Abstract
Satellite cells (SCs) are adult muscle stem cells capable of repairing damaged and creating new muscle tissue throughout life. Their functionality is tightly controlled by a microenvironment composed of a wide variety of factors, such as numerous secreted molecules and different cell types, including blood vessels, oxygen, hormones, motor neurons, immune cells, cytokines, fibroblasts, growth factors, myofibers, myofiber metabolism, the extracellular matrix and tissue stiffness. This complex niche controls SC biology – quiescence, activation, proliferation, differentiation or renewal and return to quiescence. In this review, we attempt to give a brief overview of the most important players in the niche and their mutual interaction with SCs. We address the importance of the niche to SC behavior under physiological and pathological conditions, and finally survey the significance of an artificial niche both for basic and translational research purposes.
Satellite cells
Over the past half a century, the focus of research on muscle regeneration has shifted from other myogenic cells of muscle tissue to satellite cells (SCs), from developmental myogenesis to adult muscle regeneration, from cell-intrinsic properties of SCs to the relevance of extrinsic factors delivered by their niche. SCs, small, inactive cells wedged between the myofiber and the surrounding extracellular matrix (ECM), have attracted the attention of scientists since their discovery 56 years ago (1). The astonishing translational potential of SCs continues to fascinate, and the ever expanding knowledge of SCs and their microenvironment paves the way for the development of novel cell and gene therapies, in vitro disease models and preclinical drug testing paradigms. Here, we discuss different aspects of SC biology and the niche in health and disease. For a more detailed assessment of the particularities of SCs and the SC niche, we direct readers to several recent reviews focusing on the extracellular matrix (2), blood vessels (3), bioengineering (4), SC function from a cell-intrinsic perspective (5) and an extensive review on SC biology (6).
Skeletal muscle regeneration and muscle stem cells
Comprising approximately 40% of body weight, skeletal muscle can be considered as the largest organ in the human body (7). Muscle not only supports breathing and movement, but is also a very important metabolic and endocrine organ. It comes as no surprise that skeletal muscle has a remarkable capability to repair damage caused by injuries or simple everyday wear-and-tear. As numerous animal studies demonstrate, skeletal muscle is able to regain near original morphology and functionality within several weeks of serious damage caused by injection of myotoxic agents (e.g. cardiotoxin, bupivacaine, barium chloride or notexin), freezing, crushing, or complete mincing and re-transplantation (8–12). However, aging, traumatic injuries in humans resulting in volumetric muscle loss and various myopathies result in impaired functionality and inability of the tissue to regain homeostatic conditions.
SCs are the main cells responsible for sustaining skeletal muscle morphology and functionality throughout the lifetime of an individual. They are largely lineage-committed adult stem cells located at the periphery of muscle fibers, situated between the sarcolemma (the myofiber membrane) and basal lamina (BL) (1), in close proximity to blood vessels (3) and the neuromuscular junction (13). This specific environment surrounding SCs is known as the SC niche.
Under resting conditions, SCs are in the G0 phase (non-cycling state) and quiescent, with a heterochromatic nucleus and a thin rim of cytoplasm containing scarce organelles. These cells are most commonly distinguished by the expression of the paired box transcription factor Pax7. SCs have a tremendous myogenic potential and self-renewal capabilities, as demonstrated by single-fiber (14) as well as single cell (15) implantation in irradiated muscles of immunodeficient mice.
The classical cascade of regeneration resembles that of prenatal skeletal muscle development (16). In response to injury or other stimuli, SCs become activated, increase in size and begin proliferation. The majority of the progeny reduces Pax7 and induces MyoD expression. After several rounds of proliferation, these myoblasts start to express myogenin and exit the cell cycle as myocytes. The myocytes subsequently fuse in order to form new or repair existing myofibers (depending on the severity of injury). The myofibers then express MRF-4 and grow, supported by hypertrophy, until reaching their pre-injury size. At the same time, a part of the SC progeny reacquires high Pax7 levels and returns to quiescence, thereby replenishing the SC pool and maintaining sufficient reserves for future rounds of regeneration.
Besides SCs, several other cell types, such as muscle side population cells, muscle-derived stem cells, bone marrow stem cells, PW1+ interstitial cells, CD133+ cells, mesoangioblasts and pericytes, can successfully regenerate muscles and some can even reconstitute the niche upon transplantation into damaged muscle (17). However, the contribution of these cells seems to be very low under physiological conditions and dependent on SCs, which are essential for skeletal muscle regeneration and therefore represent the true stem cells of muscle tissue (18–21).
According to their gene expression profiles and their characteristics in vitro, SCs stemming from different muscle groups (e.g. head vs. limb muscles) are heterogeneous. Nevertheless, SCs from the masseter muscle (head) are able to regenerate the extensor digitorum longus (EDL) muscle (limb) as efficiently as SCs from the EDL muscle (22), attesting to the enormous influence of the in vivo microenvironment on the behavior and functionality of SCs, which in some cases can overcome the intrinsic differences between SCs.
The heterogeneity of satellite cells and its dependence on the niche
Several studies have addressed the heterogeneity of SC populations in regard to their renewal potential. Interestingly, SC heterogeneity was not only reported between different muscle beds, but also observed between SCs on the same muscle fibers, thereby implicating additional factors besides ontogeny and composition of the fiber type as possible causes. According to these studies, only a small proportion of SCs are bona fide stem cells, whereas the vast majority are committed progenitors with limited stemness. For example, Chakkalakal et al. discovered heterogeneity among SCs based on their proliferative history, suggesting that cells that cycle less frequently have higher self-renewal potential (23). On a related note, Rocheteau et al. evaluated differential DNA strand segregation, where one daughter cell retains the template strands, stays in the niche and returns to quiescence, while the other daughter cell receives newly synthesized DNA strands, continues to proliferate and finally differentiates (24). It was suggested that such DNA strand segregation would prevent accumulation of proliferation-associated mutations in the stem cell, and therefore provide a lifelong supply of progenitors. Similarly, in a lineage tracing experiment with Myf5-Cre/ROSA-YFP mice, Kuang et al. found that the majority of SCs are Pax7+/Myf5+, and only small subset are Pax7+/Myf5- cells (25). Upon isolation and transplantation, both cell populations are capable of proliferating and differentiating, but only Myf5- SCs occupy the niche in the transplanted muscle. In addition, after in vivo activation, Pax7+/Myf5+ (committed progenitors) are exclusively prone to symmetrical division, giving rise to more committed progenitors, whereas Pax7+/Myf5- (true stem cells) on the other hand can divide both symmetrically and asymmetrically, producing uncommitted and committed daughter cells. Mechanistically, the asymmetrical distribution of the Par complex results in p38α/β MAPK activation and MyoD expression only in the committed daughter (26). Importantly, the capability to control the orientation of the cell division is tightly coupled to the SC niche. Following asymmetric division, the uncommitted progenitor remains in the niche in contact with the BL, whereas the committed progenitor is pushed towards the muscle fiber, thus losing contact with the niche. In contrast, both daughter cells retain contact with the BL and the myofiber during a stem cell pool expansion through symmetric division of Pax7+/Myf5-cells.
The satellite cell niche in quiescence and regeneration
SC quiescence, activation, proliferation, differentiation and renewal are intricately connected to the niche. There is a plethora of cell-cell and cell-matrix interactions, numerous paracrine and endocrine molecules (e.g. growth factors and cytokines), as well as biophysical properties of muscle that have a direct effect on the SC. However, this communication is bidirectional, as the SCs themselves also influence their local environment.
The extracellular matrix
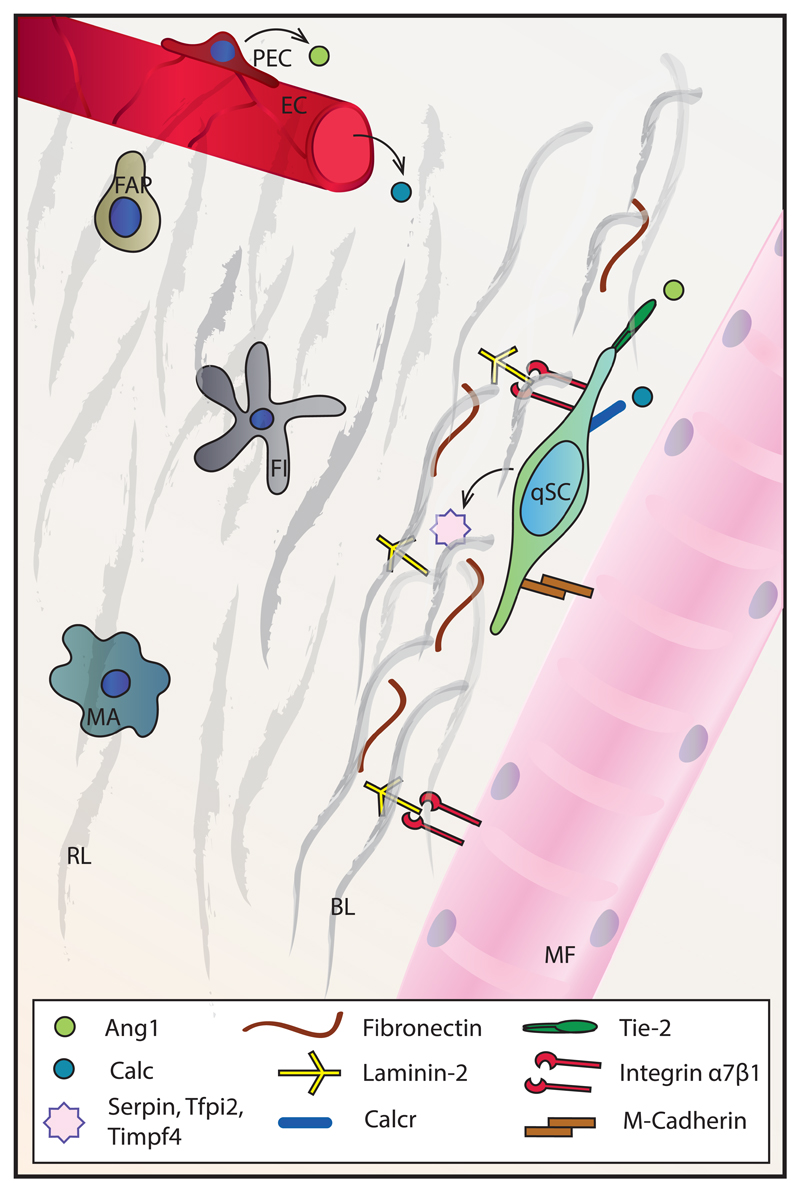
In homeostatic conditions, SCs are situated just outside the muscle fiber, in direct contact with the sarcolemma and the ECM. The ECM surrounding muscle fibers is called the basal membrane (BM) and it consists of two parts – the reticular lamina (RL) and the BL, the latter being in direct contact with the fiber. The BM is a mesh composed of various glycoproteins and proteoglycans with sequestered growth factors. The main components of the RL are fibrillar collagens, whereas the main components of the BL are laminin-2 (α2β1γ1) and non-fibrillar collagen IV (27). The laminins and collagen of the BL self-assemble into networks that are cross-linked by the glycoprotein nidogen. This network provides binding sites for components of the RL on one, and the sarcolemma and SC membrane on the other side. In addition, proteoglycans such as perlecan are anchored to the main BL mesh and bind polypeptidic growth factors with their glycosaminoglycan chains. These growth factors, including fibroblast growth factors (FGFs), epidermal growth factor (EGF), insulin-like growth factors (IGFs) and hepatocyte growth factor (HGF), are secreted by various components of the niche, such as muscle fibers, interstitial cells and SCs, or can be delivered to the niche by blood vessels.
