Abstract
Aim
Atorvastatin, a HMG-CoA reductase inhibitor, used in the treatment of hypercholesterolemia, has been previously shown to regulate ABCB1 expression in vivo and in vitro. We hypothesized that the statin could regulate gene expression of ABCB1 transporter via microRNAs.
Methods
Expression of microRNAs and ABCB1 mRNA were examined in atorvastatin-treated and control cells using real-time PCR. miR-491-3P mimic and inhibitor were transfected in Caco-2 and ABCB1 expression was monitored by western blot and real-time PCR.
Results
In HepG2 cells, none of the microRNAs predicted to target ABCB1 3′UTR were regulated by atorvastatin treatment. In agreement with this, ABCB1 3′UTR activity was not modulated in HepG-2 cells after 48h-treatment as measured by luciferase assay. In Caco-2 cells, atorvastatin treatment provoked a decrease in luciferase activity and, accordingly, miR-491-3p was up regulated about 2.7 times after 48h-statin treatment. Luciferase analysis of miR-491-3p with a mimetic or inhibitor of miR-491-3p revealed that this microRNA could target ABCB1 3′UTR, as after miR-491-3p inhibition, ABCB1 levels were increased by twofold, and miR-491-3p super expression decreased ABCB1 3′UTR activity. Finally, functional analysis revealed that treatment with miR-491-3p inhibitor could reverses atorvastatin attenuation of ABCB1 (Pg-p) protein levels.
Conclusion
Our results suggest atorvastatin control ABCB1 expression via miR-491-3p in Caco-2 cells. This finding may be an important mechanism of statin drug-drug interaction, since common concomitant drugs used in the prevention of cardiovascular diseases are ABCB1 substrates.
Keywords: HMG-CoA inhibitors, ABCB1, microRNAs, efflux drug transporters
Graphical Abstract
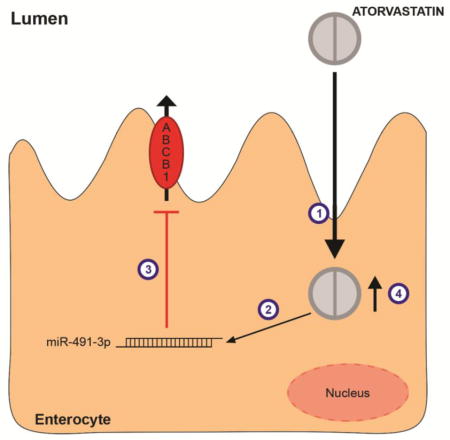
1. INTRODUCTION
Solute transporters are membrane proteins essential for homeostasis of all types of cells. These protein control the influx of essential nutrients and efflux of cellular waste, environmental toxins, drugs, and other xenobiotics (Giacomini and Sugiyama, 2011). In considering drug transport, most of the researchers focus on two major families ABC (ATP-binding cassette) or SLC (solute carriers) transporters. P-glycoprotein (P-gp, encoded by ABCB1 gene) is localized in brush-border membrane of intestinal cells limiting drug absorption and in the canalicular membrane of hepatocytes, where it mediates the efflux (excretion) of drugs and their metabolites (Leslie et al., 2005). This transporter has been characterized to transport statins (for review, see (Rodrigues, 2010)).
Statins are 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors used for treatment of hypercholesterolemia. Intestinal absorption appears to have an important role in disposition of statins, although only 30% of atorvastatin is absorbed after oral ingestion and its oral bioavailability is around 14% (Shitara and Sugiyama, 2006). Atorvastatin pharmacokinetics (PK) and pharmacodynamics (PD) have been related to ABCB1 expression. Plasma total cholesterol reductions after statin therapy was inversely correlated to ABCB1 expression (Rebecchi et al., 2009; Rodrigues et al., 2006), and down-regulation of ABCB1 was observed both in hepatocytes and intestinal cells after statin exposure (Rodrigues et al., 2009a, 2006).
The mechanism responsible for atorvastatin-induced ABCB1 transporter down-regulation is not known. Previously, we found that decreased mRNA stability by atorvastatin treatment might explain the decrease in ABCB1 transcript levels (Rodrigues et al., 2009b). Because atorvastatin reduces ABCB1 mRNA half-life, we hypothesized that statins might be acting through epigenetics mechanisms such as microRNAs.
MicroRNAs are short (~21 nt long), noncoding RNAs that control the post-transcriptional expression of target genes (Bartel, 2004). Specifically in the regulation of ABCB1 drug transporters, microRNAs are newly recognized molecules capable of regulating its mRNA translation (Feng et al., 2011; Kovalchuk et al., 2008; Zhu et al., 2008). In addition, the expression of some microRNAs were shown to be altered by exposure to xenobiotics agents, and could be potential targets for pharmacological therapy, mainly because they revealed to be largely dysregulated in cancer cells (for review, (Yu, 2009)).
In the present study, we have investigated the role of statins on the expression of ABCB1 regulatory microRNAs in HepG2, HuH-7 and Caco-2 cells. This may be a relevant mechanism for variability in statin PK/PD and for the clinically observed drug-drug interactions, since many cardiovascular drugs are substrates for ABCB1.
