Abstract
Objectives
We aimed to examine 1) whether specific glucose response curve shapes during oral glucose tolerance tests are predictive of type 1 diabetes development and 2) the extent to which the glucose response curve is influenced by insulin secretion.
Methods
Autoantibody positive relatives of patients with type 1 diabetes whose baseline OGTT met the definition of a monophasic or biphasic glucose response curve were followed for the development of type 1 diabetes (n=2627). A monophasic curve was defined as an increase in OGTT glucose between 30 and 90 minutes followed by a decline of ≥0.25 mmol/L between 90 and 120 minutes. A biphasic response curve was defined as having a decrease in glucose after the initial increase, which is then followed by a second increase of ≥0.25 mmol/L. Associations of type 1 diabetes risk with glucose curve shapes were examined using cumulative incidence curve comparisons and proportional hazards regression. C-peptide responses were compared with and without adjustments for potential confounders.
Results
The majority had a monophasic curve at baseline [n=1732 (66%)] vs. [n=895 (34%)]. The biphasic group had a lower cumulative incidence (p<0.001) of type 1 diabetes, which persisted after adjustments for age, sex, BMI z-score and number of autoantibodies (p<0.001). Among the monophasic group, the risk of type 1 diabetes was greater for those with a glucose peak at 90 minutes than for those with a peak at 30 minutes; the difference persisted after adjustments (p<0.001). Compared to the biphasic group, the monophasic group had a lower early C-peptide (30-0 minutes) response, a lower C-peptide index (30-0 minute C-peptide/30-0 minute glucose), as well as a greater 2-hr C-peptide level (p<0.001 for all).
Conclusions
Those with biphasic glucose curves have a lower risk of progression to type 1 diabetes than those with monophasic curves, and the risk among the monophasic group is increased when the glucose peak occurs at 90 minutes than at 30 minutes. Differences in glucose curve shapes between the monophasic and biphasic groups appear to be related to C-peptide responses.
Keywords: Oral glucose tolerance test, type 1 diabetes, glucose curve shape
INTRODUCTION
The 2-hr oral glucose tolerance test (OGTT) is traditionally used for the diagnosis of diabetes, as well as for classifying glucose tolerance status into normal or impaired [1]. However, little attention is paid to the glucose changes at different time points as well as the morphology of the glucose curve during the 2-hour interval. Most non-diabetic individuals demonstrate a monophasic 2-hour OGTT pattern in which there is an initial increase in glucose that peaks at 30 or 60 minutes, followed by a decline without a secondary increase [2–4]. Although a secondary increase (or biphasic pattern) occurring in the minority has long been observed, its implications are still not fully understood.
The relationship between the shape of the glucose response curve during an OGTT and risk for type 2 diabetes development has been well studied in both adolescents and adults [5–12]. Specifically, those with a monophasic response curve were shown to have more evidence of metabolic abnormalities as well as a higher risk for the development of type 2 diabetes compared to those with a biphasic response curve.
The relevance of the shape of the glucose response curve during a 2-hr OGTT to the development of type 1 diabetes has not yet been studied. Moreover, the role of insulin secretory patterns in generating the various glucose shapes of OGTTs is not well understood. Thus, we have assessed whether specific shapes of glucose response curves during 2-hr OGTTs are predictive of the development of type 1 diabetes in autoantibody positive (Ab+) relatives of type 1 diabetes patients. In addition, we examined the relation of the glucose response curve to C-peptide secretory patterns.
METHODS
Participants
Data from autoantibody positive [for at least one autoantibody: glutamic acid decarboxylase antibody (GADA), insulinoma-associated-2 antibody (IA-2A), micro insulin autoantibodies (mIAA), and/or islet cell cytoplasmic autoantibody (ICA)] relatives of patients with type 1 diabetes were analyzed. Those relatives were followed in the TrialNet Pathway to Prevention (PTP) study for the development of type 1 diabetes. The PTP study is a longitudinal observational study that has been previously described [13]. Data was available for a total of 3842 participants. Subjects with diabetes at baseline were excluded from the analyses (n=278). Further, those who did not follow the definitions of a monophasic or biphasic glucose curve (n=937) were excluded from the analyses. Oral glucose tolerance data from the remaining 2627 participants was analyzed. Institutional Review Board approval of the study was obtained at all participating sites, and written informed consent and assent, as applicable, were obtained.
Procedures
Oral glucose tolerance tests were performed at baseline and at all follow-up visits. For participants entered into the PTP before 2012, the interval for those visits has been every 6 months. However, since 2012 those entered into the PTP have been followed either every 6 months or annually depending on the degree of risk [14]. Following a 10-hour overnight fast, fasting levels for glucose and C-peptide were obtained, which was then followed by the ingestion of a 1.75 g/kg oral glucose load (maximum 75 g). Venous blood samples were obtained at 30-minute intervals after the glucose load for 2 hours for determination of both plasma glucose and C-peptide levels.