Integrins on the SC membrane and the sarcolemma bind to laminins in the BL, forming focal adhesions and contributing to mechanical stability between the ECM and intracellular cytoskeleton. However, these interactions also have important signaling functions. The main integrin isoforms on SCs are α7 and β1, which bind to laminin-2 on the BL side (28). After SC activation, the expression of integrins on the SC membrane changes, along with the preference for binding partners in the BL. For example, activated, but not quiescent SCs express the β3 integrin isoform, which probably binds to fibronectin in a complex with the αv chain (29). Both quiescent and activated SCs also express the transmembrane heparin sulfate proteoglycans syndecan-3 and syndecan-4. These proteins form complexes with different tyrosine kinases such as c-Met and FGF receptor (FGFR) on the SC membrane and are consequently important not only for cell adhesion to the BL, but also for SC activation (30).
Expression profiles of quiescent and activated SCs suggest that SCs actively contribute to maintaining niche quiescence while remaining highly sensitive to activating stimuli (31). Quiescent SCs express the protease inhibitors Serpin and Tfpi2 as well as metalloprotease inhibitor Timpf4. Upon activation, however, these genes become downregulated, and instead, SCs start expressing the matrix metalloproteases MMP-2 and MMP-9 (32). MMPs are major enzymes responsible for ECM degradation.
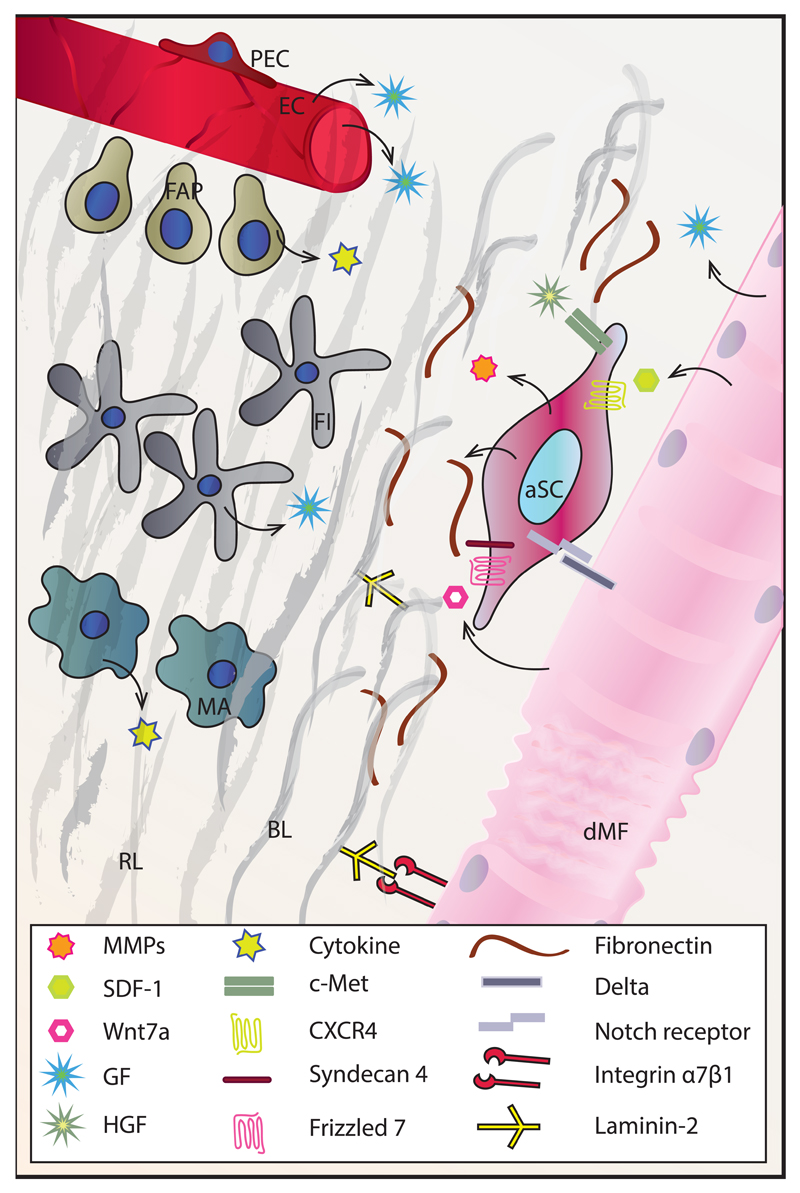
Activated SCs also produce fibronectin (FN), an ECM glycoprotein whose role in SC maintenance by enabling their attachment to the niche has recently been demonstrated (33). SC-produced FN potentiates Wnt7a signaling through the receptor complex syndecan-4/Frizzled-7, thereby supporting symmetric division of SCs and expansion of the stem cell pool (34). Specific knock-down of FN in SCs leads to a drastic reduction in symmetric division, in particular in the Pax7+/Myf5- population, leading to a drop in SC numbers during regeneration.
Collagen VI is another BL component essential for preserving the SC pool. Fibroblasts are the prime producers of this protein as well as many other BL components. Collagen VI knock-out mice exhibit reduced regeneration and an inability to maintain SC numbers following injury. This defect is, however, rescued by transplanting wild-type fibroblasts, demonstrating the critical importance of non-SC-autonomous ECM factors in SC maintenance (35).
The muscle fiber
On the apical side, SCs are bound to a muscle fiber, and M-cadherin is the main adhesion protein supporting the connection between these two cell types. Myofibers are important regulators of SC state: for example, myofiber damage or stretch induces nitric oxide (NO) synthesis in the BL, which is able to activate MMPs, and through that action liberate ECM-bound HGF, allowing its binding to the c-Met receptor on SCs. This HGF signaling through c-Met has been proposed as an initial activation signal for SCs (36).
SCs are furthermore affected by the Notch and Wnt signaling pathways in regard to quiescence, activation, proliferation and differentiation (6). Proof-of-concept was provided in different studies, e.g. by ablation of RBP-Jκ, a downstream mediator of Notch., This ablation leads to spontaneous activation and differentiation of SCs without a proliferative phase, precipitating depletion of the SC pool and thus indicating that Notch signaling is essential for SC quiescence (37, 38). Upon injury, damaged fibers express Delta, a ligand of the Notch receptor, which stimulates SC proliferation. In addition, regenerating fibers synthesize Wnt7a, which induces SC symmetrical cell division by binding to the Frizzled7 receptor (39).
In regeneration, myofibers secrete stromal cell-derived factor-1 (SDF-1), which binds to the receptor CXCR4 on SCs and induces SC chemoattraction (40). Injured fibers and other cells of the niche also secrete FGFs, EGF and IGFs, which further regulate SC proliferation and differentiation. For instance, FGF-2 induces proliferation and represses differentiation of progenitor cells by binding to the tyrosine kinase FGFR and activating the Ras/MAPK pathway (41). Likewise, IGF-II supports proliferation, while the pleiotropic functions of IGF-I include stimulation of SC proliferation, differentiation, migration and anti-inflammatory effects on the niche (reviewed in (42). These effects of IGF-I are mediated through several signal transduction pathways, all initiated by IGF-I binding to the tyrosine receptor kinase IGF1R. The situation is further complicated by the existence of multiple IGF-I isoforms, as well as IGF binding proteins (IGFBPs) secreted by activated SCs, whose function is to transport IGFs and modulate their half-life (reviewed in (43). On the other hand, myofibers also secrete myostatin (Mstn), a member of the transforming growth factor β (TGF-β) family and negative regulator of muscle growth that has been implicated in reducing SC activation and self-renewal (44).
Much attention has been given to metabolic reprograming of SCs, that is, the effects of the metabolism of a SC on its fate (45). Some research proposes that in quiescence, SCs primarily rely on fatty acid oxidation (46), whereas upon activation, they increase substrate utilization through glycolysis, and finally switch to oxidative phosphorylation during differentiation (47). Other studies suggest that activated SCs depend more on oxidative phosphorylation (45, 48, 49). It also remains unclear how metabolic substrate utilization in skeletal fibers (the SC niche) influences the SC state. Experiments with caloric restriction have suggested that the increased fatty acid oxidation and mitochondrial activity in the fiber in this context probably induce SC activation through increased oxidative phosphorylation (49).
Effects of fiber metabolism on SCs are furthermore implied by the observation that resting SC numbers are considerably higher in oxidative slow-twitch compared to glycolytic fast-twitch myofibers (50). Moreover, a similar difference in SC numbers can be achieved by endurance exercise, which promotes a switch from glycolytic to oxidative fibers (51, 52). Although a conclusive explanation for the correlation between SC numbers and the oxidative fiber type remains elusive, the metabolic properties and the vascularization have been linked to this observation. The existence of a denser blood vessel network in slow fibers is of particular interest given close vicinity of the majority of SCs to blood vessels (3). However, this simple view has recently been challenged. Namely, mice with myofiber-specific overexpression of peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α), a nodal modulator of oxidative metabolism, exhibit both a switch to oxidative fibers and increased capillarity (53), but nevertheless have fewer SCs, albeit with an increased myogenic capacity (54). In fact, in regard to most metabolic and contractile traits, PGC-1α transgenic, bona fide oxidative and endurance-trained muscles are indistinguishable. Interestingly, the muscle fiber PGC-1α transgene affects expression of BM components FN and tenascin-C (54), which might account for the increased myogenic potential of the SCs. However, a possible influence of other differences in the microenvironment, for instance the increased percentage of M2 macrophages in resting conditions in these animals, should not be overlooked (11, 55). Therefore, an alternative explanation for the correlation between SC number and fiber type could be a difference in ECM organization. For example, the slow soleus muscle has double the amount of collagen IV and half the amount of laminin-2 compared to the fast rectus femoris in rats (56). However, the link between SC number and fiber type-specific ECM composition is still poorly understood and thus awaits further research.
