SUMMARY
We used local field potentials (LFPs) and spikes to investigate representations of visual space in prefrontal cortex and the dynamics of those representations during eye movements. Spatial information contained in LFPs of the frontal eye field (FEF) was differentially distributed across frequencies, with a majority of that information being carried in alpha and high-gamma bands and minimal signal in the low-gamma band. During fixation, spatial information from alpha and high-gamma bands and spiking activity was robust across cortical layers. Receptive fields (RFs) derived from alpha and high-gamma bands were retinocentrically organized, and they were spatially correlated both with each other and with spiking RFs. However, alpha and high-gamma RFs probed before eye movements were dissociated. Whereas high-gamma and spiking RFs immediately converged toward the movement goal, alpha RFs remained largely unchanged during the initial probe response, but they converged later. These observations reveal possible mechanisms of dynamic spatial representations that underlie visual perception during eye movements.
In Brief
Chen et al. report dissonant representations of visual space in prefrontal cortex, carried in the spiking activity and the local field potentials (LFPs), which ultimately converge toward the goal of saccadic eye movements. These observations have important implications for the neural mechanisms underlying visual perception during eye movements.
INTRODUCTION
Local field potentials (LFPs) are low-frequency extracellular voltage fluctuations that reflect multiple sources (Buzsáki et al., 2012; Einevoll et al., 2013), including synaptic activity (Haider et al., 2016; Mitzdorf, 1985), action potentials (Berens et al., 2011; Ray and Maunsell, 2011), and intrinsic voltage-dependent membrane responses (Silva et al., 1991). The study of LFPs thus provides a unique window into synaptic processes and neural networks at multiple temporal and frequency scales. In the primate brain, LFPs have been extensively studied and linked to visual stimulus coding (Henrie and Shapley, 2005; Katzner et al., 2009; Liu and Newsome, 2006; Ray and Maunsell, 2010), visual attention (Buschman and Miller, 2009; Fries et al., 2001; Gregoriou et al., 2014), and perceptual reports (Gail et al., 2004; Wilke et al., 2006; Wimmer et al., 2016; Womelsdorf et al., 2006), and they have been demonstrated to provide additional information over the spiking activity about the underlying neural mechanisms. For example, recent evidence suggests that, in cortex, lower LFP frequencies (6–16 Hz, which include the alpha band) reflect synaptic inputs from distal subcortical and cortical sources (Belitski et al., 2008; Saalmann et al., 2012; van Kerkoerle et al., 2014; Vijayan and Kopell, 2012), whereas higher frequencies (80–150 Hz, high-gamma band) reflect processes from more local sources (Ray et al., 2008; Ray and Maunsell, 2010). LFPs can thus provide information about the underlying neural circuitry of visual processing.
The neural representation of visual space relies on the receptive field (RF) organization of neurons throughout the visual system. A number of studies have demonstrated that the RFs of visual neurons can change due to behavioral demands (Connor et al., 1996; Merrikhi et al., 2017; Moran and Desimone, 1985; Reynolds et al., 1999; Tolias et al., 2001). In particular, neuronal responses tend to be enhanced for stimuli that are covertly attended (Fries et al., 2001; Kastner and Ungerleider, 2001; Luck et al., 1997; Reynolds and Chelazzi, 2004) or are the targets of saccadic eye movements (Chelazzi et al., 1993; Desimone et al., 1994; Fischer and Boch, 1981; Moore et al., 1998; Moore and Zirnsak, 2017; Sheinberg and Logothetis, 2001). In the latter case, RF changes appear to emerge shortly before movement onset. Saccades provide a severe challenge to the visual system as they lead to substantial displacements of the retinal image. Paradoxically, although perception is stable across saccades, strong perceptual distortions occur during these movements when probed experimentally, and these distortions are thought to provide clues about the mechanisms underlying the illusion of stability (Burr et al., 2010). Recently, we reported that the RFs of neurons within prefrontal cortex (frontal eye field [FEF]) converge toward the location of eye movement targets prior to the movement (Zirnsak and Moore, 2014; Zirnsak et al., 2014). This convergence provides a neuronal correlate of the overriding perception of target space during saccades (Zhao et al., 2012), and it suggests a basis for the illusion of stability during such movements (Deubel et al., 1996).
The FEF is heavily interconnected with most extrastriate areas within posterior visual cortex, and thus the origin of its RF dynamics remains an important question. To address this question, we analyzed the LFPs in the FEF, together with the spiking activity, both during fixation and during saccadic eye movements. LFPs provide a means to investigate the interaction of local neuronal networks with other networks in distal subcortical and cortical areas, which provide synaptic input to this network (Einevoll et al., 2013) and, thus, can illuminate the origin of RF dynamics in the FEF. We first asked how much visual spatial information can be derived from different frequency bands of FEF LFPs across cortical layers. Next, we asked whether the RFs derived from LFPs were retinocentrically organized and whether they correlated with those derived from the spiking activity. Finally, we examined the dynamics of LFP-derived RFs during eye movements.
RESULTS
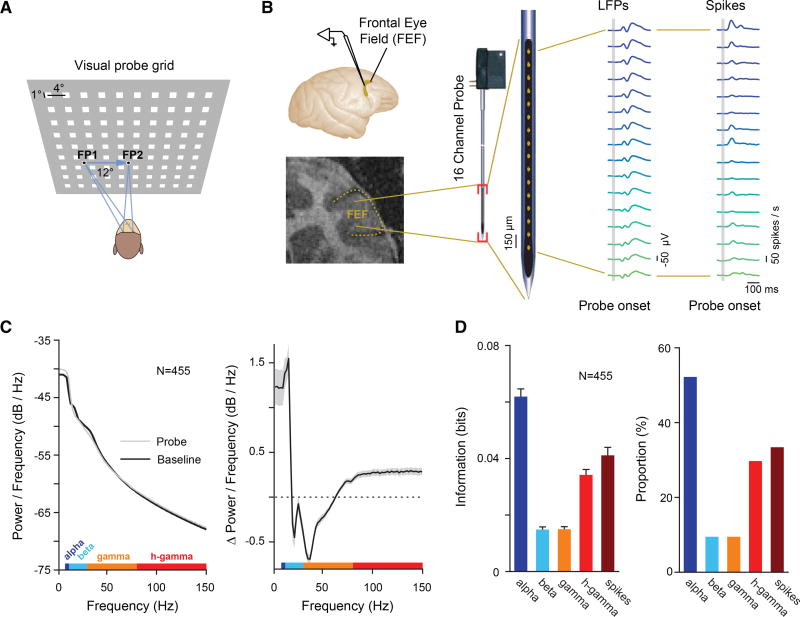
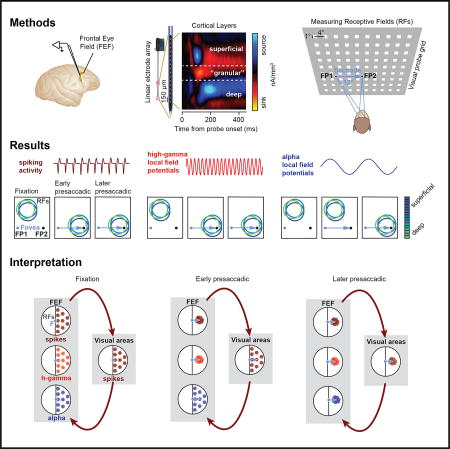
We measured LFP and spiking responses to brief (25-ms) visual probe stimuli during fixation at a first fixation point (FP1; fixation 1), during fixation at a second fixation point (FP2; fixation 2), as well as shortly before saccadic eye movements from FP1 to FP2 (presaccadic) (Figure 1A). The location of probe stimuli (90) was pseudorandomly varied across a 36 × 32-degree of visual angle (dva) part of visual space. We recorded LFPs and spiking activity within the FEFs of two monkeys (Macaca mulatta) with 16-channel laminar electrodes (U-Probes, Plexon) (Figure 1B). In total, LFPs and multi-unit spiking activity were recorded from 455 FEF sites (Experimental Procedures).
Figure 1. Measuring Visual Spatial Representation across LFP Frequency Bands and Spiking Activity during Fixation and Saccadic Eye Movements.
(A) A probe stimulus (white square) was briefly presented while monkeys maintained stable fixation (at FP1 or FP2) or while they prepared an eye movement from FP1 to FP2.
(B) FEF neural responses were recorded with 16-channel linear arrays. Top left shows location of the FEF in the macaque brain. Bottom left shows coronal magnetic resonance image of the FEF in monkey N.
(C) The left graph shows the LFP power spectra for baseline (black) and visual responses (gray). The right graph shows the difference between the two power spectra. The gray shaded area denotes the SEM. LFP frequency bands (alpha, beta, low gamma, and high gamma) used for analyses are shown color coded on top of the x axis.
(D) The left graph shows the average MI about probe location contained within the LFP responses for a given frequency band (Figure S1) and contained within the spikes, both as measured during fixation. Error bars denote the SEM. The right graph shows the percentage of electrode sites across all experimental sessions yielding statistically significant MI for the different LFP bands and spikes.
Visual Spatial Information in FEF LFPs Is Differentially Distributed across Frequency Bands
We first analyzed visual responses in the LFPs by comparing the power spectrum before and after the presentation of probes during fixation (Figure 1C). Our analysis of LFPs focused on four candidate frequency bands known to contain visual information (Einevoll et al., 2013; Tremblay et al., 2015), specifically the low-frequency bands, centered on alpha (8–12 Hz), the beta band (12–30 Hz), the low-gamma band (30–80 Hz), and the high-gamma band (80–150 Hz). Results were qualitatively identical when using frequency bands defined on the basis of signal correlations (Experimental Procedures). In contrast to previous studies in posterior visual areas, where enhanced power (P) in response to visual stimulation was observed in beta and low-gamma bands (Burns et al., 2010; Liu and Newsome, 2006), we observed increased power in the alpha ( , p < 10−5) and high-gamma bands ( , p < 10−5) and suppressed power in the beta ( , p < 10−5) and low-gamma bands ( , p < 10−4).
