Abstract
Background:
Despite increased attention for palliative care in dementia, recent studies found burdensome symptoms and unmet family caregiver needs in the last phase of life. Feedback is being used to improve the quality of palliative care, but we do not know how effective it is.
Aim:
To assess the effect of two feedback strategies on perceived quality of end-of-life care and comfort in dying nursing home residents with dementia.
Methods:
In a cluster-randomized controlled trial, the End-of-Life in Dementia–Satisfaction With Care and the End-of-Life in Dementia–Comfort Assessment in Dying scales were completed by bereaved family caregivers of residents with dementia of 18 Dutch nursing homes. Two feedback strategies, generic feedback with mean End-of-Life in Dementia-scores and feedback with individual (patient-specific) End-of-Life in Dementia-scores, were compared to no feedback provided. The intervention groups discussed End-of-Life in Dementia-ratings in team meetings and formulated actions to improve care. Multi-level analyses assessed effects.
Results:
A total of 668 families rated the End-of-Life in Dementia–instruments. Compared to no feedback, the generic strategy resulted in lower quality of end-of-life care in unadjusted (B = −1.65, confidence interval = −3.27; −0.21) and adjusted analyses (B = −2.41, confidence interval = −4.07; −0.76), while there was no effect on comfort. The patient-specific strategy did not affect the quality of end-of-life care, but it increased comfort in unadjusted analyses (only, B = 2.20, confidence interval = 0.15; 4.39; adjusted: B = 1.88, confidence interval = −0.34; 4.10).
Conclusion:
Neither feedback strategy improved end-of-life outcome. Perhaps, skills to translate the feedback into care improvement actions were insufficient. Feedback with favorable family ratings might even have triggered opposite effects. Trial number: NTR3942.
Keywords: Palliative care, dementia, quality improvement, nursing homes, quality of health care
What is already known about the topic?
Audit and feedback is a proven method to evaluate and improve quality of palliative care, although not in the context of palliative care for nursing home residents dying with dementia.
Despite some promising trends in research of improved levels of comfort among nursing home residents dying with dementia, the literature found symptom management in the last phase of life still to be suboptimal as well as family caregivers reporting unmet needs.
What this paper adds?
The Feedback on End-of-Life in Dementia (FOLlow-up) study developed, tested, and compared two active feedback strategies for nursing home professionals to improve family caregivers’ perceptions of quality of care and quality of dying in dementia.
Our results showed that providing nursing homes professionals with a feedback report based on generic performance scores that were compared with a norm together with instructions to improve care performance that scored lower than the norm had no or even opposite effect on family caregivers’ perceptions of quality of care and quality of dying with dementia.
Providing nursing home professionals with patient-specific feedback of family caregivers’ perceptions of quality of care and quality of dying was found to have a small but not clinically relevant effect on quality of dying.
Implications for practice, theory, or policy
More research is needed to develop and test feedback strategies that are more adequate for nursing home professionals to change their behavior and that are robust to the contextual challenges of nursing homes.
Our findings and recommendations may guide future research initiatives to implement and test feedback strategies, as well as inspire care professionals to systematically evaluate and improve care quality using audit and feedback not only in the context of palliative care but also in other care fields.
Introduction
Research on end-of-life care increasingly addresses dementia care. Encouraging trends of improved care and outcomes have been reported, such as improved family satisfaction with care over time,1 decreased use of tube feeding,2 and lower levels of discomfort in pneumonia.3 However, longitudinal studies show that burdensome symptoms, such as pain, agitation, and pressure ulcers frequently occur during the last phase of life with dementia.4–7 Furthermore, studies on family caregivers of nursing home residents with advanced dementia report unmet needs, for example, with respect to communication with physicians and support of shared decision-making.8
Feedback is a well-known strategy to improve professional care practice. In specialist palliative care settings, feedback with patients’ reports of their own health and well-being with standardized and validated questionnaires is widely used. A systematic review of Etkind et al.9 reported audit and feedback to be an effective tool to improve care processes such as better symptom recognition, improved patient-physician communication, and improved caregiver outcomes. The literature assumes audit and feedback to be more effective when accompanied by interventions, whether active or passive (such as educational outreach or publication of performance), but more research that compares different feedback strategies is needed.10 In the nursing home context, Castle11 and Vandenberg et al.12 reported about the usefulness of reporting care performance outcomes to health professionals for care quality improvement. These studies were not about dying with dementia; they employed a passive feedback strategy, and the study design was based on within-group comparisons. To our best knowledge, the effectiveness of feedback to improve end-of-life outcomes for nursing home residents dying with dementia has not been studied.
