Abstract
This study evaluated the effects of inhaled nitric oxide (iNO) therapy combined with intravenous (IV) corticosteroids on hemodynamics, selected cytokines, and kidney messenger RNA toll-like receptor 4 (mRNA TLR4) expression in ischemia–reperfusion injury animal model. The primary endpoint was the evaluation of circulatory, respiratory, and renal function over time. We also investigated the profile of selected cytokines and high-mobility group box 1 (HMGB1) protein, as well as renal mRNA TLR4 activation determined by quantitative real-time polymerase chain reaction analysis. Pigs (n = 19) under sevoflurane AnaConDa anesthesia/sedation were randomized and subjected to abdominal laparotomy and alternatively suprarenal aortic cross-clamping (SRACC) for 90 min or sham surgery: Group 1 (n = 8) iNO (80 ppm) + IV corticosteroids (25 mg ×3) started 30 min before SRACC and continued 2 h after SRACC release, followed with decreased iNO (30 ppm) until the end of observation, Group 2 (n = 8) 90 min SRACC, Group 3 (n = 3)—sham surgery. Renal biopsies were sampled 1 hr before SRACC and at 3 and 20 h after SRACC release. Aortic clamping increased TLR4 mRNA expression in ischemic kidneys, but significant changes were recorded only in the control group (P = 0.016). Treatment with iNO and hydrocortisone reduced TLR4 mRNA expression to pre-ischemic conditions, and the difference observed in mRNA expression was significant between control and treatment group after 3 h (P = 0.042). Moreover, animals subjected to treatment with iNO and hydrocortisone displayed an attenuated systemic inflammatory response and lowered pulmonary vascular resistance plus increased oxygen delivery. The results indicated that iNO therapy combined with IV corticosteroids improved central and systemic hemodynamics, oxygen delivery, and diminished the systemic inflammatory response and renal mRNA TLR4 expression.
Keywords: inhaled nitric oxide, ischemia–reperfusion injury, kidney, steroid, toll-like receptor 4
Background
Reperfusion following prolonged organ ischemia, as a result of effective medical intervention, is an expected and a necessary event in order to restore organ function. Alongside alterations in perfusion, an inflammatory reaction is also a natural phenomenon that occurs as part of the total adaptation to such a challenge.1 The main clinical problem in this case is the ability to control the magnitude of both these processes and to minimize the side effects. Surgical reconstruction of the abdominal aorta with suprarenal aortic cross-clamping (SRACC) is an excellent example of a clinical situation where the ischemia–reperfusion (I/R) injury—not only to the organ tissues located below the surgically involved aorta but also other distal organs—is subjected to a generalized inflammatory response, causing distant or remote organ injury.2 The underlying mechanisms are complex and often triggered by damage-associated molecular patterns (DAMPs), factors directly released from the primary injured tissues, or indirectly by factors from activated leukocytes or other inflammatory cells. Endogenous ligands released from damaged tissues can be signaled through different receptors, with a pivotal role assumed by toll-like receptors (TLRs).3 These ligands include high-mobility group box 1 (HMGB1) protein, heat-shock proteins, hyaluronan, fibronectin, heparan sulfate, and so on.4 It was shown that the activation of the toll-like receptor 4 (TLR4) by these ligands may be a major trigger of different organ inflammation in response to ischemia.5 Additionally, TLR4 signaling in intrinsic kidney cells plays a dominant role in mediating kidney damage.6,7
There is an abundance of experimental and clinical evidence on the various methods of organ protection in the course of an I/R injury.8–10 An important role is attributed to nitric oxide (NO) and its donors. However, there are fundamental problems with the intravenous (IV) use of NO donors, including maintaining blood flow, systemic hypotension, and tolerance development.11
Several reports on the use of inhaled NO have documented a broad spectrum of effects outside the lung. Remote effects are typically dose dependent, and the mechanisms responsible for these effects are not completely understood. It is already known that many NO effects are independent of the classic cyclic guanosine monophosphate-dependent pathway. New evidence has indicated that NO inhalation leads to the formation of new compounds which may be carried as thiol groups attached to proteins in the blood or which may act indirectly through nitrite metabolites.12,13 NO in tissue has been shown to exert anti-inflammatory effects and inhibit the expression of cytokines, adhesion molecules, interleukins, and other inflammatory mediators.14 Taken together, these data support the concept that inhaled NO could be a novel treatment option for a disease characterized by systemic endothelial dysfunction. In addition, steroids have been shown to ameliorate both sepsis and I/R-induced kidney injury through different mechanisms, including glucocorticoid receptor activation and protection.15
It has been previously reported that a combination of inhaled nitric oxide (iNO) and IV steroids attenuates endotoxin-induced organ damage in a porcine endotoxemia model.16 We have shown in a preliminary study that therapy with iNO alone or IV steroids alone did not improve organ function in the porcine endotoxemia model; however, a tendency toward protecting kidney function was noted when a combination of both iNO and IV steroids was used.17 Since the inflammatory response during an I/R injury plays a pivotal role in the development of multi-organ injury, we aimed to evaluate the combined treatment of iNO and IV steroids in a SRACC piglet model study, including a 20-h post-clamping observation period. The primary endpoint of the study was the evaluation of circulatory, respiratory, and renal function over time. We also investigated the profile of selected cytokines and HMGB1 protein, as well as renal mRNA TLR4 activation determined by quantitative real-time polymerase chain reaction (PCR) analysis.
Materials and methods
Animals, approval
All animals were housed and handled according to the Guide for the Care and Use of Laboratory Animals (EU Directive 2010/63/EU for animal experiments). The Animal Research Ethics Committee of the Wroclaw University of Environmental and Life Sciences approved the study (permission no. 27/2009). Experiments were conducted at the Department of Surgery at Wroclaw University of Environmental and Life Sciences.
