Abstract
Aim
The combination of tumor-targeting IL2 and TNF based antibody-cytokine fusions has exhibited encouraging results in mouse and men. Here, we studied their combination to assess efficacy and mechanism of action in four different immunocompetent mouse models of cancer.
Methods
Mice receiving a single intratumoral injection of F8-IL2, F8-TNF or the combination were investigated for tumor-infiltrating leucocytes and rechallenged when cured.
Results
In three models, a proportion of treated animals could be cured, most probably by infiltrating NK and CD8+ T cells. Most of the cured mice did not acquire protective immunity when rechallenged with the same tumor cell line.
Conclusion
Immunocompetent mouse tumor models may not be adequate enough to predict the search for more efficacious therapy regimens.
Keywords: Immunocytokines, interleukin-2, tumor necrosis factor, intratumoral injection, EDA domain of fibronectin
Introduction
The intralesional administration of therapeutics to injectable cancer lesions has been studied over many decades as a possible alternative to surgical resection. Most clinical studies have been performed in melanoma patients, because of the accessibility of skin metastases[1, 2]. However, intralesional pharmacological treatment modalities have been proposed for many other tumor entities, including lymph node and liver metastases [1], head&neck tumors[3], bladder cancer [4] and high-grade astrocytomas[5], to name just a few. A number of clinical trials using intratumoral therapeutics derived from pathogen-associated molecular patterns, cytokines, dendritic cells, viruses, monoclonal antibodies or adoptive cell transfer are ongoing and summarized in a review recently published by Aznar and colleagues[6].
The intralesional treatment of patients with Stage III and Stage IV melanoma includes the administration of Bacille Calmette-Guerin, electrochemotherapy or cytokines[1]. This latter group of therapeutic agents has attracted considerable attention, as cytokines are capable of potently activating an immune response at the site of disease, but can be toxic even at low doses, when administered systemically[7]. Clinical-stage intralesional anti-cancer strategies also include the use of Rose Bengal[8, 9] and of antibodies against immunological check-point inhibitors[10].
For many intralesional immunotherapeutic approaches, the strategy to “treat locally, act globally”[11] aims at boosting anti-cancer immunity. Intratumoral injections ensure that drugs reach the tumor, while considerable pharmacodelivery challenges (i.e., low tumor uptake) are typically associated with systemically-administered protein based therapeutics[12].
Among the many pro-inflammatory cytokines, which have been investigated for cancer therapy, interleukin-2 (IL2) has attracted substantial interest, thanks to the pioneering work of the group of Claus Garbe in Tübingen (Germany). Over a period of 11 years, the authors documented the treatment of 72 patients with Stage III or Stage IV melanoma. Administering doses between 0.3 and 6 MIU of IL2 depending on lesion size three times a week for an average of 6.5 weeks, Garbe and colleagues reported a complete response in 66.7 % of patients[13–15]. However, the onset of a systemic anticancer activity was not directly investigated. Furthermore, an increase in progression-free survival compared to historical controls was observed in patients with Stage IIIB melanoma, but not in Stage IIIC or Stage IV disease[15].
In an attempt to improve the efficacy of intralesional IL2 therapy, we developed an antibody-IL2 fusion protein (“L19-IL2”) specific to a splice isoform of fibronectin, with the goal to increase the residence time of the cytokine payload at the site of disease and, hence, therapeutic activity. In a Phase II clinical trial, 25 patients with stage IIB/IIIC melanoma were treated with intralesional injections of L19-IL2. After receiving a maximum dose of 10 MIU/week for 4 weeks, 6 patients showed a complete response, 5 of them even with a long-lasting response[16]. A substantial increase in progression-free survival was observed, compared to historical controls.
The combination of L19-IL2 with a second clinical-stage immunocytokine (L19-TNF) led to encouraging preclinical results, as a single intralesional administration of the two agents could eradicate tumors, which could not be cured by the individual products[17]. The efficacy of the combination treatment was confirmed in a Phase II clinical trial, involving 20 melanoma patients[18]. Interestingly, in patients for which some lesions were not treated, an objective response was observed in 70% of the non-injected lesions. These results laid the foundations for a Phase III trial in patients with fully-resectable Stage IIIB,C melanoma, which has recently started (EUdraCT Number: 2015-002549-72).
Alternative combination strategies, aimed at improving the activity of intratumoral IL2, included the addition of anti-CD40 antibodies [19] or of anti-CTLA-4 products[10]. Interestingly, the group of Delia Nelson has reported that combination of IL2 with the anti-CD40 antibody leads to a systemic anticancer response after local administration in a two-tumor model[19]. In the clinical setting, the combination of intratumoral IL2 with the anti-CTLA-4 antibody ipilimumab was also investigated. Activity on a proportion of non-injected lesions was reported, but not all injected melanomas responded to therapy[10].
