0.97, 1.57) |
| Pivmecillinam | 52 (10.8) | 268 (4.7) | 2.30 (1.70, 3.11) |
| Nitrofurantoin | 35 (7.3) | 272 (4.8) | 1.52 (1.08, 2.16) |
| Nasal or cough and cold preparations | |||
| Mucolytic combinations | 31 (6.4) | 218 (3.8) | 1.68 (1.15, 2.47) |
| Opium derivatives and expectorants | 36 (7.5) | 249 (4.4) | 1.71 (1.21, 2.43) |
| Phenylpropanolamine | 26 (5.4) | 235 (4.1) | 1.31 (0.89, 1.94) |
| Other medications | |||
| Paracetamol | 88 (18.2) | 167 (2.9) | 6.24 (4.81, 8.11) |
| Levothyroxine sodium | 77 (15.9) | 282 (4.9) | 3.24 (2.50, 4.19) |
| Omeprazole | 50 (10.4) | 129 (2.3) | 4.59 (3.27, 6.45) |
| Codeine, combinations excluding psycholeptics | 35 (7.3) | 132 (2.3) | 3.14 (2.18, 4.52) |
| Promethazine | 34 (7.0) | 288 (5.0) | 1.40 (0.97, 2.03) |
| Clemastine | 33 (6.8) | 276 (4.8) | 1.42 (0.98, 2.06) |
| Carbamide | 27 (5.6) | 67 (1.2) | 4.77 (2.97, 7.68) |
Prevalence ratio (PR) and 95% CI.
Most common medications are those with a prevalence ≥5% during pregnancy.
Reference = SLE pregnancies.
95% CIs account for multiple pregnancies per woman.
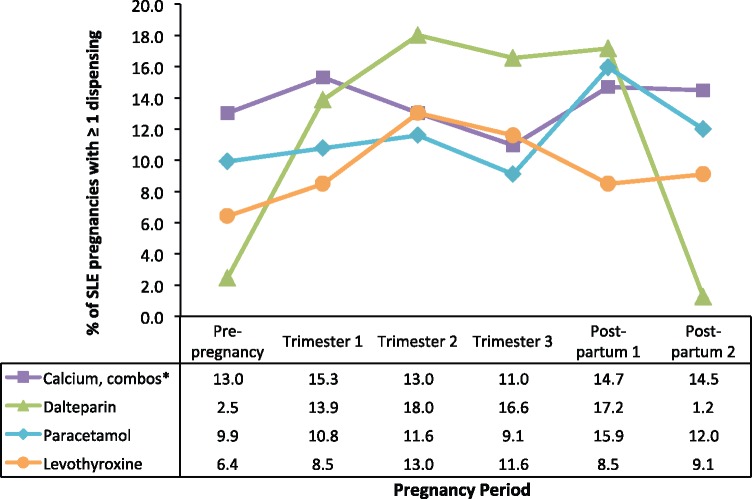
The prevalence estimates for medications dispensed to at least 15% of SLE pregnancies are plotted in Fig. 2 according to pregnancy period (see supplementary material Table S2, available at Rheumatology Online, for all commonly used medications). Dalteparin had the greatest change; from a high of 18.0% in the second trimester to a low of 1.2% in the second postpartum period. Levothyroxine prevalence was highest in the second and third trimesters.
Fig. 2.
Non-SLE-related medications dispensed to at least 15% of SLE patients, by pregnancy period
*Calcium, combinations with vitamin D or other drugs.
Groups with fourth level ATC codes that did not directly overlap with fifth level ATC codes included the following: heparin group (27.5% SLE vs 1.2% non-SLE); penicillins with extended spectrum (13.7 vs 7.1%); proton pump inhibitors (12.0 vs 2.6%); iron bivalent, oral preparations (10.4 vs 1.5%); phenothiazine derivatives (9.1 vs 7.7%), for example, promethazine; natural opium alkaloids (8.1 vs 2.4%), for example, codeine; aminoalkyl ethers (6.8 vs 4.9%), for example, clemastine; mucolytics (6.8 vs 4.1%), for example, acetylcysteine; and caries prophylactic agents (5.4 vs 1.6%), for example, sodium fluoride.