Anesthesia and monitoring
The piglets were fasted overnight prior to the study, with only water given according to preference. The induction of anesthesia was performed with intramuscular injection of zolazepam/tiletamine 4 mg kg−1 dissolved in medetomidine of 0.08 mg kg−1. The trachea was intubated, and the piglets were ventilated in a pressure-controlled mode using a Servo 900C ventilator (Siemens-Elema AB, Solna, Sweden) at an inspired fraction of oxygen (FIO2) of 0.3 and a positive end expiratory pressure (PEEP) of 5 cm H2O. The inspiratory pressure was set to keep the piglet normo-ventilated. Inhaled anesthesia/sedation was administered with the anesthetic-conserving device AnaConDa™ (Sedana Medical™, Danderyd, Sweden), which is a modified heat and moisture exchanger, placed between the Y-piece and the endotracheal tube. This allowed the delivery of volatile anesthetics via a miniaturized vaporizer directly into the breathing circuit of conventional ICU ventilators. The gas is instilled in liquid form through a 50 mL syringe linked to a standard perfusion pump. For sevoflurane infusion to the AnaConDa, a syringe infusion pump Ivac P7000 (Alaris Medical Systems™, Wokingham, UK) was used. An absorbent charcoal membrane integrated within the AnaConDa stored the expired anesthetic gas (to 90%) and facilitated the recirculation of gas during inspiration. The expired sevoflurane passing the filter was released through the expiratory outlet of the ventilator to an active coal scavenging system (CONTRAfluran™, ZeoSys Pneumatik, Berlin, Germany). The sevoflurane infusion rate was initially set to 2 mL/h and then adjusted to reach an adequate sedation depth by changing the rate in steps of 0.1 mL/h, according to the department’s routine sedation protocol. Expiratory gas concentrations and vital signs were monitored using the General Electric health care AS/3 Instrumentarium OY (GE, Helsinki, Finland). The sevoflurane pump infusion rate was adjusted to the initial target of end-tidal (ET) sevoflurane, which was 0.7%. In addition to sevoflurane anesthesia/sedation to maintain anesthesia during all the experiments, the animals received a continuous IV infusion of fentanyl (0.8–1.3 µg kg−1 h−1) during the surgical preparation. Anesthetic doses were increased during instrumentation and lowered closer to standard sedation doses for the remainder of the study period. The adequacy of anesthesia during maintenance was assessed based on hemodynamic responses and additional IV fentanyl (25–50 µg) doses were given if the animals were judged to be inadequately anesthetized.
Instrumentation involved the placement of an arterial line to the carotid artery (BD Careflow artery catheter®, Becton Dickinson, Singapore) and a trans-abdominal urinary bladder catheter (mini-laparotomy, Rüsch catheter, Kernen, Germany). We also placed, via an internal jugular cut down, both a central venous catheter (CVC) (BD Careflow central venous catheter®, Becton Dickinson, Wokingham, UK) and a balloon-tipped flotation catheter with a thermistor (PAC) (CritiCath SP5105H TD catheter®, Becton Dickinson, Wokingham, UK). The latter was advanced into the pulmonary artery. The piglets then underwent a 1-h recovery period after which baseline data were registered and the initial blood specimen was drawn from the arterial catheter.
Protocol
Experimental groups and treatments
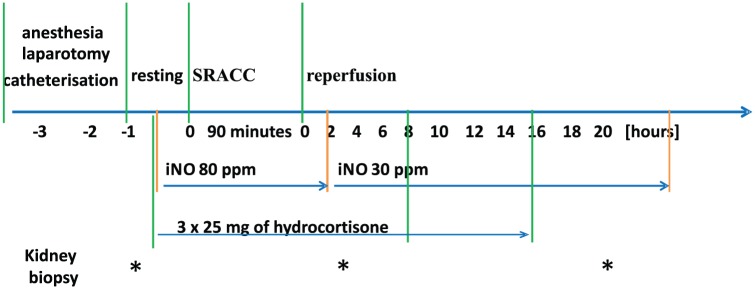
Domestic piglets (n = 19, 3–4 months old, 20–25 kg body weight, 60% male) were randomized into three groups and observed for 20 h as follows (Figure 1):
Figure 1.
The study design included instrumentation, resting, suprarenal aortic cross-clamping (SRACC), and reperfusion.
Kidney biopsies were performed by an open biopsy 1 h before the start of the experiment, 3 h after aortic clamp release, and during the autopsy at the end of the experiment.
Group 1 (treatment group, n = 8) received administration of iNO (Pulmonox-Messer Griesheim 800 ppm NO in 9000 nitrogen), which was delivered by a Pulmomix Mini (Messer Griesheim, Gumpoldskirchen, Austria) to the inspiratory limb of the ventilator as previously described by this department.17 Inhaled NO (80 ppm) was started 30 min before SRACC and continued for 2 h after SRACC release, followed by decreased iNO (30 ppm) until the end of a 20 h observation period. Also, 25 mg of hydrocortisone was given via IV 30 min before SRACC, at the commencement of the iNO, and at 8 and 16 h after the start of SRACC.
Group 2 (control group, n = 8) received 90 min of SRACC, followed by a 20-h observation period.
Group 3 (sham group, n = 3) received sham surgery, followed by a 20-h observation period.
Surgical technique
Under anesthesia and aseptic conditions (shaved skin, sterile surgical fields), a median laparotomy was performed to expose the abdominal aorta. A clamp was placed across the aorta under direct visualization above the renal and superior mesenteric arteries but below the celiac trunk, thereby starting the ischemic period. The SDF (Sidestream Dark Field) instrument (Microscan, Microvision Medical, Amsterdam, Holland) was used to confirm renal blood flow arrest. After 90 min, the clamp was released, and the reperfusion period was started; observation continued for 20 h. In all animals, hemostasis was accomplished, and the final closure of the abdominal cavity and the abdominal wall was made after another kidney biopsy in the third hour of reperfusion from the time of the aortic clamp release. In the sham group, the animals were subjected to midline laparotomy to expose the abdominal aorta; the surgical procedure was the same as in the other two treatment groups with the exception of aortic clamping and renal biopsy after 3 h.
Management protocol
Throughout these experiments, the animals received a constant fluid substitution of 0.9% saline/5% glucose (1:1) at 15 mL kg−1 h−1. At clamp release, sodium bicarbonate 50 mEq was administered via IV in all animals, along with a 250 mL bolus of 0.9% sodium chloride. During the reperfusion period, the animals in the control and iNO + steroid groups received additional IV boluses of saline if the mean arterial pressure (MAP) was less than 60 mmHg and pulmonary capillary wedge pressure (PCWP) was less than 6 mmHg in order to reach a PCWP of 8 mmHg. If the PCWP was above 8 mmHg and the MAP was less than 60 mmHg, an IV infusion of norepinephrine from 0.025 μg/kg−1 min−1 was started and slowly increased until a MAP was achieved above 60 mmHg. The maximal norepinephrine dose was limited to 0.6 μg/kg−1 min−1. If the MAP was maintained above 60 mmHg, norepinephrine was slowly phased out if possible.
In addition, all animals received an antibiotic, cefuroxime (GlaxoSmithKline, Solna, Sweden) 750 mg, intravenously before instrumentation and every 8 h to counter accidental bacterial contamination. Sodium heparin of 0.5 mg/kg was given intravenously to all animals before aortic clamping, including the sham surgery group. Body temperature was monitored by the PAC thermistor in order to keep the animals normothermic (37°C–38°C) using heating blankets or external cooling if needed. The animals were turned from side to side every 4 h. After 20 h of reperfusion, the animals were killed by IV administration of sodium pentobarbital (Morbital, Biowet) of 133.3 mg/mL, 0.6 mL/kg−1, and tissue samples from the kidneys were collected and fixed in RNAlater® RNA Stabilization Solution (Thermo Fisher Scientific, Waltham, MA, USA).
