In the past four decades, substantial progress has been made in breast cancer survival in part because of advances in early detection and treatment following diagnosis.1,2 Further, recent studies3,4 have reported that the observed improvement in breast cancer mortality and survival between the 1970s and 2000s is also the result of changes in the distribution of tumor characteristics, which include the identification of the human epidermal growth factor receptor 2 and the development of the targeted agent such as trastuzumab that extends survival in both the adjuvant and metastatic settings for a range of 15%–25% of patients with human epidermal growth factor receptor 2–positive tumors. For example, in 2012, the annual age-adjusted breast cancer mortality was approximately 21 per 100,000 and 5-year relative survival exceeded 90%.5 Despite this progress, not all age, racial/ethnic, or socioeconomic groups have benefited equally, and disparities in incidence and mortality still exist.6 During the past four decades, incidence of breast cancer was much higher in older women (aged >50 years) and the survival rate was lower in younger women (aged <50 years).7 This is in part because breast cancer in women aged 15–44 years (henceforth referred to as younger women) is often characterized by aggressive tumor subtypes that are less likely to be amenable to treatment at the time of diagnosis and have poorer survival outcomes.8–11 As a result, these breast cancers could result in more devastating health outcomes and economic burden to younger women, their families, and society.
In recent years, there has been increased interest in breast cancer among younger women. In 2009, the Education and Awareness Requires Learning Young Act, which is presented in detail in Section 10413 of the Patient Protection and Affordable Care Act,12 authorized CDC to conduct research and develop initiatives that increase knowledge in evidence-based approaches to advance understanding and awareness of breast health and breast cancer among younger women. To provide information to support resource allocation decisions that effectively increase awareness and support among younger women diagnosed with breast cancer, the authors set out to quantify the economic burden in this population. To date, there have been no national studies specifically quantifying the economic burden of breast cancer in younger women in the U.S. The available estimates are at the aggregate level regardless of specific age group. For instance, a study13 reported economic burden on breast cancer care for all women to be $16.5 billion in 2010 dollars. Although these are useful data for decision making, they mask information on the burden of breast cancer outcomes and economic costs in younger women. Therefore, gaps exist in knowledge about the health outcomes and economic burden of breast cancer in this population. Historically, economic burden studies have proven to be useful for providing a basis for decision making in program planning and evaluating interventions such as cancer screening programs.
The five articles in this theme issue attempt to address the economic burden of breast cancer among younger women. In this issue, economic burden was defined as changes in health-related quality of life (HRQoL), a measure of resources used for medical care and loss of economic resources associated with the diagnosis and treatment of breast cancer in this population. Given this definition, the articles presented in this theme issue consist of two categories:
assessment of health outcomes; and
assessment of economic impacts.
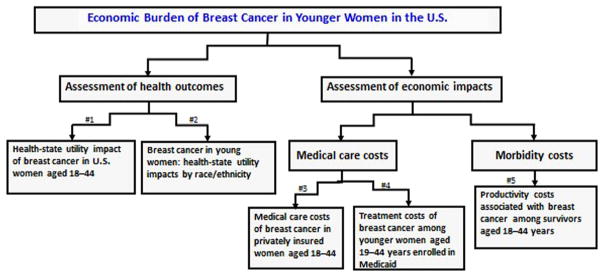
Figure 1 illustrates the conceptual framework of how the articles fit together to provide up-to-date data that could be used to inform budget, clinical, and health promotion decisions in this population.
Figure 1.
Illustration of the conceptual framework of the articles in this theme issue.
Note: # means the number of articles in the theme issue. Health state utility (HSU) values are scaled to a single 0 (dead) to 1 (best health) cardinal index. HSU is a special health-related quality of life measure that represents global health-related well-being, is based on preference-based tradeoffs, and is used in economic evaluations to value improvements in morbidity and mortality from interventions (e.g., quality-adjusted life-years).