Discussion
This descriptive population-based study demonstrates that pregnant women with SLE are a highly medicated group. In nearly half of SLE pregnancies, women were dispensed DMARDs and CSs. Compared with term SLE pregnancies, SLE pregnancies with preterm delivery had higher prevalence estimates for CSs across each pregnancy period. Postpartum CS prevalence was particularly high for pregnancies with preterm delivery in the 90 days postpartum; three out of five were dispensed a CS. Women with preterm births may have had more severe disease, and the increase in CS prevalence during the postpartum period for women with preterm births may reflect the need to treat disease flares. In two out of five SLE pregnancies, women were dispensed aspirin, primarily during the first and second trimesters. There were major differences in prevalence estimates between commonly used medications and supplements in SLE pregnancies vs non-SLE pregnancies.
HCQ prevalence during pregnancy was higher in this study than in several previous reports [8, 11, 13, 14, 27]. The previous studies included several years prior to the 2006 recommendation that endorsed continuation of HCQ treatment during pregnancy and, consequently, time trends could contribute to the discrepancies. In the present study, HCQ prevalence was higher in later study years. Compared with previous studies, CS prevalence during pregnancy in Sweden was lower than reports from single hospital cohorts in Asia, Saudi Arabia and Argentina (71–89%) [6, 8, 12, 14, 27] and was similar to reports from hospital cohorts in the USA and Denmark (36–48%) [11, 13]. Aspirin prevalence varied greatly across previous studies of SLE pregnancies (9–60%) [6–8, 11, 13, 14], and the estimate in this study was similar to that from the hospital-based cohort in Denmark (39%) [11]. In previous studies, anti-hypertensives, as a broad therapeutic class, have been reported to have a relatively high prevalence (13–29%) [6, 11, 13]. In this cohort of pregnancies, anti-hypertensive medications, at the fourth or fifth ATC level, had prevalence estimates of < 5% during pregnancy. MTX and mycophenolic acid are teratogenic exposures and are contraindicated during pregnancy [28]. Only rarely was a MTX dispensing during pregnancy observed in this cohort.
This study provides population-based snapshots of medications dispensed across the antenatal and postpartum periods in SLE pregnancies. Pregnancies with preterm delivery had an average gestational length that was 44 days shorter than term pregnancies. The shortened opportunity to obtain prescriptions contributed to the decreased prevalence of DMARDs and CSs that was observed among preterm deliveries only in the third trimester. If the majority of individuals were given a 30 day supply for all prescriptions, we may have not observed as large a decrease in the final trimester. Overall, DMARD prevalence estimates increased from pre-pregnancy to the first trimester, decreased in the third trimester and increased postpartum, and CS prevalence estimates increased from pre-pregnancy to the first postpartum period, with the exception of a dip during the third trimester.
Fifteen of the commonly dispensed medications that were identified among SLE pregnancies had prevalence estimates that were at least 50% higher among SLE pregnancies vs non-SLE pregnancies and many reflect treatment for conditions or symptoms that co-occur with SLE. Dalteparin and tinzaparin were among the most commonly dispensed medications. These low-molecular weight heparins, among others, are indicated for women with aPL and a history of obstetric complications [29]. The prevalence of levothyroxine sodium during pregnancy was 3.2-fold higher among SLE pregnancies compared with general population pregnancies, which is consistent with the higher prevalence of hypothyroidism among women with SLE [30]. Omeprazole prevalence was 4.6-fold higher during pregnancy among SLE pregnancies compared with non-SLE pregnancies, which is consistent with heartburn being a common symptom among individuals with SLE [31]. Prevalence estimates for penicillin antibiotics, that is, phenoxymethylpenicillin and pivmecillinam, and nitrofurantoin were higher among SLE pregnancies compared with pregnancies from the general population, which reflects the increased susceptibility of SLE patients to infection owing to both abnormal immunological response and immunosuppressive treatments [32]. Paracetamol prevalence was 6.2-fold higher among SLE pregnancies and codeine prevalence 3.1-fold higher among SLE pregnancies compared with non-SLE pregnancies. The prevalence estimates for several supplements (calcium combinations, folic acid, ferrous sulphate and cyanocobalamin) were much higher in the SLE population than the in non-SLE population. Women with SLE are at increased risk for osteoporosis, and calcium with vitamin D is recommended for women treated with heparin during pregnancy [33, 34]. Furthermore, a high prevalence of anaemia and decreased serum B12 levels have been observed among non-pregnant individuals with SLE [35]. It is possible that women with SLE are more likely to have reached the annual high-cost threshold [23] and receive all of their prescriptions free compared with women without SLE. Women who meet the high-cost threshold have an incentive to obtain over-the-counter medications as prescriptions. Consequently, the observed imbalance in medications that are also available over the counter, for example, paracetemol and supplements, between women with SLE and women without SLE could be attributed in part to women with SLE obtaining these medications by prescription.