Kidney biopsy
Open biopsies were performed during surgery at T0 (1 h before the start of the experiment) and T3 (3 h after aortic clamp release). TruGuide® BARD biopsy needles (Bard Biopsy Systems, Tempe, AZ, USA) were used. At the end of the experiment (T20), the renal cortex was sampled during autopsy.
Hemodynamic, respiratory, and biochemistry data collection
Animals were monitored continuously with a three-point electrocardiogram (ECG), as well as recordings of heart rate (HR) and mean arterial pressure (MAP), mean pulmonary arterial pressure (MPAP), and central venous pressure (CVP). The stroke volume index was calculated as the cardiac index divided by HR. Global oxygen delivery was calculated as the arterial oxygen content multiplied by the cardiac index. Global oxygen uptake was calculated as the arterial oxygen content subtracted by the venous oxygen content multiplied by the cardiac index. The oxygen content was calculated with the standard formula of pulse oximetry (SpO2), fraction of inspired oxygen (FiO2), and end-tidal carbon dioxide (EtCO2) concentration. PCWP (mmHg) and thermodilution cardiac output (CO, L min−1) were measured at the baseline and at 15 min and 1, 10, and 20 h of reperfusion. The pulmonary vascular resistance index (PVRI) and systemic vascular resistance index (SVRI) vascular resistance indices (dynes s−1 cm−5) were calculated. Arterial and mixed venous bloods were analyzed in a blood gas machine at baseline and during the reperfusion period (15 min and 1, 5, 10, and 20 h). Venous blood samples for full blood count and lactate measurement were sampled at baseline and 1, 10, and 20 h of reperfusion. Urine volumes were determined at every 10 h of reperfusion.
Laboratory parameters
Blood samples were collected for complete blood count and biochemistry tests, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine, urea, sodium (Na), potassium (K), and chloride (Cl); all tests were performed within 1 h after collection. Point-of-care analysis was done for blood gases (ISTAT, Abbot, East Windsor, USA), including pH, PaCO2, PaO2, bicarbonate (HCO3–), lactate, and base excess (BE). High or low potassium levels and acidosis were not treated, except for the sodium bicarbonate bolus given at SRACC release.
Inflammatory mediators and HMGB1 protein
The arterial blood for mediator assays was collected at baseline and 3, 10, and 20 h. Blood was drawn from the arterial blood catheter into tubes containing ethylenediaminetetraacetic acid (EDTA) as an anticoagulant and immediately centrifuged at 4000 r/min for 15 min. Plasma samples were stored at −70°C until assays were performed. Tumor necrosis factor-alpha (TNF-alpha), interleukin 10 (IL-10), and interleukin 6 (IL-6) were measured with Quantikine porcine enzyme-linked immunosorbent assay (R&D Systems, Abingdon, UK) with a sensitivity of 5, 5.5, and 4.3 pg/mL, respectively. HMGB1 protein was measured with an immunoassay (IBL, Hamburg, Germany) with a sensitivity of 0.2 ng/mL. Concentrations of mediators were measured in duplicate with appropriate controls, according to the manufacturer’s instructions.
RNA isolation and real-time reverse transcription PCR
Total RNA from a porcine kidney and heart tissue was extracted with RNeasy Mini Kit (Qiagen, Hilden, Germany) in accordance with the manufacturer’s instructions. Total RNA (2 µg) was used to synthesize cDNA using an oligo (dT) primer and SuperScript™ III Reverse Transcriptase (200 U/µL) in the presence of Synthesis Mix of 10x RT buffer, 25 mM MgCl2, 0.1 mM dithiothreitol, and enzyme RNaseOUT™ (40 U/µL). The reaction mixtures were incubated for 50 min at 50°C and terminated for 5 min at 85°C. Finally, the mixtures were treated with Escherichia coli RNase for 20 min at 37°C. PCR amplifications of porcine total cDNA were carried out for 30 cycles, and a variable amount of the cDNA was used in a total volume of 20 µL of a PCR mixture using SuperScript III First-Strand Synthesis System for RT-PCR (real-time polymerase chain reaction) (Invitrogen, Carlsbad, CA, USA). Specific TaqMan primers and probes for TLR4 (Ss03389780_ml) and for YWHAZ (Ss03216374_m1) were obtained from Applied Biosystems (Applied Biosystems, Foster City, CA, USA). cDNA was amplified in 1× Universal Master Mix (Applied Biosystems) with gene-specific primers and a probe on the 7500 Real-Time PCR System (Applied Biosystems, USA), according to the manufacturer’s instructions. Thermal cycling conditions were as follows: 50°C for 2 min and 95°C for 10 min, followed by 45 cycles of 95°C for 15 s and 60°C for 1 min. Data were analyzed using the 7500 Real-Time PCR System (Applied Biosystems). The expression of each gene was normalized against mRNA expression of the housekeeping gene GAPDH (glyceraldehyde-3-phosphate dehydrogenase). Real-time PCR experiments for each gene were performed on three separate occasions.
Statistics
Data are presented as the mean and standard deviation or the median and interquartile ranges (25th and 75th percentiles), respectively. The distribution of the variables was not normal based on a Shapiro–Wilk test. For the RQ (relative quantification) TLR4, IL-6, IL-10, TNF-alpha, and HMGB1 data, the Box–Cox transformation was applied to stabilize variances. Repeated measures analysis of variance (ANOVA) and post-hoc analysis were used to investigate changes in recorded parameters over time points. The one-way ANOVA and post-hoc test were used to determine whether there are any statistically significant differences between the means of three independent groups (sham, control, and treatment groups). A P-value of <0.05 was considered statistically significant. Categorical variables were analyzed using Pearson’s chi-squared test or Fisher’s exact test when necessary. Data were analyzed with Statistica 12.0 (StatSoft Inc., Tulsa, OK, USA).
Results
General findings
All animals survived the preparation and challenge with aortic clamping for 90 min, as well as the entire observation period. During clamping, the animals were hypertensive; during reperfusion, all animals exhibited some degree of hypotension, which was counteracted by fluids and inotropic support. Additional fluids were given to target filling pressures above 6 mmHg, which was similar in the treatment and control groups. Animals in each group needed variable doses of norepinephrine in order to maintain a MAP over 60 mmHg.
Circulatory effects and oxygen transport
The baseline hemodynamic variables were similar in the three groups (Table 1). The aortic clamp release resulted in a significant increase in pulmonary vascular resistance in the control animals and a decreased cardiac index with sustained wedge pressure, and this tendency continued until the end of the observation period. Significantly, lower PVRI was noted in the treatment after 10 h of observation. In addition, the treatment group animals exhibited an increased cardiac index after 10 h, which was sustained until the end of the observation period. The cardiac index in this group was significantly higher compared to the control group. Despite a drop in systemic vascular resistance, the mean arterial pressure was essentially maintained with no difference between groups. Additionally, the treatment group animals exhibited a significantly higher DO2 (oxygen delivery) after 10 h. These differences occurred although there were no differences in the static preload indices, pulmonary artery wedge pressure, and CVP (Table 1). No significant difference between the control and treatment groups was noted in the cumulative noradrenaline dosage (Table 2).