We next measured the visual spatial information contained in the probe-driven responses within the selected frequency bands. To do this, we computed the mutual information (MI) between the responses and the probe locations (Experimental Procedures). Consistent with the observed enhancement of alpha and high-gamma power in the visual response, we found that most of the spatial information was contained within those bands ( ; ANOVA, p < 10−88) (Figure 1D, left). Furthermore, the proportion of recording sites with significant MI was greatest in the alpha (52.20%) and high-gamma (29.74%) bands (Figure 1D, right). Thus, alpha and high-gamma bands showed the strongest positive visual responses and contained most of the information about probe location.
Alpha and High-Gamma RFs Are Retinocentrically Organized during Fixation and Spatially Correlated with Spiking Activity and Each Other
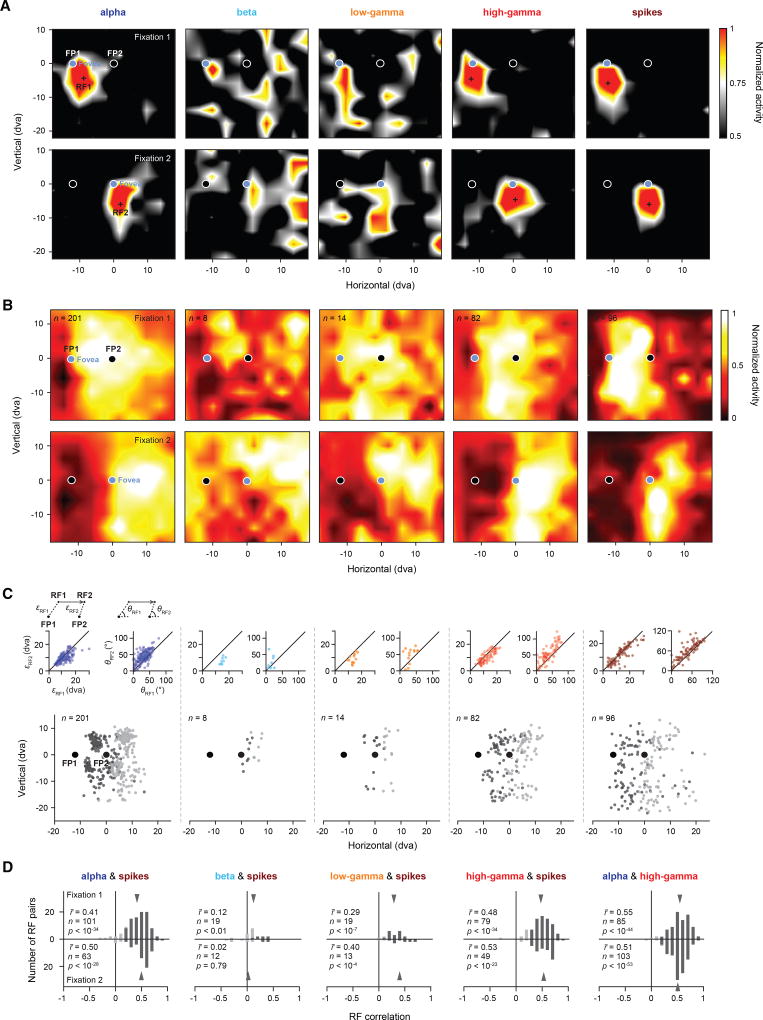
We next tested whether visual RFs could be derived from the different LFP frequency bands. Figure 2A shows an example recording in which the MI analysis revealed significant spatial information in the alpha and high-gamma bands (MIalpha = 0.093 bits, p < 0.001; MIhigh gamma = 0.055 bits, p < 0.001), but not the beta and low-gamma bands (MIbeta = 0.007 bits, p = 0.717; MIlow gamma = 0.000 bits, p = 0.681). Consistent with that observation, clear RFs could be derived from the former two bands, but not the latter. Across the two fixation locations, the LFP-derived RFs exhibited a retinocentric pattern; that is, the RFs and their centers were largely independent of the orbital position of the eye, and they were displaced in an amount approximately equal to the displacement of the fovea between fixations (Figure 2A, first and fourth columns), similar to the RFs derived from the spiking activity (Figure 2A, fifth column).
Figure 2. Retinocentricity of LFP- and Spiking-Derived RFs.
(A) Five example response maps derived from different LFP frequency bands (alpha, beta, low gamma, and high gamma) and spikes recorded simultaneously from one recording site. Response maps shown in the top row were measured during stable fixation at FP1 (fixation 1) (Experimental Procedures; Figure 4A). The cross in each map indicates the estimated RF1 center. Response maps shown in the bottom row were measured during fixation at FP2 (fixation 2). The cross in each map indicates the estimated RF2 center. The RFs have been spatially interpolated and smoothed for visualization.
(B) Population RFs based on all significant RFs from individual electrode sites as measured during fixation 1 (top row) and fixation 2 (bottom row). The population RFs have been spatially interpolated and smoothed for visualization.
(C) Populations of RF centers across fixations (RF1s in dark gray and RF2s in light gray). Insets on the top left of each graph show correlations between the distance from RF1 to FP1 (εRF1) and the distance from RF2 to FP2 (εRF2) for the respective population of RF centers. Solid lines depict the line of unity. Insets on the top right of each graph show the correlation between the two smallest absolute angles θRF1 and θRF2 formed by the horizontal meridian and εRF1, respectively ε′RF2 (Experimental Procedures). Solid lines depict the line of unity.
(D) Histograms of correlations between LFPs and spikes across frequency bands, based on pairs of significant RFs measured during fixation 1 (top row) and fixation 2 (bottom row). Rightmost histogram shows the correlation between alpha and high gamma. Darker bars indicate statistically significant correlations. Gray arrows indicate the mean of the respective distribution.
To examine the retinocentricity of RFs across the population of recordings and within each LFP band, we analyzed all recordings with significant visual spatial information (Experimental Procedures) during both fixation 1 and fixation 2. Figure 2B shows the population RFs (Experimental Procedures) for all significant recordings of each LFP band. Similar to the spiking population RFs, those in the alpha and high-gamma bands were displaced across the two fixations in a manner roughly equal to the change in fixation. In contrast, the displacement pattern across the two fixations was less clear for the beta- and low-gamma-derived population RFs. A similar pattern of results was obtained when we only examined the centers of each RF (Figure 2C). To quantify this result, we computed the correlation between the eccentricity (ε) and direction (θ) of each RF during fixation 1 and fixation 2 (Experimental Procedures). Similar to the eccentricities and directions of RFs derived from the spiking activity, eccentricities, and directions of RFs derived from alpha and high-gamma activity were significantly correlated across fixations (alpha: rε = 0.67, p < 10−26, rθ = 0.66, p < 10−25; high gamma: rε = 0.80, p < 10−18, rθ = 0.81, p < 10−19; spikes: rε = 0.86, p < 10−28, rθ = 0.80, p < 10−21). However, there were no significant correlations observed for beta- and low-gamma-band RFs (beta: rε = 0.70, p = 0.06, rθ = 0.07, p = 0.87; low gamma: rε = 0.48, p = 0.08, rθ = 0.44, p = 0.12), presumably because there were too few significant RFs in those bands to begin with.
In the example recording (Figure 2A), alpha- and high-gamma-band RFs were significantly correlated with the RF derived from the spiking activity (fixation 1: ralpha, spikes = 0.25, p < 0.01; rhigh gamma, spikes = 0.63, p < 0.001; fixation 2: ralpha, spikes = 0.32, p < 0.001; rhigh gamma, spikes = 0.48, p < 0.001). This pattern of results was also observed across the population of recordings. RFs derived from the alpha and high-gamma bands were significantly correlated with the spiking-derived RFs in 89.90% (90/101) and 96.20% (76/79) of all significant recordings during fixation 1 and 92.06% (58/63) and 95.92% (47/49) during fixation 2, respectively (fixation 1: r̄alpha, spikes = 0.41, p < 10−34; r̄high gamma, spikes = 0.48, p < 10−34; fixation 2: r̄alpha, spikes = 0.50, p < 10−28; r̄high gamma, spikes = 0.53, p < 10−23) (Figure 2D). Across the two fixations, the correlation between spiking RFs and high-gamma RFs was slightly greater than between spiking RFs and alpha RFs (r̄high gamma, spikes − r̄alpha, spikes = 0.05, p = 0.03). Very few of the recordings showed significant correlations with the spiking RFs in the beta (fixation 1: 7/19; fixation 2: 4/12) and low-gamma bands (fixation 1: 17/19; fixation 2: 11/13).
Consistent with their correlations with the spiking RFs, alpha and high-gamma RFs were also correlated with each other. In the example (Figure 2A), the correlation amounted to ralpha, high gamma = 0.62 (p < 0.001) for fixation 1 and to ralpha, high gamma = 0.40 (p < 0.001) for fixation 2. Across the population of recordings, 95.2% (81/85) showed significant correlations during fixation 1 (r̄alpha, high gamma = 0.55, p < 10−44) and 96.1% (99/103) showed significant correlations during fixation 2 (r̄alpha, high gamma = 0.51, p < 10−53) (Figure 2D, right).
