SUMMARY
Neurofibromatosis type 1 (NF1) is a common autosomal-dominant disorder associated with attention deficits and learning disabilities. The primary known function of neurofibromin, encoded by the NF1 gene, is to downregulate Ras activity. We show that nf1-deficient zebrafish exhibit learning and memory deficits and that acute pharmacological inhibition of downstream targets of Ras (MAPK and PI3K) restores memory consolidation and recall but not learning. Conversely, acute pharmacological enhancement of cAMP signaling restores learning but not memory. Our data provide compelling evidence that neurofibromin regulates learning and memory by distinct molecular pathways in vertebrates and that deficits produced by genetic loss of function are reversible. These findings support the investigation of cAMP signaling enhancers as a companion therapy to Ras inhibition in the treatment of cognitive dysfunction in NF1.
INTRODUCTION
Neurofibromatosis type 1 (NF1) is associated with a broad range of clinical characteristics, including a predisposition to develop benign and malignant tumors, pigmentation defects, and cognitive deficits (Cichowski and Jacks, 2001). As many as 50%–70% of children with NF1 exhibit attention deficits and learning disabilities that contribute to scholastic underachievement and impaired social development (Hyman et al., 2005, 2006; Levine et al., 2006). Genetic and pharmacological experiments performed in mice and Drosophila support a role for the Ras-GTPase activating domain (GRD), which functions to downregulate Ras activity in protein-synthesis-dependent memory (Costa et al., 2002; Cui et al., 2008; Guilding et al., 2007; Ho et al., 2007; Li et al., 2005; Silva et al., 1997). However, cognitive dysfunction in NF1 has been linked to mutations throughout the NF1 gene that do not cluster in the region encoding the GRD, leading to the proposal that neurofibromin serves additional cellular functions (Fahsold et al., 2000). Studies performed in Drosophila suggest that neurofibromin can also stimulate adenylyl cyclase (AC), cAMP production, and PKA to promote learning and memory (Guo et al., 2000; Hannan et al., 2006; The et al., 1997; Tong et al., 2002). Nf1-deficient Drosophila brains show reduced cAMP levels, and expression of a C-terminal neurofibromin fragment lacking the GRD is sufficient to rescue learning (Ho et al., 2007; Tong et al., 2002). Similarly, brains of Nf1+/− mice exhibit reduced cAMP levels (Brown et al., 2010, 2012; Hegedus et al., 2007) and cAMP regulation of dopaminergic function in the hippocampus is disrupted (Diggs-Andrews et al., 2013). The mechanism by which neurofibromin regulates AC remains controversial, and both Ras-dependent and Ras-independent pathways have been suggested (Guo et al., 1997; Hannan et al., 2006; Tong et al., 2002). Studies in Drosophila models of NF1 further argue that the resulting elevation in Ras activity, mediated through the upstream activation of neuronal dAlk, is responsible for observed decreases in cAMP signaling (Gouzi et al., 2011; Walker et al., 2006, 2013). Neurofibromin is also known to modulate both neural and glial development from neuroglial progenitors, and both Ras and cAMP have been implicated (Hegedus et al., 2007). Recent studies suggest that pharmacological activation of the cAMP pathway may enhance cognition in murine models (Jayachandran et al., 2014; Peng et al., 2014; Richter et al., 2013). However, it remains unclear whether NF1-dependent cAMP signaling is critical for learning or memory in vertebrates. Furthermore, the contributions of developmental and structural abnormalities to learning and memory deficits in NF1 have not yet been clearly defined (Armstrong et al., 2012; Karlsgodt et al., 2012; Shilyansky et al., 2010).
