Abstract
The fusariotoxins Enniatin B (Enn B) and Beauvericin (Bea) have recently aroused interest as food contaminants and as potential anticancer drugs. However, limited data are available about their toxic profile. Aim of this study was to investigate their pharmacological behavior in vivo and their persistence in mice. Therefore, liquid chromatography tandem mass spectrometry (LC-MS/MS) was used to analyze the distribution of Enn B and Bea in selected tissue samples and biological fluids originating from mice treated intraperitoneally with these cyclohexadepsipeptides. Overall, no toxicological signs during life time or pathological changes were observed. Moreover, both fusariotoxins were found in all tissues and serum but not in urine. Highest amounts were measured in liver and fat demonstrating the molecules´ tendency to bioaccumulate in lipophilic tissues. While for Bea no metabolites could be detected, for Enn B three phase I metabolites (dioxygenated-Enn B, mono- and di-demethylated-Enn B) were found in liver and colon, with dioxygenated-Enn B being most prominent. Consequently, contribution of hepatic as well as intestinal metabolism seems to be involved in the overall metabolism of Enn B. Thus, despite their structural similarity, the metabolism of Enn B and Bea shows distinct discrepancies which might affect long-term effects and tolerability in humans.
Keywords: enniatin B, beauvericin, in vivo, metabolism, LC-MS/MS
1. Introduction
Enniatins (Enns) and Beauvericin (Bea) belong to a group of cyclic hexadepsipetides which are produced mainly by Fusarium fungi that invade and grow on crops. They are resistant to heat, acids as well as digestion and are stable during commercial processing like brewing, melting, hot drying or ensiling. Consequently, these cyclodepsipeptides play an important role as contaminants of grain and grain-based products (Faeste et al., 2011; Jestoi, 2008). The most important contributors to chronic dietary exposure to Enns and Bea are especially bread and rolls, fine bakery wares and pasta. Usually, toddlers are the population group with the highest dietary chronic and acute exposure to both fusariotoxins (Panel, 2014). Recently, both fusariotoxins were shown to be capable of crossing the human skin barrier and reaching the viable epidermis and dermis (Taevernier et al., 2015b). Moreover, immunological disorders were suggested in humans after ingestion of these alimentary toxins (Ficheux et al., 2013; Juan et al., 2014).
Interestingly, Enns and Bea also evoked interest because of their pharmacological properties. In 1953 a mixture of Enn A, A1, B and B1 (called fusafungine) was originally patented as local antibiotic for the topically treatment of nose and throat infections. Fusafungine is currently marketed under the trade names Locabiotal®, Bioparox®, Locabiosol® and Fusaloyos®. However, only limited data are available about its bioavailability. Recently, Taevernier et al. demonstrated that Enns are capable of permeating porcine buccal mucosa and suggested that Enn-based solutions for oromucosal use in the treatment of innocent upper respiratory tract infections should be questioned, because Enns potentially will reach significant systemic concentrations (Taevernier et al., 2015a). Additionally, Bea is used in traditional Chinese medicine as a constituent in preparations with reputed anticonvulsant and antineoplastic actions. Moreover, a patent has been issued for a Bea tablet (containing 5 mg Bea) to lower blood cholesterol levels (Jestoi, 2008).
Nevertheless, data regarding the toxicology, toxicokinetics and toxicodynamics of Enns and Bea are still fragmentary. So far, no reports of adverse events in humans or animals due to contaminated food or feed exist (Jestoi, 2008; Panel, 2014). Moreover, subchronic (28 days) feeding experiments with Wistar rats using a repeated dose of 20.91 mg Enn A/kg b.w./day showed no adverse effects (Manyes et al., 2014). Acute toxicity was reported in the literature after oral administration with an LD50 ≥ 100 mg/kg b.w. for Bea and 350 mg/kg b.w. for fusafungine (Panel, 2014).
On the contrary, despite their low in vivo toxicity, both fusariotoxins evoked considerable toxicity in diverse in vitro assays (Behm et al., 2012; Dornetshuber et al., 2009a; Fornelli et al., 2004; Ivanova et al., 2006). Noteworthy, their cytotoxic potential was especially prominent in malignant cells derived from a wide range of cancer types as compared to non-malignant cells (Dornetshuber et al., 2007). Thus, both cyclic hexadepsipeptides came into focus of interest as potential anticancer drugs and for Enn B in vivo anticancer activities against cervical cancer were recently reported by our research group (Dornetshuber-Fleiss et al., 2015).
Consequently, the observed difference of in vitro and in vivo toxicity might have its origin in the low bioavailability. On the one hand, peptides are generally not considered to be orally well absorbed because of significant enzyme degradation in the digestive tract (Chan et al., 1997). Low oral bioavailability may be caused by impaired uptake from the gastrointestinal tract because of low compound water solubility (Blais et al., 1992) and interaction with efflux pumps (Dornetshuber et al., 2009b; Ivanova et al., 2011). On the other hand, oral bioavailability of N-methyl peptides like cyclosporine A is known, which is structurally related to Enns and Bea (Cooney et al., 1997). Hence, the apparent mean bioavailability in CaCo-2 cells for Enn A, A1, B, and B1 was assessed by an average of 80% (Meca et al., 2012). Moreover, in vivo trials using pigs demonstrated even a higher bioavailability of 91% for Enn B (Devreese et al., 2014). Therefore, other mechanisms like rapid elimination from the systemic circulation because of metabolic reactions might explain the low acute oral in vivo toxicity (Faeste et al., 2011). Accordingly, recent in vitro metabolism studies of Enn B with rat, dog and human liver microsomes reported twelve biotransformation products suggesting that the reduced in vivo toxicity is based on an extensive hepatic metabolism (Faeste et al., 2011; Ivanova et al., 2011). Additionally, an in vivo study by Manyes et al. reported intestinal degradation products and adducts for Enn A in Wistar rats (Manyes et al., 2014). For Bea, less data are available in this regard. However, at least inhibitory effects on cytochrome P450 enzymes were shown in human and rat liver microsomes (Mei et al., 2009).
Therefore, to fill gaps in toxicology-related knowledge and considering the evaluation of the two fusariotoxins as potential anticancer drug candidates and as emerging food-born toxins, this in vivo study aims: (i) to investigate the pharmacological behavior of Enn B and Bea in vivo by histochemistry studies and, (ii) to evaluate their persistence in selected tissues and biological fluids of mice after intraperitoneal administration by using a sensitive and specific liquid chromatography tandem mass spectrometric (LC-MS/MS) analytical method.
2. Experimental procedures
2.1. Chemical and reagents
Bea, Cremophor, DMSO, LC gradient grade methanol, acetonitrile as well as MS grade ammonium acetate and glacial acetic acid (p.a.) were purchased from Sigma–Aldrich (Vienna, Austria). A Purelab Ultra system (ELGA LabWater, Celle, Germany) was used for further purification of reverse osmosis water. Enn B for animal studies was purified from F. oxysporum ETH 1536/9 as described previously (Dornetshuber-Fleiss et al., 2015). Additionally, the cytotoxic potential was compared to Enn B obtained from Sigma Aldrich GmbH (St. Louis, MO, USA) and shown to be in a comparable range in all cell lines tested.
2.2. Analytical standards
Analytical standards of Enn B and Bea (>95% HPLC purity) were purchased from Sigma–Aldrich (Vienna, Austria). Of each substance, a stock solution (1 mg/mL DMSO) was prepared and working standard solutions were obtained by serial dilution of the respective stock and stored at -20°C.
2.3. Animal treatment and sample extraction
Sixteen male CB-17 scid/scid (severe combined immunodeficiency) mice with an average weight of 25 g at the age of eight weeks were purchased from Harlan Laboratories (San Pietro al Natisone, Italy). Animals were kept under pathogen-free conditions and all procedures were performed in the laminar flow hood. All experiments were approved by the Austrian Ethics committee (BMWF-66.009/0084-II/36/1013) and conducted in line with the ARRIVE guidelines for animal care and protection as well as the Austrian and the Federation of European Laboratory Animal Science Associations (FELASA).