RFs and Spatial Information from Alpha- and High-Gamma-Band and Spiking Activities Were Robust across Cortical Layers
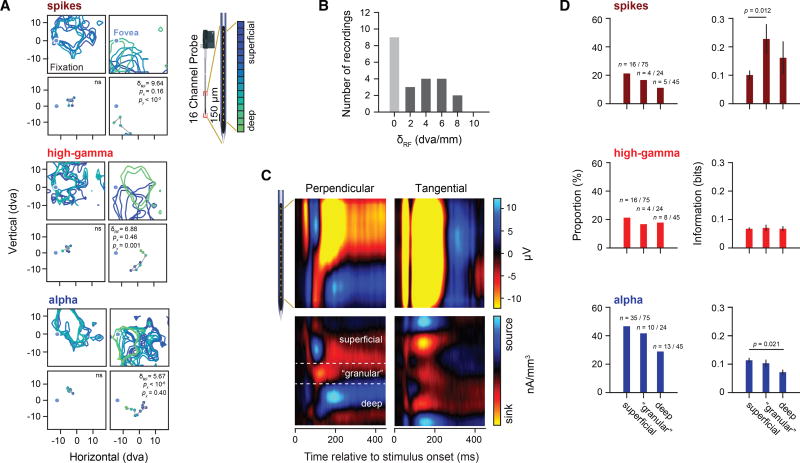
Next, we asked whether LFP-derived RFs and spatial information are differently distributed across FEF cortical layers. Since the majority of the FEF is buried within the arcuate sulcus, it is more difficult to position microelectrodes perpendicular to the cortical surface than it is in other cortical areas that lie largely on the surface (Buffalo et al., 2011; Chandrasekaran et al., 2017; Godlove et al., 2014; Nandy et al., 2017). However, at least some portions of the FEF extend onto the gyral surface (Bruce et al., 1985; Gerbella et al., 2010; Huerta et al., 1987; Schall et al., 1995), where perpendicular electrode penetrations are possible. Indeed, we found that some of our linear array recordings were obtained from largely perpendicular penetrations based on the lack of visual RF displacement across electrode array channels, as observed in previous studies within visual cortex (Engel et al., 2016; Nandy et al., 2017) (Figure 3A). We observed clear examples of recordings with little or no RF displacement of LFP or spike-derived RFs, as well as recordings in which the RFs were significantly displaced across channels (Figure 3A). We found that 9 of the 29 recordings were made perpendicular to the cortical surface (Figure 3B) (Experimental Procedures). For these putative perpendicular recordings, we then used current source density (CSD) analysis (Mitzdorf, 1985; Nicholson and Freeman, 1975) to identify superficial layers, an input (granular) layer (Bruce et al., 1985), and deeper layers of the FEF (Figure 3C) (Experimental Procedures), similar to studies in other cortical structures (Godlove et al., 2014; Nandy et al., 2017; Schroeder et al., 1998).
Figure 3. Visual Spatial Information across FEF Layers.
(A) Examples of perpendicular (left) and tangential (right) recordings plotted separately for spike, alpha-, and high-gamma-band RFs. RF contours indicate plus 1 SD after Z-transformation of the individual RFs. The bottom row of each panel depicts the corresponding RF centers. RF contours and RF centers are plotted as a function of channel depth. Recordings with significant RF displacements across electrodes (δRF) were classified as tangential with respect to the cortical layers, whereas recordings with non-significant RF displacements were classified as perpendicular.
(B) Histogram showing the number of perpendicular (light gray) and tangential recordings (dark gray).
(C) LFP responses across all probe locations during fixation and corresponding CSDs for a perpendicular and tangential recording. The CSD map has been spatially smoothed for visualization.
(D) Proportion of significant RFs and amount of MI for significant recordings during fixation across cortical compartments. Error bars denote the SEM.
Using data obtained solely from perpendicular penetrations, we analyzed the amount of visual spatial information contained within the three identified cortical compartments during fixation for the alpha, high-gamma, and spiking activities (Figure 3D). Although there was a trend toward fewer significant RFs in deeper layers, particularly for the spiking and alpha activities, there was no significant difference in the proportions of RFs across layers (Fisher’s exact test, pspikes = 0.46, phigh gamma = 0.88, and palpha = 0.16). However, for recordings yielding significant RFs, we observed a significant difference in MI across cortical layers for the spiking and alpha activities (ANOVA, pspikes = 0.02 and palpha = 0.02) (Figure 3D, right column). Post hoc pairwise comparisons revealed a significant difference between superficial and granular layers for the spiking-derived RFs, with higher MI contained within the granular layers (p = 0.012, t-test with Bonferroni correction). For the alpha-band-derived RFs, we observed greater MI contained within the superficial layers as compared to deep layers (p = 0.021, t-test with Bonferroni correction).
Dissociation of Alpha and High-Gamma RFs during Eye Movements
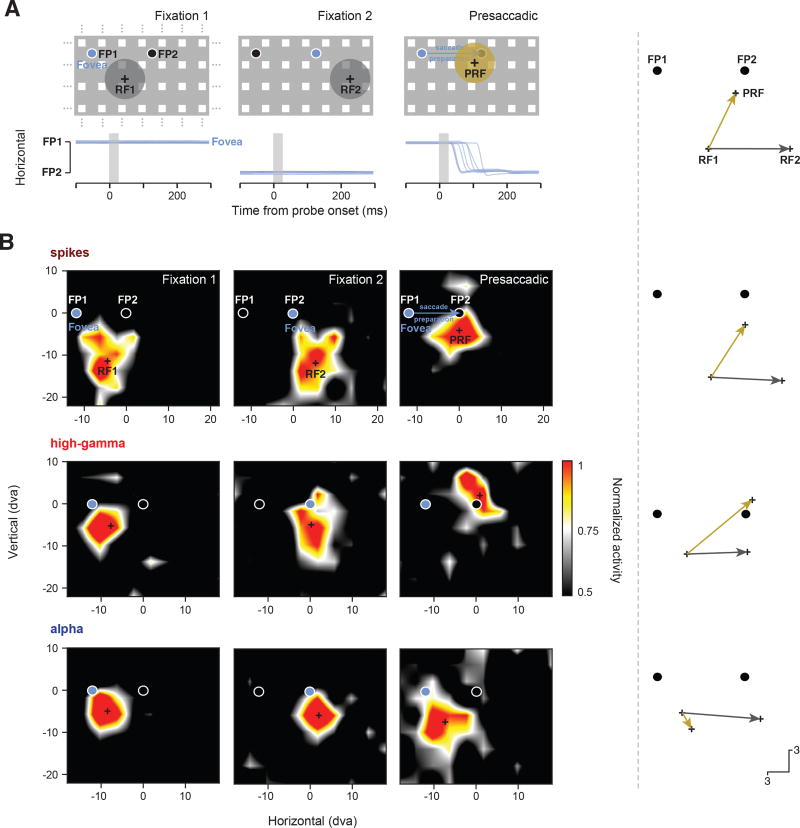
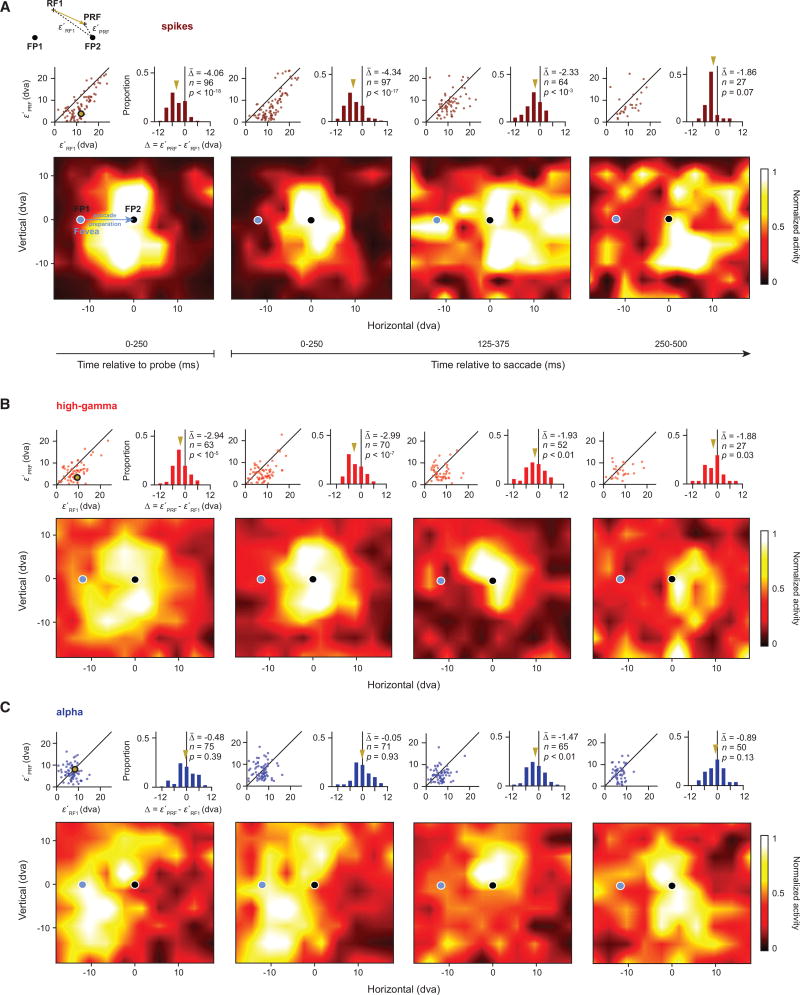
We previously reported that RFs based on the spiking activity of FEF neurons converge toward the saccadic target prior to movement onset (Zirnsak et al., 2014). We therefore tested whether the RFs derived from alpha and high-gamma bands also converge toward the target during saccade preparation (Experimental Procedures; Figure S2). For these tests, we combined perpendicular and tangential recordings as both yielded the same pattern of results, with no clear trend across cortical layers. First, for comparison, we computed spiking RFs and their centers using responses to probes flashed during fixation 1 (RF1) and fixation 2 (RF2) and during the presaccadic period (PRF) using responses to probes flashed shortly before the movement (Experimental Procedures). Figure 4A illustrates the pattern of results for FEF neurons described previously in which the PRFs shift toward the saccadic target (FP2). The example spiking RF shown in Figure 4B (top row) demonstrates both the retinocentricity of spiking RFs (gray arrow) and the shift of the PRF toward the target (gold arrow). The latter effect differed between RFs derived from alpha and high-gamma bands. In the same time period in which spiking RFs exhibited shifts toward the saccade target ([0, 250] ms from probe onset), we found that high-gamma band RFs showed the same shifts (Figure 4B, middle row). In contrast, during that period, alpha RFs remained largely at the RF1 location (Figure 4B, bottom row). Thus, in the presaccadic period, alpha and high-gamma RFs were spatially dissociated.
