Abstract
The effectiveness of biologic therapies now means that remission or low disease activity are realistic targets for treatment. However, after achieving remission/low disease activity, the next steps remain unclear. The aim of this publication was to conduct a broad systematic literature review to evaluate dosing down of biologics. After screening papers and abstracts for relevance and application of inclusion/exclusion criteria, a structured extraction process was used to collect information on the included studies. Fifty-two papers were included in the analysis across rheumatic disease. In patients who discontinue therapy, remission is not typically sustained, with reported rates of relapse and flare across early RA (48–54%), established RA (2–84%), axial spondyloarthritis (11–53%) and PsA (44.9%). In many cases, an acceptable disease activity can be regained upon retreatment. More research is needed to understand the long-term impacts of these strategies on efficacy, safety and cost.
Keywords: systematic review, biologic therapy, dose tapering, dosing down, treatment withdrawal, dose spacing, rheumatoid arthritis, axial spondyloarthritis, psoriatic arthritis
Rheumatology key messages
Even in established RA, careful and controlled dose reduction appears possible for some individuals.
Examination of disease state and personal characteristics is needed for determining suitability for dosing down.
Most patients respond well on reintroduction of treatment following flare after dose reduction.
Introduction
Biologic treatment for RA, PsA and axial spondyloarthritis (axial SpA) is now commonplace. International guidelines recommend biologics in patients whose RA or PsA is not adequately controlled by traditional DMARDs [1, 2], or whose axial SpA is not controlled by NSAIDs [3].
The effectiveness of biologic therapies now means that remission or low disease activity (LDA) are realistic targets for treatment for RA [4], PsA and AS [5]. Despite intense debate over definitions, remission rates in RA have doubled in the decade 2000–10 [6]. However, it is unclear whether the dose should be maintained, titrated down or withdrawn. In addition, if dose titration or withdrawal is carried out, how the patient should be monitored for ‘flare’ is debated [7–10].
Most data have been provided by randomized clinical trials (RCTs), although observational studies as well as data from registries are available. A Cochrane review of down-titration and discontinuation strategies of TNF blockers in RA that included seven clinical trials of etanercept and adalimumab concluded that dose reduction of etanercept 50 mg weekly to 25 mg weekly, after at least 3–12 months of LDA, seemed as effective as continuing the standard dose, and discontinuation was inferior to continuation of treatment [11].
The purpose of our review was to evaluate evidence from all published literature in order to provide clinicians with an overview of the practical uses of dose-reduction strategies. We attempted to answer seven core questions through auditing and analysing the literature, as well as to create a research agenda for future projects and studies.
Literature search
Search strategy
We searched PubMed (January 2000 to October 2014), Embase (January 2000 to October 2014), Cochrane Library (January 2000 to October 2014), ACR abstracts (2013–14), EULAR abstracts (2013–14) and International Congress on Spondyloarthropathies abstracts (2014). Due to the nature of the ACR and International Congress on Spondyloarthropathies databases, hand-searching for relevant abstracts was conducted. Searches of the EULAR database were carried out with [all words] in the search tool. Table 1 gives a list of the 26 primary search terms and 21 secondary search terms used. Searches were performed using a combination of a single primary term in conjunction with each secondary term to form combinations of [primary term AND secondary term].
Table 1.
Terms used in literature search
| Primary search terms | Secondary search terms |
|---|---|
| RA | Dose titration |
| Axial spondyloarthritis | Dose reduction |
| AS | Dose de-escalation |
| Non-radiographic axial spondyloarthritis | Dose tapering |
| PsA | Spacing |
| Biologics | Cessation |
| TNF | Stopping |
| TNF | Interval widening |
| Anti-TNF | Dosing down |
| Anti-TNF | Treatment holiday |
| Adalimumab | Dose interval increase |
| Humira | Drug withdrawal |
| Etanercept | Variable dosing |
| Enbrel | Flexible dosing |
| Infliximab | Dose adjustment |
| Remicade | Disease flare |
| Abatacept | Discontinuation |
| Orencia | Stepwise decrease |
| Certolizumab | Remission |
| Certolizumab pegol | Optimization |
| Cimzia | On-demand treatment |
| Golimumab | |
| Simponi | |
| Tocilizumab | |
| Actemra | |
| RoActemra | |
| Rituximab | |
| Rituxan |
We included studies published in English, with primary data on adults with RA, PsA or axial SpA. An additional inclusion criterion was that the study must relate to dose tapering, with this term, or a variation of it, contained within the title or abstract. Exclusion criteria included meeting abstracts without sufficient publicly available details, studies with fewer than five patient cases and studies investigating side-effect profiles.
Screening was performed by one assessor and subsequently refined by an additional assessor. Related citations to relevant topics were also searched. Hits were manually de-duplicated across databases. To be included in the final analysis, articles had to report primary data from studies conducted in adults with RA, PsA or axial SpA; be related to a therapy listed as a primary search term in Table 1; and the issue of biologic tapering had to be mentioned in the title or abstract.
The authors discussed findings of the initial literature search and observed that some key studies were not found as a result of search terms or human error of the hand-searching of databases. In addition, key studies recently published were discussed and included although they fell outside the period of the initial search. Reasons for inclusion included further real-world evidence and biologics that were not identified in the original search, as well as additional studies in those inflammatory diseases that had few studies in the initial reference list.
Data extraction
Two authors independently extracted data from each study using a data extraction form adapted from the Cochrane Systematic Review Extraction Form template. Differences were resolved by discussion and consensus. For the purposes of this systematic review, the definitions in Table 2 were used.