2. MATERIAL AND METHODS
2.1.Chemicals and Materials
Atorvastatin was kindly provided by Pfizer (Guarulhos, SP). MEM and fetal bovine serum (FBS) were bought from Hyclone (Waltham, MA). Oligonucleotide primers were synthesized by Integrated DNA Technologies (Skokie, IL). SyBR Green was obtained from Invitrogen (Carlsbad, CA), and GoTaq master mix, RNAsin, M-MLV RT enzyme, and dNTPs were purchased from Promega (Madison, WI). miRIDIAN microRNA mimics and hairpin inhibitors for miR-491-3p were purchased from GE Dharmacon (Chicago, IL). Cells lines were bought from ATCC (Manassas, VA).
2.2. Cell Culture
Human colorectal adenocarcinoma (Caco-2), human hepatoma (HepG2) and hepatocellular carcinoma (HuH-7) cells were cultured in MEM medium supplemented with 10% fetal bovine serum (FBS). The cells were grown at 37°C in a humidified atmosphere with 5% CO2/95% air. Cell culture media consisted of 10,000 units/mL penicillin and 10,000 units/mL streptomycin. Culture medium was replaced three times a week and cells were trypsinized and subcultured every 7 days.
2.3.Drug Treatment
One day before the treatment, cells were seeded at a density of 1.0 × 105 (HuH-7 and HepG2) and 2.0 × 105 (Caco-2) cells per well of a 24-well plate, respectively. The final concentration of methanol in the culture medium did not exceed 0.1%. Preliminary experiments with these concentrations of methanol did not show cytotoxicity. Cells were treated with vehicle control (methanol 0.1%) or atorvastatin 1μM for 24–72h.
2.4.In Silico Identification of Putative miRNA Binding Sites
The 3′UTR sequence of human ABCB1 (GenBank sequence NM_000927.3) was searched for antisense matches to individual miRNAs using TargetScan (http://www.targetscan.org/) and PITA (http://genie.weizmann.ac.il/index.html) softwares (Kertesz et al., 2007; Lewis et al., 2005).
2.5.Stem loop Reverse Transcription and Real-time PCR
All the experiments were essentially conducted as described in Rodrigues et al., 2011. Primers sequences for miR-27a and 451 and small nucleolar RNA U74 (used as a reference gene for normalization) were previously described (Rodrigues et al., 2011). All the others primer sequences are described in table 1.
Table 1.
Sequences of primers used in Stem loop RT-qPCR analyses
| microRNA | Sequence (5′→3′) | |
|---|---|---|
| miR-129-5p | RT | GTC GTA TCC AGT GCA GGG TCC GAG GTA TTC |
| GCA CTG GAT ACG ACG CAA GC | ||
| Forward | GCC CCT TTT TGC GGT CTG G | |
| miR-221 | RT | GTC GTA TCC AGT GCA GGG TCC GAG GTA TTC |
| GCA CTG GAT ACG ACG AAA CC | ||
| Forward | GCC CAG CTA CAT TGT CTG CTG | |
| miR-222 | RT | GTC GTA TCC AGT GCA GGG TCC GAG GTA TTC |
| GCA CTG GAT ACG ACA CCC AG | ||
| Forward | GCC CAG CTA CAT CTG GCT A | |
| miR-223 | RT | GTC GTA TCC AGT GCA GGG TCC GAG GTA TTC |
| GCA CTG GAT ACG ACT GGG GT | ||
| Forward | GCC CTG TCA GTT TGT CAA AT | |
| miR-593 | RT | GTC GTA TCC AGT GCA GGG TCC GAG GTA TTC |
| GCA CTG GAT ACG ACA GAA AC | ||
| Forward | GCC CTG TCT CTG CTG GG | |
| miR-491-3p | RT | GTC GTA TCC AGT GCA GGG TCC GAG GTA TTC |
| GCA CTG GAT ACG ACG TAG AA | ||
| Forward | GCC CCT TAT GCA AGA TTC CC | |
| Universal reverse | GTGCAGGGTCCGAGGT |
2.6.Western Blotting
Crude membrane fractions were prepared as previously described (Rodrigues et al., 2009b). Protein concentrations were determined using the BCA Protein Assay Kit (Pierce, Rockford, IL). Crude membrane fractions proteins (20 μg) were separated on 8.0% SDS-polyacrylamide gels (PAGE) and electrophoretically transferred onto nitrocellulose membranes (Invitrogen, Grand Island, NY), which were then incubated overnight with an anti-ABCB1 monoclonal antibody 1:500 diluted (Santa Cruz biotechnology). After further incubation with a horseradish peroxidase rabbit anti-mouse IgG (BD Bioscience, San Jose, CA) the proteins were visualized with Odyssey ClX scanning system (LI-COR). Image acquision and densitometric analyses were conducted using Image Studio software version 4.0 (LI-COR). β-actin (Sigma-Aldrich) was used as a loading control.
2.7.Plasmids
ABCB1 3′UTR segment (0–611 nt, from stop codon), which was amplified from HepG2 cells genomic DNA with primers with XbaI/FseI sites (Fw: 5′-CGT CTA GAA CTC TGA CTG TAT GAG ATG TTA AAT ACT TT-3′; Rv: 5′-ATA GGC CGG CCA TGT GGA AAT GCA AGA ATC AGC-3′), was cloned into XbaI/FseI-digested pGL3 vector (Promega). The 3′UTR segment was inserted downstream of the Firefly luciferase gene (Luc). The insert was confirmed by direct DNA sequencing on the ABI PRISM® 310 Genetic analyzer (Applied Biosystems, EUA).