RESULTS AND DISCUSSION
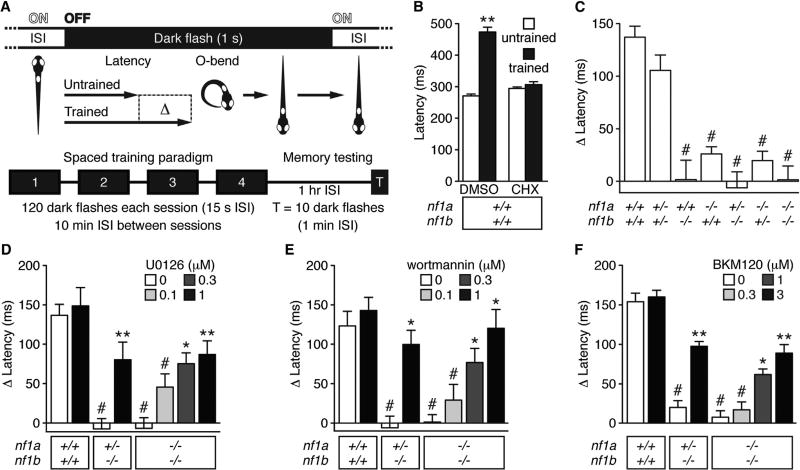
We utilized a zebrafish model of NF1 that harbors null alleles in the NF1 orthologs nf1a and nf1b (Shin et al., 2012) to evaluate molecular signaling pathways that control NF1-dependent learning and memory in vertebrates. Larval zebrafish show a remarkable capacity for behavioral plasticity in response to visual and acoustic stimuli, including habituation (Roberts et al., 2013; Wolman et al., 2011), as evidenced by a progressive decline in responsiveness to repeated, inconsequential stimuli (Thompson and Spencer, 1966). The duration of habituated behavior provides a metric for nonassociative learning (short-term habituation) and memory formation and recall (longer-term, protein-synthesis-dependent habituation). Importantly, habituation reflects a highly conserved form of attention-based learning and memory that is similar to the type of cognition impairment found in NF1 children (Hyman et al., 2005; Isenberg et al., 2013; Levine et al., 2006). We tested 5-day-old larvae for protein-synthesis-dependent visual habituation to evaluate memory formation and recall. After a period of light adaptation, exposing the larvae to a sudden absence of light, termed a dark flash, elicited a highly stereotyped yet habituatable reorientation maneuver known as an O-bend (Movie S1; Burgess and Granato, 2007a). Delivering repetitive dark flashes through a spaced training paradigm elicited protein-synthesis-dependent memory formation (Figures 1A and 1B). One hour after training, wild-type larvae showed a near doubling in the latency time period before initiating an O-bend compared with responses prior to training (Figure 1B). Treatment with the protein synthesis inhibitor cycloheximide (CHX, 10 µM) abolished this increase (Figure 1B), consistent with a requirement for protein synthesis (Beck and Rankin, 1995; Davis and Squire, 1984). Larvae null for nf1a or nf1b showed impaired memory (Figure 1C). This memory deficit is consistent with cognitive impairment observed in NF1 patients and in other animal models of NF1, and supports the use of nf1 mutant zebrafish to probe the mechanisms of NF1-dependent cognition.
Figure 1. nf1 Mutant Larvae Exhibit Reduced Memory Recall.
(A) Schematic representation of the visual memory assay. ISI, interstimulus interval.
(B–F) Mean O-bend latency (B) or latency change (C–F) 1 hr after spaced training (test) versus untrained controls (n = 26–130 O-bend maneuvers per genotype/treatment). #p < 0.001 versus wild-type untreated (C) or DMSO-treated (B and D–F) larvae. *p < 0.01, **p < 0.001 versus same genotype, DMSO-treated larvae. One-way ANOVA. Error bars denote SEM.
See also Figures S2 and S3.
Memory impairment in Drosophila and mouse NF1 models is due at least in part to elevated Ras signaling (Costa et al., 2002; Cui et al., 2008; Hannan et al., 2006; Li et al., 2005). Since nf1 mutant larvae also show increased Ras activity (Shin et al., 2012), we asked whether acute pharmacological inhibition of the Ras effectors MAPK and PI3K could improve memory recall in nf1 mutants. Small molecules readily cross the developing blood-brain barrier of larval zebrafish until at least 8 days of age (Fleming et al., 2013), facilitating pharmacogenetic approaches for identifying signaling pathways that underlie biological processes and screening of potential therapeutics for neuropsychiatric disorders such as NF1. We treated wild-type, nf1a+/−; nf1b−/−, and nf1a−/−; nf1b−/− larvae with inhibitors of MAPK (U0126) or PI3K (wortmannin, BKM120) for 30 min before and throughout training and testing for memory recall. Each compound improved memory recall in nf1 mutant larvae in a dose-dependent manner (Figures 1D–1F). Treatment with 1 µM wortmannin restored memory to wild-type levels, and 1 µM U0126 or 3 µM BKM120 yielded significant memory improvement. Although each of these Ras pathway antagonists exhibits known off-target effects, their different selectivity profiles (Bain et al., 2007; Liao and Laufs, 2005; Maira et al., 2012) suggest that nonspecific effects are unlikely to underlie the observed increase in memory recall. Therefore, these results support a conserved function for the neurofibromin GRD domain in regulation of memory formation through the Ras/MAPK/PI3K signaling pathway.