Table 2.
Definitions used in this sysyematic review
| Discontinuation | Complete withdrawal of the biologic |
|---|---|
| Tapering by dose reduction | Maintaining the same frequency of dose, but reducing the quantity of the drug per administration |
| Tapering by injection/ infusion frequency reduction | Maintaining the same quantity of drug per administration, but increasing the time in between injections/infusions |
| Progressive stepwise | Initially tapering by dose reduction or tapering by injection/infusion frequency reduction, and then further tapering again by dose reduction or frequency reduction (i.e. initially 50 mg/7 days then 25 mg/7 days then 25 mg/14 days) |
| Disease activity– driven tapering | The decision is made whether or not to dose-down based on the patient’s disease activity |
| Flare | Considered in the paper as synonymous with relapse or loss of remission/LDA or failure of the tapering strategy |
Risk of bias assessment
RCTs were assessed for methodological quality using the Cochrane Risk of Bias tool [12]. The checklist of Downs and Black was used to assess the risk of bias in observational studies [13]. The risk of bias assessment was conducted by one reviewer. A second reviewer checked the results against the source document and any discrepancies were resolved by discussion and consensus.
Data analysis
We assembled a group of five rheumatologists with an interest of dose optimization to create core questions based on what clinical rheumatologists might commonly ask when considering dosing down a biologic. Having developed seven key questions during face-to-face meetings, we used these to direct a systematic literature review to describe the evidence behind these questions (Table 3).
Table 3.
Seven core questions to be answered
| Question No | Question |
|---|---|
| 1 | Does tapering of biologics occur, and what are the various strategies adopted? |
| 2 | Which disease and patient characteristics are helpful in deciding on a dose-down strategy? |
| 3 | Which therapies can be dosed down, and how should this occur? |
| 4 | How should flare be defined, and what is the risk of relapse? |
| 5 | How should patients be monitored while on tapered doses of biologics? |
| 6 | How should patients be managed long term in terms of retreatment and response? |
| 7 | What are patients’ perspectives regarding tapering of biologics and its various aspects? |
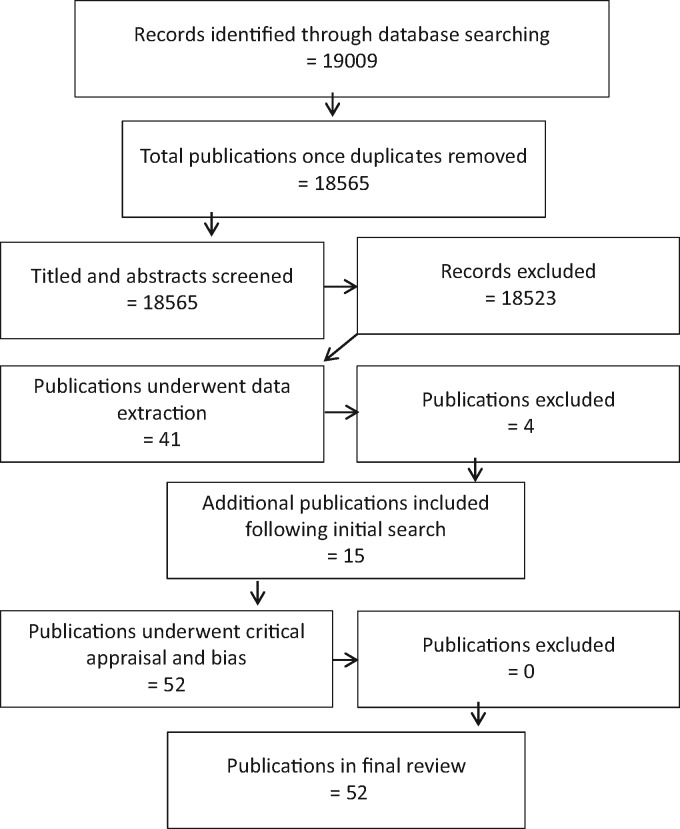
A total of 52 studies underwent data extraction (Fig. 1). Among the publications we included six reporting on the BeSt Study [14–19], four on STRASS [20–23] and two each on HONOR [24, 25], van der Ven et al. [26, 27] and the Southampton group [28, 29]. Results obtained from the analysis were then used to help answer a number of questions (supplementary Table S1, available at Rheumatology Online)—each of the questions has been answered within the context of the disease type in which dosing down was studied: early RA; established RA; axial SpA; and PsA. One study had a cross-indication population, which has been taken into consideration.
Fig. 1.
Selection of studies flowchart
Early RA and established RA
Does tapering of biologics occur, and what are the various strategies adopted?
Early RA
In the case of early RA, we found only RCTs that had investigated dosing down (50%; decrease in dose; one RCT) and discontinuation (three RCTs) [14, 30–32]. No RCT had yet investigated the strategy of injection spacing. In the case of the PRIZE study, patients who initially dosed down were given the option of continuing within the trial, then withdrawing treatment completely in a step-down process (results not discussed here) [31]. As all the studies in early RA were RCTs, the protocols adhered to were fixed with specific time points for dosing down and withdrawal [32].
Established RA
For established RA, nine RCTs were identified, one non-randomized trial, two placebo-controlled pilot studies, five prospective studies, nine observational studies, three reports from registries and two retrospective reports (supplementary Table S1, available at Rheumatology Online). A large number of different strategies have been adopted that fall within three overarching strategies: discontinuation of therapy; dose reduction—dosing down through decreasing the dose, in the majority of cases this is by 50%, but in some cases 25 or 33%; and injection frequency reduction—dosing down through increasing the spacing between individual doses, which leads to a typical decrease in dose of 50%.
