Abstract
Background
Insertion of a central venous catheter (CVC) is common practice in critical care medicine. Complications arising from CVC placement are mostly due to a pneumothorax or malposition. Correct position is currently confirmed by chest x-ray, while ultrasonography might be a more suitable option. We performed a meta-analysis of the available studies with the primary aim of synthesizing information regarding detection of CVC-related complications and misplacement using ultrasound (US).
Methods
This is a systematic review and meta-analysis registered at PROSPERO (CRD42016050698). PubMed, EMBASE, the Cochrane Database of Systematic Reviews, and the Cochrane Central Register of Controlled Trials were searched. Articles which reported the diagnostic accuracy of US in detecting the position of CVCs and the mechanical complications associated with insertion were included. Primary outcomes were specificity and sensitivity of US. Secondary outcomes included prevalence of malposition and pneumothorax, feasibility of US examination, and time to perform and interpret both US and chest x-ray. A qualitative assessment was performed using the QUADAS-2 tool.
Results
We included 25 studies with a total of 2548 patients and 2602 CVC placements. Analysis yielded a pooled specificity of 98.9 (95% confidence interval (CI): 97.8–99.5) and sensitivity of 68.2 (95% CI: 54.4–79.4). US examination was feasible in 96.8% of the cases. The prevalence of CVC malposition and pneumothorax was 6.8% and 1.1%, respectively. The mean time for US performance was 2.83 min (95% CI: 2.77–2.89 min) min, while chest x-ray performance took 34.7 min (95% CI: 32.6–36.7 min). US was feasible in 97%. Further analyses were performed by defining subgroups based on the different utilized US protocols and on intra-atrial and extra-atrial misplacement. Vascular US combined with transthoracic echocardiography was most accurate.
Conclusions
US is an accurate and feasible diagnostic modality to detect CVC malposition and iatrogenic pneumothorax. Advantages of US over chest x-ray are that it can be performed faster and does not subject patients to radiation. Vascular US combined with transthoracic echocardiography is advised. However, the results need to be interpreted with caution since included studies were often underpowered and had methodological limitations. A large multicenter study investigating optimal US protocol, among other things, is needed.
Electronic supplementary material
The online version of this article (10.1186/s13054-018-1989-x) contains supplementary material, which is available to authorized users.
Keywords: Central venous catheter, Ultrasound, CVC malposition, Iatrogenic complications, Chest x-ray, Pneumothorax, Meta-analysis
Background
Most patients admitted to an intensive care unit (ICU) undergo central venous catheterization. In the United States, over 5 million central venous catheter (CVC) placements are performed each year [1]. Although central venous catheterization offers multiple advantages, the procedure is associated with adverse events that could be hazardous for patients. Adverse events can be divided into immediate complications and delayed complications. Immediate complications arise directly after introducing a CVC and consist of mechanical complications and malposition. The most common mechanical complications include arterial puncture, hematoma, and pneumothorax [2, 3]. Delayed complications consist of infectious and thrombotic adverse events and may be provoked by malposition of a CVC [4]. Additionally, malposition of the CVC tip into the right atrium could cause arrhythmias and atrial perforation [5].
To date, the most commonly used reference standard to detect CVC malposition and pneumothorax is post-procedural chest x-ray (CXR). A disadvantage of CXR, however, is that the patient is exposed to radiation. Moreover, performing and interpreting the CXR are often time consuming. Replacing or omitting CXR could reduce healthcare costs and minimize the delay until catheter use [6].
To confirm correct intravascular catheter position and to detect pneumothorax it has been suggested that ultrasound (US) may be a suitable alternative diagnostic modality. Major advantages of US over CXR are that it is often performed faster and does not subject a patient to radiation [7]. Furthermore, using US to guide cannulation is considered as best practice nowadays and, compared with the traditional ‘blind’ landmark method, it reduces failed catheterizations and complications [8, 9]. To verify correct CVC placement, the accuracy of bedside US as an alternative diagnostic modality has been analyzed by a number of small studies. However, these studies used different US protocols and reported a wide range of diagnostic accuracy. To address this problem we performed a systematic review and meta-analysis on these studies.
The primary aim of our study was to investigate whether intravascular CVC misplacement and pneumothorax can be reliably detected by US. A secondary aim was to compare the diagnostic outcomes of the studies to their respective US protocol. Outcomes were compared to a reference standard, e.g., CXR or transesophageal echocardiography (TEE).
Methods
Study design
This is a systematic review and meta-analysis.
To improve the quality of this systematic review we followed the PRISMA guidelines (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) [10] (Additional file 1). The protocol was registered at PROSPERO International prospective register of systematic reviews, registration number CRD42016050698.
Selection of studies
We aimed to include all studies that investigated the accuracy of bedside US in detecting CVC misplacement and other iatrogenic complications, e.g., pneumothorax. In these studies, US is compared to any diagnostic modality that detects CVC malposition. To select eligible studies, a medical librarian who is experienced in organizing systematic reviews was consulted to define and perform a robust search strategy. The search was implemented in MEDLINE via PubMed, EMBASE, the Cochrane Database of Systematic Reviews, and the Cochrane Central Register of Controlled Trials. The initial search was performed on 4 October 2016 and a second search was performed on 9 January 2017 (Additional file 2).
Inclusion of studies
Titles and abstracts were evaluated by one independent reviewer (JMS) while a full-text analysis was performed by two independent reviewers (JMS and RR). References of the selected studies were screened and potentially eligible studies were evaluated and included or excluded afterwards. Inclusion and exclusion criteria are described in Additional file 3. For the removal of duplicates, Covidence® (systematic review software) was used. Disagreement was resolved by consensus meetings with a third reviewer (PRT).
Data extraction
Data were extracted independently by two reviewers (JMS and RR) using Covidence®. The following study characteristics from the included studies were collected: first author and year of publication, study design and period, setting and country, number of patients and CVCs, utilized US protocol, reference standard, primary outcome and secondary outcome of individual studies, number of performing operators, and number of experienced operators. To be classified as experienced, US operators were required to have completed an IC US course and at least 20 practice studies [11]. In addition, characteristics of patients and outcome parameters were collected, including gender, age, weight/body mass index (BMI) and CVC insertion site. These characteristics are described in Additional file 4. To calculate specificity and sensitivity, a 2 × 2 contingency table was constructed based on the raw data from the included literature. Raw data comprise the number of true positives, true negatives, false positives, and false negatives of the included studies concerning US. If additional information was required, attempts were made to contact the authors of the article.
Outcomes
Our primary outcome was to evaluate the accuracy of bedside US in detecting CVC misplacement. If an alternative primary outcome rather than CVC malposition, for example CVC tip visualization or inter-observer agreement, was reported by the included studies, raw data were adjusted or reversed to meet our outcome specifications. The specificity and sensitivity of the included studies were calculated by extracting the raw data and implementing it in a 2 × 2 contingency table. A ‘true positive’ result was defined as a US-suggested aberrant position of the CVC (catheter tip in any other vein than the superior vena cava, outside the venous system, or positioned in the right atrium), confirmed by a reference test. If bedside US correctly ruled out an aberrant position of the catheter tip, as confirmed by a reference standard, it was considered to be a ‘true negative’ result.
The feasibility of US was defined as the percentage of patients in whom cardiac US images could be obtained during transthoracic echocardiography (TTE). Both feasibility and accuracy of lung US to detect pneumothorax were regarded as secondary outcome measures. Since CXR may be an inadequate reference standard, we could not accurately determine accuracy parameters of lung US to identify pneumothorax [12]. Moreover, to detect pneumothorax a recent meta-analysis showed a better sensitivity and a similar specificity for lung US in comparison to CXR [13]. Therefore, the prevalence of pneumothorax was reported instead. Finally, the time to perform the US examination and time to perform or interpret CXR (whichever was reported) were regarded as a secondary outcome measure.
US protocols of included studies could be divided into four separate US protocols, consisting of 1) vascular US and TTE; 2) TTE combined with contrast-enhanced US (CEUS); 3) a combination of 1 and 2; or 4) supraclavicular US (SCU).
CEUS is defined as a flush of the CVC with agitated or non-agitated saline during TTE. A subgroup analysis was conducted on these four protocols. In addition, it was noted whether the US examination was performed during central venous cannulation and therefore the advancement of the guidewire was primarily visualized, or whether the examination was performed post-procedural and CEUS was utilized to identify catheter tip position. Finally, accuracy of US to detect intra-atrial CVC misplacement was investigated. To perform this analysis, a distinction was made between intra- and extra-atrial misplacement [14–16]. Extra-atrial misplacements were considered to be all vascular misplacements other than intra-cardiac position of the CVC tip. An additional subgroup analysis was conducted in these two groups.