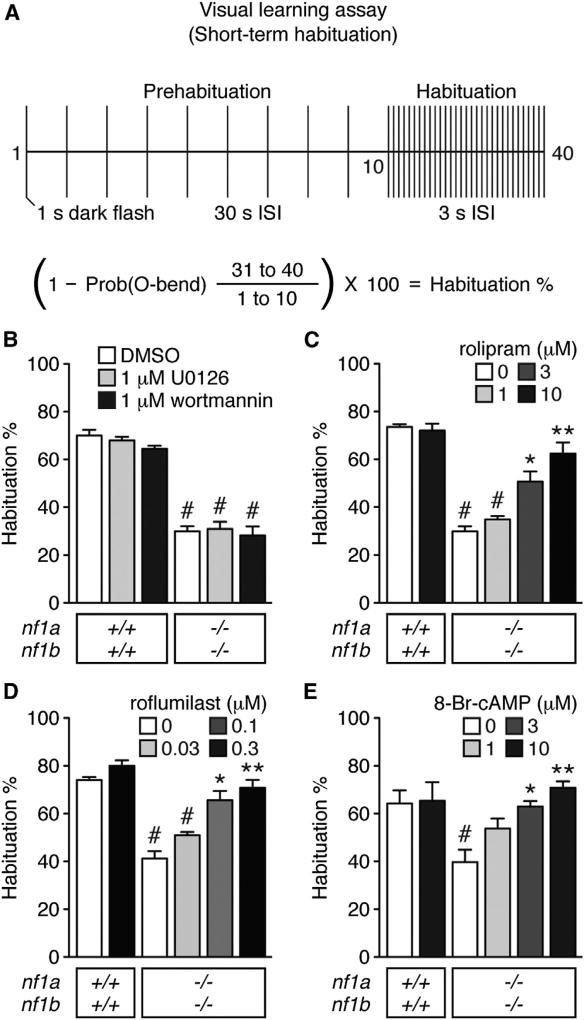
Learning (the acquisition of information) is critical for establishing memory. We evaluated learning by exposing larvae to dark-flash stimuli delivered at 3 s interstimulus intervals (ISIs) and measuring short-term habituation, as indicated by a reduction in the probability of initiating an O-bend response (Figure 2A). nf1a−/−; nf1b−/− larvae showed markedly reduced short-term visual (Figure 2B) and acoustic (Figures S1A and S1B; Shin et al., 2012) habituation compared with wild-type controls. Notably, nf1a−/−; nf1b−/− larvae showed some capacity for learning, which likely accounts for their potential to form memories in the presence of Ras pathway inhibitors (Figures 1D–1F). Larvae with at least one wild-type allele of either nf1a or nf1b did not show a learning deficit, despite dramatic memory deficits (Figure 1C; M.A.W. and E.D.d.G., unpublished data; Shin et al., 2012). It is possible that our nonassociative habituation assay lacks the necessary sensitivity to detect relatively subtle learning deficiencies in larvae with these genotypes. Attenuating Ras signaling by acute pharmacological inhibition of MAPK (U0126) or PI3K (wortmannin) failed to improve the learning deficit of nf1a−/−; nf1b−/− larvae (Figures 2B and S1B), suggesting that a distinct pathway mediates NF1-dependent learning.
Figure 2. cAMP Signaling Mediates nf1-Dependent Visual Learning.
(A) Schematic representation of the visual learning assay.
(B–E) Mean percentage of habituation to repeated dark-flash stimulation (n = 3 groups of 15–20 larvae for all genotype/treatment groups). #p < 0.001 versus DMSO-treated wild-type larvae. *p < 0.01, **p < 0.001 versus DMSO-treated nf1a−/−; nf1b−/− larvae. One-way ANOVA. Error bars denote SEM.
See also Figures S1 and S3.
Whole larval lysates revealed reduced cAMP levels in nf1a−/−; nf1b−/− mutants compared with wild-type controls (nf1a−/−; nf1b−/−: 33 fmol ± SEM 2.3 versus wild-type: 79 fmol ± SEM 7.8, p < 0.001). To determine whether reduced cAMP signaling contributed to the learning deficits in nf1a−/−; nf1b−/− mutants, we tested whether enhancing cAMP signaling by acute pharmacological inhibition of phosphodiesterase 4 (PDE4) or stimulation of PKA could improve learning. Inhibition of PDE4 by rolipram or roflumilast, or PKA stimulation by 8-Br-cAMP improved learning behavior in nf1a−/−; nf1b−/− mutants in response to both repetitive visual (Figures 2C–2E) and acoustic (Figures S1C and S1D) stimuli. Treatment with at least 10 µM rolipram, 0.1 µM roflumilast, or 3 µM 8-Br-cAMP improved habituation to wild-type levels. These results provide evidence that cAMP signaling regulates NF1-dependent learning in a vertebrate system.
We next asked whether cAMP signaling regulates NF1-dependent memory in addition to learning. We tested nf1a−/−; nf1b−/− larvae, which show reduced learning and a failure to recall memory, and nf1a+/−; nf1b−/− larvae, which learn normally but fail to form memory, and compared them with wild-type controls. Treatment with 10 µM 8-Br-cAMP, a sufficient dose to restore learning in nf1a−/−; nf1b−/− larvae (Figures 2E and S1D), failed to improve memory recall in either nf1a+/−; nf1b−/− or nf1a−/−; nf1b−/− larvae (Figure S2). These results suggest that cAMP signaling regulates NF1-dependent learning but not memory. Moreover, these results indicate that the memory defects in nf1a−/−; nf1b−/− mutants are not simply attributable to their learning deficit. These data strongly imply that molecularly distinct pathways that control learning and memory are affected in NF1.