Table 1.
Systemic hemodynamics and oxygen transport parameters recorded at baseline (T0), at 15 min, and at 1, 10, and 20 h after reperfusion.
| Parameter | Sampling time | |||||
|---|---|---|---|---|---|---|
| Group | Baseline T0 | Reperfusion 15 min | Reperfusion 1 h | Reperfusion 10 h | Reperfusion 20 h | |
| HR (beat/min) | Treatment | 97.0 ± 11.7 | 121.6 ± 33.1 | 119.9 ± 34.3 | 108.5 ± 32.4 | 104.8 ± 27.3 |
| Control | 95.4 ± 6.6 | 109.8 ± 26.6 | 115.5 ± 30.4 | 112.3 ± 26.2 | 89.9 ± 33.6 | |
| Sham | 103.7 ± 9.2 | 103.7 ± 9.2 | 89.3 ± 1.5 | 96.7 ± 15.1 | 90.3 ± 16.5 | |
| MAP (mmHg) | Treatment | 78.5 ± 25.3 | 73.3 ± 15.9 | 64.9 ± 14.9 | 74.1 ± 19.9 | 71.3 ± 13.5 |
| Control | 94.9 ± 17 | 74.3 ± 15.7 | 67.1 ± 10.4 | 75.3 ± 15 | 72 ± 19.8 | |
| Sham | 80.3 ± 13.7 | 80.3 ± 13.7 | 81 ± 4.6 | 73 ± 6.6 | 76.3 ± 9.10 | |
| MPAP (mmHg) | Treatment | 19.0 ± 5.4 | 18.0 ± 6.1 | 18.0 ± 6.1 | 20.5 ± 4.5 | 19.5 ± 4.2 |
| Control | 17.6 ± 3.9 | 21 ± 4.2 | 21 ± 4.2 | 25.4 ± 7.3 | 23 ± 7.1 | |
| Sham | 17.7 ± 0.5 | 20.3 ± 4.0 | 20.3 ± 4.0 | 19.7 ± 1.5 | 22 ± 3.6 | |
| CVP (mmHg) | Treatment | 7.4 ± 3.0 | 6.9 ± 2.9 | 7.5 ± 3.1 | 7.0 ± 2.5 | 9.4 ± 2.5 |
| Control | 7.2 ± 2.4 | 5.9 ± 2.1 | 5.4 ± 2.3 | 7.1 ± 2.9 | 9.1 ± 3.4 | |
| Sham | 8.3 ± 2.5 | 8.3 ± 2.5 | 6.7 ± 0.6 | 8.3 ± 3.2 | 10.7 ± 4.6 | |
| PCWP (mmHg) | Treatment | 9.1 ± 3.2 | 9.4 ± 3.9 | 7.6 ± 3.4 | 9.6 ± 2.3 | 11.1 ± 2.9 |
| Control | 8.3 ± 1.8 | 9.3 ± 2.6 | 8.4 ± 5.2 | 9.4 ± 3.7 | 12.0 ± 4.7 | |
| Sham | 9.7 ± 1.5 | 9.7 ± 1.5 | 10.0 ± 1.0 | 8.3 ± 1.5 | 11.5 ± 2.1 | |
| CI (L/min/m2) | Treatment | 3.1 ± 0.8 | 2.9 ± 0.8 | 2.4 ± 0.9 | 3.3 ± 0.8* | 3.9 ± 1.0* |
| Control | 3.3 ± 0.6 | 2.3 ± 0.3 | 2.4 ± 0.7 | 2.4 ± 0.6 | 2.5 ± 1.3 | |
| Sham | 2.7 ± 0.2 | 2.7 ± 0.2 | 3.1 ± 0.5 | 3.2 ± 0.3 | 3.5 ± 0.9 | |
| SVI (mL/m2) | Treatment | 32.3 ± 9.5 | 25.2 ± 10.6 | 22.2 ± 9.5 | 32 ± 8.9* | 38.9 ± 10.7 |
| Control | 34.2 ± 6.1 | 22.4 ± 5.7 | 21 ± 5.1 | 21.6 ± 6.2 | 27.1 ± 8.0 | |
| Sham | 26 ± 4.4 | 26 ± 4.4 | 34.8 ± 5.3 | 33.4 ± 6.7 | 39.1 ± 6.1 | |
| SVRI (dyn s/cm5/m2) | Treatment | 1912 ± 773 | 1965 ± 728 | 1973 ± 594 | 1717 ± 628 | 1304 ± 365* |
| Control | 2168 ± 353 | 2352 ± 840 | 2228 ± 603 | 2449 ± 840 | 2499 ± 1691 | |
| Sham | 2193 ± 658 | 2193 ± 658 | 1939 ± 314 | 1632 ± 98 | 1510 ± 155 | |
| PVRI (dyn s/cm5/m2) | Treatment | 268 ± 96 | 259 ± 106 | 354 ± 158 | 256 ± 83* | 180 ± 84 |
| Control | 238 ± 129 | 405 ± 190 | 467 ± 191 | 606 ± 298 | 433 ± 300 | |
| Sham | 240 ± 33 | 240 ± 33 | 277 ± 159 | 286 ± 26 | 245 ± 80 | |
| DO2I (mL/min/m2) | Treatment | 412 ± 62 | 394 ± 92 | 358 ± 114 | 462 ± 75* | 464 ± 117 |
| Control | 416 ± 78 | 330 ± 26 | 377 ± 97 | 357 ± 86 | 329 ± 165 | |
| Sham | 350 ± 20 | 350 ± 20 | 407 ± 42 | 403 ± 31 | 403 ± 103 | |
| VO2I (mL/min/m2) | Treatment | 104 ± 29 | 115 ± 51 | 93 ± 42 | 105 ± 46 | 162 ± 79 |
| Control | 115 ± 23 | 85 ± 20 | 109 ± 57 | 84 ± 34 | 98 ± 41 | |
| Sham | 126 ± 39 | 126 ± 39 | 121 ± 53 | 113 ± 9 | 199 ± 131 | |
Treatment: ischemia with treatment iNO + IV steroid; Control: ischemia only; Sham: surgery but no ischemia period; HR: heart rate; MAP: mean arterial pressure; MPAP: mean pulmonary arterial pressure; CVP: central venous pressure; PCWP: pulmonary capillary wedge pressure; CI: cardiac index; SVI: stroke volume index; SVRI: systemic vascular resistance index; PVRI: pulmonary vascular resistance index; DO2I: oxygen delivery index; VO2I: oxygen consumption index; iNO: inhaled nitric oxide; IV: intravenous.
Values given as means ± standard deviations.
P < 0.05, the difference between the treatment and control groups.
Table 2.