Quality assessment
The QUADAS-2 tool (Quality Assessment of Diagnostic Accuracy Studies) was utilized by two independent reviewers (JMS and RR) for quality assessment of the included studies. Disagreement was resolved by consensus meetings with a third reviewer (PRT). Additional file 5 contains a complete overview of the quality assessment.
Statistical analysis and data synthesis
Specificity and sensitivity were first estimated separately for each study, together with their 95% exact confidence interval (CI). In the event that specificity and sensitivity were estimated as 100%, a one-sided 97.5% exact CI was calculated instead. These analyses were performed in Stata 14. A pooled estimate for specificity and a 95% CI was obtained using separate generalized estimating equations (GEEs) on individual patient data assuming an exchangeable correlation structure for outcomes of patients included in the same study. The same procedure was used for sensitivity. Forest plots were made using the calculated 95% CIs. GEE analyses were performed and plots were made in SPSS 22. Publication bias was assessed using Deek’s test. The secondary outcomes of mean time of US performance, mean time to CXR performance, and mean time to CXR interpretation were summarized by weighted means, together with their 95% CIs where weights were set equal to the inverse of the standard error of the mean reported in the study.
Results
Details regarding search and study selection are presented in Fig. 1. Of the initial 4983 articles identified, 25 articles, with a total of 2548 patients and 2602 CVC placements, met the inclusion criteria.
Fig. 1.
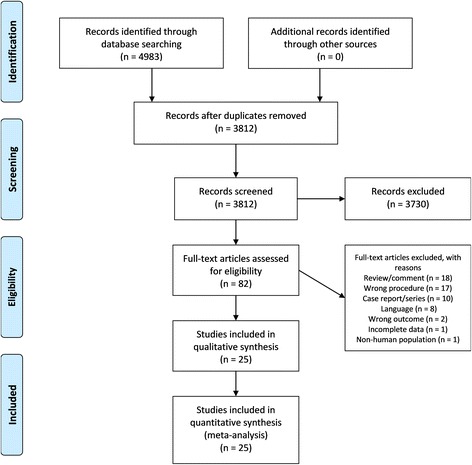
PRISMA flow diagram of search strategy and study selection. Depicted in the flow diagram are the number of identified records, the number of screened records, the number of articles assessed for eligibility with reasons for exclusion, and the number of studies included in the qualitative and quantitative syntheses
Study characteristics
Study characteristics are shown in Table 1. CVC position was evaluated prospectively in 21 studies; furthermore, there were three pilot studies and one retrospective study. The majority of studies used CXR as a reference test to evaluate CVC position; additionally, TEE was used in two studies, additional intra-fluoroscopy in one, and TEE was used exclusively in one study. US protocols used were a combination of vascular US and TTE (n = 6), a combination of TTE and CEUS (n = 11), a combination of vascular US, TTE, and CEUS (n = 5), and a SCU approach in the remaining studies (n = 3). In four studies advancement of the guidewire was assessed by US, whereas in the remaining 21 studies the CVC was visualized by US directly after placement. In one study the accuracy of CXR to detect CVC malposition was investigated and US was used as a reference standard. Here, we reversed the outcome and we used the raw data in our meta-analysis [17]. Most studies had less than three operators. Exact operator experience was stated in 19 studies. Patient characteristics are shown in Additional file 4. Deek’s test did not show evidence of strong publication bias (p = 0.91 for all 25 studies and p = 0.37 for 18 studies in which both specificity and sensitivity could be estimated; Fig. 2 and Fig. 3).
Table 1.
Study characteristics
| Author (year) | Study design (period)1 | Setting (country) | Patients (CVCs), n | Ultrasoundprotocol | Reference standard | Primary outcome (secondary outcome) | Performing operators (experienced operators), n |
|---|---|---|---|---|---|---|---|
| Killu et al. (2010) [47] | Prospective pilot study | Surgical ICU (United States) | 5 (5) | Supraclavicular ultrasounda | CXR | Malposition (time advancement guidewire) | 1 (1) |
| Kim et al. (2015) [26] | Prospective pilot study (Jul 2012–Oct 2012) | Operating theatres (Germany) | 51 (51) | Supraclavicular ultrasoundb | CXR/TEE | Malposition (time until confirmation) | 2 (2) |
| Kim et al. (2016) [25] | Prospective pilot study(Jun 2014–Aug 2014) | Operating theatres (Germany) | 20 (20) | Supraclavicular ultrasoundb | CXR | Malposition (time advancement guidewire) | 1 (1) |
| Baviskar et al. (2015) [48] | Prospective study (Apr 2013–Jan 2014) | Surgical ICU (India) | 25 (25) | TTE and CEUSa | CXR | Time until confirmation (malposition) | 1 + (1+)3 experienced staff |
| Cortellaro et al. (2014) [49] | Prospective study | Emergency department (Italy) | 71 (71) | TTE and CEUSa | CXR | Malposition (time until confirmation) | 3 (2) |
| Duran-Gehring et al. (2015) [50] | Prospective study (Dec 2012–Nov 2013) | Emergency department (United States) | 50 (50) | TTE and CEUSa | CXR | Time until confirmation (malposition, pneumothorax) | 2 (2) |
| Gekle et al. (2015) [51] | Prospective study (Dec 2012–Mar 2014) | Emergency department (United States) | 81 (81) | TTE and CEUSa | CXR | Malposition, pneumothorax (time until confirmation) | Unclear |
| Kamalipour et al. (2016) [52] | Prospective study (Aug 2013–Jan 2014) | Operating theatres (Iran) | 116 (116) | TTE and CEUSa | CXR | Malposition | 1 (1) |
| Lanza et al. (2006) [53] | Prospective study (Nov 2004–Sep 2005) | Pediatric ICU (Italy) | 107 (107) | TTE and CEUSa | CXR | Malposition, pneumothorax | 1 (1) |
| Salimi et al.2 (2015) [17] | Prospective study | Nephrology department (Iran) | 82 (82) | TTE and CEUSa | CXR | Malposition, pneumothorax | 1 (1) |
| Santarsia et al. (2000) [54] | Prospective study | Nephrology department (Italy) | 158 (158) | TTE and CEUSa | CXR | Malposition | Unclear |
| Weekes et al. (2014) [55] | Prospective study (Jan 2013–Apr 2013) | Emergency department and ICU | 147 (152) | TTE and CEUSa | CXR | Malposition | 5 (5) |
| Weekes et al. (2016) [56] | Prospective study (Nov 2013–Mar 2015) | Emergency department and ICU | 156 (156) | TTE and CEUSa | CXR | Malposition, pneumothorax (time until confirmation) | Unclear, by or under supervision of study investigator |
| Wen et al. (2014) [57] | Retrospective study (Jun 2011–Jul 2012) | Nephrology department (Germany) | 202 (219) | TTE and CEUSa | CXR | Malposition (time until confirmation) | 2 + (2+)3 |
| Alonso-Quintela et al. (2015) [58] | Prospective study (Jan 2012–Jan 2014) | Pediatric ICU (Spain) | 40 (51) | Vascular ultrasound and TTEa | CXR | Malposition (time until confirmation) | 1 (1) |
| Maury et al. (2001) [59] | Prospective study (Mar 1999–Sep 1999) | ICU (France) | 81 (85) | Vascular ultrasound and TTEa | CXR | Malposition, pneumothorax (time until confirmation) | 3 (0) |
| Miccini et al. (2016) [46] | Prospective study (Jan 2012–Dec 2014) | Operating theatres (Italy) | 302 (302) | Vascular ultrasound and TTEa | IF/CXR | Malposition, pneumothorax | 2 (2) |
| Park et al. (2014) [60] | Prospective study | Pediatric ICU (United States) | 108 (108) | Vascular ultrasound and TTEa | CXR | Malposition (insertion depth CVC) | 3 (3) |
| Arellano et al. (2014) [27] | Prospective study | Operating theatres (Canada) | 100 (100) | Vascular ultrasound and TTEb | TEE | Malposition | 4 (2) |
| Bedel et al. (2013) [24] | Prospective study (Jan 2010–Nov 2010) | ICU (France) | 98 (101) | Vascular ultrasound and TTEb | CXR | Malposition (pneumothorax, time until confirmation) | 1 (1) |
| Blans et al. (2016) [18] | Prospective study (Jan 2015–Sep 2015) | ICU (The Netherlands) | 53 (53) | Vascular ultrasound, TTE and CEUSa | CXR | Malposition, pneumothorax (time until confirmation) | 2 (2) |
| Matsushima and Frankel (2010) [19] | Prospective study (Nov 2004–Sep 2005) | Surgical ICU (United States) | 69 (83) | Vascular ultrasound, TTE and CEUSa | CXR | Malposition, pneumothorax (time until confirmation) | 1 (0) |
| Meggiolaro et al. (2015) [32] | Prospective study (Jan 2013–Sep 2013) | Operating theatres (Italy) | 105 (105) | Vascular ultrasound, TTE and CEUSa | CXR | Malposition, pneumothorax (timing bubble test, time until confirmation) | 1 (1) |
| Vezzani et al. (2010) [31] | Prospective study (Apr 2008–Aug 2008) | ICU (Italy) | 111 (111) | Vascular ultrasound, TTE and CEUSa | CXR | Malposition, pneumothorax (time until confirmation, cost analysis) | 1 (1) |
| Zanobetti et al. (2013) [61] | Prospective study (Jan 2009–Dec 2011) | Emergency department (Italy) | 210 (210) | Vascular ultrasound, TTE and CEUSa | CXR | Malposition, pneumothorax (time until confirmation) | 4 + (4+)3 |
CEUS contrast enhanced ultrasound, CVC central venous catheter, CXR chest x-ray, ICU intensive care unit, IF intra-fluoroscopy, TEE transesophageal echocardiography, TTE transthoracic echocardiography
aThe CVC is primarily visualized
bThe advancement of the guidewire is primarily visualized
1All studies were observational in design
2Accuracy CXR investigated; TTE used as reference standard
3Possible more than described amount of operators
Fig. 2.