2.8. Luciferase assay
Lipofectamine 2000 (Thermo-fisher) was used for only plasmid transfection, following the manufacturer’s instructions. In particular, 2 μL of Lipofectamine 2000 diluted in Opti-Mem I reduced serum was mixed with the desired plasmids diluted in Opti-Mem for each well in 24-well plates (final volume of 100 μL). Caco-2 or HepG2 cells were co-transfected with pGL3-ABCB13′UTR plasmid (800 ng) or pGL3 control (800 ng) and pRL-CMV plasmid (20 ng) that expresses Renilla luciferase. For dual transfection protocol in Caco-2 cells, 0.5 μL of dharmafect duo reagent was mixed with pGL3-ABCB13′UTR plasmid (100ng), pRL-CMV plasmid (2.5ng), and miR-491-3p miRIDIAN microRNA mimic (50nM) or miRIDIAN hairpin inhibitor (25nM) and their negative controls (Dharmacon).
For both transfections assays, in the next day, cells were treated with atorvastatin (1μM) or vehicle control, and luciferase activities were assayed 24h after treatment using the Dual-Luciferase Reporter Assay System (Promega). Hexaplicate transfections were tested. Firefly luciferase activity was normalized to Renilla luciferase activity and compared among the different treatments.
2.9. miR-491-3p functional analysis
All transfection experiments were conducted with Dharmafect 1 reagent (GE-Dharmacon), following the manufacturer’s instructions. Caco-2 cells were seeded in 6-well plates at a density of 5 × 105 cells, 24h before the transfection. At the day of the transfection, 0.8 μL of dharmafect was mixed with miRNA-491-3p miRIDIAN hairpin inhibitor for each well in a 6-well plate. Caco-2 cells (50% confluent) were transfected with miRNA-491-3p miRIDIAN hairpin inhibitor (25 nM) or miRIDIAN hairpin inhibitor negative control (25nM) in a total volume of 2.0 mL. After 6h of transfection, the media was changed for fresh growth culture medium and, in the next day, cells were treated for 48h with atorvastatin (1μM) or its vehicle (methanol 0.1%). ABCB1 protein levels were measured by Western Blotting after 72h of the transfection.
2.10.Statistical analysis
Each set of experiments were performed in triplicate and repeated two times in cells pertaining to different passages. All values were expressed as mean ± S.E.M. Different treatments were compared by unpaired Student’s t test or One or Two-Way ANOVA, followed by Bonferroni post-test when more than two groups were analyzed. Statistical analyses were carried out using GraphPad Prism version 5.00 for Windows (GraphPad Software Inc., San Diego, CA, USA). Significance level was set at p < 0.05.
3. RESULTS
3.1. ABCB1 3′UTR activity
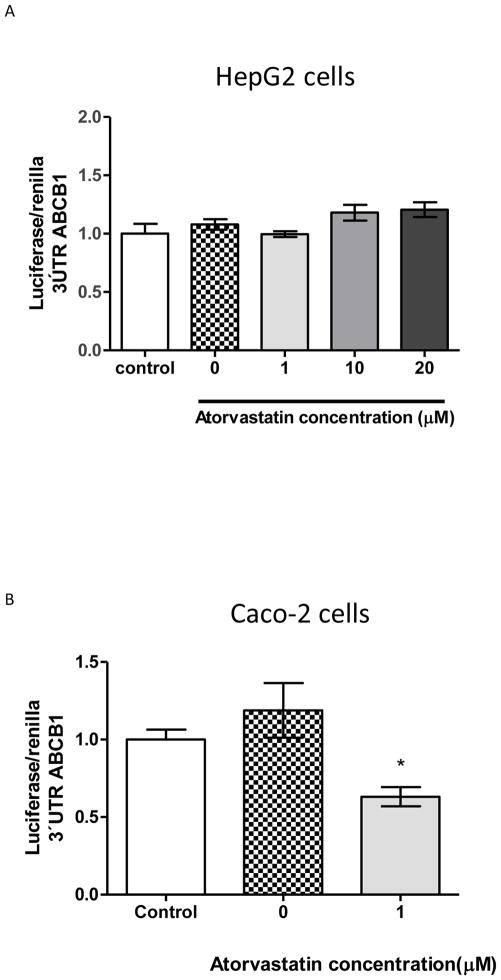
Initially, luciferase reporter assay was used to measure ABCB1 3′UTR activity after atorvastatin treatment in HepG2 and Caco-2 cells (Figure 1). Transient transfection using this pGL3-ABCB1-3′UTR in HepG2 cells showed no significant effect of reporter activity in response to different concentrations of atorvastatin (Figure 1A). Differently, ABCB1 3′UTR-luciferase activity decreased approximately 50% when Caco-2 cells were treated with atorvastatin 1μM for 24h (figure 1B), which is consistent with atorvastatin attenuation of ABCB1 levels reported previously by our group (Rodrigues et al., 2009a, 2006). We did not used higher concentrations in Caco-2 cells because doses higher than 1μM has been previously shown to decrease cell proliferation and viability (Rodrigues et al., 2009a). HepG2 cells were treated with concentrations of atorvastatin found in the blood levels after an oral dose of 10 to 80 mg per day ((Mohammadi et al., 1998).