In some studies a step-wise disease activity-driven protocol was adopted whereby, should the patient continue to be in remission, then an additional reduction in the dose or an increase in intervals between injections was carried out [21]. In other cases, the patients were asked to continue to increase the interval between doses until their disease flared or they could be considered for complete discontinuation of the biologic [33].
Which disease and patient characteristics are helpful in deciding on a dose-down strategy?
Early RA
These RCTs had a number of criteria that had to be met in order to allow the patient to dose down their biologic treatment, but most focused around the DAS28 score. In the case of the OPTIMA and PRIZE studies, both required patients to have a DAS28 </⩽ 3.2 [31, 32]. PRIZE then went on to specify that the patients should have a DAS28 < 2.6 in order for them to withdraw treatment, regardless of the three arms they had been in during the second period of treatment (25 mg etanercept + MTX; MTX; no treatment) [31]. Minimal duration of remission or LDA was requested in only one study, the BeSt study: DAS44 ⩽2.4 for a minimum of 4 months [14].
Established RA
The majority of RCTs used LDA (DAS28 < 3.2) or clinical remission (DAS28 ⩽ 2.6) to define entry to dose reduction. Some studies required other DASs or multiple criteria to be met prior to dose reduction. These included: absence of synovitis on power Doppler US, absence of radiographic progression on X-ray, low or no swollen or tender joint count (SJC/TJC) compared with baseline, and no CS use [21, 26, 28].
Large variation in rates of flare/failure to prolong remission or LDA following dose-down, was observed across longstanding and early disease. Almost all studies monitored the DAS scores, with a large number taking SJC/TJC measurements and HAQ.
Which therapies can be dosed down and how does this occur?
Early RA
The four RCTs investigated the dosing down of infliximab (one RCT), adalimumab (two RCTs) and etanercept (one RCT). As discussed above, the most common approach to dosing down was discontinuation in the case of all three of these biologics, with etanercept the only biologic to have been investigated in the case of a dose decrease of 50% with the same treatment interval.
Established RA
Across all the studies investigating dosing down approaches in the established RA population, a large spectrum of the biologics available for RA treatment have been investigated: etanercept, infliximab, adalimumab, certolizumab pegol, golimumab, tocilizumab, rituximab and abatacept. In one registry study with rituximab, the initial dose of rituximab was not clear [34]. In the RETRO trial [35], both conventional synthetic DMARD (csDMARD) and biologic DMARD (bDMARD) were tapered together. The EULAR guidelines recommend to start with tapering steroids, then bDMARD, then csDMARD [1].
How should flare be defined, and what is the risk of relapse?
Early RA
In the four trials, relapse was defined as loss of remission or LDA. In the case of adalimumab, discontinuation led to 54% of patients no longer being in remission (defined in the OPTIMA study as DAS28-CRP <3.2) at week 78 [32]. In the case of etanercept, a decrease of 50% in dose led to 37% of patients no longer having a DAS28 <2.6, compared with 60 and 77% of patients who had either etanercept, or etanercept and MTX discontinued [31], respectively. In the BeSt study with infliximab, 48.1% of patients flared according to the study definition of DAS28 >2.4, with a median time of 17 months [14].
Established RA
Flares after stopping therapy for RA ranged between 2 and 84%. Definitions of flare included the rheumatologist’s personal discretion and measurements of disease activity passing the threshold for which the patient could no longer be defined as in remission or LDA; however, the use of US has been used as an assessment of flare.
RCTs assessing biologic tapering in patients with RA, using either a discontinuation, dose reduction or increasing injection intervals strategy, have shown varying success rates and varying relapse rates. Similar discontinuation relapse rates have been seen with infliximab and adalimumab. Likewise, the HONOR RCT [24] showed that 52% of the 52 patients who discontinued their adalimumab treatment did not sustain remission (DAS28 <2.6) at 1 year. Flares were reported in as many as 84% of cases within a year of discontinuation [36].
Increasing the interval between biologic injections has been studied by van Herwaarden et al., who increased the interval of etanercept and adalimumab injections every 3 months until flare or discontinuation of drug. An investigation as to whether serum drug levels could predict the success of dose reduction or discontinuation regime found that at 18 months, dose reduction was no longer possible in 36% of patients receiving etanercept and 50% of patients receiving adalimumab.
It appears that some disease characteristics may reduce the success of the dosing-down regimen. These include a higher level of initial disease activity and presence of RF or ACPA [21, 28, 35]. DAS below 2.1 or 2.2 was shown as a predictor of success in the RRR and HONOR studies [24, 37].
RA registry data for over 700 patients showed that 72.4% of patients were no longer feeling the clinical benefit of the initial therapy after the drug had been discontinued for 36 months [38].
How should patients be monitored while on tapered doses of biologics?
Early RA and established RA
A number of disease activity measures, including DAS28, along with scores of functional activity using HAQ, have been assessed. The frequency of study assessment varied from monthly to 6-monthly, and studies lasted up to 12 or 18 months [14, 31, 32].
How should patients be managed long term in terms of retreatment and response?
Early RA
In the case of the BeSt study, as more long-term data are available than are available for other studies, once infliximab was re-introduced, 100% of patients achieved a DAS44 ⩽2.4. The BeSt trial utilized varying strategies, and patients had to go through several different treatment regimens to reach biologic treatment before then reaching a threshold such that infliximab could be withdrawn [14].