In this study, we aimed at exploring the activity of intralesional administrations of two immunocytokines (F8-IL2 and F8-TNF) in four immunocompetent mouse models of cancer. The F8 antibody recognizes its cognate antigen (the alternatively-spliced EDA domain of fibronectin) with identical affinity in mouse and man[20]. The EDA domain of fibronectin is virtually undetectable in normal adult tissues (exception made for placenta, endometrium and some vessels in the ovaries), but is strongly expressed in the majority of solid tumors[21], of lymphomas[22] and also in the bone marrow of acute leukemias[23]. Human IL2 is active in the mouse, while the F8-TNF fusion protein consisted of the murine TNF payload, as human TNF does not cross-react with the cognate mouse receptor. The cloning, expression and purification of F8-IL2 and F8-TNF has been published before[24, 25]. In three models of cancer (WEHI-164, F9 and Lewis Lung Carcinoma), cancer cures were predominantly observed when F8-TNF or the combination F8-TNF plus F8-IL2 was used. By contrast, none of the therapeutic regimens cured TIB-49 tumors.
Materials and Methods
Cell lines and tumor models
F9 teratocarcinoma cells (ATCC), WEHI-164 fibrosarcoma cells (ATCC), Lewis Lung Carcinoma cells (ATCC) and acuted myeloid leukemia TIB-49 cells (ATCC) were cultured according to the supplier protocol. For tumor growth at the right flank, tumor cells were injected subcutaneously into 8 weeks old female 129/Sv mice (15 x 106 F9 cells), BALB/c mice (5 x 106 WEHI-164 cells) or C57BL/6 mice (1 x 106 LLC or 1 x 106 TIB-49 cells). For the study of therapeutic activity in mice carrying two subcutaneous lesions in the flanks, F9 teratocarcinoma or Lewis Lung Carcinoma cells were injected subcutaneously (cell numbers as indicated above). Mice were controlled daily, tumors were measured with a caliper and tumor mass was calculated using the formula width x width x length x 0.5 (mm3). Tumor studies were performed under a project license granted by the Veterinäramt des Kantons Zürich, Switzerland (27/2015).
Therapy schedule
The fusionproteins F8-TNF and F8-IL2 were produced and purified as earlier described by our group[24, 25] and diluted in PBS for tumor injection. When tumors reached an average size of 100 mm3, mice were non-blindly randomized in groups of n=4. Each mouse received a single intralesional injection of 6 μg F8-TNF, 60 μg F8-IL2 or the combination of both. Control mice received a single intralesional injection of PBS. Cured mice were rechallenged 40 days after the first tumor cell injection with the same cell number and monitored for tumor growth.
Immunhistochemistry
Twenty-four hours after intralesional injection, one mouse of each group was sacrificed and tumors were excised. Tumor tissue was frozen in cryoembedding medium and cryostatsections of 10 μm were made. Sections were fixed with ice-cold acetone, blocked with 3% FBS and stained for CD4 (clone GK1.5, Biolegend), CD8 (clone 53-6.7, Biolegend), F4/80 (clone BM8, eBioscience) and anti-asialoGM1 (clone 986-10001, Wako) and labeled with Alexa Fluor 594 (A21208, Invitrogen). Endothelial cells were stained with an anti-CD31 antibody (AF3628, R&D Systems) and labeled with Alexa Fluor 488 (A11058, Invitrogen). Stainings were mounted with Dako Mounting Medium and analyzed with an Axioskop2 mot plus microscope (Zeiss). The scale bar represents 50 μm.
Statistical analysis
In vivo data were analyzed using Prism6 (Graph Pad Software Inc.). Data are represented as mean + SEM. A two-way ANOVA test followed by a Bonferroni post-test with a p-value < 0.05 was performed to determine statistical significance. Significance towards the PBS, control group is shown for the last day of the therapy. (**=p < 0.01, *** = p < 0.001, n.s. = non significant).
Results
Tumor models and immunohistochemistry
Four tumor models were established by subcutaneous injection of tumor cells in immunocompetent mice: (i) F9 teratocarcinoma in 129/Sv mice; (ii) WEHI-164 fibrosarcoma in BALB/c mice; (iii) Lewis Lung Carcinoma in C57BL/6 mice and (iv) acute myeloid leukemia TIB-49 in C57BL/6 mice.
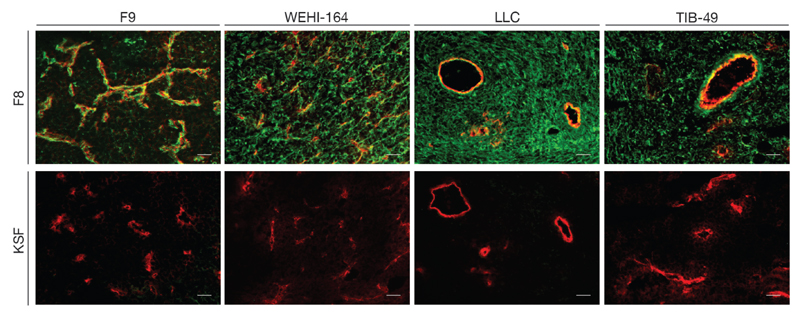
Figure 1 shows immunofluorescence findings using the F8 antibody (specific to the alternatively-spliced EDA domain of fibronectin)[20] and the KSF antibody (specific to hen egg lysozyme, thus serving as negative control)[26]. Blood vessels were stained in red, using an anti-CD31 antibody. The F8 antibody exhibited a selective and intense staining of the perivascular extracellular matrix in all four tumor types. In addition, a diffuse staining of the interstitium was observed for WEHI-164, Lewis Lung Carcinoma and TIB-49 tumors.