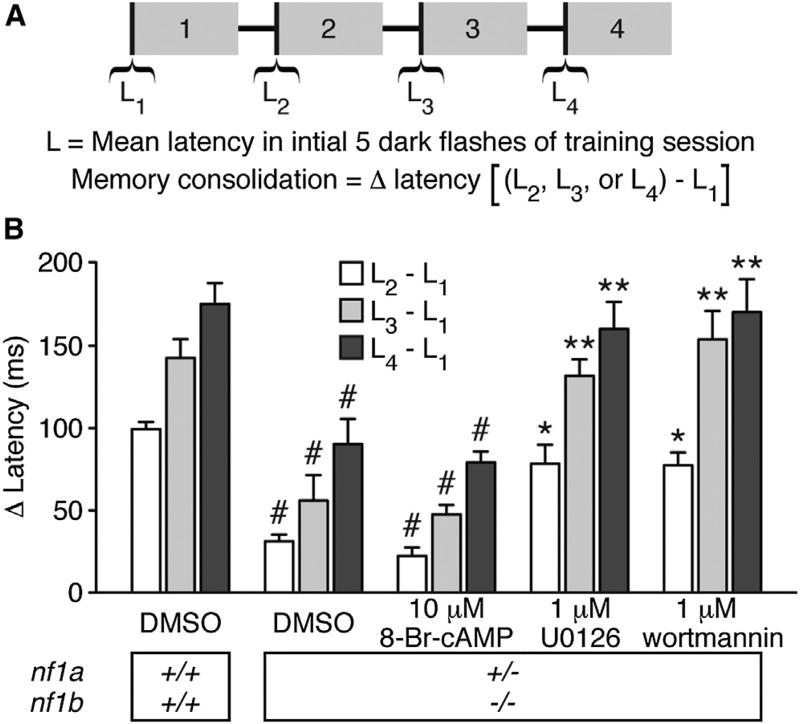
Learned behavior requires consolidation to form stable memory. Despite consensus that defective neurofibromin function can result in learning and memory impairments, whether impaired consolidation contributes to memory deficits remains unclear. nf1a+/−; nf1b−/− larvae learn normally (M.A.W. and E.D.d.G., unpublished data; Shin et al., 2012) but show reduced memory recall (Figure 1C). Therefore, we asked whether reduced memory was due to a consolidation deficit. We determined memory consolidation by calculating the difference between the mean O-bend latency in response to the first five dark-flash stimuli of training session 1 and subsequent training sessions (Figure 3A). Long ISIs between training sessions promote memory consolidation, and therefore spaced training paradigms elicit more stable memory than do massed training paradigms (Beck and Rankin, 1997; Ebbinghaus, 1885). After each session, nf1a+/−; nf1b−/− larvae showed reduced consolidation compared with wild-type larvae (Figure 3B), suggesting that the memory-recall deficit observed in nf1a+/−; nf1b−/− larvae (Figure 1C) may be due to a defect in memory consolidation.
Figure 3. Inhibition of Ras Signaling Improves Memory Consolidation Deficits in nf1 Mutants.
(A) Schematic representation of visual memory consolidation measurement.
(B) Mean O-bend latency change comparing responses to dark-flash stimuli 1–5 of sessions 2–4 versus stimuli 1–5 of session 1 (n = 30–139 O-bend maneuvers per genotype/treatment). #p < 0.001 versus DMSO-treated wild-type larvae. *p < 0.01, **p < 0.001 versus DMSO-treated nf1a+/−; nf1b−/− larvae. One-way ANOVA. Error bars denote SEM.
See also Figure S3.
To determine the contribution of cAMP and Ras signaling to NF1-dependent memory consolidation, we attempted to improve consolidation in nf1a+/−; nf1b−/− larvae by pharmacologically enhancing cAMP or attenuating Ras. Enhancing cAMP in nf1a+/−; nf1b−/− larvae by treatment with 10 µM 8-Br-cAMP did not increase consolidation (Figure 3B). Pharmacological inhibition of MAPK (1 µM U0126) or PI3K (1 µM wortmannin) improved memory consolidation in nf1a+/−; nf1b−/− larvae to levels indistinguishable from those observed in DMSO-treated wild-type larvae (Figure 3B). These results reveal that deficits in memory consolidation contribute to the etiology of memory dysfunction in NF1 and support a specific role for Ras signaling in mediating NF1-dependent memory formation.