Figure 4. Changes in RFs at the Time of Saccadic Eye Movements.
(A) Illustration of previous observations on presaccadic RF changes of FEF neurons (Zirnsak et al., 2014). Following an eye movement from fixation point 1 (FP1) to fixation point 2 (FP2), FEF RFs (gray disks) remain fixed in retinocentric coordinates when measured during fixation (fixation 1 and fixation 2). When measured during saccade preparation to FP2, while the animal is still fixating at FP1, FEF RFs (gold disk) shift toward the eye movement target. Blue traces indicate horizontal eye position from one animal over time for a number of example trials. The gray shaded area indicates the timing and duration of the probe. Crosses in each panel indicate the respective fixation RF centers (RF1 and RF2) and the presaccadic RF center (PRF). The vectors on the right show the displacement of the RF center across fixations (gray) and the presaccadic shift toward the saccadic target (gold).
(B) The top row shows an example spiking RF as measured during the three experimental periods (fixation 1, fixation 2, and presaccadic). Crosses in each panel indicate the RF centers as used in the vector plots. The middle row shows an example high-gamma RF and the bottom row shows an example alpha. RFs have been spatially interpolated and smoothed for visualization.
The dissociation of alpha and high-gamma RFs was also observed across the population of significant RFs (Figure 5). As expected, PRFs derived from the spiking activity aligned to probe onset ([0, 250] ms) exhibited clear shifts toward the saccadic target (Figure 5A). That is, the PRFs were significantly closer to the target (FP2) than their corresponding RF1s (ε′PRF − ε′RF1 = −4.06 degrees, p < 10−18) (Figure S3 contrasts the PRFs and RF2s). In addition, these shifts were evident in the spiking activity aligned to saccade onset, although the shift did not reach significance in the last time bin, corresponding to the decrease in number of measurable RFs ([0, 250] ms: −4.34 degrees, p < 10−17; [125, 375] ms: −2.33 degrees, p < 10−3; [250, 500] ms: −1.86 degrees, p = 0.07). Similar to the PRF shifts in the spiking activity, we observed early shifts of PRFs derived from high-gamma LFPs (Figure 5B). In the probe-aligned responses, high-gamma PRFs were significantly closer to the target than their corresponding RF1s (ε′PRF − ε′RF1 = −2.94 degrees, p < 10−5). High-gamma PRF shifts were also evident in the responses aligned to saccade onset. Moreover, these shifts were significant across all three saccade-aligned time bins ([0, 250] ms: −2.99 degrees, p < 10−7; [125, 375] ms: −1.93 degrees, p = 0.004; [250, 500] ms: −1.88 degrees, p = 0.03). Thus, the high-gamma RFs exhibited clear shifts toward the target when probed during saccade preparation, and they did so in a manner similar to the RFs derived from the spiking activity.
Figure 5. Dissociation of Alpha and High-Gamma RFs at the Time of Eye Movements.
(A) Spiking population RFs during saccade preparation. Each map shows the population RF based on responses at different times relative to the eye movement (from left to right, population RF aligned to probe onset [0–250] ms, population RFs aligned to saccade onset [0–250] ms, [125–375] ms, and [250–500] ms). The insets on top of each population RF compare the distance between the presaccadic RF centers (PRFs) and the saccade target (FP2) to the distance between the fixation 1 RF centers (RF1s) and the FP2 for all significant presaccadic RFs of a given time (Experimental Procedures; Figure S3). Solid lines in the scatterplots depict the line of unity. The gold dot corresponds to the example RF shown in Figure 3B. Gold arrows in the histograms indicate the mean of the population.
(B) High-gamma population RFs. The same conventions were used as in (A).
(C) Alpha population RFs. The same conventions were used as in (A). All population RFs and RF centers are based on, respectively from, statistically significant individual presaccadic RFs. Figure S4 shows the same results for all presaccadic RFs independent of their statistical significance. Figure S5 shows the PRF versus RF2 comparison for this RF population. Population RFs have been spatially interpolated and smoothed for visualization.
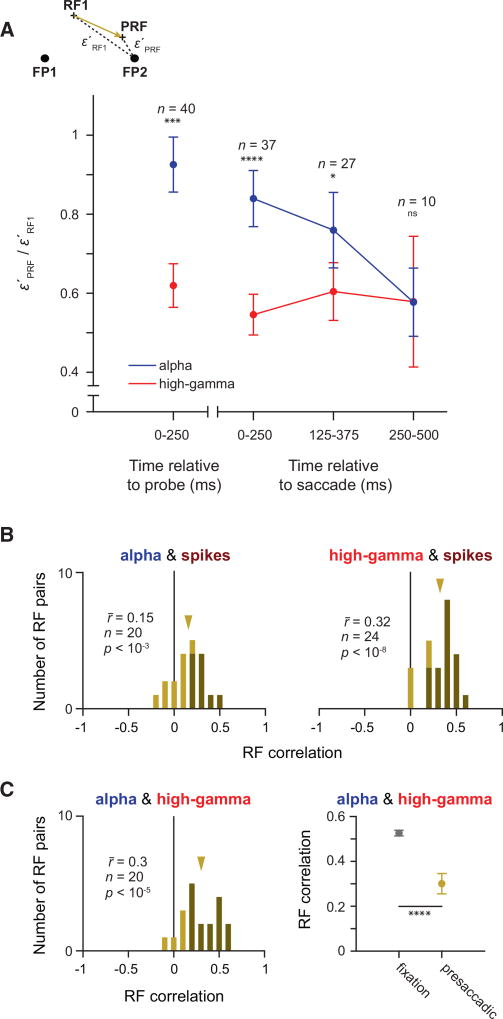
During the initial presaccadic probe response, in contrast to PRFs in the high-gamma band, alpha-band PRFs exhibited no significant shifts (Figure 5C). Instead, the distance between alpha PRFs and the target remained equal to the distance between the RF1 and the target across the population of RFs. There was no evidence of a shift in either the probe-aligned PRFs (ε′PRF − ε′RF1 = −0.48 degree, p = 0.39) or in the initial saccade-aligned PRFs (0.05 degree, p = 0.93). However, by the second, saccade-aligned time bin ([125, 250] ms), the PRF shift toward the target emerged (−1.47 degrees, p < 0.01). This shift was similar in magnitude to the shift evident in the same time bin of the high-gamma PRFs (Figure 5B). In the final time bin, the alpha-band PRF shift was still apparent but no longer significant (−0.89 degree, p = 0.13). Thus, the alpha-band and high-gamma-band PRFs were dissociated from each other during saccade preparation; high-gamma PRFs shifted first toward the target, similar to the spiking PRFs, followed then by alpha PRFs. The sequence of PRF shifts was also evident in a subset of recording sites with significant RFs in both the alpha and high-gamma bands (paired RFs, Figure 6A). In this dataset, the probe-aligned high-gamma PRFs were closer to the saccadic target than their corresponding alpha PRFs (ε′alpha PRF/ε′alpha RF1 − ε′high-gamma PRF/ε′high-gamma RF1 = 0.302, p = 0.001). For the saccade-aligned responses, this difference was largest in the earliest time bin ([0, 250] ms: 0.270, p < 10−3; [125, 375] ms: 0.151, p = 0.047), but it was no longer significant in the last one ([125, 375] ms: 0.006, p = 0.979). This dissociation between alpha and high-gamma PRFs cannot be explained by a lack or delay of signal in the alpha band, as the probe-evoked activity emerges earlier and is stronger in the alpha band as compared to the high-gamma band (matching pursuit decomposition; Chen et al., 2010; Ray et al., 2008) (Figure S6).
Figure 6. Asynchronous RF Convergence of Alpha and High-Gamma RFs and Correlations with Spiking RFs.
(A) Ratio of (distance between the PRF and FP2) to (distance between the RF1 and FP2) for paired alpha (blue) and high-gamma (red) RFs from the same recording site. Ratios are plotted for the same time epochs relative to the eye movement as in Figure 4. Asterisks indicate a significant difference between the alpha and high-gamma band (*p < 0.05, ***p < 10−3, and ****p < 10−4). Error bars denote the SEM.
(B) Correlation histograms based on pairs of significant presacadic RFs within different LFP bands. Darker bars indicate statistically significant correlations. Gold arrows indicate the mean of the respective distribution.
(C) Correlation between presaccadic alpha and high-gamma RFs (left) was reduced compared to the fixation period (right). Error bars denote the SEM.
Next, we analyzed the presaccadic shifts separately for perpendicular recordings and tangential recordings, as well as presaccadic shifts across different cortical layers. Consistently, for both set of recordings, as for the complete set of recordings, we found that in the initial presaccadic probe response ([0, 250] ms, saccade aligned), RFs derived from spikes and from the high-gamma band converged immediately toward the saccadic target (perpendicular recordings: spikes −3.65 degrees, p = 0.009; high gamma −3.45 degrees, p = 0.04; tangential recordings: spikes −6.07 degrees, p = 4.35 × 10−11; high gamma −4.58 degrees, p = 1.52 × 10−4), whereas RFs derived from the alpha band did not (perpendicular recordings: 0.58 degree, p = 0.70; tangential recordings: 0.43 degree, p = 0.64). In the subsequent time period ([125,375] ms), however, the alpha RFs converged as well (perpendicular recordings: −2.73 degrees, p = 0.009; tangential recordings: −2.33 degrees, p = 0.03). Thus, we observed the same overall dissociation between the alpha-band RFs and high-gamma-band and spiking-derived RFs in our perpendicular recordings as we did for the complete dataset. Finally, we looked for differences in the magnitude of the RF shifts across cortical layers. Although some trends could be observed, e.g., stronger mean shift in spiking RFs measured within the granular layer, none of the cross-laminar comparisons reached statistical significance (ANOVA, 0–250 ms: spikes p = 0.42, high gamma p = 0.53, alpha p = 0.72; 125–375 ms: spikes p = 0.14, high gamma p = 0.43, alpha p = 0.19). However, it is possible that with a much larger number of significant RFs, differences between laminar compartments might emerge.