Pharmaceutical dispensing data are useful to understand not only what physicians prescribed during pregnancy but also what prescriptions patients filled. Compared with prescribing information, dispensing information is more similar to real use. The date of dispensing does not necessarily mean that the drug was taken on the day when it was dispensed, or at all, but it can be used as a proxy for exposure. For some drugs this may be more accurate than for others. For example, one study found that agreement between self-reported and dispensed immunosuppressant therapy was high, but for CSs the agreement was low [5]. Furthermore, the data in the present study do not include infusions, such as some biological DMARDs, nor the number of days of medication supplied for each dispensing.
Besides the potential for exposure misclassification, this study has some additional limitations to consider. First, there could be misclassification of SLE because of our reliance on ICD codes to identify SLE, although this is likely to be minimal considering the findings from previous studies [18, 19]. Second, there may be measurement error of the pregnancy time windows because the windows are based on the estimated date of the LMP. However, for the majority of pregnancies, the date of the LMP was estimated using ultrasound. Third, medications that cause fetal harm may be underestimated because pregnancies ending in spontaneous abortions and terminations are not included.
To our knowledge, this is the first population-based study to describe the prevalence of medications used to treat SLE before, during and after pregnancy. Our study is also novel because it identifies the most common medications in SLE pregnancies, not only the medications that are used to treat SLE. As such, it provides practitioners with a robust picture of medication use during pregnancy among SLE patients, beyond the medications they typically prescribe.
Moreover, our study provides perspective by contrasting prevalence estimates with non-SLE pregnancies. A major strength of our study is that data were collected prospectively throughout pregnancy and avoids recall problems. We anticipate that our results are generalizable to most other populations because some medications used to manage SLE in the absence of pregnancy are contraindicated, leaving few treatment options in pregnancy. In future research, the population-based approach to study medication use among pregnant women with SLE should be implemented in other counties.
Pregnant women with SLE are commonly dispensed medications. This includes not only DMARDs and CSs to treat and/or prevent flares, but numerous other medications to treat co-morbidities associated with SLE. For clinicians, it is crucial to consider the risks and benefits of all medications used in SLE pregnancies. For researchers, medications that are commonly used among women with SLE should be accounted for when studying associations between SLE-related medications and pregnancy outcomes. Research regarding the benefits and risks of these commonly used medications and their combinations on SLE pregnancies, breast milk and long-term outcomes for offspring is needed. We plan to study the associations between medication exposures and pregnancy outcomes within this cohort.
Supplementary data
Supplementary data are available at Rheumatology Online.
Supplementary Material
Acknowledgements
K.P. is supported by a career development award from the Eunice Kennedy Shriver National Institute of Child Health & Human Development, National Institutes of Health [K99HD082412]. J.F.S. is partly supported by the Strategic Research Program in Epidemiology at Karolinska Institutet.
Funding: This work was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health [K01-AR06687801].