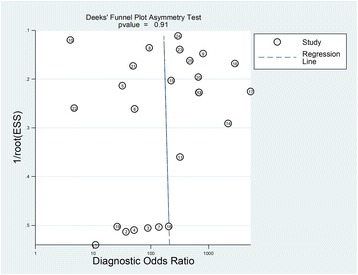
Deek’s funnel plot asymmetry test for all 25 studies. The risk of bias when all 25 studies are included in Deek’s funnel plot asymmetry test (p = 0.91). ESS effective sample size
Fig. 3.
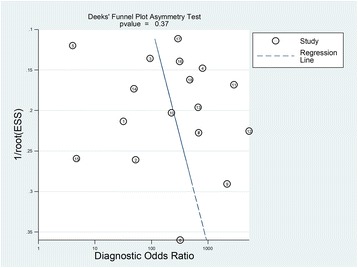
Deek’s funnel plot asymmetry test for 18 studies in which both specificity and sensitivity could be estimated. The risk of bias when only the 18 studies are included in Deek’s funnel plot asymmetry test for which both sensitivity and specificity could be estimated (p = 0.37). ESS effective sample size
Outcomes
The results of primary and secondary outcomes of included studies are presented in Table 2. Pooled specificity was 98.9 (95% CI: 97.8–99.5), and the lowest specificity reported was 91.2 (95% CI: 80.7–97.1) by Salimi et al. [17]. Pooled sensitivity was 68.2 (95% CI: 54.4–79.4), and the lowest sensitivity reported was 0 (95% CI: 0–70.8) by Blans et al. [18]. Pooled specificity and sensitivity of US for detection of CVC misplacement are shown in a Forest plot in Fig. 4. Specificity and sensitivity show considerable statistical heterogeneity: for specificity, I2 = 83.3 (95% CI: 64.6–86.7) and, for sensitivity, I2 = 75.5 (95% CI: 77.1–90.4). On average, US examination was feasible in 96.8% of the cases. The lowest reported feasibility of 71% was reported by Matsushima and Frankel [19]. The prevalence of pneumothorax, investigated by 11 studies, was 1.1% on average. In addition, the prevalence of CVC malposition was 6.8% on average.
Table 2.
Outcomes regarding feasibility, prevalence, accuracy parameters, and time to measurement of included studies
| Study | Feasibility | Prevalence of pneumothorax (%) | Prevalence of malposition (%) | Specificity (95% CI)1 | Sensitivity (95% CI)2 | Mean time for US (min) (±SD)4 [IQR] | Mean time for CXR performance (min) (±SD)4 [IQR] | Mean time for CXR interpretation (min) (±SD)4 [IQR] |
|---|---|---|---|---|---|---|---|---|
| Killu et al. (2010) [47] | 100% | – | 0% | 100.0 (47.8–100) | – | 4.2 | – | – |
| Kim et al. (2015) [26] | 92% | – | 0% | 100 (92.0–100) | – | 11 (0.72) | 111 (31) | – |
| Kim et al. (2016) [25] | 100% | – | 0% | 100 (81.5–100) | – | – | – | – |
| Baviskar et al. (2015) [48] | 100% | – | 0% | 100 (86.3–100) | – | 0.75 (0.25) | – | – |
| Cortellaro et al. (2014) [49] | 100% | – | 8.4% | 98.5 (91.7–100) | 33.3 (4.3–77.7) | 4 (1) | – | 288 (216) |
| Duran-Gehring et al. (2015) [50] | 92% | 4.3% | 6.5% | 100 (91.8–100) | 33.3 (0.8–90.6) | 5 (0.8) | 28.2 (11.3) | 299 (90.5) |
| Gekle et al. (2015) [51] | 100% | 0% | 0% | 100 (94.7–100) | – | 8.80 (1.34) | 45.78 (8.75) | |
| Kamalipour et al. (2016) [52] | 89.7% | – | 15.4% | 97.7 (92.0–99.7) | 68.8 (41.0–89.0) | – | – | – |
| Lanza et al. (2006) [53] | 100% | 0.9% | 11.2% | 100 (96.2–100) | 83.3 (51.0–97.7) | – | – | – |
| Salimi et al.* (2015) [17] | 100% | – | 30.5% | 91.2 (80.7–97.1) | 28.0 (12.1–49.4) | – | – | – |
| Santarsia et al. (2000) [54] | 100% | – | 1.9% | 100 (93.3–100) | 100 (2.5–100) | – | – | – |
| Weekes et al. (2014) [55] | 96.6% | – | 2.7% | 100 (97.5–100) | 75.0 (19.4–99.4) | – | – | – |
| Weekes et al. (2016) [56] | 97.4% | – | 2.6% | 100 (97.5–100) | 75.0 (19.4–99.4) | 1.1 (0.7) | 20 (30) | – |
| Wen et al. (2014) [57] | 100% | – | 0.9% | 100 (98.3–100) | 100 (15.8–100) | 3.2 (1.1) | 28.3 (25.7) | – |
| Alonso-Quintela et al. (2015) [58] | 100% | – | 11.8% | 95.6 (84.9–99.5) | 100 (54.1–100) | 2.23 (1.06) | – | 22.96 (20.43) |
| Maury et al. (2001) [59] | 98.8% | 1.2% | 10.7% | 100 (95.2–100) | 100 (66.4–100) | 6.8 (3.5) | 80.3 (66.7) | – |
| Miccini et al. (2016) [46] | 100% | 1.0% | 1.3% | 100 (98.8–100) | 100 (39.8–100) | – | – | – |
| Park et al. (2014) [60] | 96.2% | – | 0% | 100 (96.4–100) | – | – | – | – |
| Arellano et al. (2014) [27] | 94% | – | 0% | 96.8 (91.0–99.3) | – | – | – | – |
| Bedel et al. (2013) [24] | 97% | 0% | 6.2% | 100 (96.0–100) | 83.3 (35.9–99.6) | 1.76 (1.3) | 49 (31) | 103 (81) |
| Blans et al. (2016) [18] | 98.1% | 0% | 5.8% | 98.0 (89.4–99.9) | 0 (0–70.8) | – | – | 24.5 [18.1–45.3] |
| Matsushima and Frankel (2010) [19] | 71% | 0% | 16.9% | 98.0 (89.4–99.9) | 50.0 (18.7–81.3) | 10.8 | – | 75.3 |
| Meggiolaro et al. (2015) [32] | 100% | 0% | 13.3% | 100 (96.0–100) | 64.3 (35.1–87.2) | 5.0 [5.0-10.0] | – | 67.0 [42.0–84.0] |
| Vezzani et al. (2010) [31] | 89.2% | 1.8% | 28.3% | 95.8 (88.1–99.1) | 92.9 (76.5–99.1) | 10 (5) | 83 (79) | – |
| Zanobetti et al. (2013) [61] | 100% | 2.0% | 4.4% | 100 (98.1–100) | 55.6 (21.2–86.3) | 5 (3) | – | 65 (74) |
| Pooled (patients, n) | (patients, n = 1267) | (patients, n = 2548) | (patients, n = 1362) | (patients, n = 749) | (patients, n = 777) | |||
| All studies (2548) | 96.8% | 1.1% | 6.8% | 98.4 (97.8–99.5) | 68.2 (54.4–79.4) | 2.83 (95% CI: 2.77–2.89) | 34.7 (95% CI: 32.6–36.7) | 46.3 (95% CI: 44.4–48.2) |
| Supraclavicular ultrasound (76) | 94.6% | – | 0% | 100 (94.4–100)3 | – | |||
| TTE and CEUS (1195) | 97.7% | 1.4% | 6.8% | 98.9 (96.1–99.7) | 68.7 (61.7–96.4) | |||
| Vascular ultrasound and TTE (729) | 98.1% | 0.8% | 3.4% | 99.0 (96.5–99.7) | 96.1 (79.7–99.4) | |||
| Vascular ultrasound, TTE and CEUS (548) | 93.3% | 1.4% | 12.3% | 98.6 (96.1–99.5) | 56.2 (32.8–77.1) |
CEUS contrast enhance ultrasound, CI confidence interval, CXR chest x-ray, IQR interquartile range, SD standard deviation, TTE transthoracic echocardiography, US ultrasound
*Accuracy CXR investigated; TTE used as reference standard
1One-sided 97.5% confidence interval in case specificity is estimated to be 100%
2One-sided 97.5% confidence interval in case sensitivity is estimated to be 100%
3Exact confidence intervals (not taking into account between-study differences); GEE model not estimable as all controls were correctly identified
4Values shown as mean (SD) or median [IQR]
Fig. 4.