For the short-time exposure experiment, both substances were dissolved in 10% DMSO. 5 mg/kg of the respective substance were injected on two (Enn B) or three (Bea) consecutive days into two mice and animals were sacrifized twenty-four hours after the last injection. For the xenograft experiments, 1x106 KB-3-1 cells were suspended in 50 µL RPMI medium (Sigma–Aldrich, Vienna, Austria) and injected subcutaneously into the right flank of 12 mice. Four animals were randomly assigned to each treatment group. After the tumor reached an approximate size of 25 mm3, the respective mice were daily treated i.p. with a dose of 5 mg/kg b.w. Enn B or Bea (dissolved in 10% DMSO). The control mice received the solvent alone. Effects of the treatment were assessed by regularly recording tumor sizes and health parameters (e.g. body weight). The day following the last treatment animals were anaesthezised. Afterwards, in both treatment groups urine was collected via bladder puncture. Additionally, blood samples were collected by heart puncture and incubated at room temperature for approximately 30 min allowing the samples to clot. Following centrifugation for 10 min at 4°C and 3000 rpm (Eppendorf Centrifuge 5417R, Hamburg, Germany) sera were recovered and, after centrifugation under the same conditions, stored at -80°C. Furthermore, organs and tumors were collected, weighed and shock-frozen in liquid nitrogen and stored at -80°C until sample preparation for LC-MS/MS was performed. For immunohistochemical experiments organs and tumors were fixed in 4% formalin/phosphate-buffered saline (Roti®-Histofix 4%, Roth, Karlsruhe, Germany).
2.4. Sample preparation
To extract the analytes of interest from the tissues collected from control and treated mice, 0.2 ± 0.05 g of sample was placed in a 2 mL Eppendorf Safe-Lock Microcentrifuge Tube and 1.5 mL of acetonitrile was added. Samples were completely grounded using an Ultra-Turrax® T8 (IKA Labortechnik, Staufen, Germany) for 1 min. Afterwards, they were centrifuged at 4000 rpm for 5 min at 4ºC (Eppendorf Centrifuge 5417R, Hamburg, Germany). The clear supernatant was transferred into an autosampler vial and 10 µL were subsequently injected. On the other hand, analytes contained in biological fluids were extracted as follows: to 50 µL of sample 1.5 mL acetonitrile was added followed by a vortex mixing (15 s) and centrifugation (4000 rpm, 5 min, 4ºC). The clear supernatant was transferred into an autosampler vial and 10 µL were subsequently injected into the LC-MS/MS apparatus.
2.5. LC-MS/MS analysis
Detection and quantification was performed with a QTrap 5500MS/MS system (Applied Biosystems, Foster City, CA) equipped with a TurboV electrospray ionization (ESI) source and a 1290 seriesUHPLC system (Agilent Technologies, Waldbronn, Germany). Chromatographic separation was performed at 25°C on a Gemini®C18-column, 150 × 4.6 mm i.d., 5 µm particle size, equipped with a C18 security guard cartridge, 4 × 3 mm i.d. (all from Phenomenex, Torrance, CA, US). Elution was carried out in binary gradient mode. Both mobile phases contained 5 mM ammonium acetate and were composed of methanol/water/acetic acid 10:89:1 (v/v/v; eluent A) and 97:2:1 (v/v/v; eluent B), respectively. After an initial time of 2 min at 100% A, the proportion of B was increased linearly to 50% within 3 min. Further linear increase of B to 100% within 9 min was followed by a hold-time of 4 min at 100% B and 2.5 min column re-equilibration at 100% A. The flow rate was 1 mL/min. ESI-MS/MS was performed in the scheduled selected reaction monitoring (sSRM) mode both in positive mode. The target scan time was set to 1 s. The settings of the ESI source were as follows: source temperature 550ºC, curtain gas 30 psi (206.8 kPa of max. 99.5% nitrogen), ion source gas 1 (sheath gas) 80 psi (551.6 kPaof nitrogen), ion source gas 2 (drying gas) 80 psi (551.6 kPa of nitrogen), ion-spray voltage +5500, collision gas (nitrogen) medium.
2.6. In-house validation
The method was validated according to the EU Commission Decision 2002/657/EC. A prior analysis of the samples from control animals was performed in order to ensure that they did not contain any of the studied compounds. These samples were selected as blank for spiking, calibration curves and recovery purposes.
Linearity was evaluated using standard solutions and matrix-matched calibrations by analyzing in triplicate six concentrations levels over a range of 0.01 – 100 µg/L. Matrix-matched calibration were prepared by spiking extracts of blank samples with Enn B and Bea. Matrix-induced signal enhancement or suppression (SSE) was assessed by comparison of the respective matrix-matched standards with the neat solvent standards as indicated by the subsequent equation:
| (Eq. 1) |
Accuracy and precision were estimated by the relative recovery from spiked samples. Blank samples were fortified with the working standard solution at 50 µg/L for each matrix. About 30 min after spiking, each sample was extracted as described in section 2.4. Intra-day precision was assessed by calculating the relative standard deviation (RSDr, %) and calculated from the results generated under repeatability conditions of three determinations in a single day. Inter-day precision was calculated by the relative standard deviation (RSDR, %) and calculated from the results generated under reproducibility conditions by triplicate determination on three days. Sensitivity was evaluated by limits of detection (LODs) and limits of quantitation (LOQs) values. LODs and LOQs were calculated as the concentrations for which signal-to-noise ratios were 3 and 10, respectively.
2.7. Histochemical stainings
To determine possible changes during the long-term toxicological tests, the tissues were formalin-fixed, dehydrated and paraffin-embedded and sectioned at 3 µm (Schlederer et al., 2014). For detailed analysis of the different tissue compartments, the sections were stained with either haematoxylin and eosin (H&E), Periodic acid–Schiff (PAS), modified Masson trichrome staining (CAB) or Perls iron staining using standard procedures.
