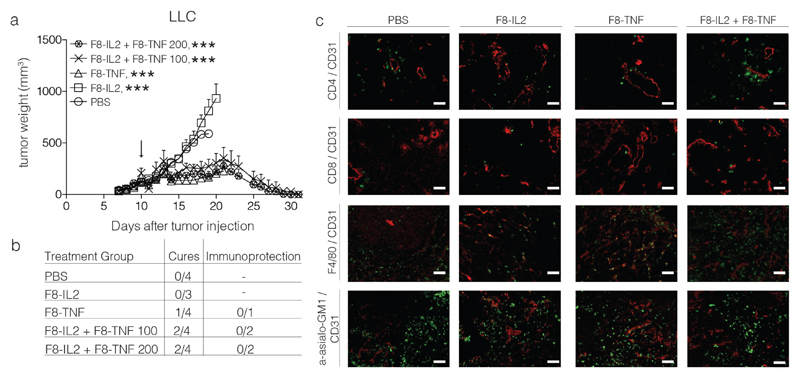
Figure 5.
Intralesional activity of F8-IL2, F8-TNF or their combination in C57BL/6 mice bearing s.c. Lewis Lung Carcinoma tumors: a) Tumor-bearing mice were treated with one intralesional administration of 60 μg F8-IL2, 6 μg F8-TNF, 60 μg F8-IL2 + 6 μg F8-TNF or PBS as control, when tumors reached a size of 100 mg. An additional group of mice bearing tumors of approximately 200 mg was treated with the combination of F8-IL2 + F8-TNF. Data are expressed as mean + SEM, statistical significance towards PBS, control group is shown on day 19 , *** = p < 0.001. b) In order to test for the development of protective anti-cancer immunity (“Immunoprotection”), cured mice were rechallenged at day 40 with the same tumor. The number of mice which did not develop tumors following the re-challenge is indicated. c) Representative immunohistochemistry stainings of tumors taken 24 h after intralesional administration, immune cell markers stained in green, CD31 staining in red.