The first two articles address issues on health outcomes that reflect women’s self-assessment of the impact of breast cancer treatment(s) on their HRQoL and well-being. The article by Brown et al.14 provides new and important data on the burden of breast cancer in younger women for use in the economic evaluation of intervention programs and public health surveillance. The authors used a measure known as the health state utility (HSU), a cardinal scale that represents a 0 (worst health) to 1 (best health) summary of preference-weighted outcomes over HRQoL outcomes. They found that the burden of breast cancer in terms of HSU was significantly larger for younger women compared with women aged ≥45 years. Additionally, the HSU impact on breast cancer was significantly larger than that found for other types of cancer. These differences underscore the importance of conducting age-specific analysis when measuring HRQoL and HSU values in economic evaluation of public health programs and when making medical decisions for cancer treatment among younger women. The results also suggest that clinicians and public health officials should consider placing greater emphasis on identification of preventive measures for younger women who may be at greater risk for breast cancer, as the HRQoL burden for a woman aged <45 years is significantly larger than that caused by other cancers.
The second article by Trogdon and colleagues15 complements Brown et al.14 by further examining the HRQoL impacts of breast cancer, measured by HSU, age of diagnosis, and race/ethnicity. Prior to this paper, little was known about the effect of breast cancer diagnosis on HRQoL among younger women. The existing literature suggests that younger women with breast cancer may face substantial HRQoL challenges, including chemotherapy-induced menopause, decreased sexual function, infertility, diminished body image, and other side effects.16–19 There is also evidence that the HRQoL effects of breast cancer vary by race/ethnicity.20–24 Trogdon and colleagues15 reported three key findings. First, the HRQoL effects of breast cancer are larger among women diagnosed at younger ages. Second, the HRQoL effects of breast cancer are concentrated in the first year after diagnosis, with larger effects among women diagnosed at younger ages. Third, there are significant differences in the HRQoL effects of breast cancer by race/ethnicity. The results highlight the need for separate quality of life adjustments for women by age at diagnosis and race/ethnicity when conducting cost-effectiveness analysis of breast cancer prevention, detection, and treatment.
The last three articles assessed economic impacts and described the direct medical care costs consumed in treating breast cancer and indirect morbidity costs associated with the diagnosis of breast cancer in this population. Two of the articles focused on direct medical care costs: Allaire et al.25 examined medical care costs of breast cancer in privately insured younger women, and Ekwueme and colleagues26 presented estimates of treatment costs of breast cancer among younger women enrolled in Medicaid. The last article in the series by Ekwueme et al.27 focused on indirect morbidity costs and presented estimates of productivity costs associated with younger breast cancer survivors. The cost estimates reported in these three articles are prevalence-based, which represent economic cost burden for all young women breast cancer survivors alive in a specific year. Prevalence cost estimates can be useful for informing the design of insurance benefits, eligibility criteria for public programs, and budgeting for future program costs.28
The study by Allaire and colleagues25 utilized the MarketScan database, which contains one of the nation’s largest administrative claims data on people who have employment-based health insurance. The database included de-identified, person-specific outpatient, inpatient, and retail pharmaceutical claims from approximately 100 large payers, representing millions of covered lives.29 Treatment costs for younger breast cancer patients were compared with a matched sample of younger women without breast cancer and for those in active treatment. Allaire et al.25 found that younger women incurred >$24,000 in additional direct medical care costs per person per year compared with younger women without breast cancer. The reported treatment cost was twice higher (>$52,000) for those in active treatment. Also, they found that nearly all (96%) of these costs occurred in an outpatient setting. These estimates indicate that breast cancer is a costly illness to treat among younger, privately insured women. The findings underscore the potential financial vulnerability of women in this age group and the importance of health insurance during this time in life.