3. Results and discussion
3.1. Method development
3.1.1. Optimization of the LC-MS/MS parameters
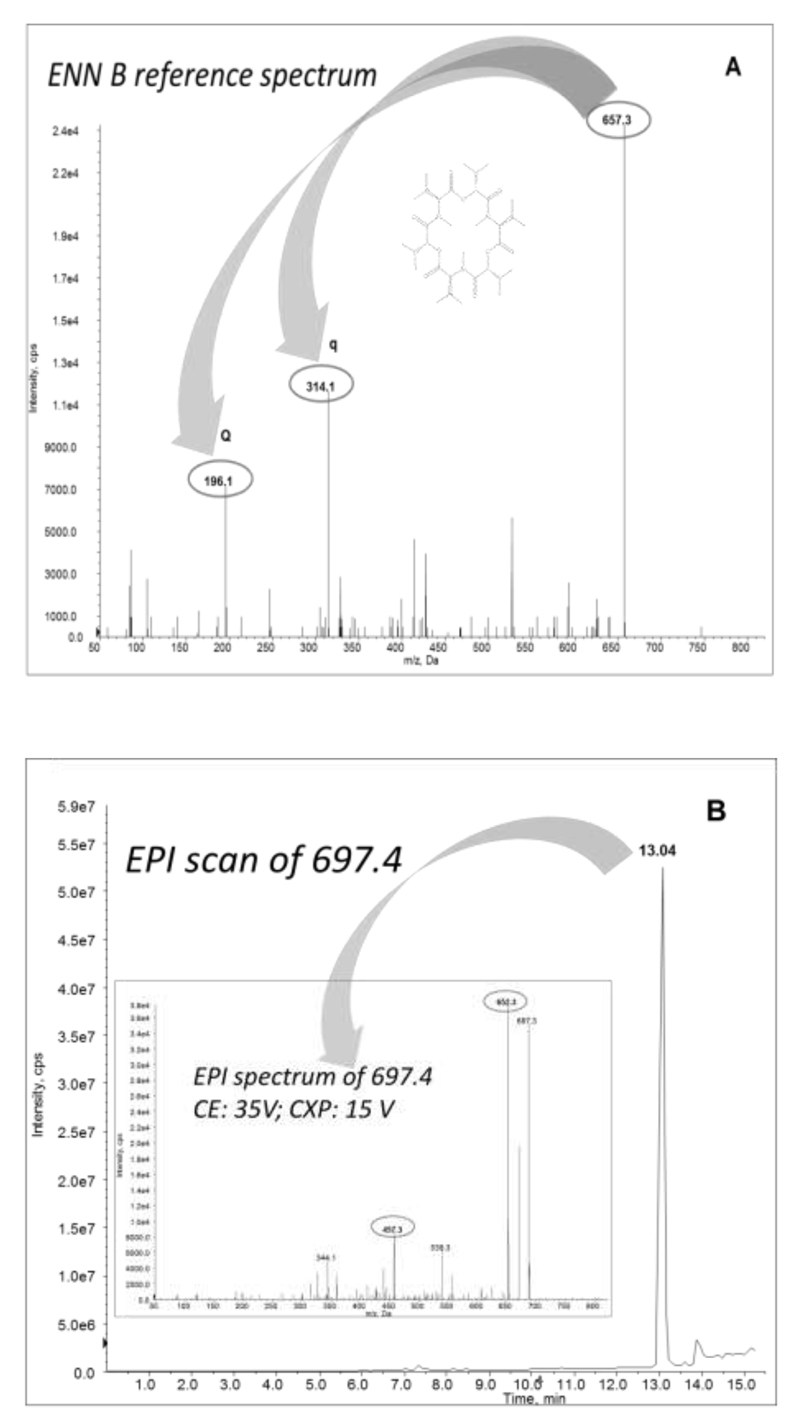
The optimization of the analyte-dependent MS/MS parameters was performed via direct infusion of standards (diluted in a 1:1 mixture of eluent A and B) into the MS source using a syringe injection at a flow rate of 10 µL/min. The MS instrument was operated in the positive electrospray mode (ESI +) as this was the most sensitive mode, confirmed by previous studies (Malachova et al., 2014). The full scan MS spectra of precursor and product ions are shown in Figure 1.
Figure 1. Enniatin B reference spectrum.
(A) LC-MS/MS spectra of Enn B showing precursor ion and products ions (Q: quantitation, q: confirmation) and (B) Enhanced product ion (EPI) scan of product degradation 697.4 (deoxygenated species) and EPI spectrum of this ion.
The in vitro fragmentation of Enn B was studied in detail by Ivanova et al. (Ivanova et al., 2011), who determined the main phase I metabolites in liver microsomes. A total of 12 microsomal Enn B metabolites were identified by liquid chromatography retention times and specific masses obtained by high-resolution tandem mass spectrometry. The molecular masses reported by Ivanova et al., regarding Enn B metabolites were targeted by Enhanced Product Ion scans to evaluate the presence or absence of these metabolites in the herein investigated biological extracts based on the m/z of the related product ions. Once a peak was obtained, optimization of the related mass spectrometric parameters (declustering potential, collision energy, and collision exit potential) was performed (Ivanova et al., 2011).
The acquisition of two sSRM-transitions per analyte allowed to confirm the identity of the positive results according to the criteria established in Document No. SANCO 12571/2013 (European Commission Health and Consumer Protection directorate-general, 2013). The product ion with the highest intensity was selected as quantifier, whereas the other was used as qualifier. The specific MS/MS parameters for each analyte are shown in Table 1.
Table 1.
Precursor ion, product ions and optimized mass spectrometric parameters for targeted analytes.
| Analyte | Precursor ion (m/z) | Product ion (m/z) | DP (V) | CE (V) | CXP (V) |
|---|---|---|---|---|---|
| Enniatin B-NH4+ | 657.3 | 196.1 | 51 | 50 | 18 |
| 314.1 | 35 | 10 | |||
| Monodemethylated- NH4+ | 643.3 | 479.1 | 45 | 20 | 10 |
| 315.1 | 35 | 15 | |||
| Didemethylated- NH4+ | 629.3 | 498.1 | 38 | 20 | 12 |
| 462.1 | 35 | 8 | |||
| Dioxygenated- NH4+ | 697.4 | 652.3 | 43 | 20 | 18 |
| 457.3 | 35 | 15 | |||
| Beauvericin-H+ | 784.4 | 244.1 | 37 | 25 | 10 |
| 262.0 | 24 | 12 |
DP: declustering potential; CE: collision energey; CXP: collision cell exit potential
3.1.2. Optimization of the sample preparation
Toxicokinetic studies may generate a large number of samples and, therefore, the use of a simple and practical sample preparation procedure is highly desirable to reduce the time and cost of the analysis. To achieve a good compromise between simplicity of extraction and sample clean-up, a generic sample preparation procedure was developed which consisted of a combination of protein precipitation with liquid extraction. To our knowledge, there is no study where the optimization of Enn B and Bea extraction from biological samples has been performed and only scarce literature regarding related compounds is available (Devreese et al., 2014; Juan et al., 2014; Manyes et al., 2014).
In this study, acetonitrile was selected as the extraction solvent to carry out the optimization of the sample preparation according to Devreese et al. (Devreese et al., 2014) who determined emerging Fusarium toxins in pig plasma and reported that acetonitrile showed the highest mycotoxin extraction recoveries regarding other organic solvents. Therefore, three different volumes were tested (0.5, 1.0 and 1.5 mL) to extract the mycotoxins spiked into a homogenized mouse liver (0.25 g) at 50 µg/L. The use of 1.5 mL of solvents gave clearer supernatant after centrifugation compared to the trials using lower volumes. This fact also influenced the method performance obtaining recoveries greater than 80% when 1.5 mL of solvent was used whereas recoveries lower than 60% were obtained with the lower volumes.
The extraction efficiency was assessed by repeating the extraction cycles of the proposed procedure. Results showed no significant differences regarding recovery data (98% ± 10, 90% ± 12 and 97%. ± 9; n = 3), for one, two and three extraction cycles, respectively. On the other hand, studies focused on reducing matrix effects were also carried out by evaluating the influence of the sample extract dilution. Therefore, both fortified liver extracts and standards in solvent at 50 µg/L were used. These solutions were injected into the LC-MS/MS system without any dilution, diluted 1:1 and diluted 1:10 with the dilution solvent (acetonitrile/water/acetic acid 20:79:1, v/v/v). Matrix effects were calculated in accordance to Eq. 1 (see Section 2.6). Results showed a reduction of matrix effects when the dilution factor was increased. In particular, SSE values of 72%, 76% and 91% were observed with the non-diluted, diluted 1:1 and diluted 1:10 extracts, respectively. Despite that, it was decided not to dilute the final extracts as a signal suppression < 30% was considered to be still acceptable. Moreover, it was possible to achieve the best sensitivity by injection of the final extract without any dilution.
3.2. Method validation
The analytical performance of the method was confirmed by the validation data reported in Table 2. Regression equations were obtained using six standard concentrations on the abscissa and the area of the chromatogram peaks as vertical coordinates. The determination coefficients (R2) of Enn B and Bea were > 0.999. Signal suppression or enhancement was evaluated during method development, since it has demonstrated to interfere with ion identification during analysis of many compounds (Decision, 2002). Moderate signal suppressions (SSE values ranging from 72 to 88%) were observed in all tissues and biological fluids tested. Thus, matrix-matched calibration curves were used for quantification purposes. Recoveries were in the range of 88–123% with RSDr and RSDR below 12% and 15%, respectively (Table 2). The sensitivity of the method was expressed in terms LODs and LOQs, which ranged between 0.025 – 0.10 µg/L and 0.05 – 0.15 µg/L, respectively (Table 2). Results showed the suitability of the developed method for the determination of trace amounts of the selected analytes in biological samples.