Figure 1. Luciferase reporter assay suggests that atorvastatin attenuation of 3′UTR ABCB1 mRNA may be regulated by microRNAs in Caco-2 cells, but not on HepG2 cells.
*p< 0.05, when compared to vehicle control (0μM). The dashed line (control) represents pGl3-3′-UTR ABCB1 with no treatments.
3.2.MicroRNA expression in human cell lines
In silico predictions revealed multiple microRNAs could bind ABCB1 3′UTR. We have chosen four microRNAs predicted to target ABCB1 with the best scores, ranked in descending order, by targetScan and PITA softwares (miR-491-3p > miR-593> miR-129-5p>miR-223), and two microRNAs already validated to target ABCB1 transcript (miR-27a and -451). Thus, we measured these miRs expression on Caco-2, HepG2 and HuH7 cells (Table 2). In HepG2 cells the following microRNAs were detected: miR-27a (Ct=26), miR-451 (Ct=30), miR-491-3p (Ct=29). Expression of miR-129-5 and miR-223 were detected at very high Ct values (Ct>35), and were considered negative. Expression of miR-593 was not detected. In HuH7 cells, miR-27a had high expression (Ct =24), miR-451 was moderately expressed (Ct =27), miR-491-3p and miR-593 had low expression (Ct=30). In Caco-2 cells, as previously reported [16], miR-27a was highly expressed (Ct values below 25), miR-491-3p was moderately expressed (Ct=28), and miR-129-5p and 451 levels had low expression (Ct values higher than 30). Expression of miR-223 and miR-593 were not detected in Caco-2 cells.
Table 2.
MicroRNA expression pattern in Caco-2, HepG2 and HuH-7 cells.
| Ct value | |||
|---|---|---|---|
|
| |||
| MicroRNA | Caco-2 cells | HepG2 cells | HuH-7 cells |
| 223 | N/D | >35 | - |
| 129-5p | 30 | >35 | - |
| 491-3p | 28 | 30 | 30 |
| 593 | N/D | ND | 30 |
| 27-a | 24 | 26 | 24 |
| 451 | 30 | 30 | 27 |
N/D: not detected. (−) microRNA expression was not performed.
3.3. Effect of atorvastatin on miRNA expression
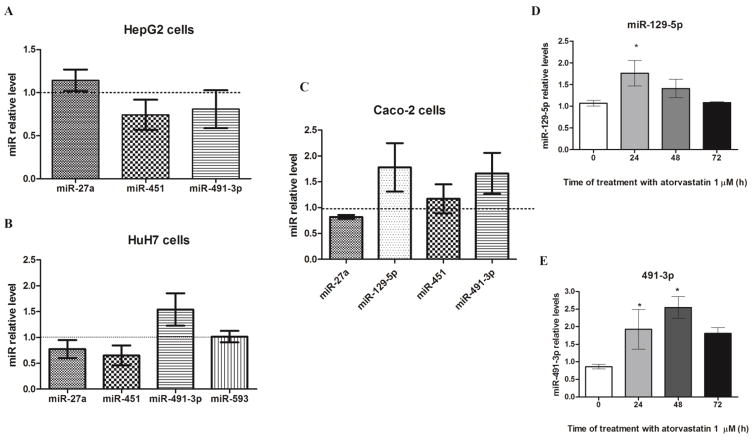
Data from HepG2, HuH-7 and Caco-2 cells treated for 24h with atorvastatin at 1μM are presented on figure 2A, 2B, and 2C, respectively. Atorvastatin treatment induced a decrease in miR-451 expression in HepG2 cells (p>0.05) (Figure 2A), and an increase in miR-491-3p in HuH-7 cells (p>0.05) (Figure 2B). The expression of miR-27a and -593 were not different from the observed in control cells after atorvastatin treatment. On the other hand, in Caco-2 cells, miR-491-3p and miR-129-5p were up regulated after a 24h-treatment (miR-129-5p: 1.02±0.07 vs 1.76±0.29, p<0.01; miR-491-3p: 1.00±0.08 vs 1.66±0.39, p<0.05) (Figure 2C). Thus, we performed a time-course to evaluate the effect of atorvastatin over time (0–72h) in Caco-2 cells. Expression of miR-129-5p returned to control levels after 48h (Figure 2D), whereas miR-491-3p expression was still elevated more than 2-fold at 48h and then start to decrease after 72h (Figure 2E). Thus, we have chosen miR-491-3p to further investigate the effect of microRNAs on ABCB1 3′UTR in Caco-2 cells.
Figure 2. MicroRNA miR-491-3p and miR-129-5p are up regulated in Caco-2 cells after atorvastatin treatment.
MicroRNAs predicted to target 3′UTR ABCB1 had their expression measured in HepG-2 cells (A), HuH7 (B) and Caco-2 cells (C) after 24h-treatment with atorvastatin and were compared to vehicle-treated cells (dashed line). *p< 0.05, when compared with vehicle control (dashed line). Next, microRNAs miR-129-5p (D) e miR-491-3p (E) were chosen for a time-course (0–72h) to evaluate a time-dependent induction. Results are expressed as relative miR levels normalized to the internal control U74 small nucleolar RNA (n=4). *p< 0.05, when compared with vehicle control (0μM).