The second medical care costs article by Ekwueme and colleagues26 used Medicaid Analytic eXtract claims and enrollment data maintained by the Centers for Medicare and Medicaid Services containing information from 50 states and the District of Columbia. The study sample was women ages 19–44 enrolled in fee-for-service Medicaid. Because much of the Medicaid Analytic eXtract population was enrolled in Medicaid for less than a full calendar year, the authors reported monthly Medicaid payments to accurately reflect the healthcare needs for each woman. The estimates were reported by mutually exclusive racial/ethnic categories and by service types. The estimates were compared with the same age group without breast cancer. The analysis found that monthly direct medical cost for breast cancer treatment among younger women enrolled in Medicaid was $5,711 per woman. The monthly cost for outpatient and inpatient services was $4,058 and $1,003, respectively, and prescription drug costs were $539. By race/ethnicity, the authors found that non-Hispanic white women had the highest monthly total medical costs, followed by Hispanic women, and then non-Hispanic women of other race. These findings can inform future planning for treatment cost of invasive breast cancer in low-income younger women enrolled in Medicaid.
The final article in this theme collection by Ekwueme et al.27 focused on productivity costs associated with younger breast cancer survivors. This article is based on the premise that cancer diagnosis and treatment may have variable effects on employment. Even if younger women who have survived do not become unemployed, there may be a degree of underemployment or loss of income. This issue has been poorly quantified in the literature, especially for younger cancer survivors, who are more at risk for longer and late effects of cancer treatment. The authors used a nationally representative sample from the National Health Interview Survey, 2000–2010, to address the following questions:
What are the costs of forgone earnings from missed work days associated with breast cancer for employed women?
What are the costs of lost home productivity associated with breast cancer?
How do the breast cancer–associated productivity costs among younger survivors compare with those of older survivors aged 45–64 years?
They found that work loss costs were higher per capita among younger employed women than older employed women. In general, the study showed that younger women with a history of breast cancer face a disproportionate share of work and home productivity losses. The authors postulated that this finding may be explained by the fact that breast cancer in younger women is often more severe, requiring more-aggressive treatment that can precipitate a range of physical, functional, and psychosocial long-term and late effects, leading to more frequent and longer absenteeism from work.30,31
Applications of the Findings
The set of articles presented in this issue have several applications. First, one of the primary goals of disease prevention is to improve HRQoL outcomes of affected individuals. However, efforts to improve HRQoL may involve a patient being treated with many cancer treatments such as surgery, chemotherapy, radiation, and hormonal therapy. These treatment regimens are associated with late and long-term health effects such as cardiotoxicity, lymphedema, sexual dysfunction, incontinence, pain and fatigue, cognitive dysfunction, and psychological distress.32 The findings in this theme issue provide data on women’s self-assessment of how breast cancer treatments have affected their lives and well-being. This is important information that could be used for evaluating treatment efficacy and effectiveness and interpreting clinical outcomes of breast cancer treatment in younger women. Further, the findings could be used to conduct cost-effectiveness or cost-utility analysis of breast cancer on primary, secondary, or tertiary prevention programs in this population.33
Second, there are multiple uses for economic data reported in this theme collection; however, most of the applications may center on two broad categories:
assessment of economic burden; and
economic evaluation of specific breast cancer interventions.
In the first category, the cost estimates in this issue take a national-level perspective, which means that the reported findings could be used to inform decisions on resource allocation in breast cancer prevention at the national level. Further, given the substantial productivity losses associated with breast cancer among younger women, the reported findings could inform the potential value of developing interventions to reduce side effects associated with cancer treatment. Many young women are surviving breast cancer, but these survivors face many challenges with medical care follow-up, managing the long-term and late effects of treatments,34 monitoring the possibility of recurrence, and an increased risk for additional cancers.34,35 In addition, as reported in this issue, these women experience limitations in their HRQoL.14,15
In the second category, the reported data in this issue can directly be used as inputs to conduct economic evaluations of specific healthcare interventions or programs related to breast cancer in younger women. For example, these findings can be used in estimating the cost-effectiveness or cost-utility analysis of breastfeeding as a primary prevention for breast cancer in younger women privately insured or publicly insured through Medicaid. In this example, the medical care costs data from privately insured reported by Allaire and colleagues25 and from Medicaid enrollees reported by Ekwueme et al.,26 along with the reported health outcomes by Brown and colleagues14 and Trogdon et al.,15 can be used to estimate the cost effectiveness of this intervention either in privately or publically insured women compared with two or more alternative programs. Similarly, an analyst could also use the presented cost data to conduct a budget impact analysis to appraise the financial impact and the feasibility and affordability of breastfeeding interventions in this population.