Established RA
Re-introduction of the biologic after flare resulted in many patients regaining LDA or remission. In the study of Takeuchi et al. [39], patients were observed to have a DAS28-CRP decrease of 1.3 within 12 weeks of the reintroduction of therapy. This speed of response was also reflected in the observational study by Brocq et al. [40], in which 100% of patients eventually achieved remission (defined as DAS28 <2.6), 86.7% of whom achieved remission within 2 months. Furthermore, in the HONOR study, 90% of patients achieved a LDA (defined as DAS28-ESR <3.2) within 6 months and 100% within 9 months [24].
This response rate following reintroduction of biologic therapy was not observed for all studies. In the case of the STRASS study, 40.8, 38.8 and 8.2% of patients achieved remission (DAS28 ⩽ 2.6), LDA (not defined) and moderate disease activity (not defined), respectively [21]. In the study of Marks et al. [28], where US remission was also one of the criteria for dosing down, 19, 19 and 47% of patients achieved DAS and US remission (DAS28 <2.6 and PDUS <1), DAS remission (DAS28 < 2.6) and LDA (DAS28 <3.2 and PDUS ⩽1), respectively [28]. Overall, following reintroduction of biologics, 19–100% of patients went on to regain remission [28, 40].
What are patients’ perspectives regarding tapering of biologics and its various aspects?
Early RA and established RA
No studies have been found in this search regarding patient preference. It is the feeling of the authors that in everyday practice, tapering is often an important patient preference.
Axial SpA
Does tapering of biologics occur, and what are the various strategies adopted?
Tapering of biologics in patients with axial SpA has been investigated in three prospective and one observational study. In the three prospective studies, the patients were listed as having AS, whereas in the observational study, the patients were listed as having axial SpA [41–44]. Across studies, various strategies were adopted, including reducing the dose by 50% or increasing the interval. In one study by Arends et al. [43], if the patients maintained LDA, a progressive step-down approach was adhered to. Although exact details were not provided in the paper by Zavada et al. [44], the authors discuss an average dose decrease of 50% being achieved by either spacing or a reduction in the dose, reflecting an average.
Which disease and patient characteristics are helpful in deciding on a dose-down strategy?
In two studies, the patients were required to have a BASDAI <4 for over 6 months of treatment on the biologic, reflecting an approach that requires sustained LDA before the physician will dose down [43, 44]. In other studies, BASDAI ⩽ or <2 were the requirements [41, 42]. Additional specifications were an absence of arthritis or enthesitis as well as a normal CRP level. As per the recommendations made by EULAR and ASAS, patients were initially treated with NSAIDs; this continued throughout treatment in some cases, and in other cases withdrawal of NSAIDs from the whole treatment paradigm was a necessity for dosing down.
Which therapies can be dosed down and how does this occur?
Across all four studies in the axial SpA population, etanercept was used in all four patient populations. In three of the four studies, a portion of the investigated population received adalimumab and infliximab. In the case of etanercept, the most common manner in which this biologic was dosed down was by a dose reduction of 50% to 25 mg every week; however, other methods were investigated, including increase in spacing [41–43]. For adalimumab, spacing leading to a reduction by 50% was utilized [42]. The Arends et al. [43] paper, which reported on a step-wise approach, involved dose reduction by increase in spacing across etanercept, infliximab and adalimumab.
How should flare be defined and what is the risk of relapse?
In the Arends et al. [43] study with the step-wise disease activity-driven approach, 26, 38, 43 and 47% of the patients no longer remained on the dose reduction method implemented as per the protocols at 6, 12, 18 and 24 months, respectively. In the other studies, the number of patients defined to have failed (varying definitions, mostly surrounding the BASDAI score that was required for the patient to be considered for dosing down) varied from 5–15% at 3 months to 16.5–29% at 6 months and 11–53% at 12 months [41, 42, 44].
How should patients be monitored while on tapered doses of biologics?
No recommendations were reported on the specific manner in which the patients should be monitored while dosing down across all four papers. However, in most cases the patients’ BASDAI, BASFI and HAQ were followed regularly across the study duration [41, 43, 44].
How should patients be managed long term in terms of retreatment and response?
In the case of Arends et al. [43], of the patients who returned to an increased dose, 88% regained a BASDAI
<4 after 6–12 months of retreatment.
What are patients’ perspectives regarding tapering of biologics and its various aspects?
No information is available to address this question.
PsA
Does tapering of biologics occur, and what are the various strategies adopted?
In the case of PsA, data are very limited regarding the dosing down of biologics. The only study found was a 2014 ACR abstract from a registry. This informs us that tapering of biologics does occur in the real-world clinical setting for PsA patients. In this case, the patients had their TNF inhibitors discontinued. In the cross-indication study, which included PsA patients, specific details were not clear for this population [45].
Which disease and patient characteristics are helpful in deciding on a dose-down strategy?
The physicians were guided by a Clinical Disease Activity Index (CDAI) ⩽10 and a skin psoriasis physician global assessment ⩽20/100 [45].
Which therapies can be dosed down, and how does this occur?
The abstract does not name specific biologics, but rather investigates the role of discontinuation in the TNF inhibitor class as a whole [45].
How should flare be defined, and what is the risk of relapse?
In the registry, patients were defined as having lost clinical benefit of the initial treatment if they encountered any of the following after therapy was discontinued: CDAI >10; skin psoriasis physician global assessment >20; increase in concomitant DMARD or prednisone; starting or restarting DMARD, prednisone or biologic therapy. It was found that 44.9% of patients were regarded as having lost the benefit within a median time of 29.2 months [45].