A fasting glucose level of ≥7.00 mmol/l (126 mg/dl) and/or a 2-hr glucose level of ≥11.01 mmol/l (200 mg/dl) prompted a repeat OGTT for confirmation. If upon repeat testing, either the fasting or the 2-hr glucose threshold was exceeded again, type 1 diabetes was diagnosed. Those who did not exceed either threshold on the confirmatory OGTT continued to be followed with OGTTs. The time of diagnosis was assigned to the date of the second confirmatory OGTT. A diagnosis could also be made without an OGTT based on the clinical presentation. Glucose was measured by the glucose oxidase method and C-peptide was measured by a two-site immunoenzymometeric assay performed on a Tosoh 600 II analyzer (Tosoh Bioscience, Inc., South San Fancisco,CA). The upper limit for the C-peptide assay is 9.99 nmol/l (30 ng/ml) and lower limit as low as 0.007 nmol/l (0.02 ng/ml) with an inter- and intra-assay coefficients of variation of <10%.
Classification of the glucose response curve
Definitions for ‘phasic’ glucose response curves were similar to those previously described by Tschritter et al. [5]. Specifically, the glucose curve shape was classified as ‘monophasic’ when plasma glucose increased after an oral glucose load to the maximum at either 30, 60, or 90 minutes and then decreased until 120 minutes with a final decline of ≥0.25 mmol/l (4.5 mg/dl) between 90 and 120 minutes. Those with glucose curves that decreased after an initial increase and then increased again by ≥0.25 mmol/l at any time were classified as ‘biphasic’. We used a more specific criterion, ≥0.25 mmol/l, than Tschritter et al. for the initial glucose rise. Based on the glucose assay used in the PTP study (mean intra- and inter-assay coefficients of variation both <2%), there is 95% confidence that the glucose change of ≥0.25 mmol/l is not due to assay variation. We further classified the biphasic group into 2 subgroups based on timing of the second glucose peak after the initial drop:
Biphasic90: if the plasma glucose at 60 minutes dropped by ≥0.25 mmol/l after the initial increase at 30 minutes and then increased again from the 60 to 90 minute time points by ≥0.25 mmol/l.
Biphasic120: if the plasma glucose at 60 minutes dropped by ≥0.25 mmol/l after the initial increase at 30 minutes and then increased again from the 90 to 120 minute time points by ≥0.25 mmol/l.
The monophasic curves were also further classified according to the timing of the peak glucose response between 30 and 90 minutes. The three monophasic subgroups were designated as: Monophasic30 (peak glucose at 30 minutes), Monophasic60 (peak glucose at 60 minutes) and Monophasic90 (peak glucose at 90 minutes). These definitions are consistent with those that have been used by Kanauchi et al. [6] for monophasic subgroups.
Measures of beta-cell function
C-peptide levels at all OGTT time points (0, 30, 60, 90 and 120 minutes) and area under the curve (AUC) C-peptide levels were studied in order to examine the possible contribution of insulin secretion to the glucose curve shape. In addition, we assessed the early (30-0 minute) C-peptide response which has been shown to correlate well with the first-phase insulin response derived from intravenous glucose tolerance tests (r= 0.50, p<0.001), [15, 16].
To further analyze the effect of C-peptide changes on glucose curve shape, the C-peptide index (30-0 minute C-peptide/30-0 minute glucose) and the AUC C-peptide/AUC glucose were evaluated. The C-peptide index and the AUC C-peptide/AUC glucose are OGTT derived indices of beta-cell function that have been previously described and validated [17].
Statistical analyses
Unpaired t-tests, the Pearson Chi-square test, and the Kruskal-Wallis test were used for comparisons. Log rank tests were used to compare cumulative incidence curves for progression to type 1 diabetes. Univariate and multivariate proportional hazards regression models were used to examine type 1 diabetes associations. Adjustments were made for age, sex, BMI z-score and autoantibodies status (single vs. multiple autoantibodies) at baseline as necessary. Non-normally distributed values were log transformed. A two-sided p-value of <0.05 was used for statistical significance. Statistical analyses were performed with SAS (version 9.4; SAS Institute, Cary, NC).
RESULTS
Baseline characteristics
More PTP participants had a monophasic than a biphasic glucose response curve at baseline [n=1732 (66%) vs. n=895 (34%), respectively]. Table 1 summarizes the baseline characteristics of participants grouped according to the overall shape of the glucose response curve. Participants with a biphasic response were younger (p<0.001) and had a lower proportion of two or more autoantibodies (p<0.001) than those with a monophasic response.
Table 1.