Weight and body surface area (BSA) of animals recorded at baseline and total diuresis and noradrenaline dose recorded at 20 h after reperfusion.
| Group | Weight (kg) | BSA (m2) | Diuresis (mL) | Total noradrenaline dosage (μg) |
|---|---|---|---|---|
| Treatment | 22.9 ± 2.1 | 0.787 ± 0.044 | 901. ± 901.9* | 3556.5 ± 4126.3§ |
| Control | 21.75 ± 2.1 | 0.759 ± 0.06 | 266.3 ± 298.03# | 3057.5 ± 3827.5# |
| Sham | 21.5 ± 0.5 | 0.760 ± 0.01 | 946.7 ± 175.0 | 520.0 ± 900.7 |
Treatment: ischemia with treatment iNO ± IV steroid; Control: ischemia only; Sham: surgery but no ischemia period; iNO: inhaled nitric oxide; IV: intravenous.
Values given as means ± standard deviations.
P < 0.05, the difference between the treatment and control groups; #P < 0.05, the difference between the control and sham groups; §P < 0.05, the difference between the treatment and sham groups.
Respiratory indices and blood gas analysis
No significant effects of iNO and IV steroid treatment were seen in respiratory indices and blood gas analyses. In both the control and treatment groups, at 15 min and 1 h after the cross-clamp release, a significant decrease in arterial pH and BE was noted, with a simultaneous increase in arterial lactate levels. A gradual return to near-baseline values was seen in the subsequent hours of observation with no differences between the cross-clamp groups (Supplementary Table 3).
Blood count and biochemistry
Hemoglobin, hematocrit, white blood cells (WBC), neutrophils, and platelet counts were similar in all three groups throughout the entire period of observation (Supplementary Table 4). Gradual increases were seen in the AST and ALT in both intervention groups, with maximal values at the end of the observation period. No significant differences were noted between study groups (Supplementary Table 4).
Renal function, electrolytes, and renal TLR4 mRNA expression
Diuresis throughout the 20 h of observation was similar in all three groups. An accurate measurement of urine output was hampered due to the presence of blood in the urine caused by the kidney biopsy. Gradual and significant increases were seen in serum creatinine concentration in both study groups with no significant difference between groups. Significant differences between intervention groups in serum potassium and sodium were noted at the end of the observation period, with extremely high potassium levels of up to 7.4 mmol/L in the control group (Supplementary Table 5).
To determine whether kidney ischemia stimulated TLR4 up-regulation, we measured the mRNA expression of TLR4 in an I/R injury kidney using real-time PCR. At baseline, TLR4 mRNA expression was low, and no significant differences between the three studied groups were noted. Aortic clamping increased TLR4 mRNA expression in ischemic kidneys; however, statistically significant changes were recorded only in the control group (P = 0.016). Treatment with iNO and hydrocortisone reduced TLR4 mRNA expression to pre-ischemic conditions, and the difference observed in mRNA expression was significant between the control and treatment groups after 3 h (P = 0.042) (Figure 2). No kidney biopsy was obtained for the sham animals after 3 h to avoid stimulation of the immune system and possible bleeding. TLR4 expression measured after the next 17 h of reperfusion, sampled during the autopsy from the cortex part of the kidney, returned to baseline values; no significant differences in TLR4 mRNA expression were noted between the three studied groups at 20 h (Figure 2).
Figure 2.
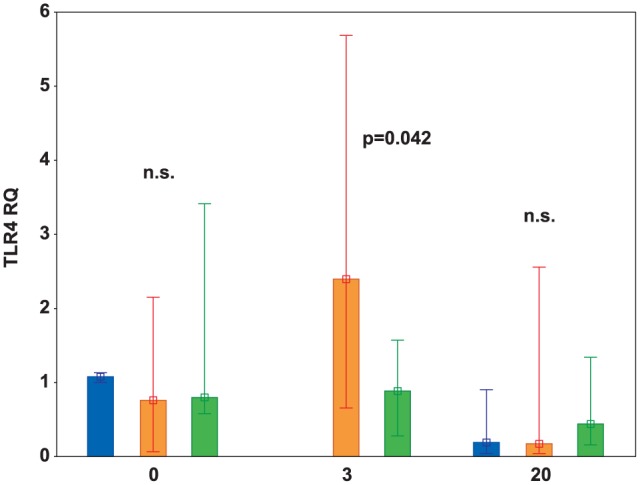
TLR4 mRNA relative expression (RQ) in sham (blue bars), control (orange bars), and treatment (green bars) groups, respectively, recorded at baseline (0), 3 h, and 20 h.
TLR4 mRNA relative expression (RQ) was significantly elevated in the control group as compared to the treatment group after 3 h of the experiment (P = 0.042). Data are expressed as median (middle point) and interquartile ranges (whiskers). A P-value at time points 0 and 20 h represents a comparison between the sham, control, and treatment groups; the P-value at 3 h represents a comparison between the control and treatment groups.
Mediators of systemic inflammatory response
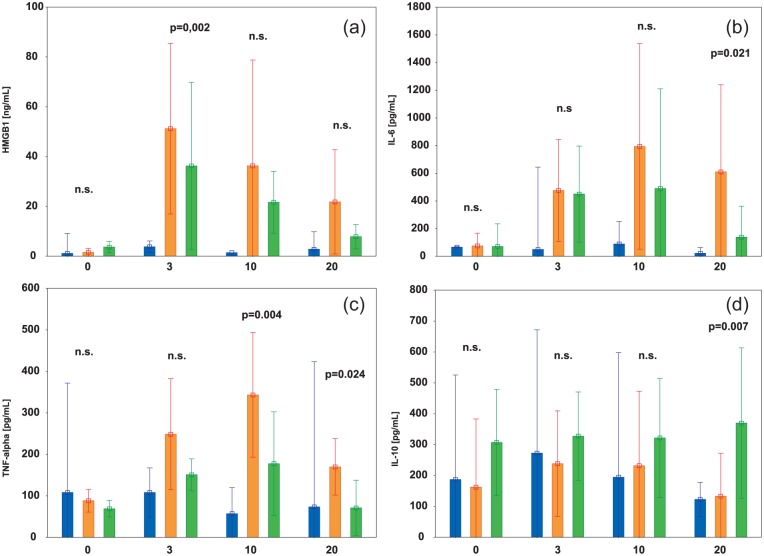
The baseline values of HMGB1, IL-6, TNF-alpha, and IL-10 were low in all study groups. The levels of these mediators remained low in the sham group but changed significantly in the control (HMGB1, P = 0.004; IL-6, P < 0.001; TNF-alpha, P < 0.001; IL-10, P < 0.001) and treatment groups (HMGB1, P = 0.005; IL-6, P < 0.001; TNF-alpha, P < 0.001; IL-10, P < 0.001) during the experiment (Figure 3).
Figure 3.