As a consequence of the early dissociation between alpha and high-gamma PRFs, we found that, across the entire presaccadic response ([0, 500] ms, saccade aligned), the correlation between the alpha PRFs and the spiking PRFs was significantly less than the correlation between the high-gamma and the spiking PRFs (r̄alpha, spikes − r̄high gamma, spikes = −0.17, p < 10−2) (Figure 6B; Figure S7A), and there were disproportionately fewer significant correlations between alpha and spiking PRFs than between high-gamma and spiking PRFs (45% versus 83%, chi-square = 4.40, p = 0.04). In addition, we compared the spiking and LFP RF correlations between the fixation (fix) and presaccadic (pre) epochs. For both the alpha and high-gamma RFs, the correlations were reduced in the presaccadic period. However, there was a significantly larger reduction in RF correlations in the alpha band ( , permutation test, p = 0.04). Finally, we sought to determine whether the dissociation of presaccadic RFs could be observed solely between alpha and high-gamma bands. Consistent with previous results, we found that the correlation between alpha PRFs and high-gamma PRFs (Figure 5C, left) was significantly lower during the presaccadic epoch than during fixation (Figure 6C, right) ( , p < 10−6; see also Figure S7B). Correspondingly, there were fewer recordings showing significant correlations between alpha and high-gamma RFs in the presaccadic epoch (75%) than in the fixation epoch (96%) (chi-square = 13.28, p < 10−3).
DISCUSSION
Distribution of Visual Spatial Information across the LFP Spectrum in Prefrontal Cortex
Previous studies have examined visual information contained in stimulus-driven LFPs within primate posterior visual cortex. These studies have generally observed that a majority of information about visual stimuli, such as contrast (Henrie and Shapley, 2005; Ray and Maunsell, 2010), size (Gieselmann and Thiele, 2008), orientation (Frien et al., 2000; Gray and Singer, 1989; Katzner et al., 2009), direction of motion (Liu and Newsome, 2006), the content of natural scenes (Belitski et al., 2008), and, like the present study, stimulus location (Eckhorn et al., 1988; Mineault et al., 2013; Self et al., 2016), is robustly contained within the low-gamma band (30–80 Hz). In contrast, our results within the FEF differ from these studies in that we observed a marked paucity of visual information in the low-gamma band. We observed not only a lack of a clear increase in low-gamma power in response to visual stimulation during fixation but also little to no visual spatial information in the low-gamma band. This result is consistent with a modeling study that predicts relatively low-gamma power in prefrontal cortex (Mejias et al., 2016), and a recent study reporting superior encoding of stimulus position from high-gamma activity within lateral prefrontal cortex (Tremblay et al., 2015). The apparent lack of visual information in the low-gamma band within prefrontal cortex could be due to fundamental differences in cytoarchitecture and connectivity between visual and prefrontal cortex (Barbas and Pandya, 1989; Gabbott and Bacon, 1996). Consistent with this possibility, evidence in rodents indicates that parvalbumin-positive (PV+) interneurons, which are less numerous in frontal cortex (Tremblay et al., 2016; Xu et al., 2010), contribute directly to low-gamma power (Cardin et al., 2009) and the encoding of sensory signals (Siegle et al., 2014). This evidence could account for the relative lack of visual spatial information in the FEF low-gamma band.
Local versus Distal Origin of RF Dissociation
We observed at least two separable dynamics of visual spatial information in the FEF around the time of eye movements, which were contained in the alpha band and in the high-gamma band and spikes. The dissociation of alpha and high-gamma spatial signals suggests possible sources of spiking RF changes within the FEF and the structures with which it connects. Here we discuss four possible scenarios that may account for our observations, based on the two frequency bands having either rather local or distal origins in this network. In two of these scenarios, alpha signals are largely generated locally within the FEF, whereas high-gamma signals are largely generated either locally or distally. Given the decorrelation of spikes and the alpha band signals during the eye movement, these two scenarios, although possible, seem unlikely. That is, the alpha signals appear more likely to be dominated by inputs from neurons located outside the FEF (Belitski et al., 2008; Saalmann et al., 2012; van Kerkoerle et al., 2014), i.e., inputs that evoke post-synaptic signals at membranes within the FEF.
In a third scenario, both the alpha and high-gamma signals may largely originate from distal sources, and they may reflect synaptic input from cortical and/or subcortical structures connected with the FEF. For example, the two different bands may reflect different input streams (e.g., synaptic inputs from dorsal and ventral visual pathways, which come together within the FEF; Schall et al., 1995; Stanton et al., 1995; Ungerleider and Desimone, 1986). Since it is known that dorsal inputs have shorter visual latencies than ventral inputs (Chen et al., 2007; Schmolesky et al., 1998), in this scenario, the slower alpha RF dynamics could originate from more ventral stream sources, whereas the faster high-gamma dynamics could originate from more dorsal stream sources. Although the nature of RF dynamics during eye movements remains controversial within posterior areas providing synaptic input to the FEF (e.g., lateral intraparietal area [LIP]) (Duhamel et al., 1992; Hamker et al., 2008; Wang et al., 2016; Zirnsak and Moore, 2014), there is evidence of RF convergence in at least area V4 (Hartmann et al., 2017; Tolias et al., 2001), which provides direct input to the FEF. Thus, in this scenario, these ventral inputs would exhibit RF dynamics subsequent to those in dorsal areas. However, as we discuss below, evidence suggests that it is unlikely that high-gamma signals are generated predominantly from distal sources of synaptic inputs. Moreover, there is no evidence yet that different visual input streams are represented in different frequency bands.
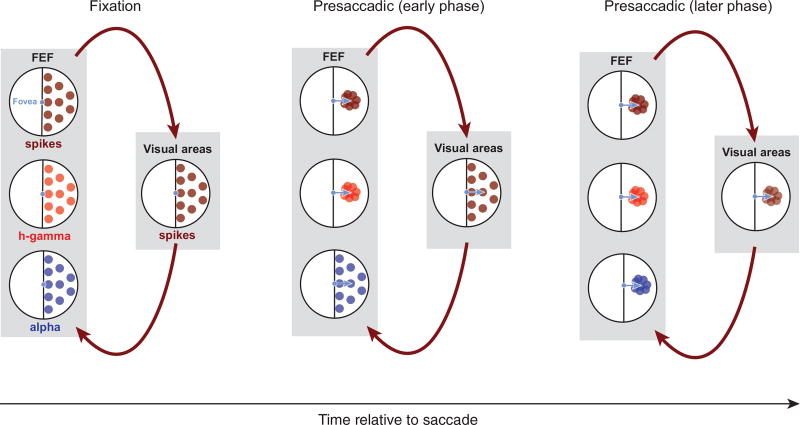
In a fourth scenario, high-gamma activity may be largely generated locally within the FEF, whereas the alpha band activity may largely reflect the synaptic input from distal structures, that is, neurons located outside the FEF. This scenario is consistent with evidence that high-gamma band power originates mostly from local sources, for example, through spike synchronization (Ray et al., 2008; Ray and Maunsell, 2011), and lower frequencies from more distal sources, which could include both cortical (Khawaja et al., 2009; van Kerkoerle et al., 2014) and subcortical structures (Belitski et al., 2008; Einevoll et al., 2013; Saalmann et al., 2012; Vijayan and Kopell, 2012). Power in the high-gamma band tends to be correlated with spikes in many cortical regions, including primary visual cortex (Rasch et al., 2008; Ray and Maunsell, 2011), the middle temporal area (Liu and Newsome, 2006), posterior parietal regions (Hwang and Andersen, 2012; Pesaran et al., 2002), and secondary somatosensory cortex (Ray et al., 2008). In contrast, low-frequency activity tends to be uncorrelated with the spiking activity in many respects. For example, in early visual cortex, low-frequency LFP power (<12 Hz) and spiking activity encode independent visual information about naturalistic stimuli (Belitski et al., 2008). In addition, low-frequency LFP power in early visual cortex is more closely related to the perception (Gail et al., 2004; Wilke et al., 2006) than the local spiking activity. If indeed alpha- and high-gamma-band activities in the present study reflect more distal and more local signals, respectively, then the observed RF dynamics contained in the alpha band, the high-gamma band, and the spiking activity could reflect an interaction between the FEF and its connected areas. In this interaction, convergence of RFs toward the movement goal could originate first from FEF neurons and be associated with a similar convergence in the high-gamma-band activity. At the same time, synaptic inputs from distal visual sources, measured in the alpha band, might largely maintain their retinocentric representation. Subsequently, as a result of recurrence with the FEF, synaptic inputs to the FEF could gradually begin to exhibit RF convergence (Figure 7). Although speculative, given the current evidence, we view this scenario as the most likely of the four.
Figure 7. Possible Basis for Separable Dynamics of Spatial Information during Eye Movements.
Black circles represent the visual field; colored dots denote RFs. Dark red dots indicate spiking RFs in the recorded visual field. Red dots indicate high-gamma RFs and blue dots indicate alpha RFs. The schematic depicts that high-gamma RFs may be largely generated locally within the FEF, whereas the alpha RFs may largely reflect the synaptic input from upstream structures. Arrows indicate the signal flow between areas. Shortly before a saccade, the convergence of RFs toward the movement goal could originate first from FEF neurons and be associated with a similar convergence in the high-gamma activity. At the same time, synaptic inputs from distal sources, reflected in the alpha band, might largely maintain their retinocentric representation. Subsequently, as a result of recurrence with the FEF, visual inputs to the FEF could gradually begin to exhibit RF convergence as well.