Demographic characteristics of participants classified according to the shape of the baseline OGTT glucose curve
| Monophasics (n=1732) |
Biphasics (n=895) |
P-valuea | |
|---|---|---|---|
| Median Age in years (IQR) | 13.00 (7.78–28.87) | 11.54 (7.80–17.78) | <0.001 |
| ≥18 yrs | 34% | 24% | <0.001 |
| Male | 48% | 50% | 0.444 |
| Median BMI z-score (IQR) | 0.50 (−0.26–1.27) | 0.50 (−0.22–1.22) | 0.495 |
| Median time to type 1 diabetes or last follow-up in years (IQR) | 1.70 (0.60–3.70) | 2.03 (0.60–3.83) | 0.193 |
| Two or more antibodies | 68% | 55% | <0.001 |
P-value: Kruskal-Wallis Test was used to compare the medians between groups; Pearson Chi-square Test was used to compare the proportions between groups.
Among the biphasic subgroups, the Biphasic90 group was significantly younger (p=0.026) with a higher median BMI z-score (p=0.007) than the Biphasic120 group [Electronic Supplementary Material (ESM) Table 1]. Among the monophasic subgroups, those with a peak glucose at 90 minutes had a higher BMI-z score (p=0.003) and a greater proportion with 2 or more autoantibodies (p=0.002) than those with a peak at 30 minutes (ESM Table 2).
Table 2.
Metabolic characteristics of participants classified according to the shape of the baseline OGTT glucose curve.
| Monophasics (n=1732) |
Biphasics (n=895) |
P-valuea | Adjusted P-valueb |
|
|---|---|---|---|---|
| Mean AUC glucose±SD (mmol/l)c | 7.84±1.36 | 6.56±1.06 | <0.001 | <0.001 |
| Mean AUC C-peptide±SD (nmol/l)c | 2.03±0.99 | 1.93±0.81 | 0.002 | 0.072 |
| Mean 2-hr C-peptide±SD (nmol/l) | 2.21±1.18 | 1.97±0.96 | <0.001 | <0.001 |
| Mean Early C-peptide response±SD (nmol/l) | 1.19±0.76 | 1.63±0.82 | <0.001 | <0.001 |
| Median C-peptide Index (Q1–Q3)d (nmol/ mmol) | 0.34 (0.21–0.53) | 0.53 (0.35–0.78) | <0.001 | <0.001 |
| Median AUC C-peptide/AUC glucose (Q1–Q3)d (nmol/ mmol) | 0.25 (0.17–0.34) | 0.28 (0.21–0.36) | <0.001 | <0.001 |
P-value: Student T Test was used to compare the means between groups.
Multiple linear regression: adjusted for age, sex, BMI z-score and number of autoantibodies (if multiple abs).
Areas under the curve (AUCs) were calculated with the trapezoidal rule. The average value of AUCs (divided by 120) was presented for AUC glucose and AUC C-peptide.
For ratios (C-peptide Index, AUC C-peptide/AUC glucose), the values were log transformed for comparisons.
Type 1 diabetes risk according to the baseline glucose response curve
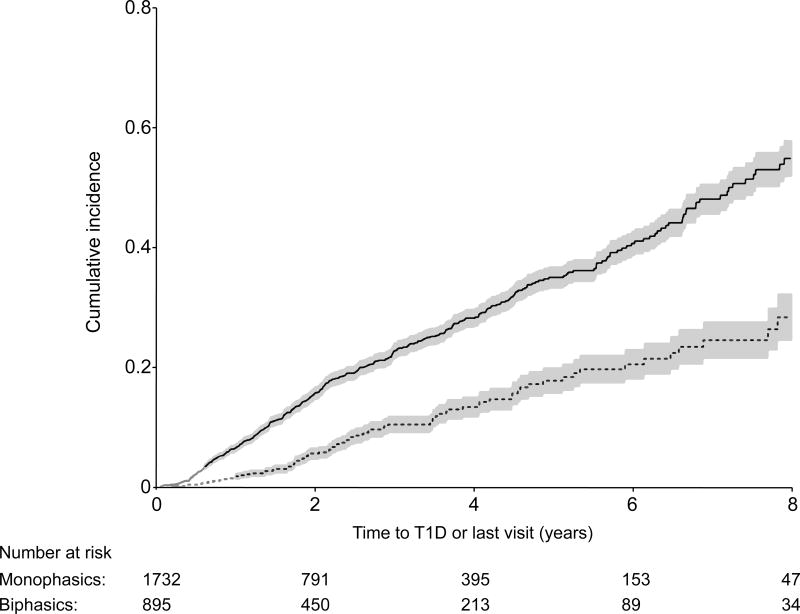
Twenty-one percent in the monophasic group developed type 1 diabetes (n=365/1732) vs. 9% in the biphasic group (n=81/895) during follow-up. Figure 1 shows the cumulative incidence curves for type 1 diabetes for the monophasic and biphasic groups. The cumulative incidence for type 1 diabetes was significantly lower for the biphasic group (p<0.001) than for the monophasic group (5-year estimates: 0.18 for biphasics vs. 0.35 for monophasics). The hazard ratio (HR) from a proportional hazards regression model (with 95% confidence intervals) reflects the lower risk of the biphasic group [HR=0.40 (0.31, 0.51); p<0.001]. After adjusting for age, sex, BMI z-score and number of autoantibodies, the association persisted [HR=0.43 (0.34, 0.55); p<0.001].