3.4. miR-491-3p targets ABCB1 mRNA in Caco-2 cells
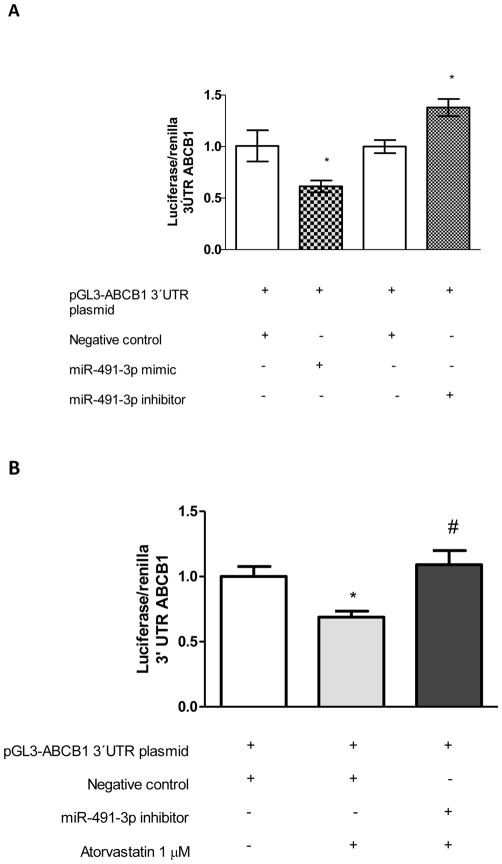
We used pGL3-ABCB1 3′UTR luciferase reporter plasmid to validate microRNA miR-491-3p response element (MRE) site. ABCB1 3′UTR luciferase activity was decreased by 40% when cells were transfected with miR-491-3p mimic, compared with negative control (Figure 3A). Co-transfection of pGL3-ABCB1 3′UTR and miR-491-3p inhibitor restored ABCB1 3′UTR luciferase activity (Figure 3A). This result also showed increasing miR-491-3p on Caco-2 cells replicates the attenuation of ABCB1 mRNA by atorvastatin. We, next, performed a loss-of-function experiment. Caco-2 cells were treated with atorvastatin with/without miR-491-3p inhibitor to evaluate if antagonist reverses attenuation of ABCB1 3′UTR luciferase activity. As predicted, loss of miR-491-3p activity blocked atorvastatin repression of ABCB1 mRNA (Figure 3B).
Figure 3. Luciferase reporter assay suggests miR-491-3p directly targets 3′-UTR of ABCB1.
A) Overexpression with miR-491-3p mimic led to a 40% decrease in ABCB1 3′-UTR-luciferase activity in Caco-2 cells. Inhibition of miR-491-3p by selective inhibitor resulted in approximately 50% increase in luciferase activity in Caco-2 cells. B) Inhibition of miR-491-3p reversed atorvastatin attenuation of ABCB1 3′-UTR – luciferase activity. *p< 0.05, compared with corresponding control, #p<0.05 as compared with atorvastatin without inhibitor (n=6 in each group).
3.5. Atorvastatin controls ABCB1 levels via miR-491-3p in Caco-2 cells
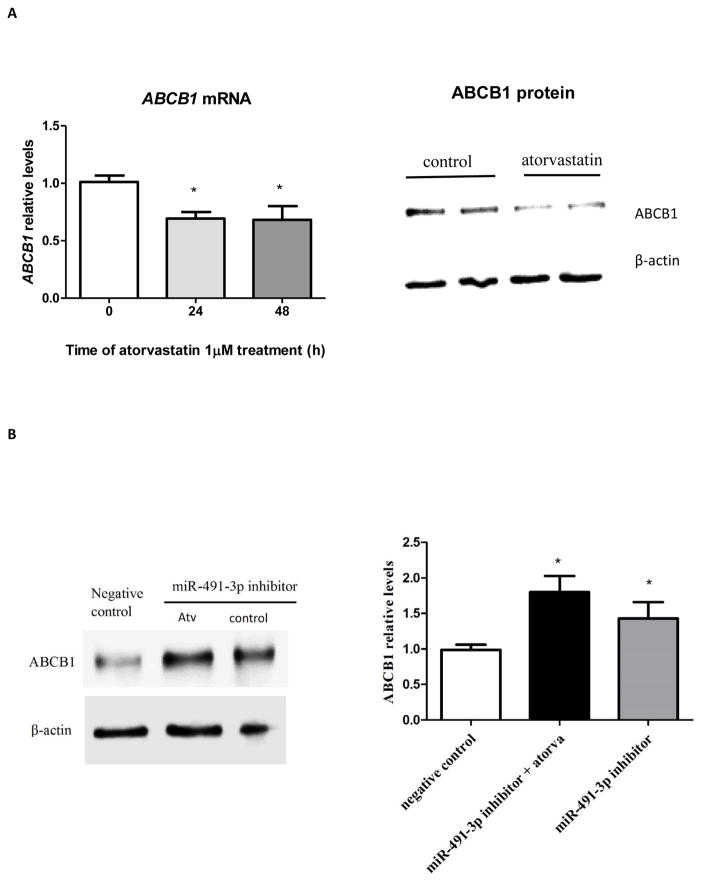
We used a loss-of-function assay to see if decreases in endogenous miR-491-3p function reverses atorvastatin attenuation of ABCB1 levels. Atorvastatin treatment for 48h reduced ABCB1 mRNA and protein by two-fold (p<0.05) in Caco-2 cells (Figure 4A), in accordance with increases of miR-491-3p found after atorvastatin treatment (Figure 2D). As expected, in the presence of miR-491-3p inhibitor, atorvastatin restores ABCB1 expression in Caco-2 cells (Figure 4B).