The aim of the Feedback on End-of-Life care in dementia (FOLlow-up) project was to test the effects of two active feedback strategies on perceived quality of end-of-life care and quality of dying (comfort) in nursing home residents dying with dementia: one strategy used generic feedback based on bereaved family caregivers’ mean ratings and the other strategy used patient-specific feedback with ratings of individual family caregivers.
Methods
Study design
The effects of the feedback strategies were tested and compared in Dutch nursing homes in a three-armed cluster-randomized controlled trial (RCT; Netherlands Trial Registration, NTR3942). The study protocol details on design and methods and has been published elsewhere.13 In brief, triplets of nursing homes were, based on previous research on factors potentially affecting nursing home resident outcome and family satisfaction with care as reported in the literature,14 matched on number of psychogeriatric beds, presence of a palliative care unit, and urban/non-urban location. The triplets were randomly assigned to the three groups: (1) the group testing the generic feedback strategy, (2) the group testing the patient-specific feedback strategy, and (3) the control group. After three nursing homes were matched, three papers with the name of the intervention group were folded and put in a blinded bag. Subsequently, a project group member, witnessed by two other project group members, drew folded papers with the group names that each of the three matched nursing homes would be assigned to. Not all nursing homes started to collect data at the same time. The pre-intervention phase started in January 2012 with the first nursing home triplets, and the intervention phase started in November 2012. In July 2014, all nursing homes concluded data collection.
Setting and study population
In total, 18 nursing homes participated in the study. The study population comprised family caregivers of nursing home residents with dementia who died on a psychogeriatric ward (almost all dementia, and residents generally stay until death). We included family caregivers who could read Dutch and whose relative had stayed in the nursing home at least 16 days in the last month of life and who had a dementia diagnosis recorded in their medical file. Around 6 weeks after death, the nursing home invited the family member who had been most involved in care during the last month of life (usually the same person since admission) to provide feedback by sending them a questionnaire along with an information letter.
The intervention
We explicitly aimed to implement feedback strategies that were “sustainable with limited external support,” as reported in the protocol article.13 The feedback strategies were implemented in Dutch nursing homes that participated in the intervention groups. Feedback was provided with bereaved family caregivers’ ratings on the End-of-Life in Dementia–Satisfaction With Care (EOLD-SWC) and the End-of-Life in Dementia–Comfort Assessment in Dying (EOLD-CAD) scales.15 We used these instruments as the (primary) outcomes because they were identified as the best instruments to evaluate the quality of end-of-life care and the quality of dying (comfort) in patients with dementia in terms of validity, reliability, and ease of use.13,16–18 These instruments allowed family caregivers to report their perception of the quality of care (EOLD-SWC) and the quality of dying (EOLD-CAD). The family reports were fed back to use it to improve the quality of end-of-life care and the quality of dying in nursing home residents with dementia.
The EOLD-SWC comprises 10 items for an after-death assessment of family members’ satisfaction with care of residents with dementia in the last month of life, with higher scores indicating higher levels of satisfaction (range: 10–40). Examples of items are “I felt fully involved in all decision making” and “The health care team was sensitive to my needs and feelings.” The 14-item EOLD-CAD instrument evaluates resident’s quality of dying and comprises the subscales Physical distress, Dying symptoms, Emotional distress, and Well-being.15 Higher scores (range: 14–42) indicate higher levels of quality of dying. We referred to the last week of life.