Figure 1.
Shown is a comparison of cumulative incidence curves (with 95% confidence intervals) for type 1 diabetes (T1D) between the Monophasic and Biphasic groups, p<0.001.
Twelve percent developed type 1 diabetes in the Biphasic90 group (n=43/346) vs. 7% in the Biphasic120 group (n=38/549) during follow-up. The cumulative incidence was significantly higher (p=0.014) in the Biphasic90 group than in the Biphasic120 group (5-year risk estimates: 0.24 vs. 0.13) (ESM Figure 1). However, in a proportional hazards regression model, there was no longer significance after adjusting for age, sex, BMI z-score and number of autoantibodies [HR=0.84 (0.54, 1.30); p=0.422].
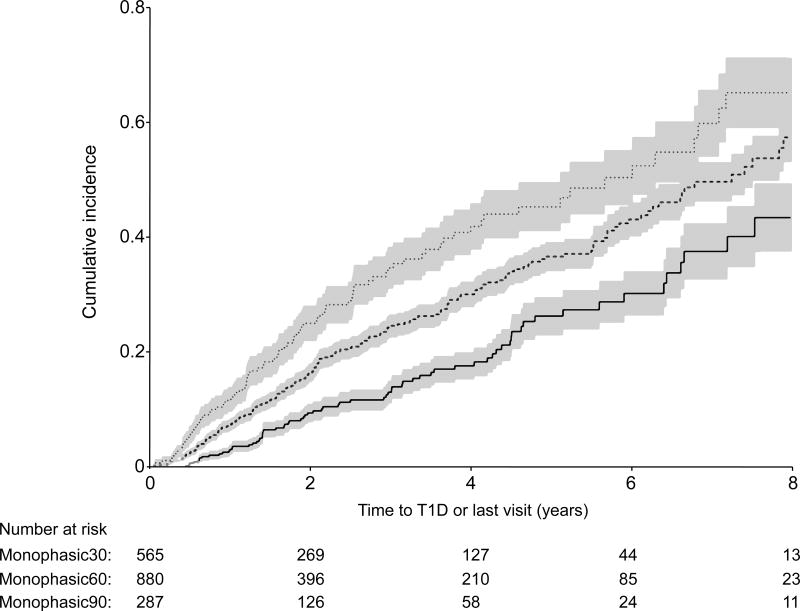
Among the monophasic subgroups, 13% developed type 1 diabetes in the Monophasic30 group (n=76/565) vs. 22% in the Monophasic60 group (n=199/880) and 31% in the Monophasic90 group (90/287) during follow-up. The cumulative incidence for type 1 diabetes development (Figure 2) was lower (p<0.001) for those in the Monophasic30 group compared to those in either the Monophasic60 or the Monophasic90 groups (5-year estimates: 0.26 vs. 0.37 vs. 0.46, respectively). Compared to the Monophasic30 group, the Monophasic90 group had a higher risk of developing type 1 diabetes [HR= 1.87 (1.37, 2.57); p<0.001] after adjusting for age, sex, BMI z-score and number of autoantibodies at baseline. Similarly, after adjustments were made, the Monophasic60 group had a higher risk of developing type 1 diabetes than the Monophasic30 group [HR= 1.57 (1.20, 2.06); p=0.001].
Figure 2.
Shown are comparisons of the Monophasic30 cumulative incidence curve (with 95% confidence intervals) for T1D with the Monophasic60 and Monophasic90 cumulative incidence curves, p<0.001 for Monophasic30 vs. Monophasic60 and p <0.001 for Monophasic 30 vs. Monophasic90.
Comparisons of glucose and C-peptide responses between the monophasic and biphasic groups
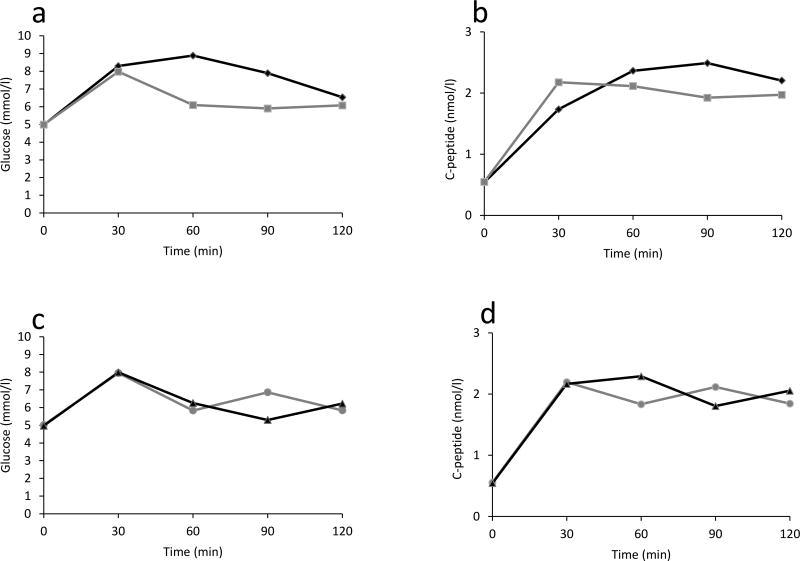
Glucose levels for the monophasic and biphasic groups according to OGTT time points are presented in Figure 3(a). The monophasic group had significantly higher glucose levels at 30, 60, 90 and 120 minutes compared to the biphasic group (p<0.001 for all). After adjusting for differences in age, sex, BMI z-score and number of autoantibodies, these differences remained significant (p<0.001 for all).