Blood vessels, oxygen, (peri)endothelial cells and secreted systemic factors
The close proximity of SCs and capillaries suggests that blood vessels are an important part of the niche. Indeed, the close correlation between a well-developed capillary network and successful skeletal muscle regeneration has been demonstrated (57, 58). This is not surprising given the fact that a myriad of factors and cells that modify the satellite cell niche, such as hormones and monocytes, are delivered by blood vessels. In addition, endothelial cells can secrete growth factors (EGF, IGF-I, bFGF) including vascular endothelial growth factor (VEGF) and platelet-derived growth factor BB (PDGF-BB), which promote SC proliferation (59). Conversely, differentiating myogenic cells also secrete VEGF, thereby stimulating angiogenesis (60). Interestingly, peri-endothelial cells, such as smooth muscle cells, secrete angiopoietin 1 (Ang1), which regulates the SC state by binding to the Tie-2 receptor that is highly expressed in resting SCs. This interaction in turn induces the expression of quiescence markers and blocks the expression of differentiation markers in SCs through ERK1/2 signaling (61), resulting in a return to quiescence at the end of regeneration.
A reduction in partial oxygen pressure has also emerged as an essential factor in SC biology. Hypoxia is a critical factor for many stem cells, with a strong link between low oxygen levels and the undifferentiated cell state (62). Myoblasts cultured under hypoxic conditions show increased quiescence and higher self-renewal efficiency upon transplantation in vivo (63).
Finally, systemic, circulating factors facilitate the adjustment of SCs to distal processes away from the niche. For example, calcitonin, a thyroid hormone that is secreted in response to high blood calcium levels, is important for SC dormancy and sub-laminar location. It exerts its effects by binding to the calcitonin receptor (Calcr), which is expressed by resting, but not by activated SCs (64). A specific knock-out of Calcr in SCs results in their relocation from the niche and loss by apoptosis (65). Likewise, SC-specific knock-out of the androgen receptor, which is expressed in this cell population (66), leads to induction of Mstn expression, a fiber-type switch and a reduction in muscle mass and strength (67).
Motor neurons, fibroblasts, fibro/adipogenic progenitors and immune cells
In slow-twitch muscles, SCs are located in close proximity to the neuromuscular junction (NMJ) (13), and the difference in SC numbers between slow and fast-twitch fibers is correlated with the pattern of neuron firing (50). When denervated, skeletal muscle fibers undergo atrophy, to which SCs initially respond with activation and proliferation similar to what is observed in damaged muscle, but after several weeks of denervation, SC number declines due to loss of proliferative capacity and apoptosis (68, 69). Conversely, it has been shown that developing muscle produces neurotrophins, which function as retrograde survival factors for the motor neuron (70), and SCs secrete the axonal guidance factor semaphorin 3A with possible implications in muscle regeneration (71). Although initially found to have a role in neuron survival, neurotrophins are emerging as important modulatory factors for various cell populations and tissues including skeletal muscle. For example, nerve growth factor (NGF) is expressed by regenerating fibers, which implies its involvement in muscle regeneration. Similarly, SC expression of brain-derived neurotrophic factor (BDNF) is important for SC maintenance, and consequently affects muscle regeneration (72, 73).
Fibroblasts contribute to the niche by secreting growth factors and structural components of the BL. Temporary thickening of the ECM coupled with an increase in the number of muscle tissue fibroblasts is a hallmark of muscle regeneration (74). Furthermore, interactions between Tcf4+ fibroblasts and SCs are necessary for successful regeneration. Selective, conditional ablation of SCs in Pax7CreERT2/+;R26RDTA/+ mice leads to insufficient proliferation of fibroblasts in the initial phases of regeneration and fibrosis at the later stages, whereas the partial ablation of fibroblasts in Tcf4CreERT2/+;R26RDTA/+ mice causes reduced proliferation and precocious differentiation of SCs, resulting in a decreased diameter of regenerated muscles and depletion of the SC pool (21).
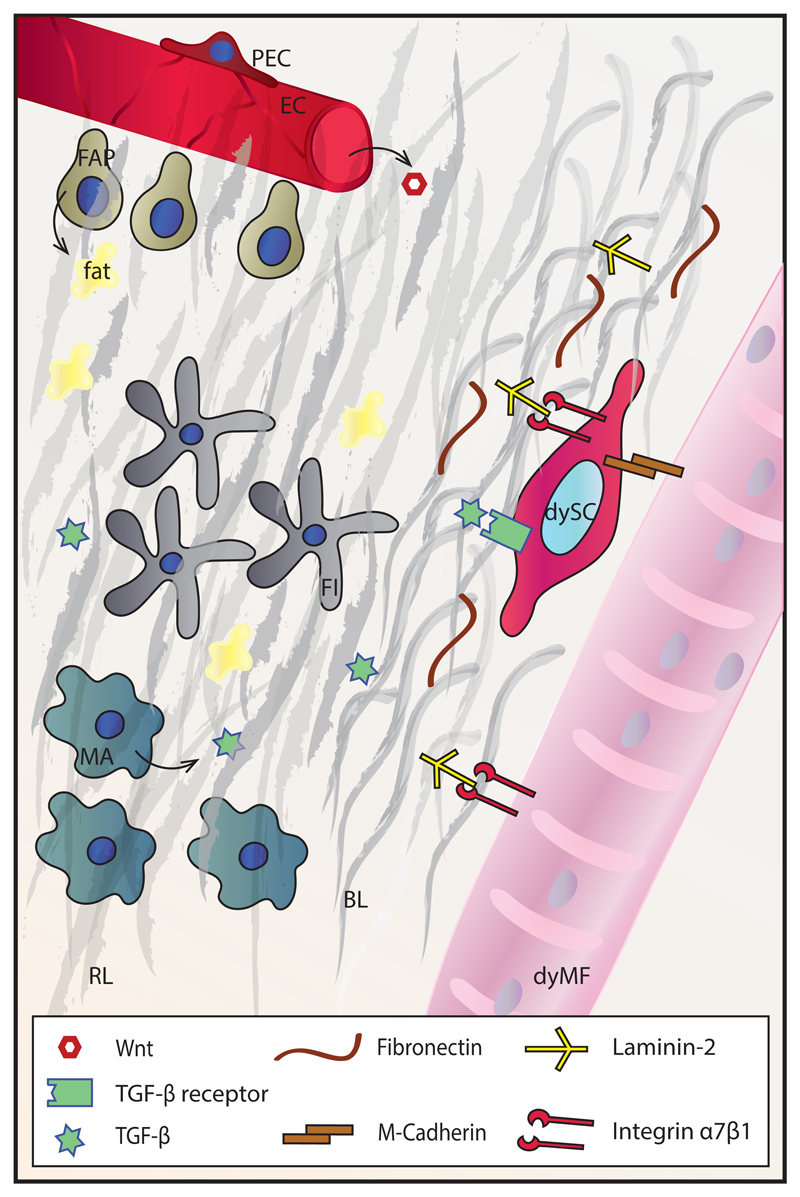
Skeletal muscle-resident mesenchymal progenitors expressing PDGFRα are known as fibro/adipogenic progenitors (FAPs) due to their ability to differentiate into adipocytes and fibroblasts (75). In homeostatic conditions, these cells are in close proximity to blood vessels (76), and their number quickly rises in the event of muscle damage. FAPs facilitate myofiber formation and myoblast differentiation by secreting specific ECM components and cytokines, respectively (77). These cells also display the ability to remove necrotic tissue (78), thereby supporting muscle regeneration. Interestingly, proper signaling from myotubes and eosinophils prevents FAP differentiation into adipocytes (75).
