Rheumatology key message
Of UK SLE patients with disease requiring biologic therapy, 13% are eligible for belimumab.
Sir, Belimumab, an anti-B lymphocyte stimulator mAb, has proven efficacy for the treatment of SLE [1, 2]. The European licence is based on post hoc analysis of randomized trials showing that predictors of better response include elevated antibodies to dsDNA, low complement and higher Safety of Estrogens in Lupus Erythematosus National Assessment - Systemic Lupus Erythematosus Disease Activity Index (SELENA-SLEDAI) scores [3, 4]. Patients with severe active LN or CNS lupus were excluded from these trials and do not form part of the patient population assessed as part of the marketing authorization [1, 2]. In June 2016, The National Institute for Health and Care Excellence recommended the use of belimumab as add-on therapy for patients with active auto-antibody-positive SLE, who have serological activity (defined as positive anti-dsDNA and low complement) and a SELENA-SLEDAI score ⩾10, despite standard treatment [5].
Since 2010, patients commencing biologic therapy for SLE in the UK have been registered in the BILAG-BR, the initial results of which are presented in a paper currently under review (McCarthy et al., manuscript under review). We sought to investigate the number of patients and the clinical characteristics of patients treated with a biologic in the BILAG-BR who would potentially have been eligible for belimumab using this guidance [5].
Of the 270 patients registered for biologic use to November 2015, 82 (33%) had evidence of both low complement and elevated anti-dsDNA antibodies at enrolment. Of these, 46 (56.1%) patients had a BILAG A in the renal (n = 29) or neuropsychiatric system (n = 17), making them ineligible for therapy. An additional four (4.9%) had a SLEDAI score <10. Thus, from 2010 to 2015, 32 patients (13%) enrolled in the BILAG-BR would have been eligible for belimumab.
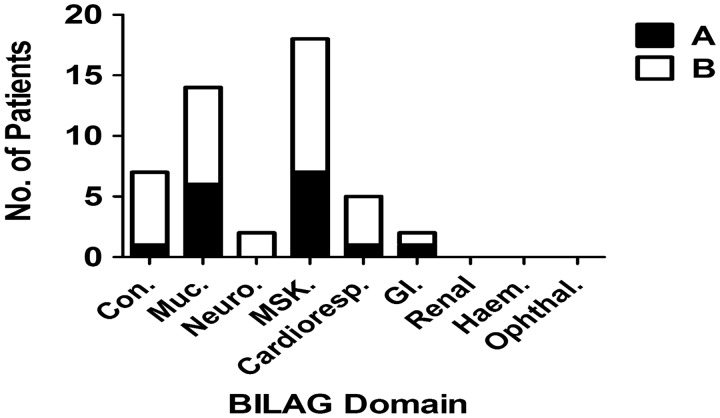
Amongst these 32 patients, the BILAG mucocutaneous (MUC) and musculoskeletal (MSK) systems had the most frequent A (MSK = 7, MUC = 6) and B scores (MSK = 11, MUC = 8; Fig. 1). Seventeen (53%) patients had a history of renal disease. The median [interquartile range (IQR)] baseline SLEDAI was 12.5 (12–15.75).
Fig. 1.
BILAG-2004 organ systems with active disease in SLE patients eligible for belimumab
Number of individual patients scoring either an A or B on BILAG-2004 scoring system across the systems assessed.
Regarding medication use, 28 (87.5%) and 27 (84.4%) patients were on an anti-malarial or oral prednisolone, respectively. The median (IQR) baseline prednisolone dose was 15 mg (10–20 mg). The median (IQR) number of prior standard immunosupressant agents was 2 (1–3). MMF was the most frequently prescribed therapy (n = 23), followed by AZA (n = 15) and CYC (n = 11).
When we assessed response to Rituximab (RTX) in this cohort who would now be eligible for belimumab, the median (IQR) SLEDAI improved from 12.5 (12–15.75) at baseline to 4 (0–8) at 6 months (P < 0.0001). The total number of BILAG A scores reduced from 16 to 2 and B scores from 33 to 9. A corresponding reduction in CS dose was also noted from 15 mg (10–20 mg) to 6 mg (5–10 mg) at 6 months (P < 0.001).
Improved access to biologic therapies will enhance physicians’ ability to control disease activity while facilitating CS tapering and preventing damage [6]. Given that the response rate to most biologic therapies in SLE is ∼50%, the addition of belimumab to UK physicians’ armamentarium is to be welcomed, especially for those patients who have not responded to conventional therapy. Our data will help inform clinicians and planners about the expected rates of usage and the clinical characteristics of patients requiring belimumab in the UK. MUC and MSK were the systems most likely to have active disease requiring belimumab. A history of renal involvement was, however, noted in ∼50% of cases, emphasizing that previous renal involvement does not exclude patients from belimumab; indeed, both the Belimumab in Subjects With Systemic Lupus Erythematosus (BLISS)-52 and 76 trials included patients with active renal disease, and a post hoc analysis suggested favourable renal outcomes in this population [7]. Our data also suggest that RTX remains a realistic therapeutic option for patients who fail to respond to belimumab.
In summary, between 2010 and 2015, 13% of patients who commenced biologic therapy for SLE in the UK would have been eligible for belimumab. Access to such treatment offers the potential of improved disease control, CS dose reduction and improved long-term outcomes for these patients.