Figure 3.
Shown are mean glucose and C-peptide response curves during 2-hr OGTTs. The monophasic group is compared with the biphasic group (a and b; p<0.001 for differences in both glucose and C-peptide levels at 30, 60, 90 and 120 minutes), and the Biphasic90 is compared with Biphasic120 (c and d; p<0.001 for differences in both glucose and C-peptide levels at 60, 90 and 120 minutes).
C-peptide levels (Figure 3b) were higher in the biphasic group at 30 minutes (p<0.001), and lower at 60, 90, and 120 minutes (p<0.001 for all). The early C-peptide response, the C-peptide index, and the AUC C-peptide/AUC-glucose were all higher in the biphasic group (p<0.001 for all) (Table 2). After adjusting for age, sex, BMI z-score and number of autoantibodies, the differences persisted (p<0.001).
Comparisons of glucose and C-peptide responses between biphasic subgroups
We compared C-peptide and glucose changes during the OGTTs between the two biphasic subgroups in Figure 3c and 3d. There were substantial differences between the groups. C-peptide levels continued to decline between 30 and 60 minutes in the Biphasic90 group, whereas C-peptide levels increased in the Biphasic120 group. Moreover, changes were in opposite directions between the groups for both glucose and C-peptide after 60 minutes. As is evident in Figure 3d, the C-peptide response also manifested a biphasic pattern in both groups.
The early C-peptide response, the C-peptide index and the AUC C-peptide/AUC-glucose were similar between the groups. Whereas the mean AUC glucose was higher in the Biphasic90 group, the mean 2-hr C-peptide was higher in the Biphasic120 group (p<0.001 and p=0.001, respectively) (Table 3).
Table 3.
Metabolic characteristics of participants with a biphasic glucose pattern classified according to the timing of their second glucose peak during the baseline OGTT
| Biphasic90 (n=346) |
Biphasic120 (n=549) |
P-valuea | Adjusted P-valueb |
|
|---|---|---|---|---|
| Mean AUC glucose±SD (mmol/l)c | 6.81±1.15 | 6.40±0.96 | <0.001 | <0.001 |
| Mean AUC C-peptide±SD (nmol/l)c | 1.94±0.81 | 1.92±0.81 | 0.783 | 0.558 |
| Mean 2-hr C-peptide±SD (nmol/l) | 1.84±0.90 | 2.05±0.99 | <0.001 | 0.001 |
| Mean Early C-peptide response±SD (nmol/l) | 1.64±0.85 | 1.62±0.79 | 0.810 | 0.828 |
| Median C-peptide Index (Q1–Q3)d (nmol/mmol) | 0.53 (0.35–0.81) | 0.53 (0.35–0.76) | 0.728 | 0.780 |
| Median AUC C-peptide/AUC glucose (Q1–Q3)d (nmol/ mmol) | 0.28 (0.20–0.36) | 0.29 (0.22–0.37) | 0.076 | 0.145 |
P-value: Student T Test was used to compare the means between groups.
Multiple linear regression: adjusted for age, sex, BMI z-score and number of autoantibodies (if multiple abs).
Areas under the curve (AUCs) were calculated with the trapezoidal rule. The average value of AUCs (divided by 120) was presented for AUC glucose and AUC C-peptide.
For ratios (C-peptide Index, AUC C-peptide/AUC glucose), the values were log transformed for comparisons.
Comparisons of C-peptide responses among monophasic subgroups
The AUC C-peptide, early C-peptide response, C-peptide index and AUC C-peptide/AUC glucose ratios were all higher in the Monophasic30 subgroup compared to the Monophasic90 subgroup (p<0.001 for all). In contrast, the mean 2-hr C-peptide response was higher in the Monophasic90 subgroup than in the Monophasic30 subgroup (p<0.001). All of the comparisons remained significant after adjustments were made for age, sex, BMI z-score and number of autoantibodies (p<0.001 for all) (Table 4). However, it is to be noted that none of the C-peptide measures showed a consistent trend across the monophasic subgroups except for the decline in the AUC C-peptide/AUC glucose ratio.
Table 4.