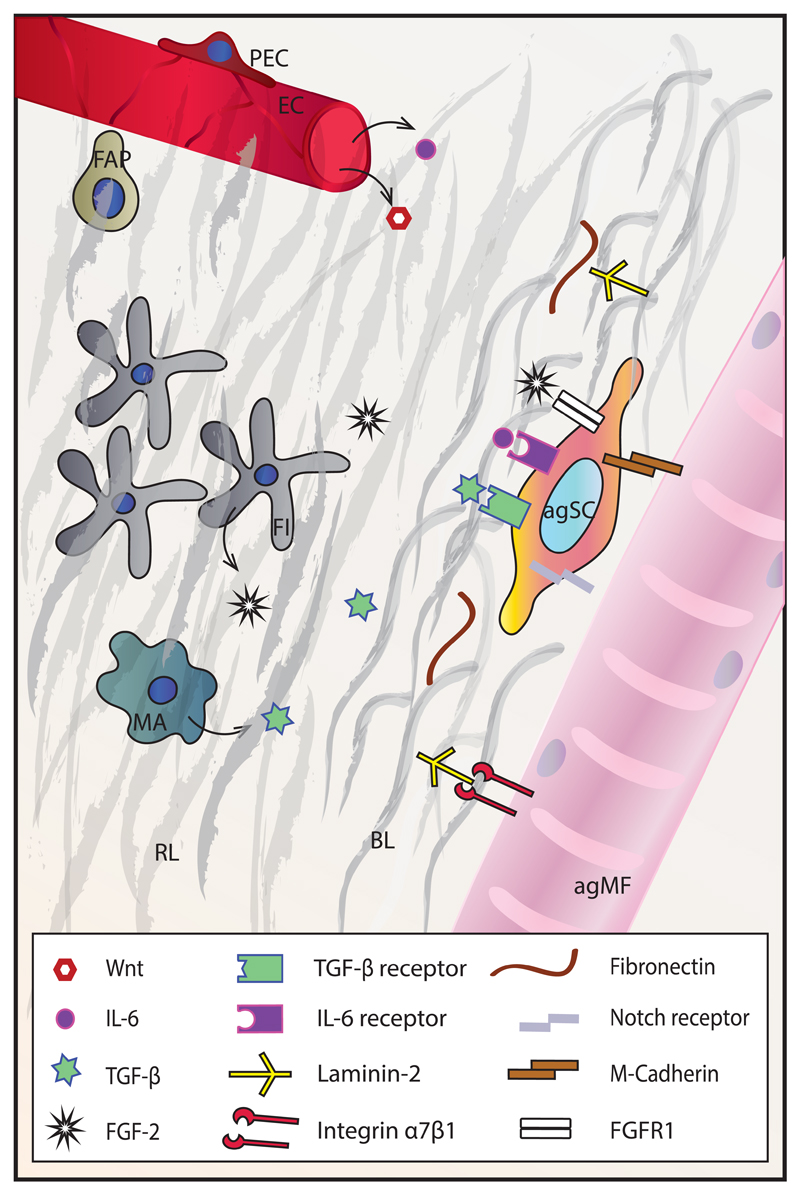
Immune cells are additional important players in defining the SC niche in regeneration. Some of these cells, like tissue macrophages and mast cells, are permanent members of the niche, but their importance in modulating the SC microenvironment in quiescence is likely limited. However, they take on an active role upon sterile injury, which induces muscle fiber damage and necrosis. Resident immune cells react by secreting cytokines and chemokines including tumor necrosis factor α (TNF-α), interleukin 6 (IL-6) and macrophage inflammatory protein 2 (MIP-2), which primarily drive the extravasation of neutrophils (79, 80). Next, neutrophils secrete MIP-1α, monocyte chemoattractant protein-1 (MCP-1) and other cytokines attracting monocytes from blood vessels, which rapidly become the most abundant inflammatory cell type in the damaged tissue (81). Depending on the milieu of inflammatory signals and immune cells present in the niche, the macrophages derived from the monocytes can acquire the M1 or M2 type. M1 macrophages secrete proinflammatory cytokines (TNF-α, IL-1β) and are characteristic of the early post-injury stages. They are essential for the removal of necrotic tissue and promote SC proliferation. Upon clearance of cellular debris, the altered conditions in the niche promote an increase in the number of M2 macrophages, which secrete anti-inflammatory cytokines (IL-4, IGF-I, TGF-β) and support the differentiation stages of regeneration (82, 83). Temporal regulation of the inflammatory cascade is crucial in the process. For example, suppression of M1 macrophages leads to reduced SC proliferation, persistence of necrosis and results in fat and fibrotic tissue accumulation. Likewise, suppression of the switch from the M1 to the M2 type negatively affects myogenesis and myofiber growth (84–86). In addition to paracrine signaling, macrophages establish direct contact with myoblasts and myotubes through cell adhesion interactions (e.g. via VCAM-1-VLA-4, ICAM-1-LFA-1, PECAM-1-PECAM-1 and CX3CL1-CX3CR1), which prevent apoptosis of myogenic cells (87). Apart from innate immunity, cells of the adaptive immune system are also central to regulating SC behavior during sterile injury. An instrumental role of T regulatory cells in proper SC expansion and muscle regeneration, as well as in the M1 to M2 macrophage switch after injury has been described (88, 89).
The biophysical properties of muscle
Aside from other factors of the niche, rigidity of the microenvironment can profoundly affect SC behavior. The elastic stiffness of uninjured skeletal muscle is ~12kPa, and ECM deposition during regeneration increases this value (90). SCs can sense and react to this biophysical property of the environment through focal adhesions (91). When cultured on rigid plastic dishes (~106kPa), SCs quickly lose their quiescence and stemness. Myoblasts cultured on hydrogels prefer a substrate stiffness of ~21kPa, while softer (~ 3kPa) and stiffer (~ 80kPa) gels reduce their proliferative rate (92). In line, SCs cultured on soft hydrogels that mimic the stiffness of natural muscle (12kPa) are able to self-renew and significantly improve their contribution to muscle regeneration upon transplantation (93).
The satellite cell niche in pathological contexts
Aging, muscle dystrophies and related pathologies invariably lead to perturbed conditions of the SC niche. These changes can cause a reduction or an expansion in the SC pool, irresponsiveness to stimuli and therefore a reduced SC activation rate, aberrant proliferation and precocious or reduced differentiation, or SC senescence and apoptosis upon activation. For example, a disproportion of symmetric and asymmetric SC division might tip the balance towards SC loss in aging and a pathological SC expansion with a reduced number of myogenic progenitors in dystrophic conditions (94). Irrespective of the dysregulation, the outcome is diminished SC regenerative capacity in both contexts.
Although some of the pathological changes are SC intrinsic, altering the niche can alleviate the underlying condition in many cases. Nevertheless, it is difficult to precisely discriminate between intrinsic and extrinsic origins of the SC pathology due to the bidirectional signaling between SCs and their microenvironment. Importantly, the niche can induce modifications in SC properties that can persist even after removal of SCs from the niche, and are hence perceived as “intrinsic”.
The satellite cell niche in aging
With advanced age, skeletal muscle mass and neuromuscular performance diminishes, a condition termed sacropenia. Decreased fiber and motor neuron numbers, reduced fiber size, a myofiber switch towards the oxidative type and loss of myonuclei resulting in an increase in myonuclear domain size are all common observations in aging, collectively resulting in a marked decrease of the efficiency of muscle regeneration (95, 96). The reduction of the SC pool has been proposed as an explanation for the underlying condition (51). However, based on conflicting results in different studies (97), the prevailing opinion is that a drop in the myogenic potential of SCs might be the causative factor of the impaired regenerative capacity.
Some changes in the aged niche are precipitated by aberrant signaling. For instance, lack of Delta upregulation by injured aged muscles leads to reduced Notch signaling in SCs and hence reduced SC proliferation – a phenotype that can be overcome by alternative Notch activation (97). Interestingly, experiments with heterochronic, parabiotic pairings (a shared circulatory system between a young and an old animal) demonstrated that systemic factors at least partially account for the perturbed SC biology, as the exposure to young blood restored otherwise reduced Notch signaling and improved SC proliferation in old mice (98). The subsequent search for rejuvenating humoral factors led to the implication of the hormone oxytocin (99) and growth factor GDF11 (100, 101) as systemic factors that decline with age and whose induction is able to revert aging-related SC pathology. However, the function of GDF11 in promoting muscle and cardiac health in aging has been largely discredited in more recent studies (102–104). Exacerbated canonical Wnt signaling due to elevated circulating Wnt activators in aged mice was also suggested as being responsible for aging-related tissue fibrosis and conversion of myoblasts into fibroblasts, a process that can be curbed by Wnt inhibitors (105). Increased NF-κB and TGF-β signaling in aged muscles are additional examples of how the immediate niche can negatively impact the regenerative potential of SCs (106, 107).
ECM deposition in the aged niche in general is thought to act as a damper and therefore exert a negative influence on the activation potential of SCs, e.g. by increasing tissue stiffness. For example, slow muscles boost the expression of collagen IV while fast muscles elevate the levels of laminin with aging (56). The ensuing imbalance in the components of the BL in old muscle disturbs the signal transduction pathways that govern SCs in the niche, such as those triggered by higher levels of TGF-β, a negative regular of SC proliferation (107), and FGF-2. FGF2 signaling through FGFR1 results in SC loss based on unmitigated cycling. Importantly, this effect can be reverted by increasing Spry1 in SCs, an inhibitor of FGF signaling and preserver of SC quiescence (23). The p38α/β MAPK pathway, downstream of FGF signaling, is consequently overactivated in aged SCs, leading to reduced asymmetric division and higher numbers of committed daughter cells, hence resulting in diminished self-renewal. Improving the SC environment by transplanting old SCs into a young host could not revert this condition, in contrast to the successful pharmacological inhibition of the p38α/β MAPK pathway in SCs (108, 109). Most likely triggered by increased IL-6 blood levels, the JAK/STAT signaling pathway is also overactivated in aged SCs and results in a reduction of symmetric division and self-renewal, which can be reverted with pharmacological inhibitors (110, 111). In geriatric mice (30 months of age), SCs lose their ability for reversible quiescence by switching to pre-senescence. At that age, the respective stimuli fail to induce SC activation and proliferation, but instead prompt senescence in a process termed geroconversion. Silencing of p16INK4a, a cell cycle inhibitor that triggers the switch to pre-senescence, is able to restore the activation and proliferation potential of SCs (112). Intriguingly, blocking autophagy in young SCs causes senescence, while its restoration in old age reestablishes the regenerative potential of SCs (113). Furthermore, loss of FN from the aged BL prevents sufficient attachment of SCs to the niche and thus disturbs signaling through focal adhesion kinase, thereby precipitating SC loss (33). In addition, mislocalization of integrin β1 on aged and dystrophic SCs leads to impaired sensitivity to FGF-2, consequently causing reduced SC proliferation and ultimately SC depletion, resulting in impaired regeneration. In both models, activation of β1-integrin reverts the impairment of SC function (114).
Hormonal and pharmacological interventions, calorie restriction as well as cell therapy have been proposed for the prevention and treatment of sarcopenia. However, to date, physical activity remains the most efficacious approach to combating this disease (115), e.g. by boosting the number and myogenic capacity of SCs (51, 116). Although an SC pathology is most likely not the only driving force for development of sacropenia, SC dysfunction contributes to impaired muscle regeneration and increased fibrosis (105). Recent advances in understanding aberrant signal transduction pathways and communication between aged SCs and their niche will potentially offer new pharmacological avenues in the treatment of sarcopenia that could circumvent the inherent problems of exercise interventions in geriatric patients.
The satellite cell niche in dystrophic conditions
Muscular dystrophies are a heterogeneous group of sporadic and inherited disorders that lead to progressive muscle wasting and weakness. Fiber size variation, fiber necrosis followed by inflammation, and muscle tissue replacement by fat and scar tissue are often hallmarks of these pathologies, depending on the severity of the dystrophy in question (117). Many dystrophies are caused by a mutation in structural proteins of the cytoskeleton, membrane or ECM, which comprise a part of the SC niche.
One of the most common and extensively studied dystrophy is Duchenne muscular dystrophy (DMD), which arises due to a genetic mutation in the structural protein dystrophin. Lack of dystrophin, a member of the membrane-bound protein complex, leads to the improper connection of the cytoskeleton to the ECM, rendering fibers more prone to mechanical damage. As a consequence, recurring rounds of degeneration and regeneration form a vicious cycle and impose proliferative pressure on SCs. It has been proposed that progressive worsening of the disease over time is at least partially due to telomere shortening and ultimately loss of the regenerative potential of SCs (118).