Table 2.
Analytical performance of the test substances in the studied tissues and fluids of Enn B- and Bea-treated SCID mice.
| Biological sample | Enn B | Bea | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Recovery of Extraction (%) | RSDr (%) | RSDR (%) | SSE (%) | LOQ (µg/kg) | Recovery of Extraction (%) | RSDr (%) | RSDR (%) | SSE (%) | LOQ (µg/kg) | |
| Liver | 108 | 12 | 15 | 72 | 0.05 | 93 | 10 | 14 | 91.3 | 0.05 |
| Kidney | 123 | 10 | 14 | 86 | 0.05 | 83 | 9 | 15 | 75.6 | 0.10 |
| Colon | 103 | 8 | 12 | 74 | 0.10 | 115 | 12 | 15 | 93 | 0.15 |
| Fat | 110 | 7 | 9 | 87 | 0.05 | 89 | 5 | 9 | 90 | 0.05 |
| Brain | 102 | 10 | 15 | 79 | 0.15 | 96 | 8 | 12 | 89 | 0.15 |
| Muscle | 104 | 8 | 12 | 86 | 0.10 | 87 | 7 | 10 | 91 | 0.15 |
| Urine | 95 | 5 | 11 | 88 | 0.10 | 90 | 6 | 13 | 92 | 0.15 |
| Serum | 88 | 7 | 9 | 80 | 0.05 | 85 | 8 | 14 | 90 | 0.10 |
| Tumor | 93 | 8 | 11 | 83 | 0.15 | 127 | 10 | 15 | 88 | 0.05 |
LOQs, limits of quantitation; RSDr: intra-day precision (n=3); RSDR: inter-day precision (n=9)
The specificity of the method was evaluated with respect to interferences from endogenous compounds. Analyzed blank samples of each tissue and fluid did not demonstrate possible interfering peaks with S/N ratio above S/N ratio of the analytes within a 2.5% margin of relative retention time, testifying the good specificity of the method.
3.3. Biological sample processing using the specifically developed LC-MS/MS
3.3.1. Evaluation of toxicological effects induced by Enn B and Bea
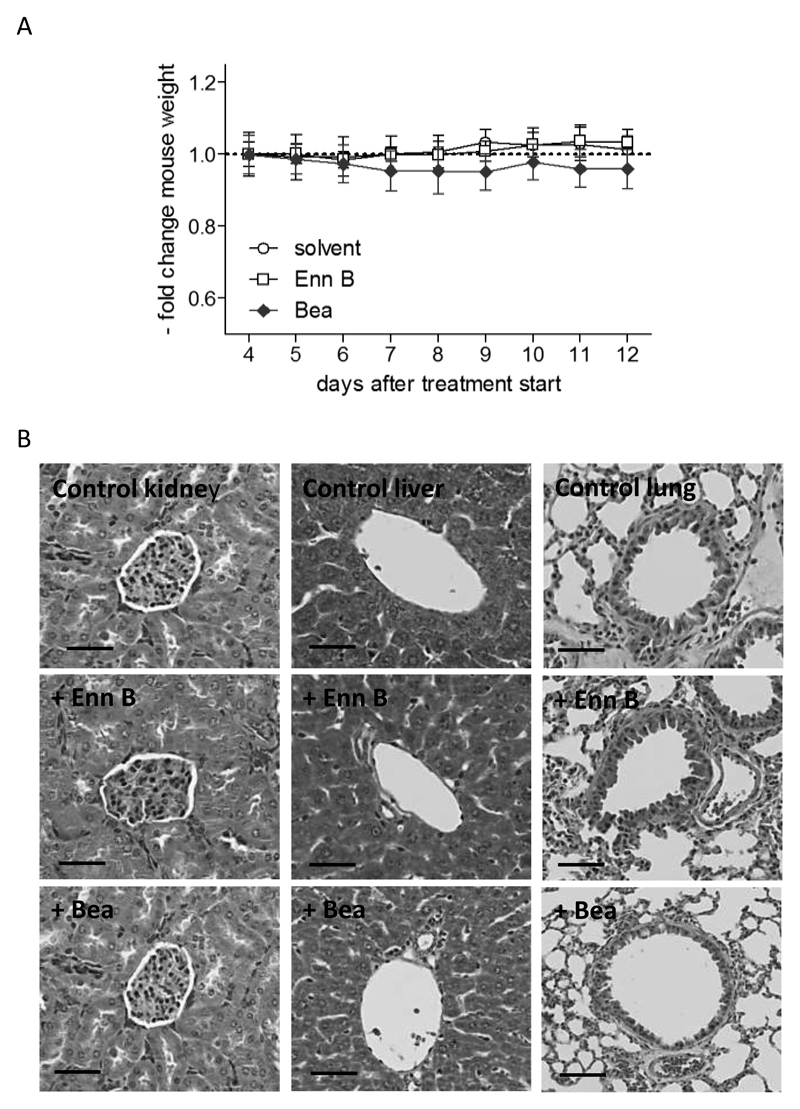
One goal of the present study was to investigate the fate of the emerging fusariotoxins Enn B and Bea in healthy mice and in a KB-3-1 cervix carcinoma xenograft model. In these preliminary experiments, a dose of 5 mg/kg was chosen as this represents a frequently used intraperitoneal dose for cyclohexadepsipeptides (Dornetshuber-Fleiss et al., 2014; Yeh et al., 2012). To assess possible toxic effects of Enn B and Bea treatments, body weight and animal behavior were monitored throughout the study. None of the treatment schedules showed apparent signs of toxicity like reduced food and fluid consumption, fatigue (data not shown) or body weight alterations (Fig. 2a). Additionally, all parenchymal organs of the animals in the different treatment groups underwent detailed histological analysis for acute or subchronic tissue damage. Macroscopically, the examined organs of all mice were intact and no change in architecture, consistency or coherence could be found during necropsy. The architecture of the organs (liver, lung and kidneys) was thoroughly investigated by different staining techniques followed by light microscopy. Histologically, all organs of the different treatment groups had an intact histological architecture and no whatsoever change due to subchronic or acute toxic damage could be observed (Fig. 2b). This is in accordance with a subchronic (28 days) feeding experiment with Wistar rats where histological analyses and biochemical blood parameters showed no adverse effects after a repeated dose of 20.91 mg Enn A/kg b.w./day (Manyes et al., 2014). In contrast, the Enn mixture fusafungine, was recently shown to induce histopathological changes such as hyperplasia, low grade dysplasia, congestion or edema in mice after treatment with a 1% solution for 10 days with two sprays daily (Yuca et al., 2006). Noteworthy, in our test settings also on the cellular level no increase of mitotic numbers, proliferation rates or apoptotic bodies were observed in the investigated tissues which indicates that the dose used to treat the animals was well within the tolerable range and was perfectly accepted by the mice. Taken together, no pathological signs in the different treatment groups could be observed, neither on macroscopical or histopathological levels. Consequently, this preliminary toxicological profile suggests that Enn B and Bea can be considered as potential therapeutic anticancer agents in the development of new chemotherapeutical drugs. However, future studies are required to explore possible chronic toxicity of these emerging fusariotoxins.
Figure 2. In vivo effects of Enn B and Bea.
SCID mice were treated 9 days with a solvent solution, Enn B and Bea (5 mg/kg; i.p.), respectively. (A) Body weight was measured at the indicated time points and expressed as change of the ratio between the initial value and the treatment value. (B) Histology of tissues after treatment with Enn B and Bea is shown by representative photomicrographs (40x objective, Scale bar = 50 µm) of H&E-stained sections of kidney (left row), liver (middle row) and lung (right row).