Implications for Visual Perception during Eye Movements
In humans and other primates, visual exploration is achieved through a series of saccadic eye movements; each movement shifting gaze to bring objects of interest to the fovea for further processing. Although these movements lead to frequent and substantial displacements of the retinal image, these displacements are not perceived. It is widely believed that the illusion of this visual stability during eye movements results from an active compensatory mechanism, such as corollary discharge (e.g., Sommer and Wurtz, 2006), which effectively reduces or eliminates the impact of visual disruptions resulting from saccades (Sommer and Wurtz, 2004; Wurtz, 2008). This compensatory mechanism is also thought to be reflected by the profound impairments in visual perception that occur at the time of saccades. For example, the ability of human observers to localize stimuli at the time of saccades is severely impaired. Typically, observers perceive the location of stimuli as being much closer to the saccadic target than their veridical location (Kaiser and Lappe, 2004; Ross et al., 1997). It has been suggested that this distortion of perceptual space can be explained by a distortion of the neuronal population response toward the saccadic target (Hamker et al., 2008). Consistent with that hypothesis, spiking-derived RFs of V4 neurons (Hartmann et al., 2017; Tolias et al., 2001) and FEF neurons (Zirnsak et al., 2014) shift closer to the saccadic target when measured around the time of the movement. Indeed, the convergence of RFs in the FEF correlates with the systematic distortions in perceptual space reported for human observers (Zirnsak et al., 2014; Zirnsak and Moore, 2014). In addition to observing a similar convergence in the LFPs, our observation of dissociated alpha- and high-gamma-band RFs, and the resulting dissonance in their spatial representations, adds perhaps another possible basis for perceptual mislocalization. Shortly before saccade onset, visual space represented in the alpha band differs dramatically from that represented in the high-gamma band, and the emergence of these dissonant visual representations, when combined, may increase the uncertainty in stimulus position contained in the FEF. Indeed, mislocalization in human observers consists both of systematic errors in perceptual judgments and of an apparent decrease in the precision of those judgments (Hamker et al., 2008). Given the FEF’s extensive connectivity with posterior visual cortex (Schall et al., 1995), and it’s clear role in visual perception (Moore and Zirnsak, 2017), it seems unlikely that such dissonant signals would have no impact on perception. Finally, the fact that both the alpha and the high-gamma representations ultimately converge toward the movement target suggests that an overrepresentation of target space emerges within the FEF and connected visual areas. This overrepresentation reflects the dominant perception of the target, a perception that appears to override the retinal displacements caused by eye movements (Deubel et al., 1996; McConkie and Currie, 1996).
EXPERIMENTAL PROCEDURES
We used two male adult monkeys (Macaca mulatta, 12 and 8 kg) in the experiments. All experimental procedures were in compliance with the US Public Health Service policy on the humane care and use of laboratory animals, the Society for Neuroscience Guidelines and Policies, and Stanford University Animal Care and Use Committee. General surgical and standard electrophysiological procedures are described in the Supplemental Experimental Procedures.
RF Measurements
We measured LFP- and spiking activity-derived RFs during stable fixation and shortly before saccades within the FEF by pseudorandomly presenting a single-probe stimulus out of a 10 × 9 probe grid extending 36 × 32 dva. In each recording session, we placed the probe grid to cover the area where we expected most of the RFs based on evoked saccade vectors by microstimulation of a given recording site. Details of the RF measurements are described in the Supplemental Experimental Procedures.
LFP- and Spiking-Derived RFs, Centers, and Their Correlations
To estimate RFs, we first computed probe-response maps (e.g., Figure 2A) as follows: for a given probe location, trial, and time interval, we obtained the mean LFP power by calculating the mean of the power spectral density (PSD) for each frequency band (alpha, 8–12 Hz; beta, 12–30 Hz; low gamma, 30–80 Hz; and high gamma, 80–150 Hz) or, in the case of the spiking activity, the spike count. We chose these frequency bands for easier comparisons to previous studies. However, the results were qualitatively identical when we instead used frequency bands (low frequency, 5–20 Hz; low-medium frequency, 20–40 Hz; medium-high frequency, 40–80 Hz; and high frequency, 80–150 Hz), based on signal correlations between different frequencies (Figure S7).
We then computed the average power or activity for each probe location as the arithmetic mean across all trials. This was done separately for the two fixation and the presaccadic conditions. Only response maps yielding significant MI (see below) were considered as significant RFs.
After assessing the statistical significance of a given RF, the RF center (xc, yc) in Cartesian coordinates was then computed as the center of mass for all locations passing the following criteria: first, the response of a given location had to be no less than the average response across all locations plus 1 SD; and second, a given location had to be surrounded by at least two directly adjacent probe locations with responses no less than the average response.
We then defined the eccentricity (ε) and the direction (θ) as the distance and angle between a given RF center to the fixation point (xf, yf):
| (Equation 1) |
| (Equation 2) |
Pearson’s r was then calculated between eccentricity and direction pairs for the RF measurements obtained during fixations 1 and 2 to quantify their retinocentricity. The correlation was calculated individually for all four LFP frequency bands and the spiking activity (Figure 2C).
In addition, 2D signal correlations were computed to quantify the similarity between two given RFs i and j. The correlation is defined as
| (Equation 3) |
where RFi,x,y and RFj,x,y denote the activity of RFi and RFj at probe location (x, y) and denotes the mean activity across all locations. In addition to quantifying the similarity between LFP RFs and spiking RFs (Figures 2 and 6), this metric was used to quantify the similarity between LFP RFs or spiking RFs with RFs at adjacent recording sites with varying distances during simultaneous recording (Figure S7).
Nonparametric permutation tests (1,000 repetitions) were used to assess the significance of a given correlation. The resulting average correlation obtained from the permutation procedure was subtracted from all reported correlation values.
Finally, population RFs were computed to visualize how the representations of visual space change before saccades. For each condition (fixation 1, fixation 2, and presaccadic), we first averaged individual RFs with adjacent centers. Visual space was divided into equally sized bins (6 × 4 dva) centered around the saccade target (FP2). RFs with centers falling inside the same bin were then simply averaged (arithmetic mean). The average RFs for each bin were then normalized and averaged again to obtain the final population RF. This method optimizes estimations of visual space representations by minimizing effects due to sampling biases of the measured RFs.
MI Analysis
To quantify the spatial information about the probe stimulus S contained within LFPs and the spiking activity, we used MI. That is, for a given recording, neural activity was measured at 90 probe locations, Sj (j∈ [1, 90]). Neural activity A, either average power in different frequency bands or average firing rate, was discretized into 6 quantiles, Qi (i∈ [1, 5]), containing equal numbers of trials. Neural activity below Q1 was classified as A1, between Qi−1 and Qi as Ai, and above Q5 as A6. The MI between neural activity and probe locations was then approximated by
| (Equation 4) |
where Mij is the number of trials classified as Ai in the response to probe Sj. Mi· and M·j are the sums of Mij over j and i, respectively. M is the number of total trials. The Bias term is computed as
| (Equation 5) |
where UAS is the number of nonzero Mij for all i and j, UA is the number of nonzero M·j for all i, and US is the number of nonzero Mi, for all j (Ito and Doya, 2009).
For each estimated MI, we performed nonparametric permutation tests (1,000 repetitions) to assess its significance. The resulting average MI obtained from the permutation procedure was subtracted from all reported MI values.
Supplementary Material
Highlights.
Spatial information is high in low and high LFP frequencies in prefrontal cortex
Spatial visual signals in LFPs and spikes are robust across cortical layers
Across fixations, LFP and spiking receptive fields (RFs) are retinocentric
During saccades, high-gamma and spiking RFs converge, followed by alpha RFs
Acknowledgments
This work was supported by NIH EY014924 (T.M.). We thank Nikos Logothetis for helpful comments on a previous version of the manuscript. We thank Eshan Govil for technical assistance.
Footnotes
Supplemental Information includes Supplemental Experimental Procedures and seven figures and can be found with this article online at https://doi.org/10.1016/j.celrep.2018.01.078.
AUTHOR CONTRIBUTIONS
Conceptualization, X.C., M.Z., and T.M.; Methodology, X.C., M.Z., and T.M.; Validation, X.C., M.Z., and T.M.; Formal Analysis, X.C.; Investigation, M.Z.; Resources, T.M.; Writing – Original Draft, X.C., M.Z., and T.M.; Writing – Review & Editing, X.C., M.Z., and T.M.; Visualization, X.C. and M.Z.; Supervision, M.Z. and T.M.; Funding Acquisition, T.M.
DECLARATION OF INTERESTS
The authors declare no competing interests.