How should patients be monitored while on tapered doses of biologics?
No information was reported on the monitoring of these patients, with no details provided on the metrics taken or when they were taken [45].
How should patients be managed long term in terms of retreatment and response?
No information was provided on the effect of retreatment following flare or loss of clinical benefit.
What are patients’ perspectives regarding tapering of biologics and its various aspects?
As above, no information was available on this.
Conclusions
Dose reduction and tapering are taking place in a number of settings, and various strategies and approaches are being adopted. There is variability in the disease and patient characteristics being used in the decision-making process, and no clear monitoring approach is in place. It seems clear that withdrawal of biologic therapy in established disease results in failure. Several guidelines and recommendations suggest cautious tapering in selected patients [1, 46], but this is not reflected in the various product licences for biologics.
It is important to understand the risks and benefits of withdrawal and dose-down strategies for biologic therapies, and the potential impacts of these approaches for both patients and health-care systems in terms of efficacy, safety and cost over both the short- and long-term. The EULAR guidelines recommend to start tapering steroids, then bDMARD, then csDMARD [1].
The results of our systematic literature review and analysis suggest that the complete withdrawal of biologic therapy in patients with established disease does not result in sustained LDA or remission, and the majority of patients will experience a flare of their disease. Flares were reported in as many as 84% of cases within a year of discontinuation [36]. However, while discontinuation of biologic therapy may not be appropriate in established disease, there may be a basis for careful and controlled dose-down or reduction in some patients because patients responded well on reintroduction of treatment. Based on these findings, it could be proposed that we define two treatment phases: a first full-dose remission-induction phase; then a remission-maintenance phase with reduced dosage or frequency, such as has been proposed in other CTDs.
In addition to standard or commonly accepted criteria for LDA or remission, we have identified several other markers that are being used to identify candidates for dose-down, such as a history of stable dosing of biologic, or a patient having no need of CSs for a defined period. Reduction in or absence of SJC, TJC or synovitis, as well as a CDAI ⩽10 and the absence of radiographic progression on X-ray have also been used as measures of eligibility. In a time of stratified medicine, RCTs often use a blanket approach to patient assessment and classification; however, some studies have allowed the evaluation of more disease activity–driven responses that are reflective of clinical practice [21, 28].
There are limitations to our analysis. The studies included are not all RCTs, and as such have been conducted with varying designs and data collection strategies, which can make drawing meaningful comparisons difficult. However, the inclusion of non-RCT data was intentional in order to capture all ideas in this rapidly changing field. As with any systematic literature review, the results will quickly be out of date with the emergence of new publications on the topic, but we hope that this offers a robust analysis of our current position and expert clinical thinking on the issues at hand.
For the future, the rise of personalized medicine calls for a bespoke approach in each patient, and the current review and analysis support the careful examination of each patient’s individual disease state and personal characteristics in order to ascertain their suitability for dose-down approaches. It is important to assess the patient’s own views and to take these into account when tailoring treatment strategies.
There are several gaps that warrant additional research in this area. Paramount is the need for assessing whether failed attempts to taper a drug cause any long-term damage in terms of immunogenicity, higher disease activity, structural damage or radiographic progression, or whether increased CRP exposure leads to a higher incidence of cardiac diseases in these patients. Of these, immunogenicity requires particular consideration, since anti-drug antibodies to TNF inhibitors are often increased in the presence of lower doses of drug, and patients with low trough levels may not represent good candidates for dose-down. Therapeutic drug monitoring is also increasingly important, and to date no study has included this in the decision criteria for dose reduction or withdrawal.
Supplementary Material
Acknowledgements
Initial drafting and subsequent medical writing support was provided by Peter Black and Isaac Bruce at Synergy (London, UK) and funded by Pfizer.
Funding: No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this manuscript.
Disclosure statement: C.J.E. has received honoraria, research support or been a member of the speakers bureau for AbbVie, Celgene, UCB, Roche, Lilly, Pfizer, Samsung, Celltrion, Napp, Mundipharma and Merck. B.F. has received research support from AbbVie, BMS, Celgene, Hospira, Lilly, Medac, MSD, Novartis, Nordic, Pfizer, Roche, SOBI and UCB. H.S.-K. has served as a consultant for and has received research grants from Pfizer. T.W.J.H./the Department of Rheumatology LUMC has received lecture fees/consultancy fees from Merck, UCB, Bristol Myers Squibb, Biotest AG, Pfizer, GSK, Novartis, Roche, Sanofi-Aventis, Abbott, Crescendo Bioscience, Nycomed, Boeringher, Takeda, Zydus, Epirus and Eli Lilly and support for travelling to EULAR/ACR meetings from Roche and Abbott. The other author has declared no conflicts in interest.
Supplementary data
Supplementary data are available at Rheumatology Online.