3.3.2. Tissue distribution of Enn B and Bea
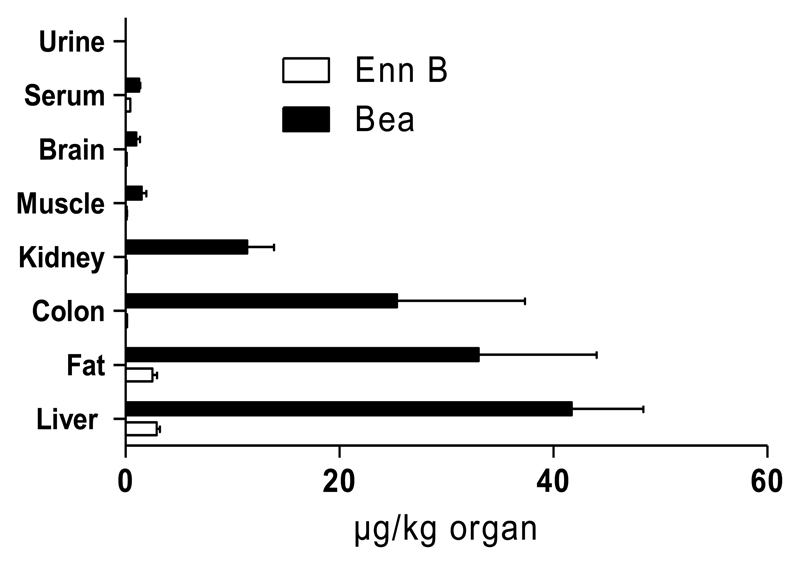
A further object of this study was to evaluate the bioavailability of the two cyclohexadepsipeptides. Therefore, the tissue distribution of Enn B and Bea in serum, urine, muscle, colon, fat, brain, kidney and liver extracts from mice treated two (Enn B) or three (Bea) consecutive days (i.p. dose: 5 mg/kg bw.) were analyzed in duplicate by the specifically established LC-MS/MS protocol. Sample concentrations were determined on the basis of peak areas using matrix-matched calibrations and are shown in Table 3a and Figure 3. As expected, none of the control samples presented detectable levels of Enn B or Bea. With regard to the treated animals, both fusariotoxins were detected in all biological samples with the exception of urine. In general, results showed that Enn B and Bea remained in their unmetabolized form in all analyzed samples but at low concentrations. Interestingly, significantly higher contents of Bea (>18-fold) were found in all tested extracts. This is in accordance with Devreese et al., showing that there is a big difference in oral absorption between the different cyclohexadepsipeptides although they have a similar chemical structure. In their study, Enn B seems to have the highest oral absorption, followed by Enn B1, A1, A and finally Bea (Devreese et al., 2014; Panel, 2014). However, previous in vitro studies by our research group showed a generally 10-fold higher cellular accumulation potential for Bea as compared to Enns in different cell types (Dornetshuber et al., 2009b). Additionally, Jestoi et al. reported higher Bea levels as compared to Enns in Finnish poultry tissues (Jestoi et al., 2007). Consequently, this suggests a distinctly different uptake of Enn subtypes and Bea probably based on their divergent lipophilic characters: Bea (N-methylphenylalanine) > Enn B1 (one N-methylisoleucine, two N-methylvaline) (Dornetshuber et al., 2009b; Ivanov, 1973; Jestoi et al., 2007; Sussmuth et al., 2011). This is in accordance with our further experiments demonstrating a tendency for both molecules to bioaccumulate in the lipophilic tissues with Bea being more prominent (2.5 ± 0.9 µg Enn B/kg and 33.0 ± 22.1 µg Bea/kg fat sample). Interestingly, although the brain consists of nearly 60% fat (Chang et al., 2009), relatively low levels of the cyclodepsipeptides were found in this organ (0.1 ± 0.02 µg Enn B/kg and 1.0 ± 0.7 µg Bea/kg brain sample). One explanation for the generally low Enn B and Bea levels in brain tissues might be insufficient drug absorption based on drug efflux by ATP-binding cassette (ABC) transporters which play a protective role by transporting toxic metabolites out of the brain back into the blood stream (Gottesman et al., 2002). This assumption is further confirmed by our previous data showing distinct interactions between ABC transport proteins and the two Fusarium metabolites (Dornetshuber et al., 2009b).
Table 3a.
Concentrations of the studied mycotoxins in the assayed biological samples in the short-term treatment study. Results expressed as mean ± SD (n = 4).
| Biological sample | Enn B (µg/kg) | Bea (µg/kg) |
|---|---|---|
| Liver | 2.9 ± 0.6 | 41.7 ± 13.4 |
| Kidney | 0.1 ± 0.01 | 11.4 ± 5.0 |
| Colon | 0.15 ± 0.02 | 25.4 ± 23.9 |
| Fat | 2.5 ± 0.9 | 33.0 ± 22.1 |
| Brain | 0.1 ± 0.02 | 1.0 ± 0.7 |
| Muscle | 0.12 ± 0.03 | 1.5 ± 0.9 |
| Urine | n.d. | n.d. |
| Serum | 0.45 ± 0.01 | 1.3 ± 0.2 |
Figure 3. Distribution pattern of Enn B and Bea in tissues and biological fluids.
SCID mice were treated i.p. with 5 mg/kg Enn B (2 days) and Bea (3 days), respectively. Tissues and biological fluids were extracted and analysed by LC-MS/MS. Sample concentrations were determined on the basis of peak areas using matrix-matched calibrations and presented µg/kg organ.
When comparing the further fate of Enn B and Bea, a remarkable distribution pattern could be observed for both compounds. Generally, the hepatobiliary system and the kidneys are the main routes by which drugs and their metabolites are excreted (van Montfoort et al., 2003). While none of the two cyclodepsipeptides could be detected in the urine, the highest concentration of both mycotoxins was found in the liver with 2.9 ± 0.6 µg/kg and 41.7 ± 13.4 µg/kg for Enn B and Bea, respectively, suggesting the liver as the principle organ of detoxification. Moreover, for Bea high levels were found in the colon (25.4 ± 23.9 µg/kg) further indicating enterohepatic recycling. Interestingly, for Enn B only trace amounts (0.15 µg ± 0.02 µg/kg) were observed in colon tissue which might be a consequence of rapid metabolisation. Moreover, only low Enn B levels (0.1 µg ± 0.01 µg/kg) were found in the kidneys. This lack of significant renal excretion of Enn B is in good agreement with the literature, where in Enn A-fed Wistar rats no Enn A was detected in kidneys and highest concentrations in the liver (Manyes et al., 2014).
Summarizing, these in vivo data suggest that Enn B and Bea can diffuse across cell membranes and are rapidly cleared from the blood stream by hepatobiliary excretion. Despite their structural resemblance, significantly higher contents of Bea compared to Enn B were found in all tested extracts indicating lower bioavailability and/or faster excretion of Enn B. Moreover, since both substances are readily soluble in fats, they tended to accumulate in fat-rich tissues such as liver and brain with Bea being more prominent. Thus, a higher pharmacological activity or chronic toxicity of Bea compared to Enn might be assumed.