Figure 4. ABCB1 expression is down regulated after 48h-atorvastatin treated cells and inhibition of miR-491-3p restores atorvastatin – induced decreases in ABCB1 levels in Caco-2 cells.
(A) Caco-2 cells were treated with atorvastatin for 48h and ABCB1 mRNA and protein expression were measured by RT-qPCR and western blot, respectively. (B) Caco-2 cells were transfected with miRNA-491-3p miRIDIAN hairpin inhibitor (25 nM) (miR-491-3p inhibitor) or miRIDIAN hairpin inhibitor negative control (25nM) (negative control) and treated or not with atorvastatin (atorva). ABCB1 expression was measured by western blot. Results are expressed as relative expression to negative control (vehicle-treated cells and transfected with miRIDIAN inhibitor negative control), *p< 0.05, compared with negative control.
4. DISCUSSION
Efflux drug transporters expression on relevant pharmacological barriers can prevent cells from toxicity. These transporters act to limit the access of drugs to tissue compartments, and to eliminate drugs and metabolites via bile (Leslie et al., 2005).
We have previously described that atorvastatin down-regulates ABCB1 through increased degradation of ABCB1 transcript in HepG2 cells (Rodrigues et al., 2009b). In the current study, we have hypothesized that atorvastatin may regulate gene expression of ABCB1 transporter via microRNAs. We have found miR-491-3p targets 3′-UTR of ABCB1 and miR-491-3p is up regulated after atorvastatin exposure in Caco-2 cells. Loss-of-function assay for miR-491-3p increased ABCB1 expression and reversed attenuation of ABCB1 expression induced by atorvastatin, suggesting this miR may be a potential target of atorvastatin to control ABCB1 efflux transporter expression.
Screening of normal human tissues and cell lines for miR-491-3p expression revealed miR-491-3p expression was higher in colon than in liver specimen. Interestingly, when cell lines were compared, Caco-2 cells (a model of colon carcinoma) had low levels whereas HuH-7 and HepG2 cells (models of hepatocellular carcinoma) had high levels of miR-491-3p (Dluzen et al., 2014). These differences observed between normal tissue and cell lines may suggest miR-491-3p contributes to tumor growth. Expression of miR-491-3p was decreased in biopsy samples from recto sigmoid area compared to ascending colon from ulcerative colitis patients. As, recto sigmoid area is the most common site of colorectal cancer development, miR-491-3p may be a tumor suppressive miRNA (Ranjha et al., 2015).
Differences between microRNAs expression between normal and cancer cell lines have also been a focus of studies on drug resistance. Considering miR-491-3p, overexpression of miR-491-3p in HuH-7 was shown to significantly inhibit UDP-glucurosyltransferase (UGT) 1A1 expression and glucuronidation activity, suggesting alterations in miR-491-3p expression may be an important mechanism controlling phase II metabolism, and consequently, modulating drug response in humans (Dluzen et al., 2014). In our study, we observed an up-regulation of miR-491-3p after atorvastatin treatment of HuH-7 cells (p>0.05). Atorvastatin acid is converted to its lactone form spontaneously or via glucuronidation mediated by UGT1A1 (Goosen et al., 2007; Prueksaritanont et al., 2002), therefore statin may induce its own metabolism through miR-491-3p.
ABCB1 has been shown to be post-transcriptionally controlled by microRNAs, such as miR-145 in Caco-2 cells (Ikemura et al., 2013), and miR-27a and -451 in multidrug resistant cancer cells (Feng et al., 2011; Zhu et al., 2008). In our prediction, we did no find miR-145 as a target of ABCB1 mRNA, probably because we have chosen microRNAs predicted by two algorithms, TargetScan and Pita, differently from Ikemura et al (2013) that used miRanda software in their study. In Caco-2 or HuH-7 cells miR-27a and -451 were not regulated by atorvastatin, however, in HepG2 cells, we did observe a down-regulation of miR-451 after treatment with statin (p>0.05). It is possible miR-451 acts indirectly to control ABCB1 in HepG2.
Atorvastin, differently from expected, did not affect the expression of the four top candidate microRNAs predicted to bind ABCB1 3′-UTR. However, we may not exclude the possibility that microRNAs control ABCB1 transcript stability in HepG2 cells, as we focused on miRNAs that target mRNA 3′-UTR, and experiments using artificial sites show that targeting can occur in 5′ UTRs and open reading frames (ORFs) (Bartel, 2009).
Poly(A) tail stabilizes mRNA directly, and changes in poly(A) tail length are used for the purpose of translational regulation (Eckmann et al., 2011). Statins have been shown to posttranscriptionally increase eNOS expression by increasing eNOS 3′polyadenilation (Kosmidou et al., 2007). Therefore, atorvastatin could induce a decrease in ABCB1 mRNA stability by poly (A) tail shortening.