References
- 1. Smolen JS, Landewé R, Breedveld FC. et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis 2014;73:492–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gossec L, Smolen JS, Gaujoux-Viala C. et al. European League Against Rheumatism. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies. Ann Rheum Dis 2012;71:4–12. [DOI] [PubMed] [Google Scholar]
- 3. Braun J, van den Berg R, Baraliakos X. et al. 2010 update of the ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann Rheum Dis 2011;70:896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Smolen JS, Aletaha D, Bijlsma JW, T2T Expert Committee et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis 2010;69:631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Smolen JS, Braun J, Dougados M. et al. Treating spondyloarthritis, including ankylosing spondylitis and psoriatic arthritis, to target: recommendations of an international task force. Ann Rheum Dis 2014;73:6–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aga AB, Lie E, Uhlig T. et al. Time trends in disease activity, response and remission rates in rheumatoid arthritis during the past decade: results from the NOR-DMARD study 2000–2010. Ann Rheum Dis 2015;74:381–8. [DOI] [PubMed] [Google Scholar]
- 7. Bartlett SJ, Hewlett S, Bingham CO III. et al. ; OMERACT RA Flare Working Group. Identifying core domains to assess flare in rheumatoid arthritis: an OMERACT international patient and provider combined Delphi consensus. Ann Rheum Dis 2012;71:1855–60. [DOI] [PubMed] [Google Scholar]
- 8. Bykerk VP, Shadick N, Frits M. et al. Flares in rheumatoid arthritis: frequency and management. A report from the BRASS Registry. J Rheumatol 2014;41:227–34. [DOI] [PubMed] [Google Scholar]
- 9. den Broeder AA, van der Maas A, van den Bemt BJ.. Dose de-escalation strategies and role of therapeutic drug monitoring of biologics in RA. Rheumatology 2010;49:1801–3. [DOI] [PubMed] [Google Scholar]
- 10. den Broeder AA, van Herwaarden N, van der Maas A. et al. Dose REduction strategy of subcutaneous TNF inhibitors in rheumatoid arthritis: design of a pragmatic randomised non inferiority trial, the DRESS study. BMC Musculoskelet Disord 2013;14:299.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Herwaarden N, den Broeder AA, Jacobs W. et al. Down-titration and discontinuation strategies of tumor necrosis factor–blocking agents for rheumatoid arthritis in patients with low disease activity. Cochrane Database Syst Rev 2014;9:CD010455. [DOI] [PubMed] [Google Scholar]
- 12. Higgins JP, Altman DG, Gøtzsche PC, Cochrane Bias Methods Group; Cochrane Statistical Methods Group et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Downs SH, Black N.. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998;52:377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Allaart CF, Lems WF, Huizinga TWJ.. The BeSt way of withdrawing biologic agents. Clin Exp Rheumatol 2013;31: S14–8. [PubMed] [Google Scholar]
- 15. van den B, Lems WF, Allaart CF.. BeSt practice: the success of early-targeted treatment in rheumatoid arthritis. Clin Exp Rheumatol 2012;30:S35–8. [PubMed] [Google Scholar]
- 16. Klarenbeek NB, van der Kooij SM, Güler-Yüksel M. et al. Discontinuing treatment in patients with rheumatoid arthritis in sustained clinical remission: exploratory analyses from the BeSt study. Ann Rheum Dis 2011;70:315–9. [DOI] [PubMed] [Google Scholar]
- 17. Markusse I, Dirven L, Han H. et al. Good adherence of rheumatologists to a 10 year treat-to-target protocol for patients with recent-onset rheumatoid arthritis (the BEST study). Presented at EULAR 2014; FRI0055. [Google Scholar]
- 18. van den Broek M, Klarenbeek NB, Dirven L. et al. Discontinuation of infliximab and potential predictors of persistent low disease activity in patients with early rheumatoid arthritis and disease activity score–steered therapy: subanalysis of the BeSt study. Ann Rheum Dis 2011;70:1389–94. [DOI] [PubMed] [Google Scholar]
- 19. Markusse I, Dirven L, vd Broek M. et al. 10 years of treat-to target therapy in rheumatoid arthritis patients (the best study): clinical and radiographic outcomes. Presented at EULAR 2014; THU0259. [Google Scholar]
- 20. Fautrel B, Gandjbakhch F, Foltz V. et al. Targeting the lowest efficacious dose for rheumatoid arthritis patients in remission: clinical and structural impact of a step-down strategy trial based on progressive spacing of TNF-blocker injections (STRASS trial). Presented at EULAR 2013; OP0066. [Google Scholar]
- 21. Fautrel B, Pham T, Alfaiate T. et al. Step-down strategy of spacing TNF-blocker injections for established rheumatoid arthritis in remission: results of the multicentre non-inferiority randomised open-label controlled trial (STRASS: Spacing of TNF-blocker injections in Rheumatoid ArthritiS Study. Ann Rheum Dis 2016;59–67. [DOI] [PubMed] [Google Scholar]
- 22. Fautrel B. Tapering TNF-blockers in established rheumatoid arthritis patients in DAS28 remission: results of a DAS28-driven step-down strategy randomized controlled trial. Presented at ACR 2012; L7. [Google Scholar]
- 23. Danré A, Gossec L, Pham T. et al. Effects of TNFα blockers tapering on occurrence of patient perceived flares in rheumatoid arthritis: a subanalysis of STRASS. Presented at EULAR 2014; THU0151. [Google Scholar]