Figure 1.
Ex vivo tumor staining of tumor sections. Upper panel: staining of ED-A+ fibronectin (green) using F8-SIP antibody and an AF488-labeled secondary antibody [procedure as described in [20]], while blood vessels were stained using an anti-CD31 antibody and an AF594-labeled secondary antibody (red). Lower panel: a green staining using the KSF-SIP antibody (specific to hen egg lysozyme) and an AF488-labeled secondary antibody was not detectable, while blood vessels were stained in red with the anti-CD31 reagents, as indicated for the upper panel. Scale bars correspond to 50 μm.
Therapy and rechallenge experiments
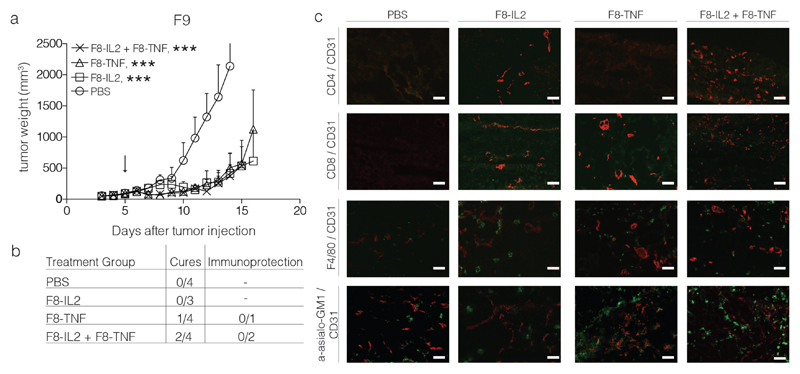
Tumor lesions were allowed to grow to a size of ~ 100 mm3, before a single intralesional injection was administered. Mice (n=4 per group) received saline, F8-IL2 (60 μg), F8-TNF (6 μg) or the combination of the two immunocytokines. Additional mice were treated and sacrificed 24 hours after the intratumoral injection, for microscopic analysis of the leukocyte infiltrate. Therapy experiments performed in the F9 teratocarcinoma model, which exhibits a predominantly vascular pattern of EDA expression, led to cures only in a portion of the mice treated with F8-TNF or with F8-TNF + F8-IL2 [Figure 2].
Figure 2.
Intralesional activity of F8-IL2, F8-TNF or their combination in 129/Sv mice bearing s.c. F9 teratocarcinoma tumors: a) Tumor-bearing mice were treated with one intralesional administration of 60 μg F8-IL2, 6 μg F8-TNF, 60 μg F8-IL2 + 6 μg F8-TNF or PBS as control, when tumors reached a size of 100 mg. Data are expressed as mean + SEM, statistical significance towards PBS, control group is shown on day 14 , *** = p < 0.001. b) In order to test for the development of protective anti-cancer immunity (“Immunoprotection”), cured mice were rechallenged at day 40 with the same tumor. The number of mice which did not develop tumors following the re-challenge is indicated. c) Representative immunohistochemistry stainings of tumors taken 24 h after intralesional administration, immune cell markers stained in green, CD31 staining in red.
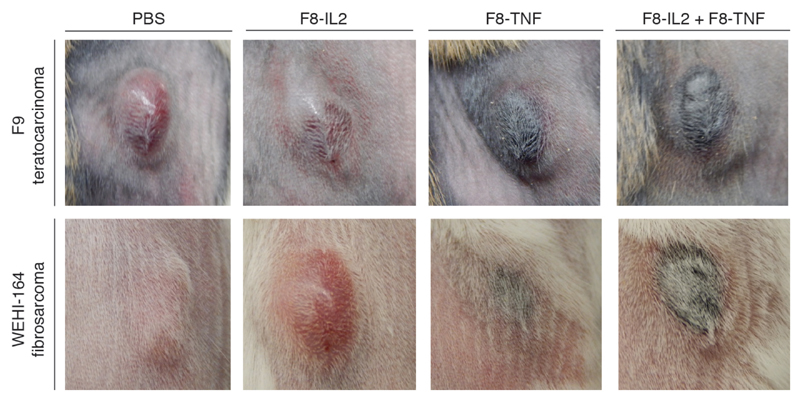
Immunofluorescence analysis of the leukocyte infiltration into the tumor mass revealed a substantial infiltration of NK cells and macrophages, while few T cells could be seen. Cures typically occurred in mice, whose tumor would form a black scab within one day after intralesional treatment [Figure 3].
Figure 3.
Tumor appearance and scab formation 24 h after intralesional injection of therapeutic products. Tumor appearance of subcutaneous F9 teratocarcinoma (upper panel) and WEHI-164 fibrosarcoma (lower panel) 24 h after intralesional injection of PBS, F8-IL2, F8-TNF or the combination of F8-IL2 and F8-TNF.