References
- Barbas H, Pandya DN. Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. J. Comp. Neurol. 1989;286:353–375. doi: 10.1002/cne.902860306. [DOI] [PubMed] [Google Scholar]
- Belitski A, Gretton A, Magri C, Murayama Y, Montemurro MA, Logothetis NK, Panzeri S. Low-frequency local field potentials and spikes in primary visual cortex convey independent visual information. J. Neurosci. 2008;28:5696–5709. doi: 10.1523/JNEUROSCI.0009-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berens P, Logothetis NK, Tolias AS. Local field potentials, BOLD, and spiking activity: relationships and physiological mechanisms. In: Kriegeskorte N, Kreiman G, editors. Visual Population Codes: Toward a Common Multivariate Framework for Cell Recording and Functional Imaging. The MIT Press; 2011. pp. 599–623. [Google Scholar]
- Bruce CJ, Goldberg ME, Bushnell MC, Stanton GB. Primate frontal eye fields. II. Physiological and anatomical correlates of electrically evoked eye movements. J. Neurophysiol. 1985;54:714–734. doi: 10.1152/jn.1985.54.3.714. [DOI] [PubMed] [Google Scholar]
- Buffalo EA, Fries P, Landman R, Buschman TJ, Desimone R. Laminar differences in gamma and alpha coherence in the ventral stream. Proc. Natl. Acad. Sci. USA. 2011;108:11262–11267. doi: 10.1073/pnas.1011284108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns SP, Xing D, Shapley RM. Comparisons of the dynamics of local field potential and multiunit activity signals in macaque visual cortex. J. Neurosci. 2010;30:13739–13749. doi: 10.1523/JNEUROSCI.0743-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr DC, Ross J, Binda P, Morrone MC. Saccades compress space, time and number. Trends Cogn. Sci. 2010;14:528–533. doi: 10.1016/j.tics.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Buschman TJ, Miller EK. Serial, covert shifts of attention during visual search are reflected by the frontal eye fields and correlated with population oscillations. Neuron. 2009;63:386–396. doi: 10.1016/j.neuron.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Anastassiou CA, Koch C. The origin of extracellular fields and currents–EEG, ECoG, LFP and spikes. Nat. Rev. Neurosci. 2012;13:407–420. doi: 10.1038/nrn3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Carlén M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran C, Peixoto D, Newsome WT, Shenoy KV. Laminar differences in decision-related neural activity in dorsal premotor cortex. Nat. Commun. 2017;8:614. doi: 10.1038/s41467-017-00715-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelazzi L, Miller EK, Duncan J, Desimone R. A neural basis for visual search in inferior temporal cortex. Nature. 1993;363:345–347. doi: 10.1038/363345a0. [DOI] [PubMed] [Google Scholar]
- Chen CM, Lakatos P, Shah AS, Mehta AD, Givre SJ, Javitt DC, Schroeder CE. Functional anatomy and interaction of fast and slow visual pathways in macaque monkeys. Cereb. Cortex. 2007;17:1561–1569. doi: 10.1093/cercor/bhl067. [DOI] [PubMed] [Google Scholar]
- Chen X, Scangos KW, Stuphorn V. Supplementary motor area exerts proactive and reactive control of arm movements. J. Neurosci. 2010;30:14657–14675. doi: 10.1523/JNEUROSCI.2669-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor CE, Gallant JL, Preddie DC, Van Essen DC. Responses in area V4 depend on the spatial relationship between stimulus and attention. J. Neurophysiol. 1996;75:1306–1308. doi: 10.1152/jn.1996.75.3.1306. [DOI] [PubMed] [Google Scholar]
- Desimone R, Chelazzi L, Miller E, Duncan J. Neural Mechanisms for Memory-Guided Visual Search. Exp. Brain Res. 1994;24:279–285. [Google Scholar]
- Deubel H, Schneider WX, Bridgeman B. Postsaccadic target blanking prevents saccadic suppression of image displacement. Vision Res. 1996;36:985–996. doi: 10.1016/0042-6989(95)00203-0. [DOI] [PubMed] [Google Scholar]
- Duhamel JR, Colby CL, Goldberg ME. The updating of the representation of visual space in parietal cortex by intended eye movements. Science. 1992;255:90–92. doi: 10.1126/science.1553535. [DOI] [PubMed] [Google Scholar]
- Eckhorn R, Bauer R, Jordan W, Brosch M, Kruse W, Munk M, Reitboeck HJ. Coherent oscillations: a mechanism of feature linking in the visual cortex? Multiple electrode and correlation analyses in the cat. Biol. Cybern. 1988;60:121–130. doi: 10.1007/BF00202899. [DOI] [PubMed] [Google Scholar]
- Einevoll GT, Kayser C, Logothetis NK, Panzeri S. Modelling and analysis of local field potentials for studying the function of cortical circuits. Nat. Rev. Neurosci. 2013;14:770–785. doi: 10.1038/nrn3599. [DOI] [PubMed] [Google Scholar]
- Engel TA, Steinmetz NA, Gieselmann MA, Thiele A, Moore T, Boahen K. Selective modulation of cortical state during spatial attention. Science. 2016;354:1140–1144. doi: 10.1126/science.aag1420. [DOI] [PubMed] [Google Scholar]
- Fischer B, Boch R. Enhanced activation of neurons in prelunate cortex before visually guided saccades of trained rhesus monkeys. Exp. Brain Res. 1981;44:129–137. doi: 10.1007/BF00237333. [DOI] [PubMed] [Google Scholar]
- Frien A, Eckhorn R, Bauer R, Woelbern T, Gabriel A. Fast oscillations display sharper orientation tuning than slower components of the same recordings in striate cortex of the awake monkey. Eur. J. Neurosci. 2000;12:1453–1465. doi: 10.1046/j.1460-9568.2000.00025.x. [DOI] [PubMed] [Google Scholar]
- Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291:1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Bacon SJ. Local circuit neurons in the medial prefrontal cortex (areas 24a,b,c, 25 and 32) in the monkey: II. Quantitative areal and laminar distributions. J. Comp. Neurol. 1996;364:609–636. doi: 10.1002/(SICI)1096-9861(19960122)364:4<609::AID-CNE2>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Gail A, Brinksmeyer HJ, Eckhorn R. Perception-related modulations of local field potential power and coherence in primary visual cortex of awake monkey during binocular rivalry. Cereb. Cortex. 2004;14:300–313. doi: 10.1093/cercor/bhg129. [DOI] [PubMed] [Google Scholar]
- Gerbella M, Belmalih A, Borra E, Rozzi S, Luppino G. Cortical connections of the macaque caudal ventrolateral prefrontal areas 45A and 45B. Cereb. Cortex. 2010;20:141–168. doi: 10.1093/cercor/bhp087. [DOI] [PubMed] [Google Scholar]
- Gieselmann MA, Thiele A. Comparison of spatial integration and surround suppression characteristics in spiking activity and the local field potential in macaque V1. Eur. J. Neurosci. 2008;28:447–459. doi: 10.1111/j.1460-9568.2008.06358.x. [DOI] [PubMed] [Google Scholar]
- Godlove DC, Maier A, Woodman GF, Schall JD. Microcircuitry of agranular frontal cortex: testing the generality of the canonical cortical microcircuit. J. Neurosci. 2014;34:5355–5369. doi: 10.1523/JNEUROSCI.5127-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray CM, Singer W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc. Natl. Acad. Sci. USA. 1989;86:1698–1702. doi: 10.1073/pnas.86.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoriou GG, Rossi AF, Ungerleider LG, Desimone R. Lesions of prefrontal cortex reduce attentional modulation of neuronal responses and synchrony in V4. Nat. Neurosci. 2014;17:1003–1011. doi: 10.1038/nn.3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider B, Schulz DPA, Häusser M, Carandini M. Millisecond Coupling of Local Field Potentials to Synaptic Currents in the Awake Visual Cortex. Neuron. 2016;90:35–42. doi: 10.1016/j.neuron.2016.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamker FH, Zirnsak M, Calow D, Lappe M. The peri-saccadic perception of objects and space. PLoS Comput. Biol. 2008;4:e31. doi: 10.1371/journal.pcbi.0040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann TS, Zirnsak M, Marquis M, Hamker FH, Moore T. Two Types of Receptive Field Dynamics in Area V4 at the Time of Eye Movements? Front. Syst. Neurosci. 2017;11:13. doi: 10.3389/fnsys.2017.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrie JA, Shapley R. LFP power spectra in V1 cortex: the graded effect of stimulus contrast. J. Neurophysiol. 2005;94:479–490. doi: 10.1152/jn.00919.2004. [DOI] [PubMed] [Google Scholar]
- Huerta MF, Krubitzer LA, Kaas JH. Frontal eye field as defined by intracortical microstimulation in squirrel monkeys, owl monkeys, and macaque monkeys. II. Cortical connections. J. Comp. Neurol. 1987;265:332–361. doi: 10.1002/cne.902650304. [DOI] [PubMed] [Google Scholar]
- Hwang EJ, Andersen RA. Spiking and LFP activity in PRR during symbolically instructed reaches. J. Neurophysiol. 2012;107:836–849. doi: 10.1152/jn.00063.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Doya K. Validation of decision-making models and analysis of decision variables in the rat basal ganglia. J. Neurosci. 2009;29:9861–9874. doi: 10.1523/JNEUROSCI.6157-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser M, Lappe M. Perisaccadic mislocalization orthogonal to saccade direction. Neuron. 2004;41:293–300. doi: 10.1016/s0896-6273(03)00849-3. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. The neural basis of biased competition in human visual cortex. Neuropsychologia. 2001;39:1263–1276. doi: 10.1016/s0028-3932(01)00116-6. [DOI] [PubMed] [Google Scholar]
- Katzner S, Nauhaus I, Benucci A, Bonin V, Ringach DL, Carandini M. Local origin of field potentials in visual cortex. Neuron. 2009;61:35–41. doi: 10.1016/j.neuron.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khawaja FA, Tsui JM, Pack CC. Pattern motion selectivity of spiking outputs and local field potentials in macaque visual cortex. J. Neurosci. 2009;29:13702–13709. doi: 10.1523/JNEUROSCI.2844-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Newsome WT. Local field potential in cortical area MT: stimulus tuning and behavioral correlations. J. Neurosci. 2006;26:7779–7790. doi: 10.1523/JNEUROSCI.5052-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ, Chelazzi L, Hillyard SA, Desimone R. Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. J. Neurophysiol. 1997;77:24–42. doi: 10.1152/jn.1997.77.1.24. [DOI] [PubMed] [Google Scholar]