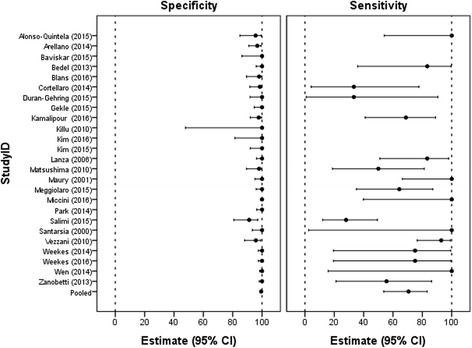
Forest plot of the specificity and sensitivity of ultrasound for detection of CVC-related complications. The pooled specificity and sensitivity as well as the specificity and sensitivity for each study individually with their respective confidence interval (CI). Studies showed significant statistical heterogeneity; for specificity, I2 = 83.3 (95% CI: 64.6–86.7) and, for sensitivity, I2 = 75.5 (95% CI: 77.1–90.4)
Subgroup outcomes
Pooled results from the subgroup analysis are shown in Table 2. The SCU group produced the highest specificity of 100% (95% CI: 94.4–100) but due to absent cases of malposition the sensitivity could not be calculated. The vascular US and TTE group yielded the highest sensitivity of 96.1% (95% CI: 79.7–99.4). The diagnostic accuracy of US to distinguish between intra- and extra-atrial malposition is shown in Table 3. Specificity of US for both intra- and extra-atrial malposition ranges from 95.6% (95% CI: 84.9–99.5) to 100% (95% CI: 98.1–100), whereas the sensitivity shows a distribution ranging from 0% (95% CI: 0–70.8) to 100% (95% CI:66.4–100), as shown in Fig. 5. A detailed description of the different US protocols with their reported respective advantages and disadvantages is given in Additional file 6. In all cases US was performed faster than CXR, with an average time of 2.83 min (95% CI: 2.77–2.89 min) for US compared to 34.7 min (95% CI: 32.6–36.7 min) and 46.3 min (95% CI: 44.4–48.2 min) for CXR performance and interpretation, respectively.
Table 3.
Results of subgroup analysis
| Study | Ultrasound protocol | Specificity1 (95% CI) | Sensitivity2 (95% CI) |
|---|---|---|---|
| All studies (pooled) | |||
| Intra-atrial | 97.4 (94.8–98.7) | 73.5 (57.2–85.3) | |
| Extra-atrial | 100.0 (98.1–100.0) | 55.6 (21.2–86.3) | |
| Total | 98.6 (97.2–99.3) | 65.4 (50.7–77.6) | |
| TTE and CEUS | |||
| Cortellaro [49] | |||
| Intra-atrial | 98.6 (92.2–100.0) | 50.0 (1.2–98.7) | |
| Extra-atrial | 100.0 (94.6–100.0) | 25.0 (0.6–80.6) | |
| Total | 98.5 (91.7–100.0) | 33.3 (4.3–77.7) | |
| Duran-Gehring [50] | |||
| Intra-atrial | 100.0 (92.3–100.0) | –4 | |
| Extra-atrial | 100.0 (91.8–100.0) | 33.3 (0.8–90.6) | |
| Total | 100.0 (91.8–100.0) | 33.3 (0.8–90.6) | |
| Kamalipour [52] | |||
| Intra-atrial | 97.8 (92.2–99.7) | 78.6 (49.2–95.3) | |
| Extra-atrial | 100.0 (96.4–100.0) | 0.0 (0.0–84.2) | |
| Total | 97.7 (92.0–99.7) | 68.8 (41.3–89.0) | |
| Lanza [53] | |||
| Intra-atrial | 99.0 (94.6–100.0) | 71.4 (29.0–96.3) | |
| Extra-atrial | 100.0 (96.4–100.0) | 80.0 (28.4–99.5) | |
| Total | 98.9 (94.3–100.0) | 75.0 (42.8–94.5) | |
| Weekes [55] | |||
| Intra-atrial | 100.0 (97.6–100.0) | –4 | |
| Extra-atrial | 100.0 (97.5–100.0) | 75.0 (19.4–99.4) | |
| Total | 100.0 (97.5–100.0) | 75.0 (19.4–99.4) | |
| Vascular ultrasound and TTE | |||
| Alonso-Quintela [58] | |||
| Intra-atrial | 94.0 (83.4–98.7) | 100.0 (2.5–100.0) | |
| Extra-atrial | 100.0 (92.7–100.0) | 100.0 (15.8–100.0) | |
| Total | 93.8 (82.8–98.7) | 100.0 (29.2–100.0) | |
| Maury [59] | |||
| Intra-atrial | 100.0 (95.4–100.0) | 100.0 (47.8–100.0) | |
| Extra-atrial | 100.0 (95.5–100.0) | 100.0 (39.8–100.0) | |
| Total | 100.0 (95.2–100.0) | 100.0 (66.4–100.0) | |
| Vascular ultrasound, TTE and CEUS | |||
| Blans [18] | |||
| Intra-atrial | 100.0 (93.2–100.0) | 0.0 (0.0–70.8) | |
| Extra-atrial | 98.0 (89.6–100.0) | 0.0 (0.0–84.2) | |
| Total | 98.0 (89.4–99.9) | 0.0 (0.0–70.8) | |
| Matsushima [19] | |||
| Intra-atrial | 98.2 (90.6–100.0) | 50.0 (1.3–98.7) | |
| Extra-atrial | 100.0 (93.2–100.0) | 50.0 (15.7–84.3) | |
| Total | 98.0 (89.4–99.9) | 50.0 (18.7–81.3) | |
| Meggiolaro3 [32] | |||
| Intra-atrial | 95.8 (88.3–99.1) | 48.5 (30.8–66.5) | |
| Extra-atrial | 100.0 (96.0–100.0) | 64.3 (35.1–87.2) | |
| Total | 100.0 (96.0–100.0) | 64.3 (35.1–87.2) | |
| Vezzani [31] | |||
| Intra-atrial | 96.0 (88.8–99.2) | 91.7 (73.0–99.0) | |
| Extra-atrial | 100.0 (96.2–100.0) | 100.0 (39.8–100.0) | |
| Total | 95.8 (88.1–99.1) | 92.9 (76.5–99.1) | |
| Zanobetti3 [61] | |||
| Intra-atrial | 89.2 (81.5–94.5) | 94.2 (87.9–97.9) | |
| Extra-atrial | 100.0 (98.1–100.0) | 55.6 (21.2–86.3) | |
| Total | 100.0 (98.1–100.0) | 55.6 (21.2–86.3) | |
CEUS contrast enhance ultrasound, TTE transthoracic echocardiography
1One-sided 97.5% confidence interval (CI) in case specificity is estimated to be 100%
2One-sided 97.5% confidence interval in case sensitivity is estimated to be 100%
3Intra-atrial tip position was reported but was not considered to be a malposition
4No intra-atrial misplacements were detected
Fig. 5.