Disclosure statement: C.D.C. receives research funding unrelated to this study from the following industry sponsors: AbbVie, Amgen Inc., Apotex, Barr Laboratories Inc., Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Janssen Pharmaceuticals, Kali Laboratories Inc., Pfizer Inc., Hoffman La Roche-Genentech, Sandoz Pharmaceuticals, Genzyme Sanofi-Aventis, Seqirus, Takeda Pharmaceutical Company Limited, Teva Pharmaceutical Industries Ltd and UCB, Inc. All other authors have declared no conflicts of interest.
References
- 1. Siegel M, Lee SL.. The epidemiology of systemic lupus erythematosus. Semin Arthritis Rheum 1973;3:1–54. [DOI] [PubMed] [Google Scholar]
- 2. Clowse ME. Lupus activity in pregnancy. Rheum Dis Clin North Am 2007;33:237–52, v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Clowse ME, Jamison M, Myers E, James AH.. A national study of the complications of lupus in pregnancy. Am J Obstet Gynecol 2008;199:127.e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Exel E, Jacobs J, Korswagen LA. et al. Depression in systemic lupus erythematosus, dependent on or independent of severity of disease. Lupus 2013;22:1462–9. [DOI] [PubMed] [Google Scholar]
- 5. Stephansson O, Granath F, Svensson T. et al. Drug use during pregnancy in Sweden – assessed by the Prescribed Drug Register and the Medical Birth Register. Clin Epidemiol 2011;3:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aggarwal N, Raveendran A, Suri V. et al. Pregnancy outcome in systemic lupus erythematosus: Asia’s largest single centre study. Arch Gynecol Obstet 2011;284:281–5. [DOI] [PubMed] [Google Scholar]
- 7. Imbasciati E, Tincani A, Gregorini G. et al. Pregnancy in women with pre-existing lupus nephritis: predictors of fetal and maternal outcome. Nephrol Dial Transplant 2009;24:519–25. [DOI] [PubMed] [Google Scholar]
- 8. Cavallasca JA, Laborde HA, Ruda-Vega H, Nasswetter GG.. Maternal and fetal outcomes of 72 pregnancies in Argentine patients with systemic lupus erythematosus (SLE). Clin Rheumatol 2008;27:41–6. [DOI] [PubMed] [Google Scholar]
- 9. Clowse ME, Magder L, Witter F, Petri M.. Hydroxychloroquine in lupus pregnancy. Arthritis Rheum 2006;54:3640–7. [DOI] [PubMed] [Google Scholar]
- 10. Georgiou PE, Politi EN, Katsimbri P, Sakka V, Drosos AA.. Outcome of lupus pregnancy: a controlled study. Rheumatology 2000;39:1014–9. [DOI] [PubMed] [Google Scholar]
- 11. Jakobsen IM, Helmig RB, Stengaard-Pedersen K.. Maternal and foetal outcomes in pregnant systemic lupus erythematosus patients: an incident cohort from a stable referral population followed during 1990–2010. Scand J Rheumatol 2015;44:377–84. [DOI] [PubMed] [Google Scholar]
- 12. Teh CL, Wong JS, Ngeh NK, Loh WL.. Systemic lupus erythematosus pregnancies: the Sarawak experience and review of lupus pregnancies in Asia. Rheumatol Int 2011;31:1153–7. [DOI] [PubMed] [Google Scholar]
- 13. Chakravarty EF, Colón I, Langen ES. et al. Factors that predict prematurity and preeclampsia in pregnancies that are complicated by systemic lupus erythematosus. Am J Obstet Gynecol 2005;192:1897–904. [DOI] [PubMed] [Google Scholar]
- 14. Koh JH, Ko HS, Kwok SK, Ju JH, Park SH.. Hydroxychloroquine and pregnancy on lupus flares in Korean patients with systemic lupus erythematosus. Lupus 2015;24:210–7. [DOI] [PubMed] [Google Scholar]