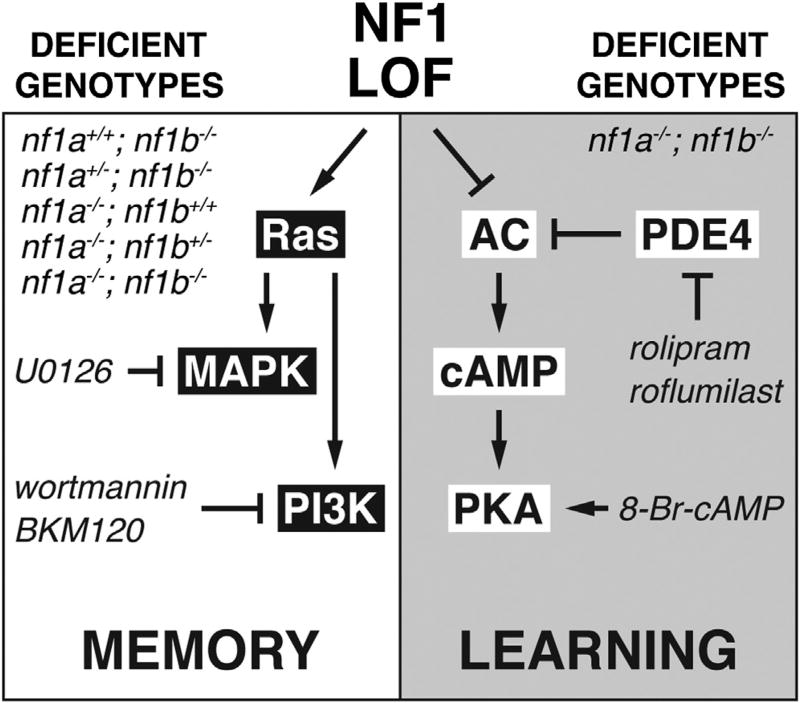
Larvae deficient for nf1 exhibit learning and memory deficits with characteristics reminiscent of those seen in human NF1 patients. We obtained strong evidence in a vertebrate system that NF1 affects at least two distinct signaling pathways that independently modulate learning and memory (Figure 4). A detailed understanding of the structure-function relationship among NF1 mutations, Ras and cAMP signaling, and phenotypes will allow for tailored and personalized therapies for cognitive defects in affected patients. It will also be interesting to determine whether the dynamic regulation of Ras or cAMP signaling in distinct areas of the brain correlates with unique behavioral outcomes. The fact that we observed robust improvements in learning and memory in our experiments even though we used only short-term treatments is encouraging for potential clinical application, and suggests that cognitive defects in this model are not developmental or irreversible. It will be exciting to determine whether these models can be validated in higher vertebrates and whether combination therapy with Ras and cAMP pathway effectors can improve the condition of some NF1 patients.
Figure 4. Effects of NF1 Loss of Function on the Ras and cAMP Pathways.
The genotypes of the zebrafish nf1 larvae that exhibited significant memory or learning deficits are shown. The pharmacological agents (italicized) that were used to improve memory or learning in these genotypes, as well as the molecular targets of the agents, are indicated. LOF, loss of function.
EXPERIMENTAL PROCEDURES
Generation and Maintenance of Zebrafish
The zebrafish (Danio rerio) larvae used in this study were generated from crosses of adults carrying the nf1aΔ5 and nf1b+10 mutant alleles (Shin et al., 2012). Embryos were raised at 28°C in a 14 hr/10 hr light/dark cycle as previously described (Burgess and Granato, 2007a) and all behavioral experiments were conducted with 5 days postfertilization (dpf) larvae. For visual behavioral experiments, larvae were PCR genotyped by clipping a small region of the caudal fin at 3 dpf and genotyping as described previously (Shin et al., 2012). Larvae tested for acoustic habituation were tested individually in a 4 × 4 grid and genotyped after testing. All animal protocols were approved by the University of Pennsylvania Institutional Animal Care and Use Committee.
Behavioral Assays and Analysis
Dark-flash-induced O-bend responses were elicited, recorded, and measured as previously described (Burgess and Granato, 2007a; Wolman et al., 2011). Larvae were trained and tested at a density of 15 larvae per 9 ml E3 in 6 cm Petri dishes and kept in the dishes during training or testing. To elicit memory formation, larvae were exposed to a training paradigm comprised of four 30 min training sessions, each consisting of exposure to a 1 s dark flash delivered every 15 s. Training sessions were separated by 10 min ISIs. After the fourth session and a 1 hr ISI, larvae were exposed to ten dark flashes with 1 min ISIs to evaluate memory recall. To calculate memory recall, the average latency to initiate an O-bend in untrained larvae was subtracted from the latency to initiate an O-bend in trained larvae. Memory consolidation was calculated by subtracting the average latency to initiate an O-bend in response to dark-flash stimuli 1–5 of training session 1 from the latency to initiate an O-bend in response to dark flashes 1–5 of sessions 2–4.
To measure visual short-term habituation, a series of 40 1 s dark flashes were delivered. Stimuli 1–10 were delivered with 30 s ISIs and stimuli 11–40 were delivered with 3 s ISIs. The percentage of habituation was calculated by dividing the mean O-bend responsiveness to stimuli 31–40 by the mean O-bend responsiveness to stimuli 1–10, subtracting this value from 1, and multiplying by 100. An acoustic short-term habituation assay was performed as previously described (Wolman et al., 2011).