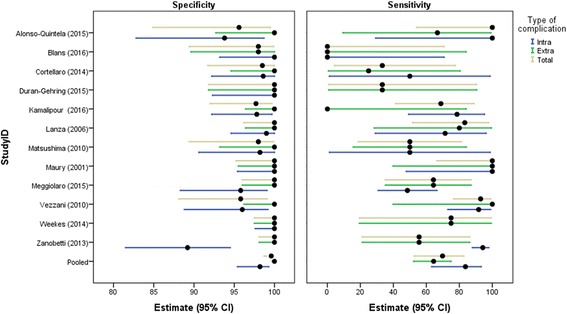
Forest plot for the specificity and sensitivity of ultrasound for detection of CVC-related complications distinguishing between intra- and extra-atrial malposition. The pooled specificity and sensitivity for intra- and extra-atrial malposition, and the specificity and sensitivity for each study individually. CI confidence interval
Quality assessment
The risk of bias and applicability concerns of the included studies are summarized in Table 4. For a more detailed description see Additional file 5. No study scored low in all domains of the bias assessment. The risk of bias within the patient selection domain was considered low in 16 studies (64%). A higher risk assessment was mainly due to inappropriate exclusion and non-consecutive patient enrollment. The risk of bias in the index test domain was deemed low in 18 studies (72%). This risk of bias was most often scored high due to the lack of a threshold when using CEUS. Only four studies (16%) had a low risk of bias in the reference standard domain, primarily because studies used CXR to detect intra-atrial CVC misplacements. Within the domain of flow and timing, 18 studies (72%) scored low due to a variety of reasons. Only three studies (12%) had low applicability concerns regarding the reference standard. The remaining studies had inadequate numbers of operators. No concerns regarding applicability were found in either the patient selection or reference standard domains.
Table 4.
Quality assessment of included studies
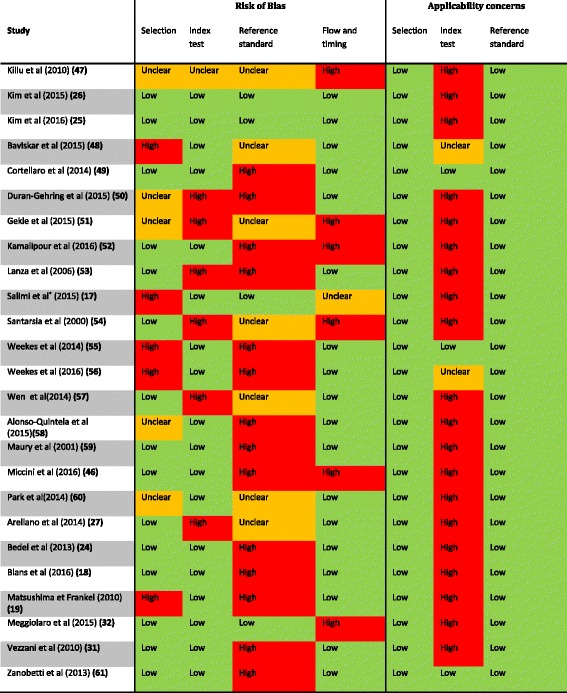
*Accuracy CXR investigated; TTE used as reference standard
Orange is unclear risk of bias or applicability concern. Green is low risk of bias or applicability concern, and red is high risk of bias or applicability concern
Discussion
The major findings of this systematic review and meta-analysis on the diagnostic accuracy of US to detect CVC malposition are a pooled specificity and sensitivity of 98.9 (95% CI: 97.8–99.5) and 68.2 (95% CI: 54.4–79.4), respectively. US was feasible in 96.8% of the cases. Furthermore, central line misplacement occurred in 6.8% and pneumothorax occurred in 1.1% of the population.
The prevalence of CVC malposition and pneumothorax in our systematic review and meta-analysis is in accordance with the published literature; the prevalence of primary CVC misplacement has been reported up to 6.7%, whereas pneumothorax normally ranges from 0.1–3.3% [3, 5, 6, 20, 21].
The limited sensitivity of US to detect CVC malposition can possibly be explained by the small a priori chance of developing post-procedural complications. Therefore, small changes in the number of false negatives could eventually cause dramatic changes in the sensitivity. This problem can persist even after pooling [22]. Another possible explanation for the low sensitivity might be an imperfect reference standard; some studies suggest that, in the absence of clinical symptoms, CXR should not be considered as a reliable diagnostic method [14]. There is a large inter-observer variability among radiologists in identifying the cavo-atrial junction on CXRs; therefore, reading of a bedside CXR alone may not be sufficiently accurate to identify intra-atrial tip position [6, 14, 15]. Off note, the risk of developing a serious complication, for example cardiac tamponade, secondary to CVC tip position in the right atrium is virtually zero [23]. Nevertheless, in spite of the low sensitivity, due to the high specificity and low prevalence the positive and negative predictive values are both excellent. Therefore, we can conclude that US is a suitable diagnostic modality to replace CXR.
Interestingly, the prevalence of CVC malposition may be reduced further by visualizing the guidewire during the insertion procedure [24–27]. In some studies echocardiography was performed during guidewire insertion in order to localize it as a hyper-echogenic line in the right atrium; subsequently, before CVC introduction the guidewire was slowly removed under US control until the “J” tip disappeared from the right atrium. If the guidewire was not visualized in the right atrium, a different view was attained and the wire was reinserted. Thus, this US protocol tends to reduce the occurrence of CVC malposition [24, 27]. The studies performed by Kim et al. incorporated a similar but slightly different per-procedural protocol (the SCU protocol as described in Additional file 6) [25, 26]. A major advantage of their protocol is that both CVC insertion and position control can be easily achieved by a single operator. Also, since the superior vena cava can readily be visualized via the right supraclavicular fossa, the advancement of the guidewire can be monitored fairly well during CVC insertion and any malposition is quickly recognized and corrected. The abovementioned protocols suggest that the rate of malposition only depends on the feasibility of US and could be as low as 0%.
CVC position, according to our meta-analysis, is best verified by vascular US combined with TTE. The SCU protocol could potentially be even better; since this protocol is performed during the insertion and procedure, misplacements rarely occur and, therefore, sensitivity could not be calculated. Theoretically, the best post-procedural protocol is an US method incorporating a scan of the jugular and subclavian vein bilaterally and visualizing the migration of the CVC tip into the heart through CEUS [28]. Surprisingly, in cases where CEUS was implemented in the study protocol an overall lower sensitivity was noted. This is probably due to the fact that studies incorporating CEUS generally deemed intra-atrial position of the catheter tip a misplacement, whereas in studies implementing only vascular US and TTE intra-atrial position was not always regarded as a malposition. Moreover, the vascular US and TTE group contained various pediatric studies where superior vena cava detection of the catheter tip is relatively easy [29, 30]. Finally, it has been debated whether the threshold of 2 s described by Vezzani and colleagues is an accurate indicator of correct CVC position [31]. More likely, the delay in appearance of microbubbles is dependent on the length of catheter used to inject the agitated saline. Subsequently, opacification of the right atrium only indicates an intravenous position of the CVC tip. Additionally, to assess CVC tip position, Meggiolaro et al. suggested that a cut-off value of 500 ms yields a better accuracy [32].
According to some ultrasound protocols two operators are required to insert the CVC and control its position at the same time with US. However, this is only necessary if either agitated saline is used to flush the line or a per-procedural protocol is being performed that visualizes the advancement of the guidewire. In any other case, one physician can insert the CVC and afterwards perform an ultrasonographic examination of the contralateral internal jugular vein, both subclavian veins, and the right atrium via the subcostal view. We suggest this information to be added to the protocol for US-guided CVC placement, recently published by Saugel and coworkers [33, 34]. We refer to Additional file 6 for a detailed description of all protocols and the number of operators needed.
Intra-atrial misplacement was more readily detected compared to extra-atrial misplacement. One possible explanation might be the fact that not all possible locations of extra-atrial malposition are detectable by US whereas the right atrium and ventricle are often easily scanned by TTE [35]. This would cause more false negatives to occur in the extra-atrial misplacement group and would therefore lead to a lower sensitivity.