3.3.3. Metabolite formation of Enn B and Bea in mouse tissue
In addition to the absolute quantification of Enn B and Bea in tissues, an additional focus was dedicated to the biotransformation of these substances. As reactive metabolites can influence the overall toxic profile, the characterization of metabolites is an important step in the safety and risk evaluation of drugs and food contaminants. Liver and kidneys are particularly susceptible to organ toxicity as they are the sites of toxin filtration and are involved in detoxification processes to render toxins less reactive and more water-soluble (Di et al., 2009). Although fusariotoxin metabolism notably happens in these organs, it can also occur in the digestive tract and more particularly in the rumen of ruminants. In general, de-acetylation and de-epoxydation processes are performed by the intestinal microflora of most species (World Health Organization/FAO, 2002). However, the metabolization pathways of Enns in vivo remain unclear and only in vitro data are available. Faeste et al. reported that after incubation of liver microsomes with Enn B the toxin undergoes extensive metabolization resulting in oxidation and N-demethylation products as characterized recently by Ivanova et al. (Faeste et al., 2011; Ivanova et al., 2011). These in vitro observations are in a good agreement with our in vivo data since three phase I Enn B-metabolites (dioxygenated-Enn B, mono- and di-demethylated-Enn B) were tentatively found in the liver of Enn B-treated animals, whereby the tentative dioxygenated-Enn B was the main metabolite. As suggested by Ivanova et al., for the oxidation reactions the isopropyl side-chains of N-methyl-valine and 2-Hydroxyisovaleric acid are the primary target whereas N-demethylation was supposed to occur via an unstable N-hydroxymethyl intermediate (Ivanova et al., 2011). However, due to the fact that there are no standards of Enn B metabolites available, it was not possible to precisely quantify the metabolites in the samples. Nevertheless, the calibration curve of the parent compound was used as an approach to quantify the detected metabolites. Therefore, based on the Enn B calibration curve, dioxygenated-Enn B was quantified in the liver at 26.9 ± 3.7 µg/kg whereas mono- and di-demethylated-Enn B were quantified at 0.7 ± 0.1 µg/kg and 1.0 ± 0.3 µg/kg, respectively (Tab. 4). Concerning other tissues, a similar trend was observed in the colon with dioxygenated-Enn B being the major metabolite. However, intensities were a factor of 10 lower than those detected in the liver (2.5 ± 0.1 µg/kg versus 26.9 ± 3.7 µg/kg). Interestingly, although the other analyzed samples such as serum, brain, muscle and kidney showed occurrence of both parental molecules in the low µg/kg range, no metabolites were found in these assayed tissues and biological fluids. Consequently, contribution of hepatic as well as intestinal metabolism might be suggested to be involved in the overall first-pass metabolism of Enn B further explaining the relatively low levels of the parent compound in the colon samples. Interestingly, despite the structural similarity of Enn B and Bea, no metabolites were detected in the assayed samples for Bea. Thus, a higher metabolic stability for Bea compared to Enn B might be suggested. However, the metabolic stability of a compound is generally reduced by the overall lipophilicity based on the lipophilic binding sites of the metabolizing enzymes which prefer higher lipophilic substrates (Nassar et al., 2004a). Hence, with regard to the higher lipophilicity of Bea, it seems to be rather unlikely that this cyclohexadepsipeptide possesses a higher metabolic stability than Enn B. On the other hand, while CYP 450 enzymes, which are responsible for the metabolic clearance of drugs, were shown to be important for Enn B metabolism in human microsomes (Faeste et al., 2011), Bea was recently presented as a potent inhibitor of diverse CYP 450 enzymes (Mei et al., 2009). Consequently, totally different metabolic processes must be considered for the two cyclohexadepsipeptides which is matter of ongoing studies.
Table 4.
Quantitation of Enn B metabolites in 2-days Enn B-treated SCID mice using Enn B calibration curves.
| Tissue | Mycotoxin (µg/kg) | Metabolites (µg/kg) | ||
|---|---|---|---|---|
| Enn B | Di-oxygenated-Enn B | Mono-demethylated-Enn B | Di-demethylated-Enn B | |
| Liver | 2.9 ± 0.6 | 26.9 ± 3.7 | 0.7 ± 0.1 | 1.0 ± 0.3 |
| Colon | 0.9 ± 0.05 | 2.5 ± 0.6 | 0.6 ± 0.09 | 2.45 ± 2.0 |
3.3.3. Enn B and Bea in tumor tissues
In the last few years Enns and Bea were also discussed as potential anticancer drugs. Both drugs were shown to exert profound in vitro antitumor activities in diverse cancer cell types such as leukemia, hepatoma, human non-small cell lung cancer and diverse other carcinomas (Chen et al., 2006; Dornetshuber et al., 2007; Dornetshuber et al., 2009a; Dornetshuber et al., 2009b; Lin et al., 2005; Watjen et al., 2008; Watjen et al., 2014). Recently, we could also show a remarkable in vivo activity for Enn B in combination with the multi-kinase inhibitor Sorafenib in a KB-3-1 cervix carcinoma xenograft model, suggesting that an Enn B/Sorafenib combination would be attractive as a novel therapeutic strategy especially for cervical cancer (Dornetshuber-Fleiss et al., 2015). Thus, we further intended to investigate the amount of Enn B and Bea as well as their respective metabolites in cervical cancer tumors of 9-days Enn B- and Bea-treated human tumor xenograft mouse models. The detected values of parental Enn B and Bea were 2.8 ± 0.8 µg/kg and 3.4 ± 0.4 µg/kg, respectively (Table 3b). Compared to serum, the tumor levels of Bea and Enn B were 1.3-fold and 0.5-fold, respectively, further confirming the higher accumulation potential of Bea. However, for both substances a promising tumor-accumulation and anticancer potential might be assumed, with Bea being more effective. On the other hand, besides direct cytotoxic anticancer effects, compounds can also exert indirect toxicity due to the formation of metabolites (Rodriguez-Antona and Ingelman-Sundberg, 2006). However, no degradation products could be found in the analyzed tumor tissues. Consequently, it seems that biotransformation products of Enn B and Bea are not responsible for the literary reported anticancer activities. However, to bring the two substances from bench to bedside of cancer therapy, one of the most important impacts on successful drug discovery is to determine, and then optimize, the exposure–activity–toxicity-relationship, and thus their suitability for advancement to development (Nassar et al., 2004b). Since no signs of toxicity could be observed in our test settings, higher concentrations of the test substances could be useful to potentiate the accumulation of Enn B and Bea in the tumor tissue and thus, their antitumor activity. Based on the data from literature of the structurally related cyclic depsipeptide FR901228 (romidepsin) (Chan et al., 1997), which was already approved for the treatment of cutaneous T-cell lymphoma by the US Food and Drug Administration (VanderMolen et al., 2011), the double dose of 10 mg/kg would be thoroughly conceivable of our future studies for successful preclinical evaluation of Enn B and Bea as chemotherapeutic substances.
Table 3b.
Concentrations of Enn B and Bea in serum and tumor samples in the long-term treatment study. Results expressed as mean ± SD (n = 4).
| Biological sample | Enn B (µg/kg) | Bea (µg/kg) |
|---|---|---|
| Tumor | 2.8 ± 0.8 | 3.4 ± 0.4 |
| Serum | 5.4 ± 0.2 | 2.6 ± 0.6 |
4. Conclusions
A sensitive and reliable method for the identification and quantification of Enn B and Bea in tissues and biological fluids from mice was developed and optimized using a simple and easy liquid extraction with acetonitrile followed by LC-MS/MS determination. The proposed methodology was in-house validated obtaining satisfactory results in terms of linearity, accuracy, precision and sensitivity. Generally, Enn B and Bea were well tolerated by the mice at the applied doses and both substances were detected not only in several organs and tissues but also in serum confirming drug absorption through the visceral peritoneum into the portal vein after i.p. administration. In more detail, the distribution pattern for Enn B and Bea were as follows: liver > fat > serum > colon ≈ muscle ≈ kidney ≈ brain, and liver > fat > colon > kidney > muscle ≈ brain ≈ serum, respectively. Additionally, distinct Enn B and Bea levels were detected in the tumor tissues further suggesting that these two cyclohexadepsipeptidic substances might be useful as novel therapeutic strategy for cancer treatment. Moreover, the here generated data suggest that both substances are rapidly cleared from the blood stream by hepatobiliary excretion. While Bea was shown to be relatively stable and highly accumulated in adipose tissue, for Enn B in addition to the parental substance, some phase I metabolites were identified mainly in liver but also in colon. Consequently, the results obtained in this study suggest a potential contribution of hepatic as well as intestinal metabolism which could be involved in the overall first-pass metabolism of Enn B but not of Bea. Additionally, a higher pharmacological activity or chronic toxicity of Bea compared to Enn B might be assumed. Future research studies focused on elucidating the phase I and phase II metabolites pathways as well as the toxicity profile of these substances are topic of ongoing studies and should help to better understand the fate of Enn B and Bea in vivo.