Changes in HMGB1 (a), interleukin 6 (IL-6) (b), tumor necrosis factor-alpha (TNF-alpha) (c), and interleukin 10 (IL-10) (d) in the sham (blue bars), control (orange bars), and treatment (green bars) groups, respectively, recorded at baseline, 3, 10, and 20 h.
Data are expressed as median (middle point) and interquartile ranges (whiskers). The P-values represent the results of the post-hoc test for the control and treatment groups. (For clarity of the figure, P-values representing the results of the post-hoc test for the sham and control and for the sham and treatment groups were not shown). Treatment: ischemia with treatment iNO + IV steroid; control: ischemia only; sham: surgery but no ischemia period.
The level of HMGB1 was higher in the control group than in the treatment group during the whole ischemia/reperfusion, but observed difference reached statistical significance only at 3 h (P = 0.002) (Figure 3(a)). Similarly, the levels of pro-inflammatory cytokines IL-6 and TNF-alpha were higher in the control group than in the treatment group; post-hoc testing showed a statistically significant difference for IL-6 only at 20 h (P = 0.021) and for TNF-alpha at 10 h (P = 0.004) and 20 h (P = 0.024) (Figure 3(b) and (c)). In contrast, an anti-inflammatory IL-10 level was higher in the treatment group than in the control, but a statistically significant difference was observed at 20 h (P = 0.007) (Figure 3(d)).
Discussion
Based on our previous experience with a prolonged lipopolysaccharide (LPS) challenge in a porcine model, a combination of both inhaled NO and IV steroids, but not therapy with iNO alone or IV steroids alone, may be associated with protection of kidney function.17 Here, we have shown that a combination of inhaled NO and IV steroids had significant multifactorial effects, expressed in part as a diminished systemic inflammatory response and renal mRNA TLR4 expression in SRACC-induced I/R injury. Additional findings demonstrated that the ischemia/reperfusion-induced pulmonary hypertension with an increase in pulmonary vascular resistance was counteracted by the perioperative combined treatment, and improved hemodynamic status was manifested by increased cardiac output and total DO2.
Our study was designed to mimic clinical conditions, with an initial ischemic insult followed by resuscitative interventions initiated in real-time over a prolonged 20-h observational study period. Our protocol allowed specific clinical interventions, including hypotension and hypovolemia corrections with designated therapeutic goals. Most of the animals required vasopressor support in addition to increased crystalloid administration in order to maintain a MAP above 60 mmHg. At present, it is not entirely clear how all those interventions influenced the possibility to detect statistically significant differences in the pathophysiology of an inflammatory response in the three studied groups.
The proper assessment of renal function and the dynamics of acute kidney injury (AKI) in our study was made challenging by a lack of optimal diuresis measurements. This problem was caused by the double renal biopsy in each animal, which caused hematuria and unwanted clot formation in the urine. Serum creatinine increased in both groups over time, but in spite of lower numerical values in the treated group, no statistical significance was seen. In contrast, the profile of electrolytes sodium and potassium at the end of the experiment clearly showed the statistically significant and beneficial effects of double treatment on renal function. In the control group, potassium concentration reached a critical level of over 7 mmol/L. Significant hyponatremia in this group was also noted.
In the context of sepsis, kidney expression of TLR4 is critical in mediating LPS-induced acute kidney failure via pro-inflammatory cytokine release and subsequent kidney damage.6 The activation of TLRs plays a pivotal role in an ischemic kidney reperfusion injury. Wu et al.18 used a mouse model of kidney I/R injury and found a significant increase in TLR4 expression by tubular epithelial cells (TECs) and infiltration of leukocytes within the kidney after ischemia. In this study, we found an increased mRNA TLR4 expression in the kidneys of ischemic animals from biopsies taken after 3 h of reperfusion. Note the significantly lower expression in the treatment group (Figure 2), suggesting the potential protective effects of the therapy. Chen et al. found that at least two different cell types express TLR4, each of which contributes to renal injury by temporally different mechanisms during ischemic AKI. Ischemic AKI triggers an inflammatory response, which exacerbates the injury and requires the increased expression of endothelial adhesion molecules. Chen and colleagues’ results showed increased mRNA TLR4 expression on endothelial cells of the vasa recta of the inner stripe of the outer medulla of the kidney 4 h after reperfusion. Furthermore, the addition of HMGB1 protein led to an increase in adhesion molecule expression on endothelia isolated from wild-type but not TLR4 knockout mice.19
The first and second kidney biopsies performed during this study included both the cortex and medulla of the renal tissue, which may explain the increase in mRNA TLR4 expression, which is consistent with the observations of others. In contrast, TLR4 mRNA expression measured from the last and third sampling performed during autopsy and taken from the cortex part of the kidney was again close to the baseline values in all our study groups. It is now known that TLR4 is constitutively expressed in both proximal and distal tubules, the thin limb of the loop of Henle, and the collecting ducts. Expression is up-regulated in these areas post I/R.20 Therefore, the observed lack of renal cortex TLR4 mRNA expression in the last samplings (20 h) in this study can be explained by the observations found in other studies.19,21 Of further note, TLR4 appeared in cortex proximal tubules and outer medulla rather late and after 24 h of reperfusion in the study by Wu et al.18 In these reports, TLR4 mRNA expression was significantly increased at days 1 and 3 after ischemia, with further up-regulation through days 5 and 9. Since our study included only 20 h of observation, this could explain the absence of TLR4 expression in the renal cortex of animals subjected to SRACC.
Our intervention of 90 min of aortic occlusion resulted in a severe inflammatory response characterized by increased activity of pro-inflammatory cytokines and accompanied by the dynamic release of HMGB1. HMGB1 can be passively released from necrotic cells or secreted by activated immune cells into the extracellular space. It was shown that HMGB1 acts as the link between the initiation of cell damage and the inflammatory cascade during renal I/R injury. Activation of TLR4 by HMGB1 causes interaction with NF-κB and facilitates the active release of other inflammatory factors, such as IL-1, -6, and -8, TGF-β, TNF-α, and MCP-1 (monocyte chemotactic protein 1). This ligand-receptor interaction forms an inflammatory cascade and released inflammatory factors bind to their receptors on renal tubular cells, causing their further damage.22–24 Serum HMGB1 release after the ischemic insult was similar in both study groups, but the inflammatory response monitored with cytokine profiles was significantly decelerated in the treatment group. Since HMGB1 has an important role in an I/R injury, as a factor of renal TLR4 activation and damage, it is unclear how this treatment protocol affected the weakening of TLR4 expression in the kidneys of the studied animals, as seen from the biopsies sampled after 3 h of reperfusion.