Concerning the detection of pneumothorax, previous studies have already shown the advantages of US in comparison to CXR [36–38]. Furthermore, due to clear advantages, US has an increasing role in the critical care setting and ICU physicians are often trained in various US techniques [7, 39–42]. By combining the techniques of lung US and TTE we show that US could be a favorable method in detecting CVC-related complications in the ICU. To perform and interpret critical care US it is suggested that the majority of learning occurs during the first 20–30 practice studies and that many learners reached a plateau in their training [11, 43]. In general, bedside US has a good concordance with and multiple advantages over portable CXR, diminishing the role of CXR in the ICU [37, 38, 44].
Recently, the ability of US to detect malposition and pneumothorax following CVC insertion was investigated by another systematic review [45]. Its design contained some important differences compared to our meta-analysis. Firstly, we included far more CVC placements (2602 vs 1553) and studies (25 vs 15). Secondly, a strength of our study was that we included studies that used alternative reference standards; TEE, computed tomography, and intra-fluoroscopy were utilized in addition to CXR [26, 27, 46]. Thirdly, our study provides an accurate overview of the various US protocols used and their accuracy. Finally, we registered our study protocol at PROSPERO which is advised by the National Institute of Health Research (NIHR). Besides study design, there were differences in results as well; we reported a similar pooled specificity of 98.9 (95% CI: 97.8–99.5) vs. 98 (95% CI: 97–99) but a considerably lower pooled sensitivity with a larger variation of 68.2 (95% CI: 54.4–79.4) vs. 82 (95% CI: 77–86). This discrepancy could be caused by the fact that we included more studies with smaller sample sizes, and studies without any positive cases.
Our meta-analysis has several limitations. Like all meta-analyses it is sensitive for publication bias. Deek’s test was not significant indicating no evidence of strong publication bias. Prevalence was low and seven studies did not have any occurrence of CVC-related complications. These studies did not provide information regarding the sensitivity. Moreover, the small number of positive cases reported in the included studies causes uncertainty and a large variation regarding the sensitivity estimates. In addition, specificity was found to be very high with no false positives in several studies. For these reasons we could not use a bivariate model as is often used in meta-analysis for diagnostic studies to jointly pool specificity and sensitivity estimates. Another limitation is the substantial amount of statistical heterogeneity concerning specificity and sensitivity; this limits the ability to interpret the pooled data. Possible explanations for this problem are small study populations, limitations in study designs, differences in US techniques, and differences in outcomes. Subgroup analyses were performed on the protocols and on the location of malposition (intra- or extra-atrial) to attenuate this problem. Another limitation is the overall high risk of bias in the reference test domain since CXR is often not reliable for detecting intra-atrial tip position.
Further research is required to establish the viability of US as a diagnostic tool. Regarding the sensitivity, this review shows a substantial amount of statistical heterogeneity, often caused by small study populations in addition to the low prevalence of complications. Due to the low prevalence it is nearly impossible to correctly power a study that investigates immediate post-procedural complications of central venous cannulation. To address this problem and assess the sensitivity correctly we suggest a larger study should be performed that uses operators of different level of experience, and the ‘SCU’ protocol or the ‘vascular US and TTE’ protocol as these showed the most promising results. Furthermore, future research should aim to investigate factors contributing to intravenous CVC malposition and pneumothorax. Identifying those factors could lead to a situation in which only cases with a high chance of complications are investigated by either US or CXR, thus reducing the required number of patients. Additionally, to detect aberrant CVC position the use of microbubbles should be re-evaluated since it is unclear whether CEUS itself produces more false negatives or that alternative factors contribute to a lower sensitivity.
Conclusion
The major findings of this systematic review and meta-analysis on the diagnostic accuracy of US to detect CVC malposition are a pooled specificity and sensitivity of 98.9 (95% CI: 97.8–99.5) and 68.2 (95% CI: 54.4–79.4), respectively. Therefore, US is an accurate and feasible diagnostic modality to detect CVC malposition and iatrogenic pneumothorax. Advantages of US over CXR are that it is performed faster and does not subject patients to radiation. Vascular US combined with transthoracic echocardiography is advised. However, results need to be interpreted with caution since included studies were often underpowered and had methodological limitations. A large multicenter study investigating optimal US protocol, among others, is needed.
Additional files
PRISMA 2009 checklist. An overview of all sections as indicated by the PRISMA guidelines with their corresponding pages in the review. (DOC 63 kb)
Search strategy. An overview of the various terms used to search the PubMed, EMBASE and Cochrane Library databases and the results from the initial search on 4 October 2016 and the secondary search on 9 January 2017. (DOCX 39 kb)
Eligibility and exclusion criteria. An overview of the eligibility and exclusion criteria used in this review. (DOCX 13 kb)
Patient characteristics. An overview of several characteristics of the included studies, namely gender, age, weight and/or BMI, CVC location, and type of catheter. (DOCX 26 kb)
Qualitative assessment of bias. An overview of the domains defined by QUADAS-2 tool to assess the risk of bias and applicability concerns for each of the included articles. (DOCX 45 kb)
Ultrasound protocols. An overview of the ultrasonographic techniques used in the various protocols. (DOCX 15 kb)
Acknowledgements
Not applicable.
Funding
Our funding was departmental.
Availability of data and materials
The datasets used to perform the meta-analysis are available from the corresponding author on reasonable request. All other data generated or analyzed during this study are included in this published article and its Additional files.
Authors’ contributions
JMS, RR, MJB, MP, PMVdV, and PRT all take responsibility for integrity of the data interpretation and analysis. All authors contributed substantially to the study design, data interpretation, and the writing of the manuscript. PMVdV performed statistical analysis and data synthesis. All authors approved the final version of the manuscript.
Abbreviations
- CEUS
Contrast-enhanced ultrasound
- CI
Confidence interval
- CVC
Central venous catheter
- CXR
Chest x-ray
- GEE
Generalized estimating equation
- ICU
Intensive care unit
- SCU
Supraclavicular ultrasound
- TEE
Transesophageal echocardiography
- TTE
Transthoracic echocardiography
- US
Ultrasound
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s13054-018-1989-x) contains supplementary material, which is available to authorized users.
Contributor Information
Jasper M. Smit, Email: j.smit6@vumc.nl
Reinder Raadsen, Email: r.raadsen@vumc.nl.
Michiel J. Blans, Email: MBlans@rijnstate.nl
Manfred Petjak, Email: Manfred.Petjak@ghz.nl.