Evidence is presented herein that, in Caco-2 cells, a model of enterocytes, atorvastatin down-regulates ABCB1 transporter, and this effect seems to be mediated by miR-491-3p. Thus, alteration of microRNAs targeting drug transporters in the intestine may contribute to the variability in oral disposition of statins, and may be an important mechanism of statin drug-drug interaction, considering combined therapy with ABCB1 inhibitors or substrates.
Acknowledgments
This work was supported in part by grants from FAPESP (2011/05876-6) and National Institutes of Health (R01DA021172). Rodrigues AC was a recipient of fellowship from FAPESP (2008/01465-9). E.A Neri, N.A. Rebouças, Hirata M.H., and Hirata R.D.C. are recipient of fellowship from CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bartel DP. MicroRNA Target Recognition and Regulatory Functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002.MicroRNA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Chen GQ, Lian WJ, Wang GM, Wang S, Yang YQ, Zhao ZW. Altered microRNA expression in skeletal muscle results from high-fat diet-induced insulin resistance in mice. Mol Med Rep. 2012;5:1362–8. doi: 10.3892/mmr.2012.824. [DOI] [PubMed] [Google Scholar]
- Dluzen DF, Sun D, Salzberg AC, Jones N, Bushey RT, Robertson GP, Lazarus P. Regulation of UDP-Glucuronosyltransferase 1A1 Expression and Activity by MicroRNA 491-3p. J Pharmacol Exp Ther. 2014;348:465–77. doi: 10.1124/jpet.113.210658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckmann CR, Rammelt C, Wahle E. Control of poly(A) tail length. Wiley Interdiscip Rev RNA. 2011 doi: 10.1002/wrna.56. [DOI] [PubMed] [Google Scholar]
- Feng DD, Zhang H, Zhang P, Zheng YS, Zhang XJ, Han BW, Luo XQ, Xu L, Zhou H, Qu LH, Chen YQ. Down-regulated miR-331-5p and miR-27a are associated with chemotherapy resistance and relapse in leukaemia. J Cell Mol Med. 2011;15:2164–2175. doi: 10.1111/j.1582-4934.2010.01213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomini KM, Sugiyama Y. Membrane Transporters and Drug Response. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 2011;Chapter 5:12e. [Google Scholar]
- Goosen TC, Bauman JN, Davis JA, Yu C, Hurst SI, Williams JA, Loi CM. Atorvastatin glucuronidation is minimally and nonselectively inhibited by the fibrates gemfibrozil, fenofibrate, and fenofibric acid. Drug Metab Dispos. 2007;35:1315–1324. doi: 10.1124/dmd.107.015230. [DOI] [PubMed] [Google Scholar]
- Ho PC, Chang KC, Chuang YS, Wei LN. Cholesterol regulation of receptor-interacting protein 140 via microRNA-33 in inflammatory cytokine production. FASEB J. 2011;25:1758–1766. doi: 10.1096/fj.10-179267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann MH, Heinrich J, Radziwill G, Radziwil G, Moelling K. A short hairpin DNA analogous to miR-125b inhibits C-Raf expression, proliferation, and survival of breast cancer cells. Mol Cancer Res. 2009;7:1635–44. doi: 10.1158/1541-7786.MCR-09-0043. [DOI] [PubMed] [Google Scholar]
- Ikemura K, Yamamoto M, Miyazaki S, Mizutani H, Iwamoto T, Okuda M. MicroRNA-145 post-transcriptionally regulates the expression and function of P-glycoprotein in intestinal epithelial cells. Mol Pharmacol. 2013;83:399–405. doi: 10.1124/mol.112.081844. [DOI] [PubMed] [Google Scholar]
- Kertesz M, Iovino N, Unnerstall U, Gaul U, Segal E. The role of site accessibility in microRNA target recognition. TL - 39 Nat. Genet. 39 VN - r. 2007:1278–1284. doi: 10.1038/ng2135. [DOI] [PubMed] [Google Scholar]
- Kosmidou I, Moore JP, Weber M, Searles CD. Statin treatment and 3′ polyadenylation of eNOS mRNA. Arterioscler Thromb Vasc Biol. 2007;27:2642–2649. doi: 10.1161/ATVBAHA.107.154492. [DOI] [PubMed] [Google Scholar]
- Kovalchuk O, Filkowski J, Meservy J, Ilnytskyy Y, Tryndyak VP, Chekhun VF, Pogribny IP. Involvement of microRNA-451 in resistance of the MCF-7 breast cancer cells to chemotherapeutic drug doxorubicin. Mol Cancer Ther. 2008;7:2152–2159. doi: 10.1158/1535-7163.MCT-08-0021. [DOI] [PubMed] [Google Scholar]
- Leslie EM, Deeley RG, Cole SPC. Multidrug resistance proteins: Role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol Appl Pharmacol. 2005 doi: 10.1016/j.taap.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Liang Z, Wu H, Xia J, Li Y, Zhang Y, Huang K, Wagar N, Yoon Y, Cho HT, Scala S, Shim H. Involvement of miR-326 in chemotherapy resistance of breast cancer through modulating expression of multidrug resistance-associated protein 1. Biochem Pharmacol. 2010;79:817–824. doi: 10.1016/j.bcp.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Mohammadi a, Macri J, Newton R, Romain T, Dulay D, Adeli K. Effects of atorvastatin on the intracellular stability and secretion of apolipoprotein B in HepG2 cells. Arterioscler Thromb Vasc Biol. 1998;18:783–793. doi: 10.1161/01.atv.18.5.783. [DOI] [PubMed] [Google Scholar]