- 24. Tanaka Y, Hirata S, Kubo S. et al. Discontinuation of adalimumab after achieving remission in patients with established rheumatoid arthritis: 1-year outcome of the HONOR study. Ann Rheum Dis 2015;74:389–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hirata S, Saito K, Kubo S. et al. Discontinuation of adalimumab after attaining disease activity score 28–erythrocyte sedimentation rate remission in patients with rheumatoid arthritis (HONOR study): an observational study. Arthritis Res Ther 2013;15:R135–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van der Ven M, Kuijper M, Gerands A. et al. Can we use ultrasound to identify rheumatoid arthritis patients in remission who can taper their medication? Presented at EULAR 2014; OP0123. [Google Scholar]
- 27. van der Ven M, Kuijper TM, Gerards AH. et al. Can we use ultrasound to identify rheumatoid arthritis patients in remission who cannot taper their medication? Presented at ACR 2014;120. [Google Scholar]
- 28. Marks JL, Holroyd CR, Dimitrov BD. et al. Does combined clinical and ultrasound assessment allow selection of individuals with rheumatoid arthritis for sustained reduction of anti-tumor necrosis factor therapy? Arthritis Care Res 2015;67:746–53. [DOI] [PubMed] [Google Scholar]
- 29. Holroyd CR, Davidson B, Bennet S. et al. A strategy for selecting individuals with RA for reduction of anti-TNF therapy using combined clinical and ultrasound assessment. Presented at ACR 2013;800. [Google Scholar]
- 30. Detert J, Bastian H, Listing J. et al. Induction therapy with adalimumab plus methotrexate for 24 weeks followed by methotrexate monotherapy up to week 48 versus methotrexate therapy alone for DMARD-naive patients with early rheumatoid arthritis: HIT HARD, an investigator-initiated study. Ann Rheum Dis 2012;72:844–50. [DOI] [PubMed] [Google Scholar]
- 31. Emery P, Hammoudeh M, FitzGerald O. et al. Sustained remission with etanercept tapering in early rheumatoid arthritis. N Engl J Med 2014;371:1781–92. [DOI] [PubMed] [Google Scholar]
- 32. Smolen JS, Emery P, Fleischmann R. et al. Adjustment of therapy in rheumatoid arthritis on the basis of achievement of stable low disease activity with adalimumab plus methotrexate or methotrexate alone: the randomised controlled OPTIMA trial. Lancet 2014;383:321–32. [DOI] [PubMed] [Google Scholar]
- 33. van Herwaarden N, van der Maas A, Minten M. et al. Randomised controlled non-inferiority study of dose reduction and withdrawal of adalimumab and etanercept in rheumatoid arthritis. Presented at ACR 2014;1843.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chatzidionysiou K, Lie E, Nasonov EK. et al. Fixed versus on-flare retreatment with rituximab in RA – Results from the Cererra collaboration. Presented at ACR 2013; Abstract 500. [Google Scholar]
- 35. Haschka J, Englbrecht M, Hueber AJ. et al. Relapse rates in patients with rheumatoid arthritis in stable remission tapering or stopping antirheumatic therapy: interim results from the prospective randomised controlled RETRO study. Ann Rheum Dis 2016;45–51. [DOI] [PubMed] [Google Scholar]
- 36. Huizinga TW, Conaghan PG, Martin-Mola E. et al. Clinical and radiographic outcomes at 2 years and the effect of tocilizumab discontinuation following sustained remission in the second and third year of the ACT-RAY study. Ann Rheum Dis 2015;35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tanaka Y, Takeuchi T, Mimori T. et al. Discontinuation of infliximab after attaining low disease activity in patients with rheumatoid arthritis: RRR (remission induction by Remicade in RA) study. Ann Rheum Dis 2010;69:1286–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kavanaugh A, Lee SJ, Solomon DH. et al. Discontinuation of tumor necrosis factor inhibitors in rheumatoid arthritis patients in low disease activity: persistent benefits. Presented at ACR 2013;1425. [Google Scholar]
- 39. Takeuchi T, Matsubara T, Ohta S. et al. Biologic-free remission of established rheumatoid arthritis after discontinuation of abatacept: a prospective, multicentre, observational study in Japan. Rheumatology 2015;54:683–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brocq O, Millasseau E, Albert C. et al. Effect of discontinuing TNFα antagonist therapy in patients with remission of rheumatoid arthritis. Joint Bone Spine 2009;76:350–5. [DOI] [PubMed] [Google Scholar]
- 41. De Stefano R, Frati E, De Quattro D, Menza L, Manganelli S.. Low doses of etanercept can be effective to maintain remission in ankylosing spondylitis patients. Clin Rheumatol 2014;33:707–11. [DOI] [PubMed] [Google Scholar]
- 42. Almirall Salman TC, Lisbona MP, Iniesta S, Maymo J.. Dosage reduction of biological therapy in patients with axial spondyloarthritis in persistent clinical remission. Presented at EULAR 2013; THU0368. [Google Scholar]
- 43. Arends S, van der Veer E, Kamps FBS. et al. Patient-tailored dose reduction of TNF-α blocking agents in ankylosing spondylitis patients with stable low disease activity in daily clinical practice. Clin Exp Rheumatol 2015;33:174–80. [PubMed] [Google Scholar]
- 44. Závada J, Uher M, Sisol K. et al. A tailored approach to reduce dose of anti-TNF drugs may be equally effective, but substantially less costly than standard dosing in patients with ankylosing spondylitis over 1 year: a propensity score-matched cohort study. Ann Rheum Dis 2016;75:96–102. [DOI] [PubMed] [Google Scholar]
- 45. Huynh DH, Etzel CJ, Cox V, Mease PJ, Kavanaugh A.. Persistence of low disease activity after tumor necrosis factor inhibitor withdrawal in patients with psoriatic arthritis. Presented at ACR 2014;1594.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Singh J, Saag K, Bridges L. et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res 2016;68:1–25. [DOI] [PubMed] [Google Scholar]