Peter M. Van de Ven, Email: p.vandeven@vumc.nl
Pieter R. Tuinman, Email: p.tuinman@vumc.nl
References
- 1.Taylor RW, Palagiri AV. Central venous catheterization. Crit Care Med. 2007;35(5):1390–1396. doi: 10.1097/01.CCM.0000260241.80346.1B. [DOI] [PubMed] [Google Scholar]
- 2.McGee DC, Gould MK. Preventing complications of central venous catheterization. N Engl J Med. 2003;348(12):1123–1133. doi: 10.1056/NEJMra011883. [DOI] [PubMed] [Google Scholar]
- 3.Parienti JJ, Mongardon N, Megarbane B, Mira JP, Kalfon P, Gros A, et al. Intravascular complications of central venous catheterization by insertion site. N Engl J Med. 2015;373(13):1220–1229. doi: 10.1056/NEJMoa1500964. [DOI] [PubMed] [Google Scholar]
- 4.Polderman KH, Girbes AR. Central venous catheter use. Intensive Care Med. 2002;28(1):1–17. doi: 10.1007/s00134-001-1154-9. [DOI] [PubMed] [Google Scholar]
- 5.Nayeemuddin M, Pherwani AD, Asquith JR. Imaging and management of complications of central venous catheters. Clin Radiol. 2013;68(5):529–544. doi: 10.1016/j.crad.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 6.Hourmozdi JJ, Markin A, Johnson B, Fleming PR, Miller JB. Routine chest radiography is not necessary after ultrasound-guided right internal jugular vein catheterization. Crit Care Med. 2016;44(9):e804–e808. doi: 10.1097/CCM.0000000000001737. [DOI] [PubMed] [Google Scholar]
- 7.Lichtenstein D, van Hooland S, Elbers P, Malbrain ML. Ten good reasons to practice ultrasound in critical care. Anaesthesiol Intensive Ther. 2014;46(5):323–335. doi: 10.5603/AIT.2014.0056. [DOI] [PubMed] [Google Scholar]
- 8.Vezzani A, Manca T, Vercelli A, Braghieri A, Magnacavallo A. Ultrasonography as a guide during vascular access procedures and in the diagnosis of complications. J Ultrasound. 2013;16(4):161–170. doi: 10.1007/s40477-013-0046-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lalu MM, Fayad A, Ahmed O, Bryson GL, Fergusson DA, Barron CC, et al. Ultrasound-guided subclavian vein catheterization: a systematic review and meta-analysis. Crit Care Med. 2015;43(7):1498–1507. doi: 10.1097/CCM.0000000000000973. [DOI] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Open Med. 2009;3(3):e123–e130. [PMC free article] [PubMed] [Google Scholar]
- 11.Millington SJ, Hewak M, Arntfield RT, Beaulieu Y, Hibbert B, Koenig S, et al. Outcomes from extensive training in critical care echocardiography: identifying the optimal number of practice studies required to achieve competency. J Crit Care. 2017;40:99–102. doi: 10.1016/j.jcrc.2017.03.020. [DOI] [PubMed] [Google Scholar]
- 12.Ebrahimi A, Yousefifard M, Mohammad Kazemi H, Rasouli HR, Asady H, Moghadas Jafari A, et al. Diagnostic accuracy of chest ultrasonography versus chest radiography for identification of pneumothorax: a systematic review and meta-analysis. Tanaffos. 2014;13(4):29–40. [PMC free article] [PubMed] [Google Scholar]
- 13.Alrajab S, Youssef AM, Akkus NI, Caldito G. Pleural ultrasonography versus chest radiography for the diagnosis of pneumothorax: review of the literature and meta-analysis. Crit Care. 2013;17(5):R208. doi: 10.1186/cc13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abood GJ, Davis KA, Esposito TJ, Luchette FA, Gamelli RL. Comparison of routine chest radiograph versus clinician judgment to determine adequate central line placement in critically ill patients. J Trauma. 2007;63(1):50–56. doi: 10.1097/TA.0b013e31806bf1a3. [DOI] [PubMed] [Google Scholar]
- 15.Chan TY, England A, Meredith SM, McWilliams RG. Radiologist variability in assessing the position of the cavoatrial junction on chest radiographs. Br J Radiol. 2016;89(1065):20150965. doi: 10.1259/bjr.20150965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wirsing M, Schummer C, Neumann R, Steenbeck J, Schmidt P, Schummer W. Is traditional reading of the bedside chest radiograph appropriate to detect intraatrial central venous catheter position? Chest. 2008;134(3):527–533. doi: 10.1378/chest.07-2687. [DOI] [PubMed] [Google Scholar]
- 17.Salimi F, Hekmatnia A, Shahabi J, Keshavarzian A, Maracy MR, Jazi AH. Evaluation of routine postoperative chest roentgenogram for determination of the correct position of permanent central venous catheters tip. J Res Med Sci. 2015;20(1):89–92. [PMC free article] [PubMed] [Google Scholar]
- 18.Blans MJ, Endeman H, Bosch FH. The use of ultrasound during and after central venous catheter insertion versus conventional chest x-ray after insertion of a central venous catheter. Neth J Med. 2016;74(8):353–357. [PubMed] [Google Scholar]
- 19.Matsushima K, Frankel HL. Bedside ultrasound can safely eliminate the need for chest radiographs after central venous catheter placement: CVC sono in the surgical ICU (SICU) J Surg Res. 2010;163(1):155–161. doi: 10.1016/j.jss.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 20.Eisen LA, Narasimhan M, Berger JS, Mayo PH, Rosen MJ, Schneider RF. Mechanical complications of central venous catheters. J Intensive Care Med. 2006;21(1):40–46. doi: 10.1177/0885066605280884. [DOI] [PubMed] [Google Scholar]
- 21.Schummer W, Schummer C, Rose N, Niesen WD, Sakka SG. Mechanical complications and malpositions of central venous cannulations by experienced operators. A prospective study of 1794 catheterizations in critically ill patients. Intensive Care Med. 2007;33(6):1055–1059. doi: 10.1007/s00134-007-0560-z. [DOI] [PubMed] [Google Scholar]
- 22.Walker E, Hernandez AV, Kattan MW. Meta-analysis: its strengths and limitations. Cleve Clin J Med. 2008;75(6):431–439. doi: 10.3949/ccjm.75.6.431. [DOI] [PubMed] [Google Scholar]
- 23.Vesely TM. Central venous catheter tip position: a continuing controversy. J Vasc Interv Radiol. 2003;14(5):527–534. doi: 10.1097/01.RVI.0000071097.76348.72. [DOI] [PubMed] [Google Scholar]
- 24.Bedel J, Vallee F, Mari A, Riu B, Planquette B, Geeraerts T, et al. Guidewire localization by transthoracic echocardiography during central venous catheter insertion: a periprocedural method to evaluate catheter placement. Intensive Care Med. 2013;39(11):1932–1937. doi: 10.1007/s00134-013-3097-3. [DOI] [PubMed] [Google Scholar]
- 25.Kim SC, Graff I, Sommer A, Hoeft A, Weber S. Ultrasound-guided supraclavicular central venous catheter tip positioning via the right subclavian vein using a microconvex probe. J Vasc Access. 2016;17(5):435–439. doi: 10.5301/jva.5000518. [DOI] [PubMed] [Google Scholar]
- 26.Kim SC, Heinze I, Schmiedel A, Baumgarten G, Knuefermann P, Hoeft A, et al. Ultrasound confirmation of central venous catheter position via a right supraclavicular fossa view using a microconvex probe: an observational pilot study. Eur J Anaesthesiol. 2015;32(1):29–36. doi: 10.1097/EJA.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 27.Arellano R, Nurmohamed A, Rumman A, Day AG, Milne B, Phelan R, et al. The utility of transthoracic echocardiography to confirm central line placement: an observational study. Can J Anaesth. 2014;61(4):340–346. doi: 10.1007/s12630-014-0111-3. [DOI] [PubMed] [Google Scholar]
- 28.Medical Advisory Secretariat. Use of contrast agents with echocardiography in patients with suboptimal echocardiography: an evidence-based analysis. Ont Health Technol Assess Ser [Internet]. 2010 May [cited 2018 02 28]; 10(13) 1-17. Available from: http://www.health.gov.on.ca/english/providers/program/mas/tech/reviews/pdf/rev_suboptimal_contrast_echo_20100601.pdf. [PMC free article] [PubMed]
- 29.Lai WW, Geva T, Shirali GS, Frommelt PC, Humes RA, Brook MM, et al. Guidelines and standards for performance of a pediatric echocardiogram: a report from the Task Force of the Pediatric Council of the American Society of Echocardiography. J Am Soc Echocardiogr. 2006;19(12):1413–30. [DOI] [PubMed]
- 30.Khouzam RN, Minderman D, D’Cruz IA. Echocardiography of the superior vena cava. Clin Cardiol. 2005;28(8):362–366. doi: 10.1002/clc.4960280804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vezzani A, Brusasco C, Palermo S, Launo C, Mergoni M, Corradi F. Ultrasound localization of central vein catheter and detection of postprocedural pneumothorax: an alternative to chest radiography. Crit Care Med. 2010;38(2):533–538. doi: 10.1097/CCM.0b013e3181c0328f. [DOI] [PubMed] [Google Scholar]
- 32.Meggiolaro M, Scatto A, Zorzi A, Roman-Pognuz E, Lauro A, Passarella C, et al. Confirmation of correct central venous catheter position in the preoperative setting by echocardiographic “bubble-test”. Minerva Anestesiol. 2015;81(9):989–1000. [PubMed] [Google Scholar]