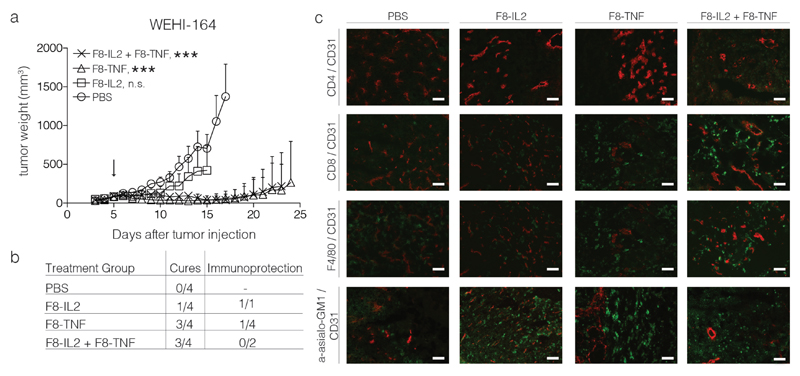
In the WEHI-164 model, three out of four mice treated with F8-TNF or with F8-TNF + F8-IL2 were cured [Figure 4]. Also in this case, as for F9 tumors, cancer cures coincided with the rapid formation of a black scab [Figure 3]. An abundant infiltration of NK cells and CD8+ T cells was observed into the tumor mass, following combination treatment with F8-TNF + F8-IL2 [Figure 4]. While mice cured from F9 tumors were not protected against a subsequent challenge with the same tumor cells [Figure 2], some of the cured mice with WEHI-164 tumors exhibited protective immunity. Surprisingly, this was observed only in the monotherapy groups, but not after combination therapy [Figure 4].
Figure 4.
Intralesional activity of F8-IL2, F8-TNF or their combination in BALB/c mice bearing s.c. WEHI-164 fibrosarcoma tumors: a) Tumor-bearing mice were treated with one intralesional administration of 60 μg F8-IL2, 6 μg F8-TNF, 60 μg F8-IL2 + 6 μg F8-TNF or PBS as control, when tumors reached a size of 100 mg. Data are expressed as mean + SEM, statistical significance towards PBS, control group is shown on day 15, n.s. = non significant, *** = p < 0.001. b) In order to test for the development of protective anti-cancer immunity (“Immunoprotection”), cured mice were rechallenged at day 40 with the same tumor. The number of mice which did not develop tumors following the re-challenge is indicated. c) Representative immunohistochemistry stainings of tumors taken 24 h after intralesional administration, immune cell markers stained in green, CD31 staining in red.
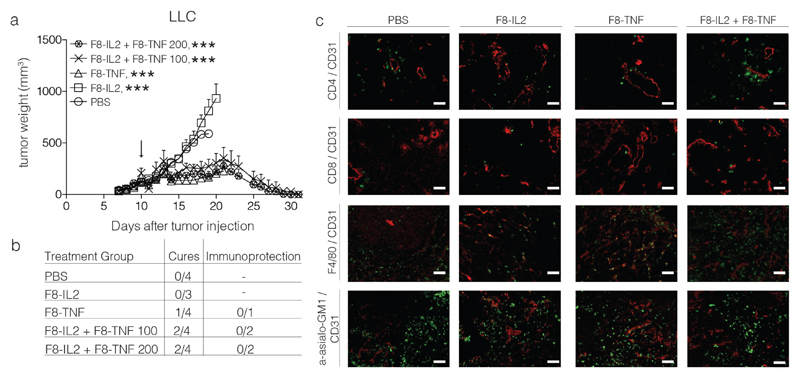
A strong but delayed (i.e., 10 days after injection) anti-cancer activity could be observed in mice, bearing subcutaneous Lewis Lung Carcinomas [Figure 5]. Activity was also observed if therapy was started when tumors had reached a size of 200 mm3. All tumors in mice treated with F8-TNF or with F8-TNF + F8-IL2 disappeared by day 30 [Figure 5]. A predominant infiltrate of NK cells and, to a certain extent, of CD4+ T cells was observed in the combination therapy group [Figure 5]. Unfortunately, cured mice did not exhibit a protective immunity, upon rechallenge with tumor cells.
Figure 5.
Intralesional activity of F8-IL2, F8-TNF or their combination in C57BL/6 mice bearing s.c. Lewis Lung Carcinoma tumors: a) Tumor-bearing mice were treated with one intralesional administration of 60 μg F8-IL2, 6 μg F8-TNF, 60 μg F8-IL2 + 6 μg F8-TNF or PBS as control, when tumors reached a size of 100 mg. An additional group of mice bearing tumors of approximately 200 mg was treated with the combination of F8-IL2 + F8-TNF. Data are expressed as mean + SEM, statistical significance towards PBS, control group is shown on day 19 , *** = p < 0.001. b) In order to test for the development of protective anti-cancer immunity (“Immunoprotection”), cured mice were rechallenged at day 40 with the same tumor. The number of mice which did not develop tumors following the re-challenge is indicated. c) Representative immunohistochemistry stainings of tumors taken 24 h after intralesional administration, immune cell markers stained in green, CD31 staining in red.