- 47. Kaine J, Gladstein G, Strusberg I. et al. Evaluation of abatacept administered subcutaneously in adults with active rheumatoid arthritis: impact of withdrawal and reintroduction on immunogenicity, efficacy and safety (Phase IIIb ALLOW study). Ann Rheum Dis 2012;71:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. van Herwaarden N, Bouman C, van der Maas A. et al. Prediction of successful dose reduction or discontinuation of adalimumab or etanercept using serum drug levels and antidrug antibody measurement. Presented at ACR 2014; 500.. [DOI] [PubMed] [Google Scholar]
- 49. Vollenhoven RV, Franck-Larsson K, Leirisalo-Repo M. et al. In rheumatoid arthritis patients with stable low disease activity on methotrexate plus etanercept, continuation of etanercept at 50 mg or 25 mg weekly are both clinically superior to discontinuation: results from a randomized, 3-arm, double-blind study. Presented at EULAR 2013; FRI0185. [Google Scholar]
- 50. Smolen JS, Nash P, Durez P. et al. Maintenance, reduction, or withdrawal of etanercept after treatment with etanercept and methotrexate in patients with moderate rheumatoid arthritis (PRESERVE): a randomised controlled trial. Lancet 2013;381:918–29. [DOI] [PubMed] [Google Scholar]
- 51. Quinn MA, Conaghan PG, O’Connor PJ. et al. Very early treatment with infliximab in addition to methotrexate in early, poor-prognosis rheumatoid arthritis reduces magnetic resonance imaging evidence of synovitis and damage, with sustained benefit after infliximab withdrawal: results from a twelve-month randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2005;52:27–35. [DOI] [PubMed] [Google Scholar]
- 52. Chatzidionysiou K, Turesson C, Teleman A. et al. Multicenter, randomized, controlled, open-label pilot study of the feasibility of discontinuation of adalimumab in rheumatoid arthritis patients in stable clinical remission. Presented at ACR 2012; Abstract 776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Raffeiner B, Botsios C, Ometto F. et al. Effects of half dose etanercept (25 mg once a week) on clinical remission and radiographic progression in patients with rheumatoid arthritis in clinical remission achieved with standard dose. Clin Exp Rheumatol 2015;33:63–8. [PubMed] [Google Scholar]
- 54. Nawata M, Saito K.. Discontinuation of infliximab in rheumatoid arthritis patients in clinical remission. Mod Rheumatol 2008;18:460–4. [DOI] [PubMed] [Google Scholar]
- 55. Iwamoto T, Ikeda K, Hosokawa J. et al. Prediction of relapse after discontinuation of biologic agents by ultrasonographic assessment in patients with rheumatoid arthritis in clinical remission: high predictive values of total gray-scale and power Doppler scores that represent residual synovial inflammation before discontinuation. Arthritis Care Res 2014;66:1576–81. [DOI] [PubMed] [Google Scholar]
- 56. Naredo E, Valor L, De la Torre I. et al. Predictive value of doppler ultrasound synovitis in relation to successful tapering of biology therapy in patients with rheumatoid arthritis. Presented at EULAR 2014; THU0250. [Google Scholar]
- 57. Kamiya M, Sohen S, Kikuchi H.. Attempting withdrawal of etanercept in patients with rheumatoid arthritis who are in clinical remission. Presented at EULAR 2013; SAT0142. [Google Scholar]
- 58. van der Maas A, Kievit W, van den Bemt BJ. et al. Down-titration and discontinuation of infliximab in rheumatoid arthritis patients with stable low disease activity and stable treatment: an observational cohort study. Ann Rheum Dis 2012;71:1849–54. [DOI] [PubMed] [Google Scholar]
- 59. Alivernini S, Peluso G, Correra M. et al. Ultrasonography as useful tool to identify rheumatoid arthritis patients in clinical remission for tapering or withdrawal TNFα blockers without disease relapse. Presented at EULAR 2014; FRI0276.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nishimoto N, Amano K, Hirabayashi Y. et al. Drug free REmission/low disease activity after cessation of tocilizumab (Actemra) Monotherapy (DREAM) study. Mod Rheumatol 2014;24:17–25. [DOI] [PubMed] [Google Scholar]
- 61. Plasencia C, Wolbink G, Krieckaert C. et al. Comparison of clinical outcomes between rheumatoid arthritis patients under TNF inhibitors using a tapering strategy or standard therapy regimen in daily clinical practice. Presented at EULAR 2014; THU0157. [Google Scholar]
- 62. Mamoto K, Koike T, Okano T. et al. Comparison of effects of standard- and low-dose etanercept on inflammatory synovitis in rheumatoid arthritis patients as assessed by ultrasonography. Presented at ACR 2013; 1449. [Google Scholar]
- 63. Greenberg JD, Shan Y, Reed GW, Bitman B, Collier D.. Comparison of switching to reduced dose vs continuation of standard dose etanercept for rheumatoid arthritis patients in the Corrona registry. Presented at EULAR 2014; THU0174. [Google Scholar]
- 64. van Herwaarden N, Herfkens-Hol S, van der Maas A. et al. Dose reduction of tocilizumab in rheumatoid arthritis patients with low disease activity. Clin Exp Rheumatol 2014;32:390–4. [PubMed] [Google Scholar]
- 65. Harigai M, Takeuchi T, Tanaka Y. et al. Discontinuation of adalimumab treatment in rheumatoid arthritis patients after achieving low disease activity. Mod Rheumatol 2012;22:814–22. [DOI] [PubMed] [Google Scholar]
- 66. Inciarte-Mundo J, Hernández MV, Rosario V. et al. Reduction of biological agent dose in rheumatic diseases: descriptive analysis of 153 patients in clinical practice conditions. Reumatol Clin 2014;10:10–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.