Pharmacology
All compounds were added to the larval media 30 min before and throughout the training and testing paradigm. Cycloheximide (C4859; Sigma-Aldrich), U0126 (9903, Cell Signaling Technology), wortmannin (9951; Cell Signaling Technology), BKM120 (S2247; Selleck Chemicals), rolipram (R6520; Sigma-Aldrich), roflumilast (S2131, Selleck Chemicals), and 8-Br-cAMP (B007; BIOLOG Life Science Institute) were dissolved in 100% DMSO and administered in a final concentration of 1% DMSO. Doses of each compound were prescreened for potential effects on baseline O-bend responsiveness to visual stimuli and short-latency C-bend responsiveness to acoustic stimuli. The defined, stereotyped kinematic parameters of both larval maneuvers were also examined (Burgess and Granato, 2007a, 2007b). Selected doses did not change baseline behavior responsiveness or kinematic performance after 30 min or 4 hr of incubation. Immunohistochemistry with anti-phospho-ERK (4377; Cell Signaling Technology) and anti-phospho-(Ser/Thr) PKA substrate (9621; Cell Signaling Technology) was performed on paraffin-embedded larval tissue after fixation in 4% paraformaldehyde, dehydration, and sectioning at 8 µMthickness in order to demonstrate the pathway specificity of the pharmacologic inhibitors (Figure S3).
Supplementary Material
Acknowledgments
We thank Tom Look, Kurt Engleka, Kurt Marsden, and Min Min Lu for helpful discussions and technical support. This work was supported by grants from the NIH (2T32 HL007843 to E.D.d.G., R01 HL062974 to J.A.E., RO1 MH092257 and HD 37975A11 to M.G., and GM086902 to T.A.J.) and the Department of Defense (NF110108 to J.A.E. and AR1101189 to T.A.J.). S.M. was supported by grants from the FRAXA Research Foundation and the University of Pennsylvania (R25 MH060490, Clinical Research Scholars Program in Psychiatry).
Footnotes
Supplemental Information includes three figures and one movie and can be found with this article online at http://dx.doi.org/10.1016/j.celrep.2014.07.054.
AUTHOR CONTRIBUTIONS
E.D.d.G. and M.A.W. designed and performed experiments together and wrote the manuscript. M.G. and J.A.E. designed experiments, supervised the work, and edited the manuscript. S.M.M. and T.A.J. contributed reagents and advice on the experimental design and approach.
References
- Armstrong BC, Le Boutillier JC, Petit TL. Ultrastructural synaptic changes associated with neurofibromatosis type 1: a quantitative analysis of hippocampal region CA1 in a Nf1(+/−) mouse model. Synapse. 2012;66:246–255. doi: 10.1002/syn.21507. [DOI] [PubMed] [Google Scholar]
- Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P. The selectivity of protein kinase inhibitors: a further update. Biochem. J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck CD, Rankin CH. Heat shock disrupts long-term memory consolidation in Caenorhabditis elegans. Learn. Mem. 1995;2:161–177. doi: 10.1101/lm.2.3-4.161. [DOI] [PubMed] [Google Scholar]
- Beck CDO, Rankin CH. Long-term habituation is produced by distributed training at long ISIs and not by massed training or short ISIs in Caenorhabditis elegans. Anim. Learn. Behav. 1997;25:446–457. [Google Scholar]
- Brown JA, Gianino SM, Gutmann DH. Defective cAMP generation underlies the sensitivity of CNS neurons to neurofibromatosis-1 heterozygosity. J. Neurosci. 2010;30:5579–5589. doi: 10.1523/JNEUROSCI.3994-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JA, Diggs-Andrews KA, Gianino SM, Gutmann DH. Neurofibromatosis-1 heterozygosity impairs CNS neuronal morphology in a cAMP/PKA/ROCK-dependent manner. Mol. Cell. Neurosci. 2012;49:13–22. doi: 10.1016/j.mcn.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess HA, Granato M. Modulation of locomotor activity in larval zebrafish during light adaptation. J. Exp. Biol. 2007a;210:2526–2539. doi: 10.1242/jeb.003939. [DOI] [PubMed] [Google Scholar]
- Burgess HA, Granato M. Sensorimotor gating in larval zebrafish. J. Neurosci. 2007b;27:4984–4994. doi: 10.1523/JNEUROSCI.0615-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichowski K, Jacks T. NF1 tumor suppressor gene function: narrowing the GAP. Cell. 2001;104:593–604. doi: 10.1016/s0092-8674(01)00245-8. [DOI] [PubMed] [Google Scholar]
- Costa RM, Federov NB, Kogan JH, Murphy GG, Stern J, Ohno M, Kucherlapati R, Jacks T, Silva AJ. Mechanism for the learning deficits in a mouse model of neurofibromatosis type 1. Nature. 2002;415:526–530. doi: 10.1038/nature711. [DOI] [PubMed] [Google Scholar]