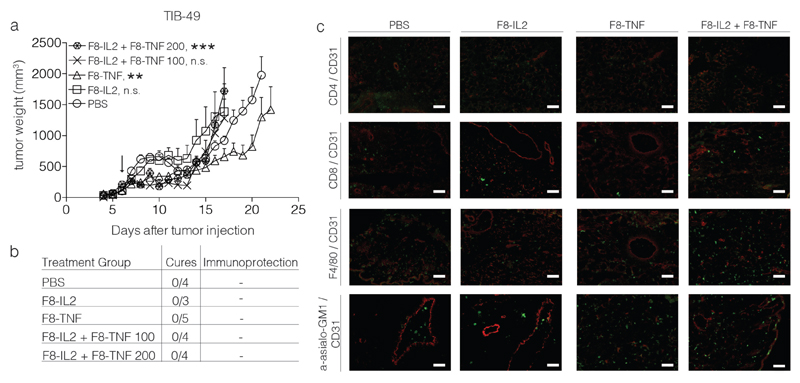
Unlike the other three tumor models studies, no tumor eradication was observed in mice, bearing TIB-49 tumors [Figure 6]. A stronger infiltration of NK cells was observed after treatment with F8-TNF or with F8-TNF + F8-IL2.
Figure 6.
Intralesional activity of F8-IL2, F8-TNF or their combination in C57BL/6 mice bearing s.c. acute myeloid leukemia TIB-49 tumors: a) Tumor-bearing mice were treated with one intralesional administration of 60 μg F8-IL2, 6 μg F8-TNF, 60 μg F8-IL2 + 6 μg F8-TNF or PBS as control, when tumors reached a size of 100 mg. An additional group of mice bearing tumors of approximately 200 mg was treated with the combination of F8-IL2 + F8-TNF. Data are expressed as mean + SEM, statistical significance towards PBS, control group is shown on day 17, n.s. = non significant, ** = p < 0.01, *** = p < 0.001. b) In order to test for the development of protective anti-cancer immunity (“Immunoprotection”), cured mice were rechallenged at day 40 with the same tumor. The number of mice which did not develop tumors following the re-challenge is indicated. c) Representative immunohistochemistry stainings of tumors taken 24 h after intralesional administration, immune cell markers stained in green, CD31 staining in red.
Additionally, we investigated the activity of intralesional immunocytokine administration in mice bearing two tumors in the flanks (F9 or LLC), of which only one lesion was injected. Tumor growth at the injected site was delayed in both models using F8-IL2, F8-TNF or the combination as therapeutic agents, but the non-injected lesions did not exhibit a growth retardation [Supplementary figure 2 and 3].
Discussion
Immunocytokines, such as F8-IL2 and F8-TNF, can display a potent anti-cancer activity in tumor bearing mice after intravenous administration[24, 25]. When used as single agents, antibody-cytokine fusions are typically unable to eradicate cancer in mouse models, but the combination of IL2- and TNF-based immunocytokines has led to cancer cures in some models[17, 27].
When administered by intravenous injection, a preferential accumulation at the tumor site can be observed for certain antibody-based products. However, in absolute terms, only a small portion of the antibody localizes to the tumor. Using antibody fragments specific to splice isoforms of fibronectin, tumor:organ ratios of approximately 10:1 can be achieved 24 hours after intravenous administration, both in mice[24, 25] and in man[12]. The absolute tumor uptake values for the same products are in the order of 5-20 % of the injected dose per gram of tumor (%ID/g) in mouse models of cancer[24, 25] and 0.001-0.01%ID/g in patients [12]. As the main portion of the injected antibody product does not reach the tumor in vivo, it is attractive to consider intratumoral administration modalities, with the aim to locally boost an immunological response, which may control both the injected lesion and distant metastases. Prior preclinical studies based on immunofluorescence microscopic analysis of tumor specimens had revealed that recombinant cytokines rapidly diffuse away from the neoplastic mass following intralesional injection, while antibody-cytokine fusions can exhibit a longer residence time at the site of disease[18, 27].
One of the most interesting observations in the clinical use of intralesional injections of L19-IL2 plus L19-TNF (two immunocytokines directed against the EDB domain of fibronectin) relates to the induction of objective responses in 70% of non-injected lesions. The induction of anti-cancer activity requires a functional immune system[17] and a lymphocyte infiltration into the tumor mass[18].
In this study, three out of four immunocompetent mouse models of cancer responded well to intralesional administration strategies based on the combination of F8-IL2 and F8-TNF. The therapeutic dose of F8-IL2 and F8-TNF used in the study corresponds to the maximal tolerated dose of those biopharmaceuticals, as previously reported by our group (i.e., 60μg for F8-IL2 [23, 28] and 6 μg for F8-TNF[25]). However, C57BL/6 mice exhibited a higher susceptibility towards immunocytokine treatment compared to 129/Sv and BALB/c mouse strains, as evidenced by transient body weight loss. A phase II study with intralesional injection of the immunocytokines L19-IL2 and L19-TNF has demonstrated a good tolerability in patients with IIIB,C melanoma patients at doses of 10 MIU/week (L19-IL2) and of 312μg/week (L19-TNF) for 4 weeks [18]. A phase III trial with this combination is currently on-going both in Europe and in the United States (NCT02938299). In mice, long-term remissions were typically only observed when tumor masses were converted into black scabs, as a result of hemorrhagic necrosis[29]. This observation suggests that a rapid killing of all cancer cells prevents tumor re-growth or that necrosis formation adequately stimulates T cells and NK cells to control minimal residual disease.