For both strategies (the generic and the patient-specific feedback strategy), we developed a document with suggestions for improvement that included evidence-based suggestions from the latest national and international literature and care guidelines in the field of end of life and palliative care—when available, we used those specific to dementia.19–22 We also developed practical suggestions based on the non-scientific national literature such as national health care guidelines. In the generic feedback strategy, nursing homes entered the family caregiver scores in a digital import program. Feedback of a minimum of 10 family caregiver questionnaires was required to generate EOLD-total and EOLD item mean scores, which were compared with a norm based on combined mean total and item scores of 372 nursing home and residential care residents with dementia collected nationwide between 2005 and 2010 in three previous Dutch studies.1 Subsequently, the digital import program compared the norm with the mean scores of a minimum of 10 family caregivers per participating nursing home using t-tests and generated a feedback report. A p-value < 0.05 was chosen to define statistically significant differences between the norm and the mean scores of a nursing home. The scores that were significantly higher or lower than the norm on item and total score level were flagged green or bold and underscored, respectively (Box 1). The lower scores were linked to the relevant suggestions, to prompt quality improvements actions by the health professionals. In cases where none of the mean EOLD item or total scores was below the norm, the feedback report included only the means, without improvement suggestions. The nursing homes were instructed to discuss the feedback reports in multi-disciplinary team meetings and to choose improvement actions from the provided suggestions or to formulate their own improvement actions. The multi-disciplinary team meetings were usually attended by elderly care physicians and nurses, and in some nursing homes also by para-medical disciplines such as physiotherapists or psychologists. No member of the research team was present at the meetings of the multi-disciplinary team.
Box 1.
Examples of (part of) a generic feedback report and improvement suggestions.
| Examples of EOLD-SWC items | Your nursing home (n = 20) | National (n = 372) | p |
|---|---|---|---|
| I felt fully involved in all decision making | 3.8 | 3.3 | <0.001 |
| All measures were taken to keep my loved one comfortable | 3.5 | 3.2 | 0.21 |
| I always knew which doctor or nurse was in charge of my loved one’s care | 3.4 | 2.9 | 0.004 |
| I felt that all medication issues were clearly explained to me | 2.8 | 3.2 | 0.005 |
| Examples of suggestions for improvement for item that scored below the norm: “I felt that all medication issues were clearly explained to me” | |||
| Suggestions | Involved disciplines | ||
| Discuss and explain any change of medication with family including its rationale and possible adverse effects. Check understanding. | Physicians | ||
| You may wish to provide a brochure on frequently used medication such as morphine (a reference to a particular brochure was included). | Management and physicians | ||
In the patient-specific strategy, nursing homes discussed in multi-disciplinary team meetings all questionnaires with family caregivers’ feedback (using the EOLD-instruments at the patient level) that were returned from the start of the intervention phase (range: between 14 and 21 questionnaires per nursing home). At the start of the intervention phase, they received the document with all improvement suggestions to inspire initiation of care improvement actions based on the feedback.13
Data collection and procedures
In the pre-intervention and in the intervention phase, all participating nursing homes sent questionnaires along with a letter that explained the study goals and procedures to the family member who was most involved according to the nursing home representative. The questionnaires were sent 6–8 weeks after the death of a nursing home resident with dementia. The questionnaire included the EOLD-instruments and items that assessed socio-demographic characteristics of both the decedent (age, gender, date of death, and length of stay in the nursing home) and the respondent (age, gender, education, and relationship with the resident) and an item on food intake dependency23 with total dependence representing Cognitive Performance Scale (CPS) 6 score.24
A nursing home staff member selected by the nursing home (usually a clerk) was instructed to register all nursing home residents who died and to send a questionnaire to every family caregiver eligible to participate along with the dates on which the questionnaires were sent out and received back. They also recorded reasons for non-eligibility and non-participation.13
After the pre-intervention phase (10 months), the nursing homes of the intervention groups received family caregivers’ feedback and the improvement suggestions according to the feedback strategy they were randomly assigned to. The nursing homes reported to the research team which improvement actions they formulated following the team meetings.
Only after data collection in the intervention phase (10 months) concluded, the nursing homes of the control condition received a feedback report that included the mean EOLD item and total scores along with a document that included all the improvement suggestions similar to the patient-specific intervention group. The research team was available to all nursing homes upon their request to provide additional support with the implementation of the intervention and the improvement actions.