- Pek SL, Tavintharan S, Woon K, Lin L, Ong CN, Lim SC, Sum CF. MicroRNAs as biomarkers of hepatotoxicity in a randomized placebo-controlled study of simvastatin and ubiquinol supplementation. Exp Biol Med (Maywood) 2015:317–330. doi: 10.1177/1535370215605588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prueksaritanont T, Subramanian R, Fang X, Ma B, Qiu Y, Lin JH, Pearson PG, Baillie TA. Glucuronidation of statins in animals and humans: A novel mechanism of statin lactonization. Drug Metab Dispos. 2002;30:505–512. doi: 10.1124/dmd.30.5.505. [DOI] [PubMed] [Google Scholar]
- Ranjha R, Aggarwal S, Bopanna S, Ahuja V, Paul J. Site-specific MicroRNA expression may lead to different subtypes in ulcerative colitis. PLoS One. 2015:10. doi: 10.1371/journal.pone.0142869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebecchi IMM, Rodrigues AC, Arazi SS, Genvigir FDV, Willrich MAV, Hirata MH, Soares SA, Bertolami MC, Faludi AA, Bernik MMS, Dorea EL, Dagli MLZ, Avanzo JL, Hirata RDC. ABCB1 and ABCC1 expression in peripheral mononuclear cells is influenced by gene polymorphisms and atorvastatin treatment. Biochem Pharmacol. 2009;77:66–75. doi: 10.1016/j.bcp.2008.09.019. [DOI] [PubMed] [Google Scholar]
- Rodrigues AC. Efflux and uptake transporters as determinants of statin response. Expert Opin Drug Metab Toxicol. 2010;6:621–632. doi: 10.1517/17425251003713519. [DOI] [PubMed] [Google Scholar]
- Rodrigues AC, Curi R, Britto LRG, Rebbechi IMM, Hirata MH, Bertolami MC, Bernik MMS, Dorea EL, Hirata RDC. Down-regulation of ABCB1 transporter by atorvastatin in a human hepatoma cell line and in human peripheral blood mononuclear cells. Biochim Biophys Acta. 2006;1760:1866–1873. doi: 10.1016/j.bbagen.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Rodrigues AC, Curi R, Genvigir FDV, Hirata MH, Hirata RDC. The expression of efflux and uptake transporters are regulated by statins in Caco-2 and HepG2 cells. Acta Pharmacol Sin. 2009a;30:956–964. doi: 10.1038/aps.2009.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues AC, Curi R, Hirata MH, Hirata RDC. Decreased ABCB1 mRNA expression induced by atorvastatin results from enhanced mRNA degradation in HepG2 cells. Eur J Pharm Sci. 2009b;37:486–491. doi: 10.1016/j.ejps.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Rodrigues AC, Li X, Radecki L, Pan YZ, Winter JC, Huang M, Yu AM. MicroRNA expression is differentially altered by xenobiotic drugs in different human cell lines. Biopharm Drug Dispos. 2011;32:355–367. doi: 10.1002/bdd.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh M, Tabuchi T, Minami Y, Takahashi Y, Itoh T, Nakamura M. Expression of let-7i is associated with Toll-like receptor 4 signal in coronary artery disease: Effect of statins on let-7i and Toll-like receptor 4 signal. Immunobiology. 2012;217:533–539. doi: 10.1016/j.imbio.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Shitara Y, Sugiyama Y. Pharmacokinetic and pharmacodynamic alterations of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors: Drug-drug interactions and interindividual differences in transporter and metabolic enzyme functions. Pharmacol Ther. 2006 doi: 10.1016/j.pharmthera.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Toscano-Garibay JD, Aquino-Jarquin G. Regulation Exerted by miRNAs in the Promoter and UTR Sequences: MDR1/P-gp Expression as a Particular Case. DNA Cell Biol. 2012;31:1358–1364. doi: 10.1089/dna.2012.1703. [DOI] [PubMed] [Google Scholar]
- Xu Y, Ohms SJ, Li Z, Wang Q, Gong G, Hu Y, Mao Z, Shannon MF, Fan JY. Changes in the expression of miR-381 and miR-495 are inversely associated with the expression of the MDR1 gene and development of multi-drug resistance. PLoS One. 2013:8. doi: 10.1371/journal.pone.0082062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YT, MS, YM, TT, TI, MN Expression of microRNA-146a/b is associated with Toll-like receptor 4 signal in coronary artery disease: Effect of renin-angiotensin system blockade and statin on microRNA-146a/b and Toll-like receptor. Eur Heart J. 2010;31:460–461. [Google Scholar]
- Yu AM. Role of microRNAs in the regulation of drug metabolism and disposition. Expert Opin Drug Metab Toxicol. 2009;5:1513–1528. doi: 10.1517/17425250903307448. [DOI] [PubMed] [Google Scholar]
- Zhu H, Wu H, Liu X, Evans BR, Medina DJ, Liu CG, Yang JM. Role of MicroRNA miR-27a and miR-451 in the regulation of MDR1/P-glycoprotein expression in human cancer cells. Biochem Pharmacol. 2008;76:582–588. doi: 10.1016/j.bcp.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]