- McConkie GW, Currie CB. Visual stability across saccades while viewing complex pictures. J. Exp. Psychol. Hum. Percept. Perform. 1996;22:563–581. doi: 10.1037//0096-1523.22.3.563. [DOI] [PubMed] [Google Scholar]
- Mejias JF, Murray JD, Kennedy H, Wang XJ. Feedforward and feedback frequency-dependent interactions in a large-scale laminar network of the primate cortex. Sci. Adv. 2016;2:e1601335. doi: 10.1126/sciadv.1601335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrikhi Y, Clark K, Albarran E, Parsa M, Zirnsak M, Moore T, Noudoost B. Spatial working memory alters the efficacy of input to visual cortex. Nat. Commun. 2017;8:15041. doi: 10.1038/ncomms15041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineault PJ, Zanos TP, Pack CC. Local field potentials reflect multiple spatial scales in V4. Front. Comput. Neurosci. 2013;7:21. doi: 10.3389/fncom.2013.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitzdorf U. Current source-density method and application in cat cerebral cortex: investigation of evoked potentials and EEG phenomena. Physiol. Rev. 1985;65:37–100. doi: 10.1152/physrev.1985.65.1.37. [DOI] [PubMed] [Google Scholar]
- Moore T, Zirnsak M. Neural Mechanisms of Selective Visual Attention. Annu. Rev. Psychol. 2017;68:47–72. doi: 10.1146/annurev-psych-122414-033400. [DOI] [PubMed] [Google Scholar]
- Moore T, Tolias AS, Schiller PH. Visual representations during saccadic eye movements. Proc. Natl. Acad. Sci. USA. 1998;95:8981–8984. doi: 10.1073/pnas.95.15.8981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran J, Desimone R. Selective attention gates visual processing in the extrastriate cortex. Science. 1985;229:782–784. doi: 10.1126/science.4023713. [DOI] [PubMed] [Google Scholar]
- Nandy AS, Nassi JJ, Reynolds JH. Laminar Organization of Attentional Modulation in Macaque Visual Area V4. Neuron. 2017;93:235–246. doi: 10.1016/j.neuron.2016.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson C, Freeman JA. Theory of current source-density analysis and determination of conductivity tensor for anuran cerebellum. J. Neurophysiol. 1975;38:356–368. doi: 10.1152/jn.1975.38.2.356. [DOI] [PubMed] [Google Scholar]
- Pesaran B, Pezaris JS, Sahani M, Mitra PP, Andersen RA. Temporal structure in neuronal activity during working memory in macaque parietal cortex. Nat. Neurosci. 2002;5:805–811. doi: 10.1038/nn890. [DOI] [PubMed] [Google Scholar]
- Rasch MJ, Gretton A, Murayama Y, Maass W, Logothetis NK. Inferring spike trains from local field potentials. J. Neurophysiol. 2008;99:1461–1476. doi: 10.1152/jn.00919.2007. [DOI] [PubMed] [Google Scholar]
- Ray S, Maunsell JH. Differences in gamma frequencies across visual cortex restrict their possible use in computation. Neuron. 2010;67:885–896. doi: 10.1016/j.neuron.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Maunsell JH. Different origins of gamma rhythm and high-gamma activity in macaque visual cortex. PLoS Biol. 2011;9:e1000610. doi: 10.1371/journal.pbio.1000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Hsiao SS, Crone NE, Franaszczuk PJ, Niebur E. Effect of stimulus intensity on the spike-local field potential relationship in the secondary somatosensory cortex. J. Neurosci. 2008;28:7334–7343. doi: 10.1523/JNEUROSCI.1588-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JH, Chelazzi L. Attentional modulation of visual processing. Annu. Rev. Neurosci. 2004;27:611–647. doi: 10.1146/annurev.neuro.26.041002.131039. [DOI] [PubMed] [Google Scholar]
- Reynolds JH, Chelazzi L, Desimone R. Competitive mechanisms subserve attention in macaque areas V2 and V4. J. Neurosci. 1999;19:1736–1753. doi: 10.1523/JNEUROSCI.19-05-01736.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J, Morrone MC, Burr DC. Compression of visual space before saccades. Nature. 1997;386:598–601. doi: 10.1038/386598a0. [DOI] [PubMed] [Google Scholar]
- Saalmann YB, Pinsk MA, Wang L, Li X, Kastner S. The pulvinar regulates information transmission between cortical areas based on attention demands. Science. 2012;337:753–756. doi: 10.1126/science.1223082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall JD, Morel A, King DJ, Bullier J. Topography of visual cortex connections with frontal eye field in macaque: convergence and segregation of processing streams. J. Neurosci. 1995;15:4464–4487. doi: 10.1523/JNEUROSCI.15-06-04464.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmolesky MT, Wang Y, Hanes DP, Thompson KG, Leutgeb S, Schall JD, Leventhal AG. Signal timing across the macaque visual system. J. Neurophysiol. 1998;79:3272–3278. doi: 10.1152/jn.1998.79.6.3272. [DOI] [PubMed] [Google Scholar]
- Schroeder CE, Mehta AD, Givre SJ. A spatiotemporal profile of visual system activation revealed by current source density analysis in the awake macaque. Cereb. Cortex. 1998;8:575–592. doi: 10.1093/cercor/8.7.575. [DOI] [PubMed] [Google Scholar]
- Self MW, Peters JC, Possel JK, Reithler J, Goebel R, Ris P, Jeurissen D, Reddy L, Claus S, Baayen JC, Roelfsema PR. The Effects of Context and Attention on Spiking Activity in Human Early Visual Cortex. PLoS Biol. 2016;14:e1002420. doi: 10.1371/journal.pbio.1002420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheinberg DL, Logothetis NK. Noticing familiar objects in real world scenes: the role of temporal cortical neurons in natural vision. J. Neurosci. 2001;21:1340–1350. doi: 10.1523/JNEUROSCI.21-04-01340.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle JH, Pritchett DL, Moore CI. Gamma-range synchronization of fast-spiking interneurons can enhance detection of tactile stimuli. Nat. Neurosci. 2014;17:1371–1379. doi: 10.1038/nn.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva LR, Amitai Y, Connors BW. Intrinsic oscillations of neocortex generated by layer 5 pyramidal neurons. Science. 1991;251:432–435. doi: 10.1126/science.1824881. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. What the brain stem tells the frontal cortex. I. Oculomotor signals sent from superior colliculus to frontal eye field via mediodorsal thalamus. J. Neurophysiol. 2004;91:1381–1402. doi: 10.1152/jn.00738.2003. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. Influence of the thalamus on spatial visual processing in frontal cortex. Nature. 2006;444:374–377. doi: 10.1038/nature05279. [DOI] [PubMed] [Google Scholar]
- Stanton GB, Bruce CJ, Goldberg ME. Topography of projections to posterior cortical areas from the macaque frontal eye fields. J. Comp. Neurol. 1995;353:291–305. doi: 10.1002/cne.903530210. [DOI] [PubMed] [Google Scholar]
- Tolias AS, Moore T, Smirnakis SM, Tehovnik EJ, Siapas AG, Schiller PH. Eye movements modulate visual receptive fields of V4 neurons. Neuron. 2001;29:757–767. doi: 10.1016/s0896-6273(01)00250-1. [DOI] [PubMed] [Google Scholar]
- Tremblay S, Doucet G, Pieper F, Sachs A, Martinez-Trujillo J. Single-Trial Decoding of Visual Attention from Local Field Potentials in the Primate Lateral Prefrontal Cortex Is Frequency-Dependent. J. Neurosci. 2015;35:9038–9049. doi: 10.1523/JNEUROSCI.1041-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay R, Lee S, Rudy B. GABAergic Interneurons in the Neocortex: From Cellular Properties to Circuits. Neuron. 2016;91:260–292. doi: 10.1016/j.neuron.2016.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider LG, Desimone R. Cortical connections of visual area MT in the macaque. J. Comp. Neurol. 1986;248:190–222. doi: 10.1002/cne.902480204. [DOI] [PubMed] [Google Scholar]
- van Kerkoerle T, Self MW, Dagnino B, Gariel-Mathis MA, Poort J, van der Togt C, Roelfsema PR. Alpha and gamma oscillations characterize feedback and feedforward processing in monkey visual cortex. Proc. Natl. Acad. Sci. USA. 2014;111:14332–14341. doi: 10.1073/pnas.1402773111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayan S, Kopell NJ. Thalamic model of awake alpha oscillations and implications for stimulus processing. Proc. Natl. Acad. Sci. USA. 2012;109:18553–18558. doi: 10.1073/pnas.1215385109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Fung CC, Guan S, Wu S, Goldberg ME, Zhang M. Perisaccadic Receptive Field Expansion in the Lateral Intraparietal Area. Neuron. 2016;90:400–409. doi: 10.1016/j.neuron.2016.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke M, Logothetis NK, Leopold DA. Local field potential reflects perceptual suppression in monkey visual cortex. Proc. Natl. Acad. Sci. USA. 2006;103:17507–17512. doi: 10.1073/pnas.0604673103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer K, Ramon M, Pasternak T, Compte A. Transitions between Multiband Oscillatory Patterns Characterize Memory-Guided Perceptual Decisions in Prefrontal Circuits. J. Neurosci. 2016;36:489–505. doi: 10.1523/JNEUROSCI.3678-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womelsdorf T, Fries P, Mitra PP, Desimone R. Gamma-band synchronization in visual cortex predicts speed of change detection. Nature. 2006;439:733–736. doi: 10.1038/nature04258. [DOI] [PubMed] [Google Scholar]
- Wurtz RH. Neuronal mechanisms of visual stability. Vision Res. 2008;48:2070–2089. doi: 10.1016/j.visres.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Roby KD, Callaway EM. Immunochemical characterization of inhibitory mouse cortical neurons: three chemically distinct classes of inhibitory cells. J. Comp. Neurol. 2010;518:389–404. doi: 10.1002/cne.22229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Gersch TM, Schnitzer BS, Dosher BA, Kowler E. Eye movements and attention: the role of pre-saccadic shifts of attention in perception, memory and the control of saccades. Vision Res. 2012;74:40–60. doi: 10.1016/j.visres.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirnsak M, Moore T. Saccades and shifting receptive fields: anticipating consequences or selecting targets? Trends Cogn. Sci. 2014;18:621–628. doi: 10.1016/j.tics.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirnsak M, Steinmetz NA, Noudoost B, Xu KZ, Moore T. Visual space is compressed in prefrontal cortex before eye movements. Nature. 2014;507:504–507. doi: 10.1038/nature13149. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.