Implications for Prevention Programs
From a public health perspective, the data reported in this collection of papers could be used to effectively develop or evaluate intervention programs in this population. For example, CDC is implementing a program on “multiple approaches to increase awareness and support among young women diagnosed with breast cancer.”36 This program is designed to develop a strategic and integrated health communication, marketing, and media approach to disseminating health messages to young women breast cancer survivors and to provide structured support services from diagnosis through post treatment. As this program is being implemented, the reported findings in this issue could provide information on the magnitude of economic burden of breast cancer on subpopulations of young women facing the disease. This information can potentially inform the allocation of resources to subgroups with disproportionate burden and effectively channel targeted interventions to this group.
In addition to the new program, CDC has recently launched a multimedia national campaign with the objectives to educate young women on the risk factors for breast cancer before age 45 years, encourage them to learn their family history of breast and ovarian cancer, inspire them to talk to their healthcare provider if they think they might be at higher risk for breast cancer, and encourage them to live healthy lifestyles and be aware of their own breast health.37 Implementing these objectives could save as well as improve the lives of these women and subsequently reduce the substantial loss in HRQoL and the huge economic burden reported in this theme issue. Because these women are at working age, a cancer diagnosis and subsequent treatment(s) may interrupt employment and have a lasting negative impact on present and future earnings, career development, and perhaps retirement decisions. Therefore, efforts to prevent breast cancer by raising awareness and risk reduction strategies such as maintaining physical activity through childhood, adolescence, and adult years38; increasing breastfeeding rates for a reasonable length of time39; and, for high-risk women, increasing access to prophylactic treatment such as surgery or use of medications like tamoxifen40,41 could eventually improve health outcomes and economic outlook in this population. These primary prevention measures are essential, particularly for younger women aged 18–44 years for whom early detection through screening is not recommended.42
Moving Forward
Together, the papers presented in this theme issue provide health outcomes and economic cost data needed to make informed clinical and health promotion decisions related to breast cancer among younger women. With the availability of these data, healthcare decision makers, public health programs, and researchers will have state-of-the-art economic burden data on breast cancer among younger women to support prevention activities.
While moving forward to ascertain the health outcomes and economic burden of breast cancer in younger women, it is important to note that more work is needed to fully understand the total economic burden of breast cancer in this population. For example, missing from the collection of papers in this issue are mortality and non-medical (financial burden) costs. Although a recent study has reported on the economic impact associated with premature death in younger women, an update of this study may be needed, specifically for women aged 18–44 years.7 In addition, studies on financial burden such as out-of-pocket/family income spent during medical care, transportation costs to and from medical care treatments, and child/dependent care costs, particularly for women at childbearing age are not included in this compilation of papers and are currently lacking in the literature.
In addition to economic burden studies, other aspects of economic analysis such as economic evaluation studies on interventions to prevent breast cancer in this population are lacking. This aspect of economic analysis compares the costs and health outcomes of an intervention (e.g., breastfeeding) with those of an alternative intervention (usually the current standard of practice). It does not describe clinical effectiveness, but addresses questions such as whether additional health benefits can be obtained from the same amount of resources or whether the same benefit can be achieved with fewer resources by use of another intervention. This type of analysis calls for the use of cost-effectiveness or cost-utility analyses of which the data reported in this issue would be used as input variables.