Infiltrating macrophages and T cells induce fibrosis through secretion of pro-fibrotic cytokines, which in chronic diseases such as muscular dystrophies result in fibrotic tissue formation at the expense of functional muscle tissue (119). For instance, in acute injury, a wave of TNFα-secreting M1 macrophages induces a reduction of the preceding FAP expansion, thereby limiting ECM accumulation. Under chronic conditions, however, loss of proper control of macrophage polarization results in exacerbated TGF-β secretion that in turn causes FAP persistence and fibrosis (120). Therefore, anti-inflammatory drugs like corticosteroids, despite their potential pro-atrophic side effects, are the current standard of care for DMD. A big portion of current DMD therapy-related research focuses on intercepting the pathways implicated in fibrotic tissue formation, namely those triggered by TGF-β and Mstn (121).
Interestingly, SC fate conversion from the myogenic to the fibrogenic lineage can contribute to fibrosis development in DMD. Thus, increased Wnt signaling in dystrophic muscle triggers TGF-β2 secretion, which in turn induces pro-fibrotic gene expression in SCs, thereby limiting their myogenic potential (122). Besides progressive fibrosis, the SC niche in DMD is affected by other events, such as alterations in the BL with differential expression of laminin α2, laminin β1 and collagen IV, which are implicated in the direct interactions with SCs (123), as well as that of decorin and biglycan, proteoglycans linked to TGF-β sequestration (124). These changes presumably also contribute to alterations in muscle stiffness, which further affects SC behavior. In addition, perturbed conditions can alter the differentiation of several multipotent progenitor populations in the muscle, including FAPs, resulting in extracellular fat accumulation (75). Of note, these alterations to the SC niche can be extrapolated to other dystrophies and muscle pathologies with prominent fibrosis and fat accumulation, even diseases such as type 2 diabetes (125, 126).
The niche has been the primary focus of research on SC dysfunction in DMD, mainly due to a body of literature suggesting that dystrophin expression is limited to differentiated myofibers. However, recent findings suggests a direct role of SCs in the pathology based on the discovery that dystrophin is also expressed in activated SCs and is important for establishing cell polarity, thus enabling asymmetric SC division (127). Lack of SC dystrophin therefore results in reduced numbers of committed progenitors and differentiated myocytes, as well as a higher numbers of Myf5- progenitors. However, both increased and decreased SC numbers have been reported in DMD, a discrepancy that could be due to the difference in age of the subjects in the studies in question (128, 129). Given the reciprocal regulation between SCs and fibroblasts (21), it will be interesting to further explore the role of SC dystrophin in fibrotic tissue accumulation and other DMD symptoms.
Dysferlinopathy is another example of a muscular dystrophy with a complex etiology. In this disease, a mutation in the structural protein dysferlin primarily prevents myotubes from patching contraction-induced small ruptures in the sarcolemma. However, dysferlinopathy also affects proper muscle regeneration, where impairment in the release of cytokines upon injury results in reduced neutrophil recruitment and leads to a prolonged inflammatory phase, creating a suboptimal environment for successful regeneration by SCs (130).
Despite extensive efforts, no treatment for most of these debilitating diseases has been found so far. Therapies are mainly symptomatic and palliative, relying on corticosteroids as well as pulmonary and cardiac management in the case of DMD (131). Experimental treatments centered on stem cell therapy (e.g. SC transplantation), gene therapy (e.g. antisense oligonucleotide exon skipping, viral delivery of mini-dystrophin, CRISPR/Cas9-mediated deletion) and pharmacology (e.g. Mstn blockade) might, however, result in therapeutic breakthroughs in the future (132–135).
Future directions – an artificial niche
Autologous SC therapy represents one of the most promising treatments both for dystrophies and sacropenia. In sacropenia, enhancement of the myogenic potential of SCs and expansion of bona fide SCs in vitro prior to their transplantation in order to boost regeneration would most likely be sufficient, while in dystrophic conditions, the approach would comprise stem cell and gene therapy, including correction of a relevant genetic mutation in vitro. However, several hurdles impede the success of such trials. For example, the inability of SCs to home in on muscle with systemic delivery (136), poor migration when delivered intramuscularly (137), as well as reduced proliferation, immediate differentiation, and apoptosis of injected cells have been reported. These effects are further compounded by the rapid and irreversible loss of SC stemness in culture, resulting in reduced myogenic potential upon transplantation (138). Thus, as expanding the stem cell population is a necessary step prior to implantation, improving the intrinsic myogenic potential of SCs, e.g. by overexpressing PGC-1α, can help to lead to enhanced early muscle tissue formation after transplantation (139). Furthermore, attempts have been made to mimic the SC niche in vitro to circumvent some of the aforementioned problems.
Bioengineering efforts have made progress in creating 3D biomimetics as acellular or cellular scaffolds for use in regenerative therapy (140). From cylindrically shaped, collagen I-based gels to various natural hydrogels and finally fibrin gels, conditions conductive to increasing cell survival, fusion and maturation are constantly improving (4). For example, in the case of trauma-induced volumetric muscle loss, acellular biodegradable materials filled with anti-fibrotic and pro-myogenic factors on one, and angiogenic and neurotrophic factors on the other hand, would possibly provide optimal conditions to tip the balance towards functional muscle tissue instead of scar tissue formation when transplanted in a timely manner (141, 142). These scaffolds would provide not only fast infiltration and proper activation of the myogenic cells of the host, but also support fast establishment of the vascular and neural network necessary to support the newly formed muscle tissue. Other conditions such as aging and dystrophies require, however, more intricate cellular approaches, with biomaterials that closely resemble the satellite cell niche in terms of stiffness and composition, enabling the cell-matrix interactions that are crucial for proper SC function. In that regard, polyethylene glycol hydrogels cross-linked with laminin have been used successfully in improving satellite cell self-renewal in vitro and engraftment in vivo (93). This substrate, in combination with pharmacological inhibition of the p38α/β MAPK pathway, was also able to reverse the age-related SC pathology (108).
Besides identification of ECM proteins as crucial components of an artificial niche, the search for extrinsic factors that would enable SC expansion in vitro without loss of cell stemness has led to the discovery of a cocktail of four cytokines. Intrigued by the role of CD4+ and CD8+ T cells in regeneration, Fu and colleagues identified T cell-derived factors that are responsible for increased SC proliferation. They defined a pro-inflammatory cytokine combination composed of IL-1α, IL-13, TNF-α and INF-γ that is sufficient and necessary to maintain SC potency in vitro (143). This combination of cytokines promoted proliferation and limited differentiation of SCs for 20 passages. The gene expression profile of cells expanded in this way suggests that these cells retain at least some of the features of freshly isolated SCs, such as high Pax7 and low MyoD expression. SCs expanded under such conditions were not only able to engraft efficiently and occupy the niche upon transplantation into muscle, but also to respond to secondary injury by undergoing activation and self-renewal (143). In addition, the transplantation efficiency of such expanded cells in vitro was comparable to freshly isolated SCs. Since the cocktail in question has been optimized for murine SCs, efforts will have to be made to find proper conditions and factors for human SCs.
Recently, Quarta and colleagues successfully mimicked the in vivo microenvironment of SCs by using a defined serum-free quiescence medium and artificial muscle fibers. A 3D microscaffold with an elasticity between 1-2kPa based on collagen, recombinant laminin and α4β1 integrin provided optimal stiffness and enabled signaling pathways to keep the cells in reversible quiescence (144). This method proved effective in keeping both murine and human SCs in a quiescent state for up to a week. With this system, the engraftment potential and self-renewal of cultured cells upon transplantation surpassed that of freshly isolated SCs and was comparable to SCs associated with their native fibers. These results confirm the importance of the niche and mimicking the in vivo microenvironment for maintaining SC stemness in vitro (144).
These studies provide crucial insights into the optimal conditions for keeping SCs in a quiescent state in vitro, SC propagation, and preservation of the stemness for subsequent in vivo transplantation. Importantly, an artificial niche not only enables disease modeling and gene therapy, but also provides an amenable experimental system for toxicology screenings of novel drugs, thereby reducing the burden of animal studies (145, 146). Together with novel imaging and cell tracking techniques (147), the increasing knowledge about SC biology, the importance of the niche, and the interplay of SCs with myofibers and other cell types will hopefully result in novel therapeutic approaches to treating sarcopenia, muscular dystrophies and other skeletal muscle-associated pathologies.