- 33.Saugel B, Scheeren TWL, Teboul JL. Ultrasound-guided central venous catheter placement: a structured review and recommendations for clinical practice. Crit Care. 2017;21(1):225. doi: 10.1186/s13054-017-1814-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steenvoorden TS, Smit JM, Haaksma ME, Tuinman PR. Necessary additional steps in ultrasound guided central venous catheter placement: getting to the heart of the matter. Crit Care. 2017;21(1):307. doi: 10.1186/s13054-017-1900-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Douglas PS, Garcia MJ, Haines DE, Lai WW, Manning WJ, Patel AR, et al. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 appropriate use criteria for echocardiography. J Am Coll Cardiol. 2011;57(9):1126–1166. doi: 10.1016/j.jacc.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 36.Lichtenstein D, Meziere G, Biderman P, Gepner A. The “lung point”: an ultrasound sign specific to pneumothorax. Intensive Care Med. 2000;26(10):1434–1440. doi: 10.1007/s001340000627. [DOI] [PubMed] [Google Scholar]
- 37.Lichtenstein DA. BLUE-protocol and FALLS-protocol: two applications of lung ultrasound in the critically ill. Chest. 2015;147(6):1659–1670. doi: 10.1378/chest.14-1313. [DOI] [PubMed] [Google Scholar]
- 38.Moreno-Aguilar G, Lichtenstein D. Lung ultrasound in the critically ill (LUCI) and the lung point: a sign specific to pneumothorax which cannot be mimicked. Crit Care. 2015;19:311. doi: 10.1186/s13054-015-1030-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peters JL, Belsham PA, Garrett CPO, Kurzer M. Doppler ultrasound technique for safer percutaneous catheterizatlon of the infraclavicular subclavian vein. Am J Surg. 1982;143(3):391–393. doi: 10.1016/0002-9610(82)90118-0. [DOI] [PubMed] [Google Scholar]
- 40.Beheshti MV. A concise history of central venous access. Tech Vasc Interv Radiol. 2011;14(4):184–185. doi: 10.1053/j.tvir.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 41.Heidemann L, Nathani N, Sagana R, Chopra V, Hueng M. A contemporary assessment of mechanical complication rates and trainee perceptions of central venous catheter insertion. J Hosp Med. 2017;12(8):646–651. doi: 10.12788/jhm.2784. [DOI] [PubMed] [Google Scholar]
- 42.International expert statement on training standards for critical care ultrasonography. Intensive Care Med. 2011;37(7):1077–83. [DOI] [PubMed]
- 43.Millington SJ, Arntfield RT, Guo RJ, Koenig S, Kory P, Noble V, et al. The Assessment of Competency in Thoracic Sonography (ACTS) scale: validation of a tool for point-of-care ultrasound. Crit Ultrasound J. 2017;9(1):25. doi: 10.1186/s13089-017-0081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Phillips CT, Manning WJ. Advantages and pitfalls of pocket ultrasound vs daily chest radiography in the coronary care unit: a single-user experience. Echocardiography. 2017;34(5):656–661. doi: 10.1111/echo.13509. [DOI] [PubMed] [Google Scholar]
- 45.Ablordeppey EA, Drewry AM, Beyer AB, Theodoro DL, Fowler SA, Fuller BM, et al. Diagnostic accuracy of central venous catheter confirmation by bedside ultrasound versus chest radiography in critically ill patients: a systematic review and meta-analysis. Crit Care Med. 2017;45(4):715-24. [DOI] [PMC free article] [PubMed]
- 46.Miccini M, Cassini D, Gregori M, Gazzanelli S, Cassibba S, Biacchi D. Ultrasound-guided placement of central venous port systems via the right internal jugular vein: are chest x-ray and/or fluoroscopy needed to confirm the correct placement of the device? World J Surg. 2016;40(10):2353–2358. doi: 10.1007/s00268-016-3574-2. [DOI] [PubMed] [Google Scholar]
- 47.Killu K, Parker A, Coba V, Horst M, Dulchavsky S. Using ultrasound to identify the central venous catheter tip in the superior vena cava. ICU Dir. 2010;1(4):220–222. doi: 10.1177/1944451610378131. [DOI] [Google Scholar]
- 48.Baviskar AS, Khatib KI, Bhoi S, Galwankar SC, Dongare HC. Confirmation of endovenous placement of central catheter using the ultrasonographic “bubble test”. Indian J Crit Care Med. 2015;19(1):38–41. doi: 10.4103/0972-5229.148642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cortellaro F, Mellace L, Paglia S, Costantino G, Sher S, Coen D. Contrast enhanced ultrasound vs chest x-ray to determine correct central venous catheter position. Am J Emerg Med. 2014;32(1):78–81. doi: 10.1016/j.ajem.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 50.Duran-Gehring PE, Guirgis FW, McKee KC, Goggans S, Tran H, Kalynych CJ, et al. The bubble study: ultrasound confirmation of central venous catheter placement. Am J Emerg Med. 2015;33(3):315–319. doi: 10.1016/j.ajem.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 51.Gekle R, Dubensky L, Haddad S, Bramante R, Cirilli A, Catlin T, et al. Saline flush test: can bedside sonography replace conventional radiography for confirmation of above-the-diaphragm central venous catheter placement? J Ultrasound Med. 2015;34(7):1295–1299. doi: 10.7863/ultra.34.7.1295. [DOI] [PubMed] [Google Scholar]
- 52.Kamalipour H, Ahmadi S, Kamali K, Moaref A, Shafa M, Kamalipour P. Ultrasound for localization of central venous catheter: a good alternative to chest x-ray? Anesth Pain Med. 2016;6(5):e38834. doi: 10.5812/aapm.38834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lanza C, Russo M, Fabrizzi G. Central venous cannulation: are routine chest radiographs necessary after B-mode and colour Doppler sonography check? Pediatr Radiol. 2006;36(12):1252–1256. doi: 10.1007/s00247-006-0307-y. [DOI] [PubMed] [Google Scholar]
- 54.Santarsia G, Casino FG, Gaudiano V, Mostacci SD, Bagnato G, Latorraca A, et al. Jugular vein catheterization for hemodialysis: correct positioning control using real-time ultrasound guidance. J Vasc Access. 2000;1(2):66–69. doi: 10.1177/112972980000100207. [DOI] [PubMed] [Google Scholar]
- 55.Weekes AJ, Johnson DA, Keller SM, Efune B, Carey C, Rozario NL, et al. Central vascular catheter placement evaluation using saline flush and bedside echocardiography. Acad Emerg Med. 2014;21(1):65–72. doi: 10.1111/acem.12283. [DOI] [PubMed] [Google Scholar]
- 56.Weekes AJ, Keller SM, Efune B, Ghali S, Runyon M. Prospective comparison of ultrasound and CXR for confirmation of central vascular catheter placement. Emerg Med J. 2016;33(3):176–180. doi: 10.1136/emermed-2015-205000. [DOI] [PubMed] [Google Scholar]
- 57.Wen M, Stock K, Heemann U, Aussieker M, Kuchle C. Agitated saline bubble-enhanced transthoracic echocardiography: a novel method to visualize the position of central venous catheter. Crit Care Med. 2014;42(3):e231–e233. doi: 10.1097/CCM.0000000000000130. [DOI] [PubMed] [Google Scholar]
- 58.Alonso-Quintela P, Oulego-Erroz I, Rodriguez-Blanco S, Muniz-Fontan M, Lapena-Lopez-de Armentia S, Rodriguez-Nunez A. Location of the central venous catheter tip with bedside ultrasound in young children: can we eliminate the need for chest radiography? Pediatr Crit Care Med. 2015;16(9):e340–e345. doi: 10.1097/PCC.0000000000000491. [DOI] [PubMed] [Google Scholar]
- 59.Maury E, Guglielminotti J, Alzieu M, Guidet B, Offenstadt G. Ultrasonic examination: an alternative to chest radiography after central venous catheter insertion? Am J Respir Crit Care Med. 2001;164(3):403–405. doi: 10.1164/ajrccm.164.3.2009042. [DOI] [PubMed] [Google Scholar]
- 60.Park YH, Lee JH, Byon HJ, Kim HS, Kim JT. Transthoracic echocardiographic guidance for obtaining an optimal insertion length of internal jugular venous catheters in infants. Paediatr Anaesth. 2014;24(9):927–932. doi: 10.1111/pan.12443. [DOI] [PubMed] [Google Scholar]
- 61.Zanobetti M, Coppa A, Bulletti F, Piazza S, Nazerian P, Conti A, et al. Verification of correct central venous catheter placement in the emergency department: comparison between ultrasonography and chest radiography. Intern Emerg Med. 2013;8(2):173–180. doi: 10.1007/s11739-012-0885-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA 2009 checklist. An overview of all sections as indicated by the PRISMA guidelines with their corresponding pages in the review. (DOC 63 kb)
Search strategy. An overview of the various terms used to search the PubMed, EMBASE and Cochrane Library databases and the results from the initial search on 4 October 2016 and the secondary search on 9 January 2017. (DOCX 39 kb)
Eligibility and exclusion criteria. An overview of the eligibility and exclusion criteria used in this review. (DOCX 13 kb)
Patient characteristics. An overview of several characteristics of the included studies, namely gender, age, weight and/or BMI, CVC location, and type of catheter. (DOCX 26 kb)
Qualitative assessment of bias. An overview of the domains defined by QUADAS-2 tool to assess the risk of bias and applicability concerns for each of the included articles. (DOCX 45 kb)
Ultrasound protocols. An overview of the ultrasonographic techniques used in the various protocols. (DOCX 15 kb)
Data Availability Statement
The datasets used to perform the meta-analysis are available from the corresponding author on reasonable request. All other data generated or analyzed during this study are included in this published article and its Additional files.