- 15. Buyon JP, Kim MY, Guerra MM. et al. Predictors of pregnancy outcomes in patients with lupus: a cohort study. Ann Intern Med 2015;163:153–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Desai RJ, Huybrechts KF, Bateman BT. et al. Brief report: Patterns and secular trends in use of immunomodulatory agents during pregnancy in women with rheumatologic conditions. Arthritis Rheumatol 2016;68:1183–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cnattingius S, Ericson A, Gunnarskog J, Källén B.. A quality study of a medical birth registry. Scand J Soc Med 1990;18:143–8. [DOI] [PubMed] [Google Scholar]
- 18. Arkema EV, Jönsen A, Rönnblom L. et al. Case definitions in Swedish register data to identify systemic lupus erythematosus. BMJ Open 2016;6:e007769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Simard JF, Sjöwall C, Rönnblom L, Jönsen A, Svenungsson E.. Systemic lupus erythematosus prevalence in Sweden in 2010: what do national registers say? Arthritis Care Res 2014;66:1710–7. [DOI] [PubMed] [Google Scholar]
- 20. Arkema EV, Simard JF.. Cohort profile: systemic lupus erythematosus in Sweden: the Swedish Lupus Linkage (SLINK) cohort. BMJ Open 2015;5:e008259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Høgberg U, Larsson N.. Early dating by ultrasound and perinatal outcome. A cohort study. Acta Obstet Gynecol Scand 1997;76:907–12. [DOI] [PubMed] [Google Scholar]
- 22. Wettermark B, Hammar N, Fored CM. et al. The new Swedish Prescribed Drug Register—opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf 2007;16:726–35. [DOI] [PubMed] [Google Scholar]
- 23. TLV. What is the high cost threshold? How it works. http://www.tlv.se/In-English/medicines-new/the-swedish-high-cost-threshold/how-it-works/ (2 October 2015, date last accessed).
- 24. Østensen M, Khamashta M, Lockshin M. et al. Anti-inflammatory and immunosuppressive drugs and reproduction. Arthritis Res Ther 2006;8:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. ATC Structure and principles. http://www.whocc.no/atc/structure_and_principles/. (15 September 2015, date last accessed).
- 26. Zou GY, Donner A.. Extension of the modified Poisson regression model to prospective studies with correlated binary data. Stat Methods Med Res 2013; 22:661–70. [DOI] [PubMed] [Google Scholar]
- 27. Al Arfaj AS, Khalil N.. Pregnancy outcome in 396 pregnancies in patients with SLE in Saudi Arabia. Lupus 2010;19:1665–73. [DOI] [PubMed] [Google Scholar]
- 28. Običan S, Scialli AR.. Teratogenic exposures. Am J Med Genet C Semin Med Genet 2011;157C:150–69. [DOI] [PubMed] [Google Scholar]
- 29. Baer AN, Witter FR, Petri M.. Lupus and pregnancy. Obstet Gynecol Surv 2011;66:639–53. [DOI] [PubMed] [Google Scholar]
- 30. Antonelli A, Fallahi P, Mosca M. et al. Prevalence of thyroid dysfunctions in systemic lupus erythematosus. Metabolism 2010;59:896–900. [DOI] [PubMed] [Google Scholar]
- 31. Ebert EC, Hagspiel KD.. Gastrointestinal and hepatic manifestations of systemic lupus erythematosus. J Clin Gastroenterol 2011;45:436–41. [DOI] [PubMed] [Google Scholar]
- 32. Sciascia S, Cuadrado MJ, Karim MY.. Management of infection in systemic lupus erythematosus. Best Pract Res Clin Rheumatol 2013;27:377–89. [DOI] [PubMed] [Google Scholar]
- 33. Ruiz-Irastorza G, Khamashta MA, Hughes GR.. Heparin and osteoporosis during pregnancy: 2002 update. Lupus 2002;11:680–2. [DOI] [PubMed] [Google Scholar]
- 34. Di Munno O, Mazzantini M, Delle Sedie A, Mosca M, Bombardieri S.. Risk factors for osteoporosis in female patients with systemic lupus erythematosus. Lupus 2004;13:724–30. [DOI] [PubMed] [Google Scholar]
- 35. Segal R, Baumoehl Y, Elkayam O. et al. Anemia, serum vitamin B12, and folic acid in patients with rheumatoid arthritis, psoriatic arthritis, and systemic lupus erythematosus. Rheumatol Int 2004;24:14–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.