In our experimental setting, the tumor infiltrate was typically dominated by an intense accumulation of NK cells, while CD8+ T cells remained scarse (with the possible exception of WEHI-164 sarcomas). Interestingly, this was the only mouse model in which cures could be observed, upon re-challenge with 5 million tumor cells, 40 days after the first injection. Future experimental studies may thus aim at performing additional combination experiments, with the aim to boost the CD8+ T cell component of the anti-cancer immune response. For example, the group of Delia Nelson has reported that a combination of anti-CD40 antibodies and intralesional IL2 was able to control both injected tumors and contro-lateral non-injected lesions in immunocompetent mice, while the single agents did not lead to cancer cures when used as individual products[19].
Our group had previously reported that the intralesional administration of L19-IL2/L19-TNF and of F8-IL2/F8-TNF combinations could eradicate cancer lesions in mice, but only when tumors were smaller than 100 mm3 [17, 27]. In the clinic, also larger melanoma lesions (e.g., subcutaneous tumor masses of 3’000 mm3) exhibited complete responses to four intralesional administrations of L19-IL2/L19-TNF[18]. Importantly, treatment with the L19-IL2/L19-TNF combination mediated objective responses in the majority of non-injected lesions[18], while a similar effect was not observed in our mouse models. This study was performed in order to investigate the therapeutic impact of locoregional immunocytokine therapy in models which are more responsive to immunotherapy (e.g. F9 teratocarcinoma and WEHI-164 fibrosarcoma) and in mouse models which are extremely difficult to cure, both with established and experimental therapeutic agents (e.g. TIB-49 acute myeloid leukemia and Lewis Lung Carcinoma).
The observation of durable complete remissions only in a portion of treated mice may relate to the difference in T-cell receptor sequences even in syngeneic mouse strains, resulting from V-D-J and V-J recombination events and potentially leading to lymphocytes with different ability to recognize tumor-associated antigens [30]. In addition to the action of T cells against mutated peptides (“neo-epitopes”) in tumors[31], the recognition of retroviral antigen by CD8+ T cells may also play a role for the anti-cancer immunity induced by targeted cytokine treatment [29]. It is becoming increasingly clear that the quantity and quality of tumor-infiltrating lymphocytes may carry considerably indifferent tumors, resulting in immunologically “hot” and “cold” malignancies, with different sensitivity to immunotherapeutic procedures[32]. We were encouraged by the observation that Lewis Lung Carcinoma, a tumor that had previously been shown to be rich in immunosuppressive cells, could be cured by a single intratumoral injection of F8-IL2 + F8-TNF[32].
It is possible that immunocompetent mouse models of cancer may not adequately predict the performance of immunotherapy in humans. For example, anti-PD-1 antibodies typically do not exhibit objective responses in the majority of established mouse models of cancer[32], while their human counterparts Nivolumab and Pembrolizumab have been reported to promote objective responses in a substantial portion of patients with more than 20 different types of malignancies[33–35].
In summary, the observation of local tumor control following immunocytokine treatment in three of the four tumor models studied in this article reinforces the concept of using intralesional procedures for cancer therapy. The lack of protective immunity, evidenced by relapse at tumor re-challenge, stimulates the search for more efficacious combination procedures and for mouse models of cancer, that may be more predictive of therapeutic activity in patients[32].
Conclusion
We conclude, that intralesional administration of immunocytokines is a promising therapeutic approach. However, not all murine tumor models seem to be well suited to predict the transferability of therapeutic activity from mouse to men.
Supplementary Material
Précis.
A single intralesional injection of two tumor-targeting antibody-cytokine fusion proteins led to tumor eradication in three out of four different tumor models. However, cured mice did not develop protective immunity upon tumor rechallenge.
Summary Points.
Intratumoral administration of tumor targeting antibody-cytokine fusions proteins F8-IL2 or F8-TNF can eradicate neoplastic lesions in WEHI-164 sarcoma, F9 teratocarcinoma and Lewis Lung carcinoma but not in TIB-49 myeloma.
Only a part of cured mice developed protective immunity after a rechallenge with the same tumor cell line.
Immunocytokines promoted an infiltration of NK cells and, to a lower and more variable extent, of CD8+ T cells.
The most striking correlation for durable complete responses was the observation of scab formation, one day after administration of F8-TNF-containing regimens.
No systematic response can be observed in mice after intratumoral treatment with F8-IL2, F8-TNF or the combination of both; however, in human melanoma patients, combination treatment with F8-IL2 and F8-TNF leads to an objective response in non-treated lesions.
Although a part of herein presented results is transferrable from mouse to men, better predictable murine tumor models are needed to search for more efficacious therapeutic regimens.