- Cui Y, Costa RM, Murphy GG, Elgersma Y, Zhu Y, Gutmann DH, Parada LF, Mody I, Silva AJ. Neurofibromin regulation of ERK signaling modulates GABA release and learning. Cell. 2008;135:549–560. doi: 10.1016/j.cell.2008.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis HP, Squire LR. Protein synthesis and memory: a review. Psychol. Bull. 1984;96:518–559. [PubMed] [Google Scholar]
- Diggs-Andrews KA, Tokuda K, Izumi Y, Zorumski CF, Wozniak DF, Gutmann DH. Dopamine deficiency underlies learning deficits in neurofibromatosis-1 mice. Ann. Neurol. 2013;73:309–315. doi: 10.1002/ana.23793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbinghaus H. Memory: A Contribution to Experimental Psychology. New York: Teachers College, Columbia University; 1885. [Google Scholar]
- Fahsold R, Hoffmeyer S, Mischung C, Gille C, Ehlers C, Kücükceylan N, Abdel-Nour M, Gewies A, Peters H, Kaufmann D, et al. Minor lesion mutational spectrum of the entire NF1 gene does not explain its high mutability but points to a functional domain upstream of the GAP-related domain. Am. J. Hum. Genet. 2000;66:790–818. doi: 10.1086/302809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming A, Diekmann H, Goldsmith P. Functional characterisation of the maturation of the blood-brain barrier in larval zebrafish. PLoS ONE. 2013;8:e77548. doi: 10.1371/journal.pone.0077548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouzi JY, Moressis A, Walker JA, Apostolopoulou AA, Palmer RH, Bernards A, Skoulakis EM. The receptor tyrosine kinase Alk controls neurofibromin functions in Drosophila growth and learning. PLoS Genet. 2011;7:e1002281. doi: 10.1371/journal.pgen.1002281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilding C, McNair K, Stone TW, Morris BJ. Restored plasticity in a mouse model of neurofibromatosis type 1 via inhibition of hyperactive ERK and CREB. Eur. J. Neurosci. 2007;25:99–105. doi: 10.1111/j.1460-9568.2006.05238.x. [DOI] [PubMed] [Google Scholar]
- Guo HF, The I, Hannan F, Bernards A, Zhong Y. Requirement of Drosophila NF1 for activation of adenylyl cyclase by PACAP38-like neuropeptides. Science. 1997;276:795–798. doi: 10.1126/science.276.5313.795. [DOI] [PubMed] [Google Scholar]
- Guo HF, Tong J, Hannan F, Luo L, Zhong Y. A neurofibromatosis-1-regulated pathway is required for learning in Drosophila. Nature. 2000;403:895–898. doi: 10.1038/35002593. [DOI] [PubMed] [Google Scholar]
- Hannan F, Ho I, Tong JJ, Zhu Y, Nurnberg P, Zhong Y. Effect of neurofibromatosis type I mutations on a novel pathway for adenylyl cyclase activation requiring neurofibromin and Ras. Hum. Mol. Genet. 2006;15:1087–1098. doi: 10.1093/hmg/ddl023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegedus B, Dasgupta B, Shin JE, Emnett RJ, Hart-Mahon EK, Elghazi L, Bernal-Mizrachi E, Gutmann DH. Neurofibromatosis-1 regulates neuronal and glial cell differentiation from neuroglial progenitors in vivo by both cAMP- and Ras-dependent mechanisms. Cell Stem Cell. 2007;1:443–457. doi: 10.1016/j.stem.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Ho IS, Hannan F, Guo HF, Hakker I, Zhong Y. Distinct functional domains of neurofibromatosis type 1 regulate immediate versus long-term memory formation. J. Neurosci. 2007;27:6852–6857. doi: 10.1523/JNEUROSCI.0933-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SL, Shores A, North KN. The nature and frequency of cognitive deficits in children with neurofibromatosis type 1. Neurology. 2005;65:1037–1044. doi: 10.1212/01.wnl.0000179303.72345.ce. [DOI] [PubMed] [Google Scholar]
- Hyman SL, Arthur Shores E, North KN. Learning disabilities in children with neurofibromatosis type 1: subtypes, cognitive profile, and attention- deficit-hyperactivity disorder. Dev. Med. Child Neurol. 2006;48:973–977. doi: 10.1017/S0012162206002131. [DOI] [PubMed] [Google Scholar]
- Isenberg JC, Templer A, Gao F, Titus JB, Gutmann DH. Attention skills in children with neurofibromatosis type 1. J. Child Neurol. 2013;28:45–49. doi: 10.1177/0883073812439435. [DOI] [PubMed] [Google Scholar]