Acknowledgements
We want to thank Sushilla van Schoonhoven for technical assistance and Gerhard Zeitler for animal care.
Funding information
This work was supported by the Spanish Minister of Economy and Competitiveness (AGL2013-43194-P). Y. Rodríguez-Carrasco thanks the F.P.U. Grant (No. AP2010-2940) provided by the Ministry of Education. Moreover, this work was supported by the Austrian Science Fund (FWF) (to R. Dornetshuber-Fleiss, project number T 451-B18) and the Johanna Mahlke, geb.-Obermann-Stiftung (to Rita Dornetshuber-Fleiss). Financial funding was also given by the center of excellence Unifying concepts in Catalysis (UniCat) granted by the German Research Council (DFG).
Abbreviations
- ABC
ATP-binding cassette
- Bea
beauvericin
- b.w.
bodyweight
- DMSO
dimethyl sulfoxide
- Enn
Enniatin
- ESI
Electrospray ionisation
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- p.a.
per analysis
- sSRM
scheduled selected reaction monitoring
- SSE
signal enhancement or suppression
- LODs
limits of detection
- LOQs
limits of quantitation
- RSDr
relative standard deviation
- SCID
severe combined immunodeficiency
Footnotes
Conflict of Interest
The authors declare that there are no conflicts of interest.
References
- Behm C, Follmann W, Degen GH. Cytotoxic potency of mycotoxins in cultures of V79 lung fibroblast cells. Journal of toxicology and environmental health Part A. 2012;75:1226–1231. doi: 10.1080/15287394.2012.709170. [DOI] [PubMed] [Google Scholar]
- Blais LA, Apsimon JW, Blackwell BA, Greenhalgh R, Miller JD. Isolation and Characterization of Enniatins from Fusarium-Avenaceum Daom-196490. Can J Chem. 1992;70:1281–1287. [Google Scholar]
- Chan KK, Bakhtiar R, Jiang C. Depsipeptide (FR901228, NSC-630176) pharmacokinetics in the rat by LC/MS/MS. Invest New Drugs. 1997;15:195–206. doi: 10.1023/a:1005847703624. [DOI] [PubMed] [Google Scholar]
- Chang CY, Ke DS, Chen JY. Essential fatty acids and human brain. Acta neurologica Taiwanica. 2009;18:231–241. [PubMed] [Google Scholar]
- Chen BF, Tsai MC, Jow GM. Induction of calcium influx from extracellular fluid by beauvericin in human leukemia cells. Biochem Biophys Res Commun. 2006;340:134–139. doi: 10.1016/j.bbrc.2005.11.166. [DOI] [PubMed] [Google Scholar]
- Cooney GF, Habucky K, Hoppu K. Cyclosporin pharmacokinetics in paediatric transplant recipients. Clinical pharmacokinetics. 1997;32:481–495. doi: 10.2165/00003088-199732060-00004. [DOI] [PubMed] [Google Scholar]
- Decision C. Commission Decision 2002/657/EC implementing Council Directive 96/23/EC concerning the Performance of Analytical Methods and the Interpretation of Results. 2002 [Google Scholar]
- Devreese M, Broekaert N, De Mil T, Fraeyman S, De Backer P, Croubels S. Pilot toxicokinetic study and absolute oral bioavailability of the Fusarium mycotoxin enniatin B1 in pigs. Food Chem Toxicol. 2014;63:161–165. doi: 10.1016/j.fct.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Di L, Kerns EH, Carter GT. Drug-like property concepts in pharmaceutical design. Current pharmaceutical design. 2009;15:2184–2194. doi: 10.2174/138161209788682479. [DOI] [PubMed] [Google Scholar]
- Dornetshuber-Fleiss R, Heilos D, Mohr T, Richter L, Sussmuth RD, Zlesak M, Novicky A, Heffeter P, Lemmens-Gruber R, Berger W. The naturally born fusariotoxin Enniatin B and Sorafenib exert synergistic activity against cervical cancer in vitro and in vivo. Biochem Pharmacol. 2014 doi: 10.1016/j.bcp.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornetshuber-Fleiss R, Heilos D, Mohr T, Richter L, Sussmuth RD, Zlesak M, Novicky A, Heffeter P, Lemmens-Gruber R, Berger W. The naturally born fusariotoxin enniatin B and sorafenib exert synergistic activity against cervical cancer in vitro and in vivo. Biochem Pharmacol. 2015;93:318–331. doi: 10.1016/j.bcp.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornetshuber R, Heffeter P, Kamyar MR, Peterbauer T, Berger W, Lemmens-Gruber R. Enniatin exerts p53-dependent cytostatic and p53-independent cytotoxic activities against human cancer cells. Chem Res Toxicol. 2007;20:465–473. doi: 10.1021/tx600259t. [DOI] [PubMed] [Google Scholar]
- Dornetshuber R, Heffeter P, Lemmens-Gruber R, Elbling L, Marko D, Micksche M, Berger W. Oxidative stress and DNA interactions are not involved in Enniatin- and Beauvericin-mediated apoptosis induction. Mol Nutr Food Res. 2009a;53:1112–1122. doi: 10.1002/mnfr.200800571. [DOI] [PubMed] [Google Scholar]
- Dornetshuber R, Heffeter P, Sulyok M, Schumacher R, Chiba P, Kopp S, Koellensperger G, Micksche M, Lemmens-Gruber R, Berger W. Interactions between ABC-transport proteins and the secondary Fusarium metabolites enniatin and beauvericin. Mol Nutr Food Res. 2009b;53:904–920. doi: 10.1002/mnfr.200800384. [DOI] [PubMed] [Google Scholar]
- European Commission Health and Consumer Protection directorate-general. Method Validation and Quality Control Procedures for Pesticide Residues Analysis in Food and Feed. 2013 SANCO/12571/2013. [Google Scholar]
- Faeste CK, Ivanova L, Uhlig S. In vitro metabolism of the mycotoxin enniatin B in different species and cytochrome p450 enzyme phenotyping by chemical inhibitors. Drug metabolism and disposition: the biological fate of chemicals. 2011;39:1768–1776. doi: 10.1124/dmd.111.039529. [DOI] [PubMed] [Google Scholar]
- Ficheux AS, Sibiril Y, Parent-Massin D. Effects of beauvericin, enniatin b and moniliformin on human dendritic cells and macrophages: an in vitro study. Toxicon. 2013;71:1–10. doi: 10.1016/j.toxicon.2013.04.024. [DOI] [PubMed] [Google Scholar]
- Fornelli F, Minervini F, Logrieco A. Cytotoxicity of fungal metabolites to lepidopteran (Spodoptera frugiperda) cell line (SF-9) Journal of invertebrate pathology. 2004;85:74–79. doi: 10.1016/j.jip.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nature reviews Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- Ivanov VT, Evstratov AV, Sumskaya LV, Melnik EI, Chumburidze TS, Portnova SL, Balashova TA, Ochinnikov YA. Sandwich complexes as a functional form of the enniatin ionophores. FEBS letters. 1973;36:65–71. [Google Scholar]
- Ivanova L, Faeste CK, Uhlig S. In vitro phase I metabolism of the depsipeptide enniatin B. Analytical and bioanalytical chemistry. 2011;400:2889–2901. doi: 10.1007/s00216-011-4964-9. [DOI] [PubMed] [Google Scholar]