References
- 1.Mauro a. Satellite cell of skeletal muscle fibers. The Journal of biophysical and biochemical cytology. 1961;9:493–5. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas K, Engler AJ, Meyer GA. Extracellular matrix regulation in the muscle satellite cell niche. Connective tissue research. 2015;56(1):1–8. doi: 10.3109/03008207.2014.947369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mounier R, Chretien F, Chazaud B. Blood vessels and the satellite cell niche. Current topics in developmental biology. 2011;96:121–38. doi: 10.1016/B978-0-12-385940-2.00005-X. [DOI] [PubMed] [Google Scholar]
- 4.Bursac N, Juhas M, Rando TA. Synergizing Engineering and Biology to Treat and Model Skeletal Muscle Injury and Disease. Annual review of biomedical engineering. 2015;17:217–42. doi: 10.1146/annurev-bioeng-071114-040640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Almada AE, Wagers AJ. Molecular circuitry of stem cell fate in skeletal muscle regeneration, ageing and disease. Nature reviews Molecular cell biology. 2016;17(5):267–79. doi: 10.1038/nrm.2016.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yin H, Price F, Rudnicki Ma. Satellite cells and the muscle stem cell niche. Physiological reviews. 2013;93(1):23–67. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. Journal of applied physiology. 2000;89(1):81–8. doi: 10.1152/jappl.2000.89.1.81. [DOI] [PubMed] [Google Scholar]
- 8.Rosenblatt JD. A time course study of the isometric contractile properties of rat extensor digitorum longus muscle injected with bupivacaine. Comparative biochemistry and physiology Comparative physiology. 1992;101(2):361–7. doi: 10.1016/0300-9629(92)90547-4. [DOI] [PubMed] [Google Scholar]
- 9.Carlson BM, Gutmann E. Development of contractile properties of minced muscle regenerates in the rat. Experimental neurology. 1972;36(2):239–49. doi: 10.1016/0014-4886(72)90020-9. [DOI] [PubMed] [Google Scholar]
- 10.Fink E, Fortin D, Serrurier B, Ventura-Clapier R, Bigard AX. Recovery of contractile and metabolic phenotypes in regenerating slow muscle after notexin-induced or crush injury. Journal of muscle research and cell motility. 2003;24(7):421–9. doi: 10.1023/a:1027387501614. [DOI] [PubMed] [Google Scholar]
- 11.Dinulovic I, et al. PGC-1alpha modulates necrosis, inflammatory response, and fibrotic tissue formation in injured skeletal muscle. Skeletal muscle. 2016;6:38. doi: 10.1186/s13395-016-0110-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warren GL, et al. Role of CC chemokines in skeletal muscle functional restoration after injury. Am J Physiol Cell Physiol. 2004;286(5):C1031–6. doi: 10.1152/ajpcell.00467.2003. [DOI] [PubMed] [Google Scholar]
- 13.Kelly AM. Perisynaptic satellite cells in the developing and mature rat soleus muscle. Anat Rec. 1978;190(4):891–903. doi: 10.1002/ar.1091900409. [DOI] [PubMed] [Google Scholar]
- 14.Collins CA, et al. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122(2):289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Sacco A, Doyonnas R, Kraft P, Vitorovic S, Blau HM. Self-renewal and expansion of single transplanted muscle stem cells. Nature. 2008;456(7221):502–6. doi: 10.1038/nature07384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bentzinger CF, Wang YX, Rudnicki Ma. Building muscle: molecular regulation of myogenesis. Cold Spring Harbor perspectives in biology. 2012;4(2) doi: 10.1101/cshperspect.a008342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Péault B, et al. Stem and progenitor cells in skeletal muscle development, maintenance, and therapy. Molecular therapy : the journal of the American Society of Gene Therapy. 2007;15(5):867–77. doi: 10.1038/mt.sj.6300145. [DOI] [PubMed] [Google Scholar]
- 18.Lepper C, Partridge Ta, Fan C-M. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development (Cambridge, England) 2011;138(17):3639–46. doi: 10.1242/dev.067595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sambasivan R, et al. Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development (Cambridge, England) 2011;138(17):3647–56. doi: 10.1242/dev.067587. [DOI] [PubMed] [Google Scholar]
- 20.McCarthy JJ, et al. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development (Cambridge, England) 2011;138(17):3657–66. doi: 10.1242/dev.068858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy MM, Lawson Ja, Mathew SJ, Hutcheson Da, Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development (Cambridge, England) 2011;138(17):3625–37. doi: 10.1242/dev.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ono Y, Boldrin L, Knopp P, Morgan JE, Zammit PS. Muscle satellite cells are a functionally heterogeneous population in both somite-derived and branchiomeric muscles. Developmental biology. 2010;337(1):29–41. doi: 10.1016/j.ydbio.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chakkalakal JV, Jones KM, Basson MA, Brack AS. The aged niche disrupts muscle stem cell quiescence. Nature. 2012;490(7420):355–60. doi: 10.1038/nature11438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rocheteau P, Gayraud-Morel B, Siegl-Cachedenier I, Blasco MA, Tajbakhsh S. A subpopulation of adult skeletal muscle stem cells retains all template DNA strands after cell division. Cell. 2012;148(1–2):112–25. doi: 10.1016/j.cell.2011.11.049. [DOI] [PubMed] [Google Scholar]
- 25.Kuang S, Kuroda K, Le Grand F, Rudnicki MA. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129(5):999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Troy A, et al. Coordination of satellite cell activation and self-renewal by Par-complex-dependent asymmetric activation of p38alpha/beta MAPK. Cell stem cell. 2012;11(4):541–53. doi: 10.1016/j.stem.2012.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanes JR. The basement membrane/basal lamina of skeletal muscle. The Journal of biological chemistry. 2003;278(15):12601–4. doi: 10.1074/jbc.R200027200. [DOI] [PubMed] [Google Scholar]
- 28.Blanco-Bose WE, Yao CC, Kramer RH, Blau HM. Purification of mouse primary myoblasts based on alpha 7 integrin expression. Experimental cell research. 2001;265(2):212–20. doi: 10.1006/excr.2001.5191. [DOI] [PubMed] [Google Scholar]
- 29.Liu H, Niu A, Chen S-E, Li Y-P. Beta3-integrin mediates satellite cell differentiation in regenerating mouse muscle. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2011;25(6):1914–21. doi: 10.1096/fj.10-170449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cornelison DD, Filla MS, Stanley HM, Rapraeger AC, Olwin BB. Syndecan-3 and syndecan-4 specifically mark skeletal muscle satellite cells and are implicated in satellite cell maintenance and muscle regeneration. Developmental biology. 2001;239(1):79–94. doi: 10.1006/dbio.2001.0416. [DOI] [PubMed] [Google Scholar]
- 31.Pallafacchina G, et al. An adult tissue-specific stem cell in its niche: a gene profiling analysis of in vivo quiescent and activated muscle satellite cells. Stem cell research. 2010;4(2):77–91. doi: 10.1016/j.scr.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 32.Guérin CW, Holland PC. Synthesis and secretion of matrix-degrading metalloproteases by human skeletal muscle satellite cells. Developmental dynamics : an official publication of the American Association of Anatomists. 1995;202(1):91–9. doi: 10.1002/aja.1002020109. [DOI] [PubMed] [Google Scholar]
- 33.Lukjanenko L, et al. Loss of fibronectin from the aged stem cell niche affects the regenerative capacity of skeletal muscle in mice. Nature medicine. 2016;22(8):897–905. doi: 10.1038/nm.4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bentzinger CF, et al. Fibronectin regulates Wnt7a signaling and satellite cell expansion. Cell stem cell. 2013;12(1):75–87. doi: 10.1016/j.stem.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Urciuolo A, et al. Collagen VI regulates satellite cell self-renewal and muscle regeneration. Nature communications. 2013 May;4 doi: 10.1038/ncomms2964. 1964- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tatsumi R, et al. Satellite cell activation in stretched skeletal muscle and the role of nitric oxide and hepatocyte growth factor. 2006;0038:1487–94. doi: 10.1152/ajpcell.00513.2005. [DOI] [PubMed] [Google Scholar]
- 37.Bjornson CRR, et al. Notch signaling is necessary to maintain quiescence in adult muscle stem cells. Stem cells (Dayton, Ohio) 2012;30(2):232–42. doi: 10.1002/stem.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mourikis P, et al. A critical requirement for notch signaling in maintenance of the quiescent skeletal muscle stem cell state. Stem cells. 2012;30(2):243–52. doi: 10.1002/stem.775. [DOI] [PubMed] [Google Scholar]
- 39.Polesskaya A, Seale P, Rudnicki Ma. Wnt signaling induces the myogenic specification of resident CD45+ adult stem cells during muscle regeneration. Cell. 2003;113(7):841–52. doi: 10.1016/s0092-8674(03)00437-9. [DOI] [PubMed] [Google Scholar]
- 40.Ratajczak MZ, et al. Expression of functional CXCR4 by muscle satellite cells and secretion of SDF-1 by muscle-derived fibroblasts is associated with the presence of both muscle progenitors in bone marrow and hematopoietic stem/progenitor cells in muscles. Stem cells. 2003;21(3):363–71. doi: 10.1634/stemcells.21-3-363. [DOI] [PubMed] [Google Scholar]
- 41.Fedorov YV, Rosenthal RS, Olwin BB. Oncogenic Ras-induced proliferation requires autocrine fibroblast growth factor 2 signaling in skeletal muscle cells. The Journal of cell biology. 2001;152(6):1301–5. doi: 10.1083/jcb.152.6.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Philippou A, Halapas A, Maridaki M, Koutsilieris M. Type I insulin-like growth factor receptor signaling in skeletal muscle regeneration and hypertrophy. Journal of musculoskeletal & neuronal interactions. 2007;7(3):208–18. [PubMed] [Google Scholar]
- 43.Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocrine reviews. 1995;16(1):3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 44.McCroskery S, Thomas M, Maxwell L, Sharma M, Kambadur R. Myostatin negatively regulates satellite cell activation and self-renewal. The Journal of cell biology. 2003;162(6):1135–47. doi: 10.1083/jcb.200207056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang AH, Rando TA. Induction of autophagy supports the bioenergetic demands of quiescent muscle stem cell activation. The EMBO journal. 2014;33(23):2782–97. doi: 10.15252/embj.201488278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ryall JG, et al. The NAD(+)-dependent SIRT1 deacetylase translates a metabolic switch into regulatory epigenetics in skeletal muscle stem cells. Cell stem cell. 2015;16(2):171–83. doi: 10.1016/j.stem.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wagatsuma A, Sakuma K. Mitochondria as a potential regulator of myogenesis. ScientificWorldJournal. 2013;2013 doi: 10.1155/2013/593267. 593267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rodgers JT, et al. mTORC1 controls the adaptive transition of quiescent stem cells from G0 to G(Alert) Nature. 2014;510(7505):393–6. doi: 10.1038/nature13255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cerletti M, Jang YC, Finley LW, Haigis MC, Wagers AJ. Short-term calorie restriction enhances skeletal muscle stem cell function. Cell stem cell. 2012;10(5):515–9. doi: 10.1016/j.stem.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gibson MC, Schultz E. The distribution of satellite cells and their relationship to specific fiber types in soleus and extensor digitorum longus muscles. Anat Rec. 1982;202(3):329–37. doi: 10.1002/ar.1092020305. [DOI] [PubMed] [Google Scholar]