Metabolic characteristics of participants with a monophasic glucose pattern classified according to the timing of their glucose peak during the baseline OGTT with comparisons performed between the Monophasic30 and the Monophasic90 subgroups.
| Monophasic30 (n=565) |
Monophasic60 (n=880) |
Monophasic90 (n=287) |
P-valuea | Adjusted P-valueb |
|
|---|---|---|---|---|---|
| Mean AUC glucose±SDc (mmol/l) | 7.19±1.12 | 8.08±1.34 | 8.36±1.41 | <0.001 | <0.001 |
| Mean AUC C-peptide±SDc (nmol/l) | 2.09 ±0.87 | 2.33±1.21 | 1.89 ±1.13 | 0.004 | <0.001 |
| Mean 2-hr C-peptide±SD (nmol/l) | 1.89±0.90 | 1.06 ±0.69 | 2.44±1.45 | <0.001 | <0.001 |
| Mean Early C-peptide response±SD (nmol/l) | 1.54±0.77 | 2.04 ±1.02 | 0.91±0.68 | <0.001 | <0.001 |
| Median C-peptide Index (Q1–Q3)d (nmol/mmol) | 0.41 (0.27–0.59) | 0.29 (0.19–0.48) | 0.30 (0.16–0.52) | <0.001 | <0.001 |
| Median AUC C-peptide/AUC glucose (Q1–Q3)d (nmol/mmol) | 0.28 (0.21–0.36) | 0.23 (0.16–0.32) | 0.20 (0.13–0.31) | <0.001 | <0.001 |
P-value: Student T test was used to compare the means between Monophasic 30 and Monophasic90.
Multiple linear regression to compare between Monophasic30 and Monophasic90: adjusted for age, sex, BMI z-score and number of autoantibodies (if multiple abs).
Areas under the curve (AUCs) were calculated with the trapezoidal rule. The average value of AUCs (divided by 120) was presented for AUC glucose and AUC C-peptide.
For ratios (C-peptide Index, AUC C-peptide/AUC glucose), the values were log transformed for comparisons.
DISCUSSION
Our findings showed that among Ab+ positive relatives of patients with type 1 diabetes, the majority had a monophasic glucose response pattern during a 2-hr OGTT, and that pattern was associated with greater risk for developing type 1 diabetes. Moreover, among the monophasic group, those with a peak glucose at 30 minutes were at lower risk than those with peaks at either 60 or 90 minutes. The differences in risk between the monophasic and biphasic groups and among the monophasic subgroups persisted after adjusting for baseline variables. The findings also showed that in comparison to the monophasic group, the biphasic group had higher early C-peptide responses.
A strength of the study was the large number of participants which allowed for sub-classifications of the biphasic and monophasic groups. The analyses of these subgroups showed heterogeneity among the monophasic group with regard to risk and C-peptide response patterns, and among the biphasic group with regard to the timing of the C-peptide response. In addition, we were able to show that the early C-peptide response contributes significantly to the shape of the glucose response curve.
There were some limitations. A longer OGTT with more frequent sampling than every 30 minutes could have resulted in a better understanding of the glucose curve shapes. Also, other factors, such as the effect of incretin hormones [18] or delayed gastric emptying on the shape of the glucose curve, were not assessed. In addition, approximately 26% of those without a diabetic OGTT at baseline did not meet the definition of a monophasic or a biphasic OGTT curve and, therefore, this limits the generalizability of our results.
Findings from several studies suggest that the shape of the glucose response curve is also an indicator of type 2 diabetes risk. Cross-sectional and longitudinal studies in adolescents and adults have shown that the form of the glucose response curve is related to risk factors for type 2 diabetes and its development [5–12]. In one study [5] of non-diabetic white adults, a biphasic response during a 2-hr OGTT was associated with lower BMI, better glucose tolerance, greater insulin sensitivity, and greater beta-cell function. Other studies in children and adolescents [10–12], and in adults [6–9], have confirmed this. No prior studies have examined the risk of type 1 diabetes in relation to shapes of glucose response curves, nor the contribution of C-peptide responses to shapes of glucose response curves in autoantibody positive individuals.
To our knowledge, the risk of type 1 diabetes has not been compared among monophasic subgroups. Our finding of an association between the risk of type 1 diabetes and the timing of the peak glucose among the monophasic group is consistent with prior findings of an association of the risk of type 2 diabetes with the timing of the peak glucose [19, 20]. Those prior studies were not limited to the monophasic glucose pattern, however. The type 1 diabetes risk differences that we observed among the monophasic subgroups indicated that the monophasic glucose patterns should not be considered a uniform entity.
The greater risk of type 1 diabetes in the monophasic group could be explained by a lower early C-peptide response, which has been shown to decline with progression to type 1 diabetes [15, 16]. Among those analyzed, there was a lower early C-peptide response at baseline in those who developed type 1 diabetes later (ESM Table 3). A lower early C-peptide response could also possibly explain the greater risk in the Monophasic90 subgroup than in the Monophasic30 subgroup. However, trends in C-peptide measures were generally inconsistent across the monophasic subgroups.