The burden of cancer affects nearly everyone in the U.S. Although many younger women are surviving breast cancer, these women have substantial medical expenditures among both those privately or publically insured, have lower HRQoL, and have lost employment and home productivity because of poor health. These findings may be difficult to identify in aggregate studies. Therefore, it is the authors’ hope that the findings reported in this theme issue fill some of the knowledge gaps and provide awareness regarding the economic burden of breast cancer among younger women.
Footnotes
The findings and conclusions in this editorial are those of the authors and do not necessarily represent the official position of CDC.
No financial disclosures were reported by the authors of this paper.
References
- 1.Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784–1792. doi: 10.1056/NEJMoa050518. http://dx.doi.org/10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 2.Elkin EB, Hudis CA. Parsing progress in breast cancer. J Clin Oncol. 2015;33(26):2837–2838. doi: 10.1200/JCO.2015.62.4890. http://dx.doi.org/10.1200/JCO.2015.62.4890. [DOI] [PubMed] [Google Scholar]
- 3.Park J-H, Andersen WF, Gail MH. Improvements in U.S. breast cancer survival and proportion explained by tumor size and estrogen-receptor status. J Clin Oncol. 2015;33(26):2870–2876. doi: 10.1200/JCO.2014.59.9191. http://dx.doi.org/10.1200/JCO.2014.59.9191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Figueroa-Magalhães MC, Jelovac D, Connolly RM, et al. Treatment of HER2-positive breast cancer. Breast. 2014;23(2):128–136. doi: 10.1016/j.breast.2013.11.011. http://dx.doi.org/10.1016/j.breast.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howlader N, Noone AM, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975–2012. National Cancer Institute; Bethesda, MD: [Accessed October 5, 2015]. www.seer.cancer.gov/csr/1975_2012/. Published 2015. [Google Scholar]
- 6.Bao Y, Fox SA, Escarce JJ. Socioeconomic and racial/ethnic differences in the discussion of cancer screening: “between-” versus “within-” physician differences. Health Serv Res. 2007;42(3 pt 1):950–970. doi: 10.1111/j.1475-6773.2006.00638.x. http://dx.doi.org/10.1111/j.1475-6773.2006.00638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ekwueme DU, Guy, Rim SH, et al. Health and economic impact of breast cancer mortality in young women, 1970–2008. Am J Prev Med. 2014;46(1):71–79. doi: 10.1016/j.amepre.2013.08.016. http://dx.doi.org/10.1016/j.amepre.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herdman R, Norton L, editors. Saving Women’s Lives: Strategies for Improving Breast Cancer Detection and Diagnosis: A Breast Cancer Research Foundation and Institute of Medicine Symposium. Washington, DC: National Academies Press; 2005. [PubMed] [Google Scholar]
- 9.Figueiredo JC, Ennis M, Knight JA, et al. Influence of young age at diagnosis and family history of breast or ovarian cancer on breast cancer outcomes in a population-based cohort study. Breast Cancer Res Treat. 2007;105(1):69–80. doi: 10.1007/s10549-006-9433-3. http://dx.doi.org/10.1007/s10549-006-9433-3. [DOI] [PubMed] [Google Scholar]
- 10.Klauber-DeMore N. Tumor biology of breast cancer in young women. Breast Disease. 2005–2006;23:9–15. doi: 10.3233/bd-2006-23103. [DOI] [PubMed] [Google Scholar]
- 11.Zabicki K, Colbert JA, Dominguez FJ, et al. Breast cancer diagnosis in women < or = 40 versus 50 to 60 years: increasing size and stage disparity compared with older women over time. Ann Surg Oncol. 2006;13(8):1072–1077. doi: 10.1245/ASO.2006.03.055. http://dx.doi.org/10.1245/ASO.2006.03.055. [DOI] [PubMed] [Google Scholar]
- 12.Breast Cancer Education and Awareness Requires Learning Young (EARLY) Act of 2009 (H.R. 1740, S. 994) www.govtrack.us/congress/bills/111/hr1740/text.