- Ivanova L, Skjerve E, Eriksen GS, Uhlig S. Cytotoxicity of enniatins A, A1, B, B1, B2 and B3 from Fusarium avenaceum. Toxicon. 2006;47:868–876. doi: 10.1016/j.toxicon.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Jestoi M. Emerging fusarium-mycotoxins fusaproliferin, beauvericin, enniatins, and moniliformin: a review. Crit Rev Food Sci Nutr. 2008;48:21–49. doi: 10.1080/10408390601062021. [DOI] [PubMed] [Google Scholar]
- Jestoi M, Rokka M, Peltonen K. An integrated sample preparation to determine coccidiostats and emerging Fusarium-mycotoxins in various poultry tissues with LC-MS/MS. Mol Nutr Food Res. 2007;51:625–637. doi: 10.1002/mnfr.200600232. [DOI] [PubMed] [Google Scholar]
- Juan C, Manyes L, Font G, Juan-Garcia A. Evaluation of immunologic effect of Enniatin A and quantitative determination in feces, urine and serum on treated Wistar rats. Toxicon. 2014;87:45–53. doi: 10.1016/j.toxicon.2014.05.005. [DOI] [PubMed] [Google Scholar]
- Lin HI, Lee YJ, Chen BF, Tsai MC, Lu JL, Chou CJ, Jow GM. Involvement of Bcl-2 family, cytochrome c and caspase 3 in induction of apoptosis by beauvericin in human non-small cell lung cancer cells. Cancer Lett. 2005;230:248–259. doi: 10.1016/j.canlet.2004.12.044. [DOI] [PubMed] [Google Scholar]
- Malachova A, Sulyok M, Beltran E, Berthiller F, Krska R. Optimization and validation of a quantitative liquid chromatography-tandem mass spectrometric method covering 295 bacterial and fungal metabolites including all regulated mycotoxins in four model food matrices. J Chromatogr A. 2014;1362:145–156. doi: 10.1016/j.chroma.2014.08.037. [DOI] [PubMed] [Google Scholar]
- Manyes L, Escriva L, Serrano AB, Rodriguez-Carrasco Y, Tolosa J, Meca G, Font G. A preliminary study in Wistar rats with enniatin A contaminated feed. Toxicology mechanisms and methods. 2014;24:179–190. doi: 10.3109/15376516.2013.876135. [DOI] [PubMed] [Google Scholar]
- Meca G, Manes J, Font G, Ruiz MJ. Study of the potential toxicity of commercial crispy breads by evaluation of bioaccessibility and bioavailability of minor Fusarium mycotoxins. Food Chem Toxicol. 2012;50:288–294. doi: 10.1016/j.fct.2011.10.055. [DOI] [PubMed] [Google Scholar]
- Mei L, Zhang L, Dai R. An inhibition study of beauvericin on human and rat cytochrome P450 enzymes and its pharmacokinetics in rats. Journal of enzyme inhibition and medicinal chemistry. 2009;24:753–762. doi: 10.1080/14756360802362041. [DOI] [PubMed] [Google Scholar]
- Nassar AE, Kamel AM, Clarimont C. Improving the decision-making process in structural modification of drug candidates: reducing toxicity. Drug discovery today. 2004a;9:1055–1064. doi: 10.1016/S1359-6446(04)03297-0. [DOI] [PubMed] [Google Scholar]
- Nassar AE, Kamel AM, Clarimont C. Improving the decision-making process in the structural modification of drug candidates: enhancing metabolic stability. Drug discovery today. 2004b;9:1020–1028. doi: 10.1016/S1359-6446(04)03280-5. [DOI] [PubMed] [Google Scholar]
- Panel EC. Scientific Opinion on the risks to human and animal health related to the presence of beauvericin and enniatins in food and feed. EFSA Journal. 2014;12:3802. [Google Scholar]
- Rodriguez-Antona C, Ingelman-Sundberg M. Cytochrome P450 pharmacogenetics and cancer. Oncogene. 2006;25:1679–1691. doi: 10.1038/sj.onc.1209377. [DOI] [PubMed] [Google Scholar]
- Schlederer M, Mueller KM, Haybaeck J, Heider S, Huttary N, Rosner M, Hengstschlager M, Moriggl R, Dolznig H, Kenner L. Reliable quantification of protein expression and cellular localization in histological sections. PloS one. 2014;9:e100822. doi: 10.1371/journal.pone.0100822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussmuth R, Muller J, von Dohren H, Molnar I. Fungal cyclooligomer depsipeptides: from classical biochemistry to combinatorial biosynthesis. Nat Prod Rep. 2011;28:99–124. doi: 10.1039/c001463j. [DOI] [PubMed] [Google Scholar]
- Taevernier L, Detroyer S, Veryser L, De Spiegeleer B. Enniatin-containing solutions for oromucosal use: Quality-by-design ex-vivo transmucosal risk assessment of composition variability. International journal of pharmaceutics. 2015a;491:144–151. doi: 10.1016/j.ijpharm.2015.06.029. [DOI] [PubMed] [Google Scholar]
- Taevernier L, Veryser L, Roche N, Peremans K, Burvenich C, Delesalle C, De Spiegeleer B. Human skin permeation of emerging mycotoxins (beauvericin and enniatins) Journal of exposure science & environmental epidemiology. 2015b doi: 10.1038/jes.2015.10. [DOI] [PubMed] [Google Scholar]
- van Montfoort JE, Hagenbuch B, Groothuis GM, Koepsell H, Meier PJ, Meijer DK. Drug uptake systems in liver and kidney. Curr Drug Metab. 2003;4:185–211. doi: 10.2174/1389200033489460. [DOI] [PubMed] [Google Scholar]
- VanderMolen KM, McCulloch W, Pearce CJ, Oberlies NH. Romidepsin (Istodax, NSC 630176, FR901228, FK228, depsipeptide): a natural product recently approved for cutaneous T-cell lymphoma. J Antibiot (Tokyo) 2011;64:525–531. doi: 10.1038/ja.2011.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watjen W, Debbab A, Hohlfeld A, Chovolou Y, Kampkotter A, Edrada RA, Ebel R, Hakiki A, Mosaddak M, Totzke F, Kubbutat MH, et al. Enniatins A1, B and B1 from an endophytic strain of Fusarium tricinctum induce apoptotic cell death in H4IIE hepatoma cells accompanied by inhibition of ERK phosphorylation. Mol Nutr Food Res. 2008;53:431–440. doi: 10.1002/mnfr.200700428. [DOI] [PubMed] [Google Scholar]
- Watjen W, Debbab A, Hohlfeld A, Chovolou Y, Proksch P. The mycotoxin beauvericin induces apoptotic cell death in H4IIE hepatoma cells accompanied by an inhibition of NF-kappaB-activity and modulation of MAP-kinases. Toxicol Lett. 2014;231:9–16. doi: 10.1016/j.toxlet.2014.08.021. [DOI] [PubMed] [Google Scholar]
- World Health Organization/FAO, G. Evaluation of Certain Mycotoxins in Food. JEFCA; 2002. pp. 1–65. [Google Scholar]
- Yeh CT, Rao YK, Ye M, Wu WS, Chang TC, Wang LS, Wu CH, Wu AT, Tzeng YM. Preclinical evaluation of destruxin B as a novel Wnt signaling target suppressing proliferation and metastasis of colorectal cancer using non-invasive bioluminescence imaging. Toxicology and applied pharmacology. 2012;261:31–41. doi: 10.1016/j.taap.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Yuca K, Cankaya H, Bayram I, Ozbek H, Kiris M. Local irritant effects of topical oral sprays on oral mucosa in mice. Advances in therapy. 2006;23:98–106. doi: 10.1007/BF02850351. [DOI] [PubMed] [Google Scholar]