Statistical evaluation
Respondent and resident characteristics were described and compared using analysis of variance (ANOVA) and chi-square tests. Change of mean EOLD-score per group was calculated by subtracting the group mean EOLD-score in the intervention phase from the group mean EOLD-score in the pre-intervention phase. We had defined a clinically relevant difference as a minimum change of 3 points on EOLD-total scores.13
Furthermore, the EOLD-scores between the intervention and control groups were compared in multi-level analyses adjusted and unadjusted for patient and family caregiver characteristics using mixed-models regression analysis with adjustment for clustering of evaluations at the nursing home level, and 95% confidence intervals (CIs) around the coefficients (B) were calculated. Intervention effects on the EOLD-CAD subscale scores were analyzed only in case of a significant intervention effect on the total EOLD-CAD score.
Ethics approval
Ethical approval for the research protocol of the FOLlow-up study was provided by the Medical Ethics Review Committee of the VU University Medical Center (number 2012/173). A returned and completed questionnaire was considered as family caregivers’ consent to participate.
Results
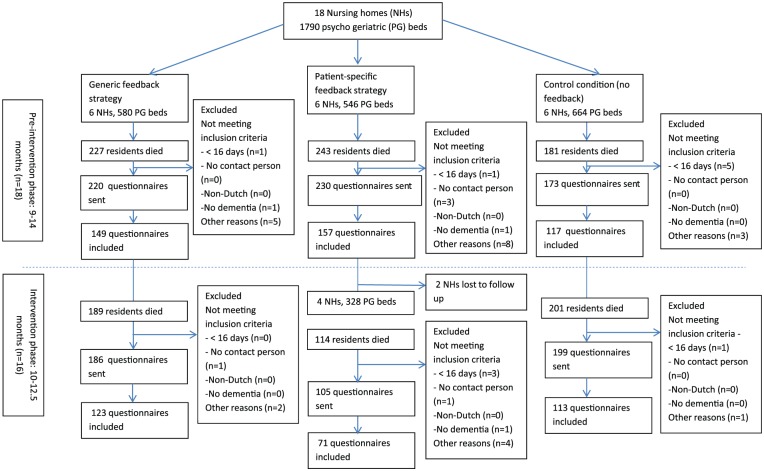
Figure 1 presents the recruitment procedure and inclusion of eligible respondents. The total response rates for the combined three groups were similar for the pre-intervention and the intervention phase (69.8% and 67.7%, respectively). In the pre-intervention phase, the response rate ranged from 67.1% (generic feedback strategy and the control condition) to 74.9% (patient-specific feedback strategy); and during the intervention phase, the response rates were 59.7% (patient-specific feedback strategy), 68.4% (generic feedback strategy), and 70.0% (control condition). However, the total number of returned questionnaires in the pre-intervention phase (range: 9–14 months, n = 18 nursing homes) was higher than the number returned during the intervention phase (range: 10–12.5 months, n = 16; 426 and 242 returned questionnaires, respectively). This difference was mainly caused by two nursing homes (in the patient-specific intervention group) that stopped sending out questionnaires after the pre-intervention phase.
Figure 1.
Flowchart: recruitment of participants.
Table 1 shows resident and respondent characteristics for the three groups in the pre-intervention and intervention phase. The majority of the residents were women, with mean length of stay between 30.3 and 35.6 months pre-intervention and between 28.2 and 33.2 months during the intervention phase. Most respondents were women around 60 years old and most were children of the residents. The characteristics of the respondents were largely similar across study phase and groups.
Table 1.
Characteristics of the residents and family caregivers enrolled in the study.