- 13.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103(2):117–128. doi: 10.1093/jnci/djq495. http://dx.doi.org/10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown DS, Trogdon JG, Ekwueme DU, et al. Health-state utility impact of breast cancer in U.S. women aged 18–44. Am J Prev Med. 2016;50(2):255–261. doi: 10.1016/j.amepre.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 15.Trogdon JG, Ekwueme DU, Chamiec-Case L, Guy GP. Breast cancer in young women: health state utility impacts by race/ethnicity. Am J Prev Med. 2016;50(2):262–269. doi: 10.1016/j.amepre.2015.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pritchard KI. Adjuvant therapy of the 370 very young woman. Breast. 2007;16:S136–S146. doi: 10.1016/j.breast.2007.07.023. http://dx.doi.org/10.1016/j.breast.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 17.Arora NK, Gustafson DH, Hawkins RP, et al. Impact of surgery and chemotherapy on the quality of life of younger women with breast carcinoma—a prospective study. Cancer. 2001;92(5):1288–1298. doi: 10.1002/1097-0142(20010901)92:5<1288::aid-cncr1450>3.0.co;2-e. http://dx.doi.org/10.1002/1097-0142(20010901)92:5<1288::AID-CNCR1450>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 18.Baucom DH, Porter LS, Kirby JS, Gremore TM, Keefe FJ. Psychosocial issues confronting young women with breast cancer. Breast Dis. 2005;23:103–113. doi: 10.3233/bd-2006-23114. [DOI] [PubMed] [Google Scholar]
- 19.Kornblith AB, Powell M, Regan MM, et al. Long-term psychosocial adjustment of older vs younger survivors of breast and endometrial cancer. Psychooncology. 2007;16(10):895–903. doi: 10.1002/pon.1146. http://dx.doi.org/10.1002/pon.1146. [DOI] [PubMed] [Google Scholar]
- 20.Janz NK, Mujahid MS, Hawley ST, et al. Racial/ethnic differences in quality of life after diagnosis of breast cancer. J Cancer Surviv. 2009;3(4):212–222. doi: 10.1007/s11764-009-0097-y. http://dx.doi.org/10.1007/s11764-009-0097-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ashing-Giwa KT, Tejero JS, Kim J, Padilla GV, Hellemann G. Examining predictive models of HRQOL in a population-based, multiethnic sample of women with breast carcinoma. Qual Life Res. 2007;16(3):413–428. doi: 10.1007/s11136-006-9138-4. http://dx.doi.org/10.1007/s11136-006-9138-4. [DOI] [PubMed] [Google Scholar]
- 22.Bowen DJ, Alfano CM, McGregor BA, et al. Possible socioeconomic and ethnic disparities in quality of life in a cohort of breast cancer survivors. Breast Cancer Res Treat. 2007;106(1):85–95. doi: 10.1007/s10549-006-9479-2. http://dx.doi.org/10.1007/s10549-006-9479-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yanez B, Thompson EH, Stanton AL. Quality of life among Latina breast cancer patients: a systematic review of the literature. J Cancer Surviv. 2011;5(2):191–207. doi: 10.1007/s11764-011-0171-0. http://dx.doi.org/10.1007/s11764-011-0171-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Von Ah DM, Russell KM, Carpenter J, et al. Health-related quality of life of African American breast cancer survivors compared with healthy African American women. Cancer Nurs. 2012;35(5):337–346. doi: 10.1097/NCC.0b013e3182393de3. http://dx.doi.org/10.1097/NCC.0b013e3182393de3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allaire BT, Ekwueme DU, Guy GP, et al. Medical care costs of breast cancer in privately insured women aged 18–44 years. Am J Prev Med. 2016;50(2):270–277. doi: 10.1016/j.amepre.2015.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ekwueme DU, Allaire BT, Arnold SE, Guy GP, Trogdon JG. Treatment costs of breast cancer among younger women aged 19 to 44 years enrolled in Medicaid. Am J Prev Med. 2016;50(2):278–285. doi: 10.1016/j.amepre.2015.