- 51.Shefer G, Rauner G, Yablonka-Reuveni Z, Benayahu D. Reduced satellite cell numbers and myogenic capacity in aging can be alleviated by endurance exercise. PLoS One. 2010;5(10):e13307. doi: 10.1371/journal.pone.0013307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilson JM, et al. The effects of endurance, strength, and power training on muscle fiber type shifting. Journal of strength and conditioning research. 2012;26(6):1724–9. doi: 10.1519/JSC.0b013e318234eb6f. [DOI] [PubMed] [Google Scholar]
- 53.Lin J, et al. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418(6899):797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- 54.Dinulovic I, et al. Muscle PGC-1alpha modulates satellite cell number and proliferation by remodeling the stem cell niche. Skeletal muscle. 2016;6(1):39. doi: 10.1186/s13395-016-0111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Furrer R, Eisele PS, Schmidt A, Beer M, Handschin C. Paracrine cross-talk between skeletal muscle and macrophages in exercise by PGC-1alpha-controlled BNP. Scientific reports. 2017;7 doi: 10.1038/srep40789. 40789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kovanen V, Suominen H, Risteli J, Risteli L. Type IV collagen and laminin in slow and fast skeletal muscle in rats--effects of age and life-time endurance training. Collagen and related research. 1988;8(2):145–53. doi: 10.1016/s0174-173x(88)80026-8. [DOI] [PubMed] [Google Scholar]
- 57.Arsic N, et al. Vascular endothelial growth factor stimulates skeletal muscle regeneration in vivo. Mol Ther. 2004;10(5):844–54. doi: 10.1016/j.ymthe.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 58.Ochoa O, et al. Delayed angiogenesis and VEGF production in CCR2-/- mice during impaired skeletal muscle regeneration. American journal of physiology Regulatory, integrative and comparative physiology. 2007;293(2):R651–61. doi: 10.1152/ajpregu.00069.2007. [DOI] [PubMed] [Google Scholar]
- 59.Christov C, et al. Muscle satellite cells and endothelial cells: close neighbors and privileged partners. Mol Biol Cell. 2007;18(4):1397–409. doi: 10.1091/mbc.E06-08-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rhoads RP, et al. Satellite cell-mediated angiogenesis in vitro coincides with a functional hypoxia-inducible factor pathway. Am J Physiol Cell Physiol. 2009;296(6):C1321–8. doi: 10.1152/ajpcell.00391.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abou-Khalil R, et al. Autocrine and paracrine angiopoietin 1/Tie-2 signaling promotes muscle satellite cell self-renewal. Cell stem cell. 2009;5(3):298–309. doi: 10.1016/j.stem.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mohyeldin A, Garzon-Muvdi T, Quinones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell stem cell. 2010;7(2):150–61. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 63.Liu W, et al. Hypoxia promotes satellite cell self-renewal and enhances the efficiency of myoblast transplantation. Development. 2012;139(16):2857–65. doi: 10.1242/dev.079665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fukada S, et al. Molecular signature of quiescent satellite cells in adult skeletal muscle. Stem cells. 2007;25(10):2448–59. doi: 10.1634/stemcells.2007-0019. [DOI] [PubMed] [Google Scholar]
- 65.Yamaguchi M, et al. Calcitonin Receptor Signaling Inhibits Muscle Stem Cells from Escaping the Quiescent State and the Niche. Cell reports. 2015;13(2):302–14. doi: 10.1016/j.celrep.2015.08.083. [DOI] [PubMed] [Google Scholar]
- 66.Sinha-Hikim I, Taylor WE, Gonzalez-Cadavid NF, Zheng W, Bhasin S. Androgen receptor in human skeletal muscle and cultured muscle satellite cells: up-regulation by androgen treatment. The Journal of clinical endocrinology and metabolism. 2004;89(10):5245–55. doi: 10.1210/jc.2004-0084. [DOI] [PubMed] [Google Scholar]
- 67.Dubois V, et al. A satellite cell-specific knockout of the androgen receptor reveals myostatin as a direct androgen target in skeletal muscle. FASEB J. 2014;28(7):2979–94. doi: 10.1096/fj.14-249748. [DOI] [PubMed] [Google Scholar]
- 68.Kuschel R, Yablonka-Reuveni Z, Bornemann A. Satellite cells on isolated myofibers from normal and denervated adult rat muscle. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 1999;47(11):1375–84. doi: 10.1177/002215549904701104. [DOI] [PubMed] [Google Scholar]
- 69.Jejurikar SS, Marcelo CL, Kuzon WM., Jr Skeletal muscle denervation increases satellite cell susceptibility to apoptosis. Plastic and reconstructive surgery. 2002;110(1):160–8. doi: 10.1097/00006534-200207000-00027. [DOI] [PubMed] [Google Scholar]
- 70.Griesbeck O, Parsadanian AS, Sendtner M, Thoenen H. Expression of neurotrophins in skeletal muscle: quantitative comparison and significance for motoneuron survival and maintenance of function. Journal of neuroscience research. 1995;42(1):21–33. doi: 10.1002/jnr.490420104. [DOI] [PubMed] [Google Scholar]
- 71.Tatsumi R, et al. Possible implication of satellite cells in regenerative motoneuritogenesis: HGF upregulates neural chemorepellent Sema3A during myogenic differentiation. Am J Physiol Cell Physiol. 2009;297(2):C238–52. doi: 10.1152/ajpcell.00161.2009. [DOI] [PubMed] [Google Scholar]
- 72.Clow C, Jasmin BJ. Brain-derived neurotrophic factor regulates satellite cell differentiation and skeltal muscle regeneration. Mol Biol Cell. 2010;21(13):2182–90. doi: 10.1091/mbc.E10-02-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Menetrey J, et al. Growth factors improve muscle healing in vivo. The Journal of bone and joint surgery British volume. 2000;82(1):131–7. doi: 10.1302/0301-620x.82b1.8954. [DOI] [PubMed] [Google Scholar]
- 74.Serrano AL, et al. Cellular and molecular mechanisms regulating fibrosis in skeletal muscle repair and disease. Current topics in developmental biology. 2011;96:167–201. doi: 10.1016/B978-0-12-385940-2.00007-3. [DOI] [PubMed] [Google Scholar]
- 75.Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nature cell biology. 2010;12(2):143–52. doi: 10.1038/ncb2014. [DOI] [PubMed] [Google Scholar]
- 76.Pretheeban T, Lemos DR, Paylor B, Zhang RH, Rossi FM. Role of stem/progenitor cells in reparative disorders. Fibrogenesis & tissue repair. 2012;5(1):20. doi: 10.1186/1755-1536-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Joe AW, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nature cell biology. 2010;12(2):153–63. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Heredia JE, et al. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell. 2013;153(2):376–88. doi: 10.1016/j.cell.2013.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang Y, Thorlacius H. Mast cell-derived tumour necrosis factor-alpha mediates macrophage inflammatory protein-2-induced recruitment of neutrophils in mice. British journal of pharmacology. 2005;145(8):1062–8. doi: 10.1038/sj.bjp.0706274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brigitte M, et al. Muscle resident macrophages control the immune cell reaction in a mouse model of notexin-induced myoinjury. Arthritis and rheumatism. 2010;62(1):268–79. doi: 10.1002/art.27183. [DOI] [PubMed] [Google Scholar]
- 81.Scapini P, et al. The neutrophil as a cellular source of chemokines. Immunological reviews. 2000;177:195–203. doi: 10.1034/j.1600-065x.2000.17706.x. [DOI] [PubMed] [Google Scholar]
- 82.Ceafalan LC, Popescu BO, Hinescu ME. Cellular Players in Skeletal Muscle Regeneration. BioMed research international. 2014;2014 doi: 10.1155/2014/957014. 957014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Arnold L, et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. The Journal of experimental medicine. 2007;204(5):1057–69. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Segawa M, et al. Suppression of macrophage functions impairs skeletal muscle regeneration with severe fibrosis. Experimental cell research. 2008;314(17):3232–44. doi: 10.1016/j.yexcr.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 85.Deng B, Wehling-Henricks M, Villalta SA, Wang Y, Tidball JG. IL-10 triggers changes in macrophage phenotype that promote muscle growth and regeneration. Journal of immunology (Baltimore, Md : 1950) 2012;189(7):3669–80. doi: 10.4049/jimmunol.1103180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Summan M, et al. Macrophages and skeletal muscle regeneration: a clodronate-containing liposome depletion study. American journal of physiology Regulatory, integrative and comparative physiology. 2006;290(6):R1488–95. doi: 10.1152/ajpregu.00465.2005. [DOI] [PubMed] [Google Scholar]
- 87.Sonnet C, et al. Human macrophages rescue myoblasts and myotubes from apoptosis through a set of adhesion molecular systems. Journal of cell science. 2006;119(Pt 12):2497–507. doi: 10.1242/jcs.02988. [DOI] [PubMed] [Google Scholar]
- 88.Castiglioni A, et al. FOXP3+ T Cells Recruited to Sites of Sterile Skeletal Muscle Injury Regulate the Fate of Satellite Cells and Guide Effective Tissue Regeneration. PLoS One. 2015;10(6):e0128094. doi: 10.1371/journal.pone.0128094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Burzyn D, et al. A special population of regulatory T cells potentiates muscle repair. Cell. 2013;155(6):1282–95. doi: 10.1016/j.cell.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Engler AJ, et al. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. The Journal of cell biology. 2004;166(6):877–87. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nature reviews Molecular cell biology. 2009;10(1):21–33. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- 92.Boonen KJ, Rosaria-Chak KY, Baaijens FP, van der Schaft DW, Post MJ. Essential environmental cues from the satellite cell niche: optimizing proliferation and differentiation. Am J Physiol Cell Physiol. 2009;296(6):C1338–45. doi: 10.1152/ajpcell.00015.2009. [DOI] [PubMed] [Google Scholar]
- 93.Gilbert PM, et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329(5995):1078–81. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chang NC, Chevalier FP, Rudnicki MA. Satellite Cells in Muscular Dystrophy - Lost in Polarity. Trends in molecular medicine. 2016;22(6):479–96. doi: 10.1016/j.molmed.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Larsson L, Ansved T. Effects of ageing on the motor unit. Progress in neurobiology. 1995;45(5):397–458. doi: 10.1016/0301-0082(95)98601-z. [DOI] [PubMed] [Google Scholar]
- 96.Faulkner JA, Larkin LM, Claflin DR, Brooks SV. Age-related changes in the structure and function of skeletal muscles. Clinical and experimental pharmacology & physiology. 2007;34(11):1091–6. doi: 10.1111/j.1440-1681.2007.04752.x. [DOI] [PubMed] [Google Scholar]
- 97.Conboy IM, Conboy MJ, Smythe GM, Rando Ta. Notch-mediated restoration of regenerative potential to aged muscle. Science (New York, NY) 2003;302(5650):1575–7. doi: 10.1126/science.1087573. [DOI] [PubMed] [Google Scholar]