The differences in the shapes of the glucose curves appear to be influenced by C-peptide responses. Approximately one third of the cohort had a biphasic and overall lower glucose response during the baseline OGTT and which was associated with a higher early C-peptide response. In addition, among the biphasic subgroups, whereas the Biphasic90 subgroup had a decline in C-peptide between 30 and 60 minutes, the Biphasic120 subgroup had an increase in the C-peptide response during the same interval (Figure 3d). This could explain the earlier second rise in glucose in the Biphasic90 group than in the Biphasic120 group. It remains unclear why there is a second increase in the glucose concentrations seen only in the biphasic group and subgroups. Further studies that could assess the effect of incretin hormones or gastric emptying and absorption on the shape of the curve should be considered. The findings have implications with regard to our understanding of the natural history of type 1 diabetes. The associations of glucose curve shapes with C-peptide measures suggests that changes in curve shape could be indicative of progression to type 1 diabetes. A better understanding of changes in shapes of OGTT curves could potentially improve the prediction of type 1 diabetes.
There are several additional unanswered questions that suggest future research. Among individuals with normal glucose tolerance, a monophasic pattern predominates [2–4], yet paradoxically, among autoantibody positive individuals, the monophasic group had the highest risk for developing type 1 diabetes. We could not explain the inconsistency between the trend of increasing risk for the monophasic glucose peaks from 30 to 90 minutes and the lack of a clear trend for C-peptide responses. Finally, it would be of interest to determine whether the shape of the glucose curve changes over time during the course of progression to type 1 diabetes development.
Conclusions
In conclusion, the present study is the first to show that shapes of plasma glucose curves during a 2-hr OGTT are predictive of the risk of progression to type 1 diabetes among Ab+ individuals. Furthermore, it shows that differences in glucose patterns appear to be explained, at least in part, by differences in C-peptide secretion patterns, particularly the magnitude and duration of the early C-peptide response to the oral glucose load.
Supplementary Material
Acknowledgments
Members of the Type 1 Diabetes TrialNet Study Group and TrialNet Affiliate Centers are listed in the online only electronic supplementary material.
Funding:
The sponsor of the trial was the Type 1 Diabetes TrialNet Pathway to Prevention Study Group. Type 1 Diabetes TrialNet Pathway to Prevention Study Group is a clinical trials network funded by the National Institutes of Health (NIH) through the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, and The Eunice Kennedy Shriver National Institute of Child Health and Human Development, through the cooperative agreements U01 DK061010, U01 DK061034, U01 DK061042, U01 DK061058, U01 DK085465, U01 DK085453, U01 DK085461, U01 DK085463, U01 DK085466, U01 DK085499, U01 DK085504, U01 DK085505, U01 DK085509, U01 DK103180, U01-DK103153, U01-DK085476, U01-DK103266 and the Juvenile Diabetes Research Foundation International (JDRF). The contents of this Article are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or the JDRF.
Abbreviations
- Ab+
autoantibody positive
- CI
Confidence interval
- DPT-1
Diabetes Prevention Trial Type 1
- ESM
- GADA
glutamic acid decarboxylase antibody
- HR
Hazard ratio
- IA-2A
insulinoma-associated-2 antibody
- ICA
islet cell cytoplasmic autoantibody
- IQR
interquartile range
- mIAA
micro insulin autoantibodies
- OGTT
oral glucose tolerance test
- PTP
TrialNet Pathway to Prevention
- T1D
type 1 diabetes
Footnotes
Contribution Statement:
HMI, PX and JMS conceptualized the analysis, analyzed and interpreted the data, and wrote the manuscript. IML, DJB, JBM, JSS and JPP contributed to the design, interpreted the data and reviewed/edited the manuscript.
HMI is the guarantor of this work, and all authors provided final approval of the manuscript prior to publishing.
Data availability:
The data was analyzed or generated during the study and is available on request from the authors.
Duality of Interest: The authors declare that there is no duality of interest associated with this manuscript.