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ekwueme DU, Trogdon JG, Khavjou OA, Guy GP. Productivity costs associated with breast cancer among survivors aged 18–44 years. Am J Prev Med. 2016;50(2):286–294. doi: 10.1016/j.amepre.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Yabroff KR, Warren JL, Banthin J, et al. Comparison of approaches for estimating prevalence costs of care for cancer patients: what is the impact of data source? Med Care. 2009;47(suppl 1):S64–S69. doi: 10.1097/MLR.0b013e3181a23e25. http://dx.doi.org/10.1097/MLR.0b013e3181a23e25 (7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marketscan Research Database: User Guide and Database Dictionary. Ann Arbor, MI: Truven Health Analytics; 2012. Truven Health Analytics. [Google Scholar]
- 30.Partridge AH, Goldhirsch A, Gelber S, Gelber RD. Chapter 85: Breast cancer in younger women. In: Harris JR, Lippman ME, Morrow M, Osborne CK, editors. Diseases of the Breast. 5. Philadelphia, PA: Lippincott Williams & Wilkins; 2014. [Google Scholar]
- 31.Anders CK, Johnson R, Litton J, Phillips M, Bleyer A. Breast cancer before age 40 years. Semin Oncol. 2009;36(3):237–249. doi: 10.1053/j.seminoncol.2009.03.001. http://dx.doi.org/10.1053/j.seminoncol.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hewitt M, Greenfield S, Stovall E. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC: National Academies Press; 2006. [Google Scholar]
- 33.Winn AN, Ekwueme DU, Guy GP, Neumann PJ. Cost-utility analysis of cancer prevention, treatment, and control: a systematic review. Am J Prev Med. 2016;50(2):241–248. doi: 10.1016/j.amepre.2015.08.009. http://dx.doi.org/10.1016/j.amepre.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ng AK, Travis LB. Second primary cancers: an overview. Hematol Oncol Clin North Am. 2008;22(2):271–289. doi: 10.1016/j.hoc.2008.01.007. http://dx.doi.org/10.1016/j.hoc.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 35.Dowling EC, Chawla N, Forsythe LP, et al. Lost productivity and burden of illness in cancer survivors with and without other chronic conditions. Cancer. 2013;119(18):3393–3401. doi: 10.1002/cncr.28214. http://dx.doi.org/10.1002/cncr.28214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.CDC. [Accessed October 5, 2015];Multiple approaches to increase awareness and support among young women diagnosed with breast cancer. www.cdc.gov/cancer/breast/young_women/young_women_foa.htm.
- 37.CDC. [Accessed October 5, 2015];Bring Your Brave Campaign. www.cdc.gov/cancer/breast/young_women/bringyourbrave/index.htm.
- 38.World Cancer Research Fund. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington, DC: AICR; 2007. [Google Scholar]
- 39.Lipworth LL, Bailey R, Trichopoulos D. History of breast-feeding in relation to breast cancer risk: a review of the epidemiologic literature. J Natl Cancer Inst. 2000;92(4):302–312. doi: 10.1093/jnci/92.4.302. http://dx.doi.org/10.1093/jnci/92.4.302. [DOI] [PubMed] [Google Scholar]
- 40.Visvanathan K, Hurley P, Bantug E, et al. Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2013;31(23):2942–2962. doi: 10.1200/JCO.2013.49.3122. http://dx.doi.org/10.1200/JCO.2013.49.3122. [DOI] [PubMed] [Google Scholar]
- 41.IOM. Breast Cancer and the Environment: A Life Course Approach. Washington, DC: National Academies Press; 2012. [Google Scholar]
- 42.Oeffinger KC, Fontham ET, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599–1614. doi: 10.1001/jama.2015.12783. http://dx.doi.org/10.1001/jama.2015.12783. [DOI] [PMC free article] [PubMed] [Google Scholar]