Acknowledgements
Dario Neri received financial support of the ETH Zürich, the Swiss National Science Foundation (Grant Nr. 310030B_163479/1), the ERC Advanced Grant “ZAUBERKUGEL” and the Federal Commission for Technology and Innovation (KTI, Grant Nr. 12803.1 VOUCHLS).
Footnotes
Conflict of Interest/Financial Disclosure:
Dario Neri is a co-founder and shareholder of Philogen (www.philogen.com), a biotech company which has developed antibody-cytokine fusion proteins into advanced clinical trials
Ethical Conduct of Research
All animal experiments were performed under a project license granted to Dario Neri by the Veterinäramt des Kantons Zürich, Switzerland (27/2015).
References
- 1.Testori A, Ribero S, Bataille V. Diagnosis and treatment of in-transit melanoma metastases. Eur J Surg Oncol. 2017;43(3):544–560. doi: 10.1016/j.ejso.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Weide B, Neri D, Elia G. Intralesional treatment of metastatic melanoma: a review of therapeutic options. Cancer Immunol Immunother. 2017 doi: 10.1007/s00262-016-1952-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Herpen CM, Van Der Voort R, Van Der Laak JA, et al. Intratumoral rhIL-12 administration in head and neck squamous cell carcinoma patients induces B cell activation. Int J Cancer. 2008;123(10):2354–2361. doi: 10.1002/ijc.23756. [DOI] [PubMed] [Google Scholar]
- 4.Calsini P, Scapicchi G, Gazzarini O, et al. Immunotherapy of bladder cancer with intralesional injection with BCG. J Exp Pathol. 1987;3(4):579–586. [PubMed] [Google Scholar]
- 5.Arista A, Sturiale C, Riva P, et al. Intralesional administration of I-131 labelled monoclonal antibodies in the treatment of malignant gliomas. Acta Neurochir (Wien) 1995;135(3–4):159–162. doi: 10.1007/BF02187762. [DOI] [PubMed] [Google Scholar]
- 6.Aznar MA, Tinari N, Rullan AJ, Sanchez-Paulete AR, Rodriguez-Ruiz ME, Melero I. Intratumoral Delivery of Immunotherapy-Act Locally, Think Globally. J Immunol. 2017;198(1):31–39. doi: 10.4049/jimmunol.1601145. [DOI] [PubMed] [Google Scholar]
- 7.Lejeune FJ, Lienard D, Matter M, Ruegg C. Efficiency of recombinant human TNF in human cancer therapy. Cancer Immun. 2006;6:6. [PubMed] [Google Scholar]
- 8.Maker AV, Prabhakar B, Pardiwala K. The Potential of Intralesional Rose Bengal to Stimulate T-Cell Mediated Anti-Tumor Responses. J Clin Cell Immunol. 2015;6(4) doi: 10.4172/2155-9899.1000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lippey J, Bousounis R, Behrenbruch C, et al. Intralesional PV-10 for in-transit melanoma-A single-center experience. J Surg Oncol. 2016;114(3):380–384. doi: 10.1002/jso.24311. [DOI] [PubMed] [Google Scholar]
- 10.Ray A, Williams MA, Meek SM, et al. A phase I study of intratumoral ipilimumab and interleukin-2 in patients with advanced melanoma. Oncotarget. 2016;7(39):64390–64399. doi: 10.18632/oncotarget.10453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marabelle A, Kohrt H, Caux C, Levy R. Intratumoral immunization: a new paradigm for cancer therapy. Clin Cancer Res. 2014;20(7):1747–1756. doi: 10.1158/1078-0432.CCR-13-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poli GL, Bianchi C, Virotta G, et al. Radretumab radioimmunotherapy in patients with brain metastasis: a 124I-L19SIP dosimetric PET study. Cancer Immunol Res. 2013;1(2):134–143. doi: 10.1158/2326-6066.CIR-13-0007. [DOI] [PubMed] [Google Scholar]
- 13.Radny P, Caroli UM, Bauer J, et al. Phase II trial of intralesional therapy with interleukin-2 in soft-tissue melanoma metastases. Br J Cancer. 2003;89(9):1620–1626. doi: 10.1038/sj.bjc.6601320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weide B, Derhovanessian E, Pflugfelder A, et al. High response rate after intratumoral treatment with interleukin-2: results from a phase 2 study in 51 patients with metastasized melanoma. Cancer. 2010;116(17):4139–4146. doi: 10.1002/cncr.25156. [DOI] [PubMed] [Google Scholar]
- 15.Weide B, Eigentler TK, Pflugfelder A, et al. Survival after intratumoral interleukin-2 treatment of 72 melanoma patients and response upon the first chemotherapy during follow-up. Cancer Immunol Immunother. 2011;60(4):487–493. doi: 10.1007/s00262-010-0957-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weide B, Eigentler TK, Pflugfelder A, et al. Intralesional treatment of stage III metastatic melanoma patients with L19-IL2 results in sustained clinical and systemic immunologic responses. Cancer Immunol Res. 2014;2(7):668–678. doi: 10.1158/2326-6066.CIR-13-0206. [DOI] [PubMed] [Google Scholar]