References
- 1.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26:S5–S20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 2.Zhao X, Peter A, Fritsche J, et al. Changes of the plasma metabolome during an oral glucose tolerance test: is there more than glucose to look at? Am J Physiol Endocrinol Metab. 2009;296:E384–93. doi: 10.1152/ajpendo.90748.2008. [DOI] [PubMed] [Google Scholar]
- 3.Knopf CF, Cresto JC, Dujovne IL, Ramos O, de Majo SF. Oral glucose tolerance test in 100 normal children. Acta Diabetol Lat. 1977;14:95–103. doi: 10.1007/BF02581396. [DOI] [PubMed] [Google Scholar]
- 4.Chandalia HB, Boshell BR. Diagnosis of diabetes. The size and nature of carbohydrate load. Diabetes. 1970;19:863–9. doi: 10.2337/diab.19.11.863. [DOI] [PubMed] [Google Scholar]
- 5.Tschritter O, Fritsche A, Shirkavand F, Machicao F, Häring H, Stumvoll M. Assessing the Shape of the Glucose Curve During an Oral Glucose Tolerance Test. Diabetes Care. 2003;26:1026–1033. doi: 10.2337/diacare.26.4.1026. [DOI] [PubMed] [Google Scholar]
- 6.Kanauchi M, Kimura K, Kanauchi K, Saito Y. Beta-cell function and insulin sensitivity contribute to the shape of plasma glucose curve during an oral glucose tolerance test in non-diabetic individuals. Int J Clin Pract. 2005;59:427–32. doi: 10.1111/j.1368-5031.2005.00422.x. [DOI] [PubMed] [Google Scholar]
- 7.Trujillo-Arriaga HM, Román-Ramos R. Fitting and evaluating the glucose curve during a quasi continuous sampled oral glucose tolerance test. Comput Biol Med. 2008;38:185–95. doi: 10.1016/j.compbiomed.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Abdul-Ghani MA, Lyssenko V, Tuomi T, DeFronzo RA, Groop L. The shape of plasma glucose concentration curve during OGTT predicts future risk of type 2 diabetes. Diabetes Metab Res Rev. 2010;26:280–6. doi: 10.1002/dmrr.1084. [DOI] [PubMed] [Google Scholar]
- 9.Tura A, Morbiducci U, Sbrignadello S, Winhofer Y, Pacini G, Kautzky-Willer A. Shape of glucose, insulin, C-peptide curves during a 3-h oral glucose tolerance test: any relationship with the degree of glucose tolerance? Am J Physiol Reg Integr Comp Physiol. 2011;300:R941–948. doi: 10.1152/ajpregu.00650.2010. [DOI] [PubMed] [Google Scholar]
- 10.Kim JY, Coletta DK, Mandarino LJ, Shaibi GQ. Glucose Response Curve and Type 2 Diabetes Risk in Latino Adolescents. Diabetes Care. 2012;35:1925–1930. doi: 10.2337/dc11-2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bervoets L, Mewis A, Massa G. The Shape of the Plasma Glucose Curve During an Oral Glucose Tolerance Test as an Indicator of Beta Cell Function and Insulin Sensitivity in End-Pubertal Obese Girls. Horm Metab Res. 2015;47:445–451. doi: 10.1055/s-0034-1395551. [DOI] [PubMed] [Google Scholar]
- 12.Nolfe G, Spreghini MR, Sforza RW, Morino G, Manco M. Beyond the morphology of the glucose curve following an oral glucose tolerance test in obese youth. Eur J Endocrinol. 2012;166:107–114. doi: 10.1530/EJE-11-0827. [DOI] [PubMed] [Google Scholar]
- 13.Mahon JL, Sosenko JM, Rafkin-Mervis L, et al. The TrialNet Natural History Study of the Development of Type 1 Diabetes: objectives, design, and initial results. Pediatr Diabetes. 2009;10:97–104. doi: 10.1111/j.1399-5448.2008.00464.x. [DOI] [PubMed] [Google Scholar]
- 14. [Last Accessed July 25, 2017]; https://clinicaltrials.gov/archive/NCT00097292/2012_02_10/changes.
- 15.Sosenko JM, Palmer JP, Rafkin LE, et al. Trends of earlier and later responses of C-peptide to oral glucose challenges with progression to type 1 diabetes in diabetes prevention trial-type 1 participants. Diabetes Care. 2010;33:620–5. doi: 10.2337/dc09-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sosenko JM, Skyler JS, Herold KC, Palmer JP, Type 1 Diabetes TrialNet and Diabetes Prevention Trial–Type 1 Study Groups The metabolic progression to type 1 diabetes as indicated by serial oral glucose tolerance testing in the Diabetes Prevention Trial-type 1. Diabetes. 2012;61:1331–7. doi: 10.2337/db11-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tura A, Kautzky-Willer A, Pacini G. Insulinogenic indices from insulin and C-peptide: comparison of beta-cell function from OGTT and IVGTT. Diabetes Res Clin Pract. 2006;72:298–301. doi: 10.1016/j.diabres.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Creutzfeldt W, Nauck M. Gut hormones and diabetes mellitus. Diabetes Metab Rev. 1992;8:149–77. doi: 10.1002/dmr.5610080206. [DOI] [PubMed] [Google Scholar]
- 19.Abdul-Ghani MA, Stern MP, Lyssenko V, Tuomi T, Groop L, Defronzo RA. Minimal contribution of fasting hyperglycemia to the incidence of type 2 diabetes in subjects with normal 2-h plasma glucose. Diabetes Care. 2010;33:557–61. doi: 10.2337/dc09-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou W, Gu Y, Li H, Luo M. Assessing 1-h plasma glucose and shape of the glucose curve during oral glucose tolerance test. Eur J Endocrinol Eur Fed Endocr Soc. 2006;155:191–7. doi: 10.1530/eje.1.02188. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.