Acknowledgements
BILAG-BR collaborators—to be indexed by The National Library of Medicine: Patrick Gordon, Department of Rheumatology, King’s College Hospital, London, UK; Steven Young-Min, Portsmouth Hospitals National Health Service (NHS) Trust, Portsmouth, UK; Robert Stevens, Department of Rheumatology, Doncaster and Bassetlaw Hospitals NHS Foundation Trust, Doncaster, UK; Athiveer Prabu, Worcestershire Acute Hospitals NHS Trust and Sandwell and West Birmingham Hospitals NHS Trust; Mike Batley, Maidstone and Tunbridge Wells NHS Trust, UK; Nagui Gendi, Basildon and Thurrock University Hospitals NHS Trust, Basildon, UK; Bhaskar Dasgupta, Southend University Hospital, Westcliff-on-Sea, Essex, UK; Munther Khamashta, St Thomas’ Hospital, London, UK; Peter Hewins, Queen Elizabeth Hospital, Birmingham, UK; Richard J. Stratton, Royal Free Hospital, London, UK; Antoni Chan, Royal Berkshire Hospital, Reading, UK; Denise De Lord, Queen Elizabeth Queen Mary Hospital, East Kent, UK; Jon King, Derriford Hospital, Plymouth, UK; Shirish Dubey, University Hospital of Coventry and Warwickshire, UK; Edmond O’Riordan, Salford Royal Foundation Trust, Manchester, UK; Shireen Shaffu, Leicester Royal Infirmary, Leicester, UK; Cathy Laversuch, Musgrove Park Hospital, Taunton, Somerset, UK; Thomas P. Sheeran, Cannock Chase Hospital, Cannock, Staffordshire, UK; Erin Vermaak, Haywood Hospital, Stoke-On-Trent, Staffordshire; Nicola Erb, Dudley Group of Hospitals NHS Foundation Trust, West Midlands, UK; Debasish Pyne, Barts Lupus Centre, Royal London Hospital, London, UK; Rachel Jeffrey, Northampton General Hospital, Northampton, UK; Hazem Youssef, Department of Rheumatology, Aberdeen Royal Infirmary, Aberdeen, UK; Wahab Al-Allaf, New Cross Hospital, Wolverhampton, UK and University of Birmingham, Birmingham, UK; Marian Regan, Royal Derby Hospital, Derby, UK; Arvind Kaul, St George’s University of London, Cranmer Terrace, London, UK.
Dr B.P. is supported by the National Institute for Health Research (NIHR) Manchester Biomedical Research Unit and the NIHR/Wellcome Trust Manchester Clinical Research Facility. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health. Professor I.N.B. is an NIHR Senior Investigator and is funded by Arthritis Research UK, the Medical Research Council, the National Institute for Health Research Manchester Biomedical Research Unit and the NIHR/Wellcome Trust Manchester Clinical Research Facility. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health. The Birmingham lupus cohort has been supported by Lupus UK, Sandwell and West Birmingham Hospitals NHS Trust and the NIHR/Wellcome Trust Birmingham Clinical Research Facility. Professor C.J.E. is supported by the Southampton NIHR Biomedical Research Centre and Southampton NIHR Wellcome Trust Clinical Research Facility. Dr Arvind Kaul has received funding support for the BILAG-BR from The National Institute for Health Research Clinical Research Network (NIHR CRN) South London. Dr Lee-Suan Teh would like to acknowledge Janice Hartley, Research and Development Department, Royal Blackburn Hospital, Blackburn, UK for help with data entry.
The register is funded by restricted income from UK pharmaceutical companies, presently Roche and GlaxoSmithKline (GSK), through a contract with the University of Manchester. The principal investigators and their team have full academic freedom and are able to work independently of pharmaceutical industry influence. All decisions regarding analyses, interpretation and publication are made autonomously of any industry contribution. The registry has also received funding support from Lupus UK, a registered charity.
Funding: No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this manuscript.
Disclosure statement: I.N.B. has received grant funding from GSK and Roche and has received speaker and consultancy fees from GSK, Roche, Medimmune and AstraZeneca. C.J.E. has received honoraria from Roche and GSK for taking part in advisory boards. D.J. has received research grants from Roche/Genentech. C.-S.Y. has provided consultancy to Bristol-Myers Squibb. D.P.D’C. reports advisory boards, consultancies and research grants from GSK, Roche, Eli Lilly, Aspreva and Bristol-Myers Squibb. E.M.V. has received honoraria from Roche and GSK and research grants paid to his employer from Roche and AstraZeneca. M.A. has received honoraria from Celgene and Astra Zeneca. All other authors have declared no conflicts of interest.
References
- 1. Navarra SV, Guzmán RM, Gallacher AE. et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet 2011;377:721–31. [DOI] [PubMed] [Google Scholar]
- 2. Furie R, Petri M, Zamani O. et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum 2011;63:3918–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van Vollenhoven RF, Petri MA, Cervera R. et al. Belimumab in the treatment of systemic lupus erythematosus: high disease activity predictors of response. Ann Rheum Dis 2012;71:1343–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. http://www.ema.europa.eu/ema/index.jsp%3Fcurl=pages/medicines/human/medicines/002015/human_med_001466.jsp%26mid=WC0b01ac058001d124%26jsenabled=true (August 2011, date last accessed).
- 5. National Institute for Health and Care Excellence. Belimumab for Treating Active Autoantibody-Positive Systemic Lupus Erythematosus. Vol. 2016; 2016. https://www.nice.org.uk/guidance/ta397.
- 6. Bruce IN, Urowitz M, van Vollenhoven R. et al. Long-term organ damage accrual and safety in patients with SLE treated with belimumab plus standard of care. Lupus 2016;25:699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dooley MA, Houssiau F, Aranow C. et al. Effect of belimumab treatment on renal outcomes: results from the phase 3 belimumab clinical trials in patients with SLE. Lupus 2013;22:63–72. [DOI] [PubMed] [Google Scholar]