TLR4 has been shown to play an essential role in post-ischemic renal IL-6 production.24 In a study by Chen et al.,19 only leukocytes from TLR 4 (+/+) mice produced IL-6 in response to HMGB1 protein. Reno-protective effects were also reported with the administration of anti-IL-6 mAb. This approach significantly inhibited the production of pro-inflammatory mediators and tissue infiltration by neutrophils.25 Similar observations have been made on the role of TNF-α as a cytokine centrally involved in the development of I/R injury-induced inflammation and apoptosis.26 IL-10 is a potent anti-inflammatory cytokine that inhibits an inflammatory response. Wan et al.27 investigated the role of IL-10 in kidney repair after a renal I/R injury, revealing an important role for IL-10 in the improvement of renal I/R injury by acting through the suppression of inflammatory mediators.
Evidence from several experimental and clinical studies supports the presence of systemic actions of iNO in various organs distant from the lungs.28–30 Recently, Junot et al.31 reported significant improvements in renal blood flow, glomerular filtration rate, and urinary output following inhalation of NO in a model of renal impairment induced by ketoprofen during general anesthesia.
Combined iNO and steroid treatment was suggested by Da et al.16 in a 6-h porcine endotoxin challenge model. The study revealed that endotoxin infusion down-regulated expression of the glucocorticoid receptor in lung, liver, and kidney tissues concomitant with the up-regulation of inflammatory markers such as NF-κB and TNF-α. Simultaneous administration of 30 ppm inhaled NO and glucocorticoid in this sepsis model inhibited the inflammatory response not only in the lungs but also in systemic organs. It may be suggested that iNO stimulates up-regulation of glucocorticoid receptors, making steroid therapy more effective in sepsis. In our previously published study, with 20 h of observation of a porcine endotoxin challenge model, we could not confirm all beneficial observations reported by Da et al. However, we found that combined early therapy with iNO 30 ppm and corticosteroids was associated with the partial protection of organ functions.17 Both studies indicated that the beneficial therapeutic effects were obtained only with the combination of iNO + steroids therapy, while steroids alone or iNO alone did not have any clinically significant effect on the improvement of organ function. In other groups of randomization, administration of steroids or iNO alone did not improve organ damage.16,17
Encouraging results of the sepsis model prompted us to test this regimen on severe ischemia induced by a 90-min SRACC pig model. Important factors concerning the use of iNO for the modulation of an I/R injury include implementation time, optimal dosage, and duration of the treatment. It is not known which doses of iNO and IV steroids are optimal for a clinical effect, and it has not been determined whether a preemptive treatment strategy would yield a stronger effect. Results from several studies suggest that iNO concentrations required to obtain systemic effects are higher than those required to obtain pulmonary vascular effects. NO in a concentration of 80 ppm administered before and during coronary reperfusion improved endocardial and epicardial blood flow in the infarct zone, improved microvascular perfusion, decreased leukocyte infiltration, reduced infarct size, and decreased cardiomyocyte apoptosis in the infarct border zone in the experimental myocardial I/R pig model.32 Ng et al.33 reported an improvement of intestinal blood flow after administration of 80 ppm iNO in the feline model of I/R. In a clinical study by Lang et al., administration of 80 ppm iNO perioperatively improved parameters of liver function after transplantation and decreased hospital length of stay.34 The same dose was used in another clinical study evaluating the potential of iNO to attenuate inflammation initiated by I/R in a human model using patients undergoing knee surgery.30
All of these considerations prompted us to use iNO as a preemptive treatment with the additional assumption that high concentrations of the gas would allow more effective loading of NO metabolites before an ischemic insult. In our experiment, 80 ppm NO was started 30 min before SRACC and continued for 2 h after SRACC release, followed by iNO decreased to 30 ppm until the end of the 20-h observation period.
We did not focus on mechanisms involved in combining iNO and IV steroid treatment. Both inhaled NO and steroids have been shown to diminish inflammatory responses independently in different models of organ I/R injury.34,35 Steroid therapy protected human kidney proximal tubular cells during an I/R injury and induced apoptosis by rapid and transient phosphorylation of extracellular signal-regulated kinase 1/2, which required the presence of the glucocorticoid receptor and activation of the endothelial NO synthase system via the phosphoinositide 3-kinase and protein kinase B pathways.36 Several experimental studies have been designed with the goal of demonstrating preserved endothelial function by increasing renal NO activity. It was shown that administration of L-arginine, the NO donor molsidomine, or the eNOS cofactor tetrahydrobiopterin can preserve renal vascular perfusion and attenuate AKI induced by an I/R injury.21 Assuming iNO can exert distant effects outside the lung through active metabolites, it is further suggested that inhaled NO extra-pulmonary activity may not have hemodynamic effects on normally perfused tissue but may increase blood flow selectively in hypoperfused tissue without any effect on systemic blood pressure. Because of these properties, iNO may serve as a rescue therapy for ischemic conditions in which collateral blood flow is important or until interventional or spontaneous reperfusion occurs.13
In conclusion, this study found that a therapeutic option of a combination of inhaled NO and IV steroids induced significant multifactorial effects with improvements in hemodynamics and oxygen transport while also diminishing the systemic inflammatory response in an experimental model of SRACC-induced I/R injury.
Supplementary Material
Acknowledgments
The authors thank Lukasz Strozecki, MSc for his help with statistical analysis.
Footnotes
Declaration of conflicting interests: Claes Frostell declares financial interest in the clinical use of iNO. The other authors declare no conflict of interest.
Funding: This work was supported by Claes Frostell Research & Consulting AB.