- Jayachandran R, Liu X, Bosedasgupta S, Müller P, Zhang CL, Moshous D, Studer V, Schneider J, Genoud C, Fossoud C, et al. Coronin 1 regulates cognition and behavior through modulation of cAMP/protein kinase A signaling. PLoS Biol. 2014;12:e1001820. doi: 10.1371/journal.pbio.1001820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsgodt KH, Rosser T, Lutkenhoff ES, Cannon TD, Silva A, Bearden CE. Alterations in white matter microstructure in neurofibromatosis-1. PLoS ONE. 2012;7:e47854. doi: 10.1371/journal.pone.0047854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine TM, Materek A, Abel J, O’Donnell M, Cutting LE. Cognitive profile of neurofibromatosis type 1. Semin. Pediatr. Neurol. 2006;13:8–20. doi: 10.1016/j.spen.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Li W, Cui Y, Kushner SA, Brown RA, Jentsch JD, Frankland PW, Cannon TD, Silva AJ. The HMG-CoA reductase inhibitor lovastatin reverses the learning and attention deficits in a mouse model of neurofibromatosis type 1. Curr. Biol. 2005;15:1961–1967. doi: 10.1016/j.cub.2005.09.043. [DOI] [PubMed] [Google Scholar]
- Liao JK, Laufs U. Pleiotropic effects of statins. Annu. Rev. Pharmacol. Toxicol. 2005;45:89–118. doi: 10.1146/annurev.pharmtox.45.120403.095748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maira SM, Pecchi S, Huang A, Burger M, Knapp M, Sterker D, Schnell C, Guthy D, Nagel T, Wiesmann M, et al. Identification and characterization of NVP-BKM120, an orally available pan-class I PI3-kinase inhibitor. Mol. Cancer Ther. 2012;11:317–328. doi: 10.1158/1535-7163.MCT-11-0474. [DOI] [PubMed] [Google Scholar]
- Peng S, Yang X, Liu GJ, Zhang XQ, Wang GL, Sun HY. The phosphodiesterase-4 inhibitor Ro 20–1724 reverses learning and memory impairments, and down-regulation of CREB in the hippocampus and cortex induced by ketamine anesthesia in immature rats. J. Neurosurg. Sci. 2014 [PubMed] [Google Scholar]
- Richter W, Menniti FS, Zhang HT, Conti M. PDE4 as a target for cognition enhancement. Expert Opin. Ther. Targets. 2013;17:1011–1027. doi: 10.1517/14728222.2013.818656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AC, Bill BR, Glanzman DL. Learning and memory in zebrafish larvae. Front Neural Circuits. 2013;7:126. doi: 10.3389/fncir.2013.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilyansky C, Karlsgodt KH, Cummings DM, Sidiropoulou K, Hardt M, James AS, Ehninger D, Bearden CE, Poirazi P, Jentsch JD, et al. Neurofibromin regulates corticostriatal inhibitory networks during working memory performance. Proc. Natl. Acad. Sci. USA. 2010;107:13141–13146. doi: 10.1073/pnas.1004829107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J, Padmanabhan A, de Groh ED, Lee J-S, Haidar S, Dahlberg S, Guo F, He S, Wolman MA, Granato M, et al. Zebrafish neurofibromatosis type 1 genes have redundant functions in tumorigenesis and embryonic development. Dis. Model. Mech. 2012;5:881–894. doi: 10.1242/dmm.009779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AJ, Frankland PW, Marowitz Z, Friedman E, Laszlo GS, Cioffi D, Jacks T, Bourtchuladze R. A mouse model for the learning and memory deficits associated with neurofibromatosis type I. Nat. Genet. 1997;15:281–284. doi: 10.1038/ng0397-281. [DOI] [PubMed] [Google Scholar]
- The I, Hannigan GE, Cowley GS, Reginald S, Zhong Y, Gusella JF, Hariharan IK, Bernards A. Rescue of a Drosophila NF1 mutant phenotype by protein kinase A. Science. 1997;276:791–794. doi: 10.1126/science.276.5313.791. [DOI] [PubMed] [Google Scholar]
- Thompson RF, Spencer WA. Habituation: a model phenomenon for the study of neuronal substrates of behavior. Psychol. Rev. 1966;73:16–43. doi: 10.1037/h0022681. [DOI] [PubMed] [Google Scholar]
- Tong J, Hannan F, Zhu Y, Bernards A, Zhong Y. Neurofibromin regulates G protein-stimulated adenylyl cyclase activity. Nat. Neurosci. 2002;5:95–96. doi: 10.1038/nn792. [DOI] [PubMed] [Google Scholar]
- Walker JA, Tchoudakova AV, McKenney PT, Brill S, Wu D, Cowley GS, Hariharan IK, Bernards A. Reduced growth of Drosophila neurofibromatosis 1 mutants reflects a non-cell-autonomous requirement for GTPase-Activating Protein activity in larval neurons. Genes Dev. 2006;20:3311–3323. doi: 10.1101/gad.1466806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JA, Gouzi JY, Long JB, Huang S, Maher RC, Xia H, Khalil K, Ray A, Van Vactor D, Bernards R, Bernards A. Genetic and functional studies implicate synaptic overgrowth and ring gland cAMP/PKA signaling defects in the Drosophila melanogaster neurofibromatosis-1 growth deficiency. PLoS Genet. 2013;9:e1003958. doi: 10.1371/journal.pgen.1003958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolman MA, Jain RA, Liss L, Granato M. Chemical modulation of memory formation in larval zebrafish. Proc. Natl. Acad. Sci. USA. 2011;108:15468–15473. doi: 10.1073/pnas.1107156108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.