- 17.Schwager K, Hemmerle T, Aebischer D, Neri D. The immunocytokine L19-IL2 eradicates cancer when used in combination with CTLA-4 blockade or with L19-TNF. J Invest Dermatol. 2013;133(3):751–758. doi: 10.1038/jid.2012.376. [DOI] [PubMed] [Google Scholar]
- 18.Danielli R, Patuzzo R, Di Giacomo AM, et al. Intralesional administration of L19-IL2/L19-TNF in stage III or stage IVM1a melanoma patients: results of a phase II study. Cancer Immunol Immunother. 2015;64(8):999–1009. doi: 10.1007/s00262-015-1704-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackaman C, Nelson DJ. Intratumoral interleukin-2/agonist CD40 antibody drives CD4+ - independent resolution of treated-tumors and CD4+ -dependent systemic and memory responses. Cancer Immunol Immunother. 2012;61(4):549–560. doi: 10.1007/s00262-011-1120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Villa A, Trachsel E, Kaspar M, et al. A high-affinity human monoclonal antibody specific to the alternatively spliced EDA domain of fibronectin efficiently targets tumor neo-vasculature in vivo. Int J Cancer. 2008;122(11):2405–2413. doi: 10.1002/ijc.23408. [DOI] [PubMed] [Google Scholar]
- 21.Rybak JN, Roesli C, Kaspar M, Villa A, Neri D. The extra-domain A of fibronectin is a vascular marker of solid tumors and metastases. Cancer Res. 2007;67(22):10948–10957. doi: 10.1158/0008-5472.CAN-07-1436. [DOI] [PubMed] [Google Scholar]
- 22.Schliemann C, Wiedmer A, Pedretti M, Szczepanowski M, Klapper W, Neri D. Three clinical-stage tumor targeting antibodies reveal differential expression of oncofetal fibronectin and tenascin-C isoforms in human lymphoma. Leuk Res. 2009;33(12):1718–1722. doi: 10.1016/j.leukres.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 23.Gutbrodt KL, Schliemann C, Giovannoni L, et al. Antibody-based delivery of interleukin-2 to neovasculature has potent activity against acute myeloid leukemia. Sci Transl Med. 2013;5(201):201ra118. doi: 10.1126/scitranslmed.3006221. [DOI] [PubMed] [Google Scholar]
- 24.Frey K, Schliemann C, Schwager K, Giavazzi R, Johannsen M, Neri D. The immunocytokine F8-IL2 improves the therapeutic performance of sunitinib in a mouse model of renal cell carcinoma. J Urol. 2010;184(6):2540–2548. doi: 10.1016/j.juro.2010.07.030. [DOI] [PubMed] [Google Scholar]
- 25.Hemmerle T, Probst P, Giovannoni L, Green AJ, Meyer T, Neri D. The antibody-based targeted delivery of TNF in combination with doxorubicin eradicates sarcomas in mice and confers protective immunity. Br J Cancer. 2013;109(5):1206–1213. doi: 10.1038/bjc.2013.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frey K, Zivanovic A, Schwager K, Neri D. Antibody-based targeting of interferon-alpha to the tumor neovasculature: a critical evaluation. Integr Biol (Camb) 2011;3(4):468–478. doi: 10.1039/c0ib00099j. [DOI] [PubMed] [Google Scholar]
- 27.Pretto F, Elia G, Castioni N, Neri D. Preclinical evaluation of IL2-based immunocytokines supports their use in combination with dacarbazine, paclitaxel and TNF-based immunotherapy. Cancer Immunol Immunother. 2014;63(9):901–910. doi: 10.1007/s00262-014-1562-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carnemolla B, Borsi L, Balza E, et al. Enhancement of the antitumor properties of interleukin-2 by its targeted delivery to the tumor blood vessel extracellular matrix. Blood. 2002;99(5):1659–1665. doi: 10.1182/blood.v99.5.1659. [DOI] [PubMed] [Google Scholar]
- 29.Probst P, Kopp J, Oxenius A, et al. Sarcoma Eradication by Doxorubicin and Targeted TNF Relies upon CD8+ T-cell Recognition of a Retroviral Antigen. Cancer Res. 2017;77(13):3644–3654. doi: 10.1158/0008-5472.CAN-16-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coulie PG, Van Den Eynde BJ, Van Der Bruggen P, Boon T. Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nat Rev Cancer. 2014;14(2):135–146. doi: 10.1038/nrc3670. [DOI] [PubMed] [Google Scholar]
- 31.Di Marco M, Peper JK, Rammensee HG. Identification of Immunogenic Epitopes by MS/MS. Cancer J. 2017;23(2):102–107. doi: 10.1097/PPO.0000000000000252. [DOI] [PubMed] [Google Scholar]
- 32.Mosely SI, Prime JE, Sainson RC, et al. Rational Selection of Syngeneic Preclinical Tumor Models for Immunotherapeutic Drug Discovery. Cancer Immunol Res. 2017;5(1):29–41. doi: 10.1158/2326-6066.CIR-16-0114. [DOI] [PubMed] [Google Scholar]
- 33.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515(7528):558–562. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 35.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.