- 98.Conboy IM, et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433(7027):760–4. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 99.Elabd C, et al. Oxytocin is an age-specific circulating hormone that is necessary for muscle maintenance and regeneration. Nat Commun. 2014;5:4082. doi: 10.1038/ncomms5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sinha M, et al. Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science. 2014;344(6184):649–52. doi: 10.1126/science.1251152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Walker RG, et al. Biochemistry and Biology of GDF11 and Myostatin: Similarities, Differences, and Questions for Future Investigation. Circulation research. 2016;118(7):1125–41. doi: 10.1161/CIRCRESAHA.116.308391. discussion 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schafer MJ, et al. Quantification of GDF11 and Myostatin in Human Aging and Cardiovascular Disease. Cell metabolism. 2016;23(6):1207–15. doi: 10.1016/j.cmet.2016.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Egerman MA, et al. GDF11 Increases with Age and Inhibits Skeletal Muscle Regeneration. Cell metabolism. 2015;22(1):164–74. doi: 10.1016/j.cmet.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Harper SC, et al. Is Growth Differentiation Factor 11 a Realistic Therapeutic for Aging-Dependent Muscle Defects? Circulation research. 2016;118(7):1143–50. doi: 10.1161/CIRCRESAHA.116.307962. discussion 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Brack AS, et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science (New York, NY) 2007;317(5839):807–10. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- 106.Oh J, et al. Age-associated NF-kappaB signaling in myofibers alters the satellite cell niche and re-strains muscle stem cell function. Aging. 2016;8(11):2871–96. doi: 10.18632/aging.101098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Carlson ME, Hsu M, Conboy IM. Imbalance between pSmad3 and Notch induces CDK inhibitors in old muscle stem cells. Nature. 2008;454(7203):528–32. doi: 10.1038/nature07034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cosgrove BD, et al. Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nature medicine. 2014;20(3):255–64. doi: 10.1038/nm.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bernet JD, et al. p38 MAPK signaling underlies a cell-autonomous loss of stem cell self-renewal in skeletal muscle of aged mice. Nature medicine. 2014;20(3):265–71. doi: 10.1038/nm.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Price FD, et al. Inhibition of JAK-STAT signaling stimulates adult satellite cell function. Nature medicine. 2014;20(10):1174–81. doi: 10.1038/nm.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tierney MT, et al. STAT3 signaling controls satellite cell expansion and skeletal muscle repair. Nature medicine. 2014;20(10):1182–6. doi: 10.1038/nm.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sousa-Victor P, et al. Geriatric muscle stem cells switch reversible quiescence into senescence. Nature. 2014;506(7488):316–21. doi: 10.1038/nature13013. [DOI] [PubMed] [Google Scholar]
- 113.Garcia-Prat L, et al. Autophagy maintains stemness by preventing senescence. Nature. 2016;529(7584):37–42. doi: 10.1038/nature16187. [DOI] [PubMed] [Google Scholar]
- 114.Rozo M, Li L, Fan CM. Targeting beta1-integrin signaling enhances regeneration in aged and dystrophic muscle in mice. Nature medicine. 2016;22(8):889–96. doi: 10.1038/nm.4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jang YC, Sinha M, Cerletti M, Dall'Osso C, Wagers aJ. Skeletal Muscle Stem Cells: Effects of Aging and Metabolism on Muscle Regenerative Function. Cold Spring Harbor Symposia on Quantitative Biology. 2011;76:101–11. doi: 10.1101/sqb.2011.76.010652. [DOI] [PubMed] [Google Scholar]
- 116.Snijders T, Verdijk LB, van Loon LJ. The impact of sarcopenia and exercise training on skeletal muscle satellite cells. Ageing research reviews. 2009;8(4):328–38. doi: 10.1016/j.arr.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 117.Emery AE. The muscular dystrophies. Lancet. 2002;359(9307):687–95. doi: 10.1016/S0140-6736(02)07815-7. [DOI] [PubMed] [Google Scholar]
- 118.Sacco A, et al. Short telomeres and stem cell exhaustion model Duchenne muscular dystrophy in mdx/mTR mice. Cell. 2010;143(7):1059–71. doi: 10.1016/j.cell.2010.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mann CJ, et al. Aberrant repair and fibrosis development in skeletal muscle. Skeletal muscle. 2011;1(1):21. doi: 10.1186/2044-5040-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lemos DR, et al. Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors. Nature medicine. 2015;21(7):786–94. doi: 10.1038/nm.3869. [DOI] [PubMed] [Google Scholar]
- 121.Bentzinger CF, von Maltzahn J, Rudnicki MA. Extrinsic regulation of satellite cell specification. Stem Cell Res Ther. 2010;1(3):27. doi: 10.1186/scrt27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Biressi S, Miyabara EH, Gopinath SD, Carlig PM, Rando TA. A Wnt-TGFbeta2 axis induces a fibrogenic program in muscle stem cells from dystrophic mice. Science translational medicine. 2014;6(267):267ra176. doi: 10.1126/scitranslmed.3008411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hayashi YK, et al. Abnormal localization of laminin subunits in muscular dystrophies. Journal of the neurological sciences. 1993;119(1):53–64. doi: 10.1016/0022-510x(93)90191-z. [DOI] [PubMed] [Google Scholar]
- 124.Fadic R, et al. Increase in decorin and biglycan in Duchenne Muscular Dystrophy: role of fibroblasts as cell source of these proteoglycans in the disease. Journal of cellular and molecular medicine. 2006;10(3):758–69. doi: 10.1111/j.1582-4934.2006.tb00435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Berria R, et al. Increased collagen content in insulin-resistant skeletal muscle. American journal of physiology Endocrinology and metabolism. 2006;290(3):E560–5. doi: 10.1152/ajpendo.00202.2005. [DOI] [PubMed] [Google Scholar]
- 126.Goodpaster BH, Wolf D. Skeletal muscle lipid accumulation in obesity, insulin resistance, and type 2 diabetes. Pediatric diabetes. 2004;5(4):219–26. doi: 10.1111/j.1399-543X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- 127.Dumont NA, et al. Dystrophin expression in muscle stem cells regulates their polarity and asymmetric division. Nature medicine. 2015;21(12):1455–63. doi: 10.1038/nm.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kottlors M, Kirschner J. Elevated satellite cell number in Duchenne muscular dystrophy. Cell Tissue Res. 2010;340(3):541–8. doi: 10.1007/s00441-010-0976-6. [DOI] [PubMed] [Google Scholar]
- 129.Jiang C, et al. Notch signaling deficiency underlies age-dependent depletion of satellite cells in muscular dystrophy. Disease models & mechanisms. 2014;7(8):997–1004. doi: 10.1242/dmm.015917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chiu YH, et al. Attenuated muscle regeneration is a key factor in dysferlin-deficient muscular dystrophy. Hum Mol Genet. 2009;18(11):1976–89. doi: 10.1093/hmg/ddp121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wagner KR, Lechtzin N, Judge DP. Current treatment of adult Duchenne muscular dystrophy. Biochimica et biophysica acta. 2007;1772(2):229–37. doi: 10.1016/j.bbadis.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 132.Chakkalakal JV, Thompson J, Parks RJ, Jasmin BJ. Molecular, cellular, and pharmacological therapies for Duchenne/Becker muscular dystrophies. FASEB J. 2005;19(8):880–91. doi: 10.1096/fj.04-1956rev. [DOI] [PubMed] [Google Scholar]
- 133.Fairclough RJ, Wood MJ, Davies KE. Therapy for Duchenne muscular dystrophy: renewed optimism from genetic approaches. Nature reviews Genetics. 2013;14(6):373–8. doi: 10.1038/nrg3460. [DOI] [PubMed] [Google Scholar]
- 134.Young CS, et al. A Single CRISPR-Cas9 Deletion Strategy that Targets the Majority of DMD Patients Restores Dystrophin Function in hiPSC-Derived Muscle Cells. Cell stem cell. 2016;18(4):533–40. doi: 10.1016/j.stem.2016.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mendell JR, Rodino-Klapac LR. Duchenne muscular dystrophy: CRISPR/Cas9 treatment. Cell research. 2016;26(5):513–4. doi: 10.1038/cr.2016.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Elster JL, et al. Skeletal muscle satellite cell migration to injured tissue measured with 111In-oxine and high-resolution SPECT imaging. Journal of muscle research and cell motility. 2013;34(5–6):417–27. doi: 10.1007/s10974-013-9368-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bentzinger CF, et al. Wnt7a stimulates myogenic stem cell motility and engraftment resulting in improved muscle strength. The Journal of cell biology. 2014;205(1):97–111. doi: 10.1083/jcb.201310035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Montarras D, et al. Direct isolation of satellite cells for skeletal muscle regeneration. Science (New York, NY) 2005;309(5743):2064–7. doi: 10.1126/science.1114758. [DOI] [PubMed] [Google Scholar]
- 139.Haralampieva D, et al. Human muscle precursor cells overexpressing PGC-1alpha enhance early skeletal muscle tissue formation. Cell transplantation. 2017 doi: 10.3727/096368917X694868. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Handschin C, Mortezavi A, Plock J, Eberli D. External physical and biochemical stimulation to enhance skeletal muscle bioengineering. Advanced drug delivery reviews. 2015;82–83:168–75. doi: 10.1016/j.addr.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sicari BM, et al. An acellular biologic scaffold promotes skeletal muscle formation in mice and humans with volumetric muscle loss. Science translational medicine. 2014;6(234):234ra58. doi: 10.1126/scitranslmed.3008085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shvartsman D, et al. Sustained delivery of VEGF maintains innervation and promotes reperfusion in ischemic skeletal muscles via NGF/GDNF signaling. Mol Ther. 2014;22(7):1243–53. doi: 10.1038/mt.2014.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fu X, et al. Combination of inflammation-related cytokines promotes long-term muscle stem cell expansion. Cell research. 2015;25(9):1082–3. doi: 10.1038/cr.2015.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Quarta M, et al. An artificial niche preserves the quiescence of muscle stem cells and enhances their therapeutic efficacy. Nature biotechnology. 2016;34(7):752–9. doi: 10.1038/nbt.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Madden L, Juhas M, Kraus WE, Truskey GA, Bursac N. Bioengineered human myobundles mimic clinical responses of skeletal muscle to drugs. eLife. 2015;4:e04885. doi: 10.7554/eLife.04885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Huh D, et al. A human disease model of drug toxicity-induced pulmonary edema in a lung-on-a-chip microdevice. Science translational medicine. 2012;4(159):159ra47. doi: 10.1126/scitranslmed.3004249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Haralampieva D, et al. Noninvasive PET Imaging and Tracking of Engineered Human Muscle Precursor Cells for Skeletal Muscle Tissue Engineering. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2016;57(9):1467–73. doi: 10.2967/jnumed.115.170548. [DOI] [PMC free article] [PubMed] [Google Scholar]