ORCID iD: Piotr Skrzypczak  https://orcid.org/0000-0002-7586-0879
https://orcid.org/0000-0002-7586-0879
References
- 1. Kalogeris T, Baines CP, Krenz M, et al. (2012) Cell biology of ischemia/reperfusion injury. International Review of Cell and Molecular Biology 298: 229–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yeung KK, Groeneveld M, Lu JJ, et al. (2016) Organ protection during aortic cross-clamping. Best Practice and Research Clinical Anaesthesiology 30: 305–315. [DOI] [PubMed] [Google Scholar]
- 3. Akira S, Takeda K. (2004) Toll-like receptor signaling. Nature Reviews Immunology 4: 499–511. [DOI] [PubMed] [Google Scholar]
- 4. Bianchi ME. (2007) DAMPs, PAMPs and alarmins: All we need to know about danger. Journal of Leukocyte Biology 81: 1–5. [DOI] [PubMed] [Google Scholar]
- 5. Arumugam TV, Okun E, Tang SC, et al. (2009) Toll-like receptors in ischemia-reperfusion injury. Shock 32: 4–16. [DOI] [PubMed] [Google Scholar]
- 6. Cunningham PN, Wang Y, Guo R, et al. (2004) Role of toll-like receptor 4 in endotoxin-induced acute renal failure. Journal of Immunology 172: 2629–2635. [DOI] [PubMed] [Google Scholar]
- 7. Rosin DL, Okusa MD. (2011) Dangers within: DAMP responses to damage and cell death in kidney disease. Journal of the American Society of Nephrology 22: 416–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Prieto-Moure B, Lloris-Carsí JM, Belda-Antolí M, et al. (2017) Allopurinol protective effect of renal ischemia by downregulating TNF-α, IL-1β, and IL-6 response. Journal of Investigative Surgery 30: 143–151. [DOI] [PubMed] [Google Scholar]
- 9. Tello D, Balsa E, Acosta-Iborra B. (2011) Induction of the mitochondrial NDUFA4L2 protein by HIF-1alpha decreases oxygen consumption by inhibiting Complex I activity. Cell Metabolism 14: 768–779. [DOI] [PubMed] [Google Scholar]
- 10. Wei Q, Liu Y, Liu P. (2016) MicroRNA-489 Induction by hypoxia-inducible factor-1 protects against ischemic kidney injury. Journal of the American Society of Nephrology 27: 2784–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Roberts BW, Mitchell J, Kilgannon JH, et al. (2013) Nitric oxide donor agents for the treatment of ischemia/reperfusion injury in human subjects: A systematic review. Shock 39: 229–239. [DOI] [PubMed] [Google Scholar]
- 12. Weitzberg E, Hezel M, Lundberg JO. (2010) Nitrate-nitrite-nitric oxide pathway: Implications for anesthesiology and intensive care. Anesthesiology 113: 1460–1475. [DOI] [PubMed] [Google Scholar]
- 13. Gozdzik W, Gozdzik A. (2012) Inhaled nitric oxide effects outside the lungs—experimental and clinical evidence. Polish Journal of Thoracic and Cardiovascular Surgery 9: 456–462. [Google Scholar]
- 14. Phillips L, Toledo AH, Lopez-Neblina F, et al. (2009) Nitric oxide mechanism of protection in ischemia and reperfusion injury. Journal of Investigative Surgery 22: 46–55. [DOI] [PubMed] [Google Scholar]
- 15. Zhang J, Yao Y, Xiao F, et al. (2013) Administration of dexamethasone protects mice against ischemia/reperfusion induced renal injury by suppressing PI3K/AKT signaling. International Journal of Clinical and Experimental Pathology 6: 2366–2375. [PMC free article] [PubMed] [Google Scholar]
- 16. Da J, Chen L, Hedenstierna G. (2007) Nitric oxide up-regulates the glucocorticoid receptor and blunts the inflammatory reaction in porcine endotoxin sepsis. Critical Care Medicine 35: 26–32. [DOI] [PubMed] [Google Scholar]
- 17. Göranson SP, Goździk W, Harbut P, et al. (2014) Organ dysfunction among piglets treated with inhaled nitric oxide and intravenous hydrocortisone during prolonged endotoxin infusion. PLoS ONE 9: e96594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu H, Chen G, Wyburn KR, et al. (2007) TLR4 activation mediates kidney ischemia/reperfusion injury. Journal of Clinical Investigation 117: 2847–2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen J, John R, Richardson JA, et al. (2011) Toll-like receptor 4 regulates early endothelial activation during ischemic acute kidney injury. Kidney International 79: 288–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Arslan F, Keogh B, McGuirk P, et al. (2010) TLR2 and TLR4 in ischemia reperfusion injury. Mediators of Inflammation 2010: 704202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Basile DP, Anderson MD, Sutton TA. (2012) Pathophysiology of acute kidney injury. Comprehensive Physiology 2: 1303–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Scaffidi P, Misteli T, Bianchi ME. (2001) Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418: 191–195. [DOI] [PubMed] [Google Scholar]
- 23. Kielar ML, John R, Bennett M. (2005) Maladaptive role of IL-6 in ischemic acute renal failure. Journal of the American Society of Nephrology 16: 3315–3325. [DOI] [PubMed] [Google Scholar]
- 24. Zhang J, Xia J, Zhang Y, et al. (2016) HMGB1-TLR4 signaling participates in renal ischemia reperfusion injury and could be attenuated by dexamethasone-mediated inhibition of the ERK/NF-κB pathway. American Journal of Translational Research 8: 4054–4067. [PMC free article] [PubMed] [Google Scholar]
- 25. Patel NS, Chatterjee PK, Di Paola R, et al. (2005) Endogenous interleukin-6 enhances the renal injury, dysfunction, and inflammation caused by ischemia/reperfusion. Journal of Pharmacology and Experimental Therapeutics 312: 1170–1178. [DOI] [PubMed] [Google Scholar]
- 26. Di Paola R, Genovese T, Impellizzeri D, et al. (2013) The renal injury and inflammation caused by ischemia-reperfusion are reduced by genetic inhibition of TNF-aR1: A comparison with infliximab treatment. European Journal of Pharmacology 700: 134–146. [DOI] [PubMed] [Google Scholar]
- 27. Wan X, Huang WJ, Chen W, et al. (2014) IL-10 deficiency increases renal ischemia-reperfusion injury. Nephron Experimental Nephrology 128: 37–45. [DOI] [PubMed] [Google Scholar]
- 28. Derwall M, Ebeling A, Nolte KW, et al. (2015) Inhaled nitric oxide improves transpulmonary blood flow and clinical outcomes after prolonged cardiac arrest: A large animal study. Critical Care 19: 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brücken A, Derwall M, Bleilevens C, et al. (2015) Brief inhalation of nitric oxide increases resuscitation success and improves 7-day-survival after cardiac arrest in rats: A randomized controlled animal study. Critical Care 19: 408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mathru M, Huda R, Solanki DR, et al. (2007) Inhaled nitric oxide attenuates reperfusion inflammatory responses in humans. Anesthesiology 106: 275–282. [DOI] [PubMed] [Google Scholar]
- 31. Junot S, Keroak S, Del Castillo JRE, et al. (2017) Inhaled nitric oxide prevents NSAID-induced renal impairment in pseudo-normovolaemic piglets. PLoS ONE 12: e0179475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu X, Huang Y, Pokreisz P, et al. (2007) Nitric oxide inhalation improves microvascular flow and decreases infarction size after myocardial ischemia and reperfusion. Journal of the American College of Cardiology 50: 808–817. [DOI] [PubMed] [Google Scholar]
- 33. Ng ES, Jourd’heuil D, McCord JM, et al. (2004) Enhanced S-nitroso-albumin formation from inhaled NO during ischemia/reperfusion. Circulation Research 94: 559–565. [DOI] [PubMed] [Google Scholar]
- 34. Lang JD, Jr, Teng X, Chumley P, et al. (2007) Inhaled NO accelerates restoration of liver function in adults following orthotropic liver transplantation. The Journal of Clinical Investigation 117: 2583-2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Checchia PA, Bronicki RA, Muenzer JT, et al. (2013) Nitric oxide delivery during cardiopulmonary bypass reduces postoperative morbidity in children: a randomized trial. Journal of Thoracic and Cardiovascular Surgery 146: 530–536. [DOI] [PubMed] [Google Scholar]
- 36. Kumar S, Allen DA, Kieswich JE, et al. (2009) Dexamethasone ameliorates renal ischemia-reperfusion injury. Journal of the American Society of Nephrology 20: 2412–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.