| Pre-intervention phase |
Intervention phase |
|||||||
|---|---|---|---|---|---|---|---|---|
| Generic feedback strategy (N = 149) | Patient-specific feedback strategy (N = 138) | No feedback (control condition) (N = 117) | p | Generic feedback strategy (N = 123) | Patient-specific feedback strategy (N = 71) | No feedback (control condition) (N = 113) | p | |
| Residents | ||||||||
| Length of stay in months, mean (SD) | 34.0 (35.5) | 30.3 (36.7) | 35.6 (38.1) | 0.53 | 28.2 (34.0) | 33.2 (30.5) | 33.2 (28.4) | 0.36 |
| Female (%) | 66.4 | 71.0 | 70.1 | 0.68 | 69.9 | 74.7 | 65.4 | 0.33 |
| Age at death, mean (SD) | 85.6 (6.5) | 85.5 (7.7) | 84.3 (8.6) | 0.29 | 86.6 (6.0) | 86.6 (5.5) | 84.3 (9.4) | 0.02* |
| Food/drink intake (%) | 0.80 | 0.43 | ||||||
| Independent | 17.7 | 13.5 | 13.8 | 18.2 | 15.6 | 17.3 | ||
| Supervision | 8.8 | 8.3 | 12.9 | 12.4 | 4.4 | 10.5 | ||
| Limited assistance | 21.8 | 22.6 | 23.3 | 23.1 | 21.1 | 21.8 | ||
| Extensive assistance | 21.1 | 25.6 | 19.8 | 17.4 | 21.1 | 24.1 | ||
| Total dependence (=CPS 6)a | 30.6 | 30.1 | 29.3 | 28.9 | 37.8 | 25.6 | ||
| Did not occur | – | – | 0.9 | – | – | 0.8 | ||
| Family caregivers | ||||||||
| Female (%) | 62.4 | 58.7 | 60.7 | 0.81 | 69.9 | 62.2 | 64.7 | 0.47 |
| Age, mean (SD) | 63.2 (12.2) | 62.1 (11.0) | 63.3 (11.7) | 0.67 | 63.2 (10.6) | 61.0 (12.0) | 62.3 (12.0) | 0.37 |
| Level of education (%) | 0.05* | 0.72 | ||||||
| Primary education/no schooling | 12.6 | 6.9 | 6.1 | 8.4 | 4.6 | 7.9 | ||
| High school, technical, or trade school | 61.5 | 59.5 | 52.6 | 55.5 | 55.2 | 59.1 |
||
| Higher education | 25.9 | 33.6 | 41.2 | 36.1 | 40.2 | 33.1 | ||
| Relation to patient (%) | 0.89 | 0.43 | ||||||
| Child | 60.4 | 63.0 | 60.7 | 65.9 | 66.7 | 64.7 | ||
| Spouse | 24.2 | 20.3 | 20.5 | 18.7 | 11.1 | 18.8 | ||
| Other | 15.4 | 16.7 | 18.8 | 15.4 | 22.2 | 16.5 | ||
SD: standard deviation; CPS: Cognitive Performance Scale.
Total dependence in eating represents the highest level of the CPS (score 6).
p < 0.05.
Effects of interventions
Table 2 compares the mean EOLD-scores of the three interventions groups and changes from the pre-intervention to the intervention phase. Positive, but no clinically relevant changes (0.2 points), were found for quality of care with the control condition and for quality of dying (comfort) (0.7 points) with the patient-specific feedback strategy. For the generic feedback intervention group, the total EOLD-SWC and EOLD-CAD mean scores decreased with 1.5 and 0.5 points, respectively. However, quality of dying also decreased in the control condition (with 1.5 point).
Table 2.
End-of-life outcomes, mean (range of nursing home means).
| Generic feedback strategy | Patient-specific feedback strategy | Control condition | |
|---|---|---|---|
| EOLD-SWC (10–40) | |||
| Pre-intervention phase | 34.6 (33.8–35.3) | 33.5 (32.7–34.3) | 33.9 (33.1–34.8) |
| Intervention phase | 33.1 (32.3–34.0) | 33.5 (32.5–34.4) | 34.1 (33.3–34.9) |
| Changea | −1.5 | 0 | +0.2 |
| EOLD-CAD (14–42) | |||
| Pre-intervention phase | 30.7 (29.7–31.6) | 30.4 (29.4–31.4) | 31.4 (30.3–32.4) |
| Intervention phase | 30.2 (29.1–31.2) | 31.1 (29.9–32.3) | 29.9 (28.8–30.9) |
| Changea | −0.5 | +0.7 | −1.5 |
EOLD-SWC: End-of-Life in Dementia–Satisfaction With Care; EOLD-CAD: End-of-Life in Dementia–Comfort Assessment in Dying.
Higher EOLD-scores and positive changes represent more favorable scores and changes.
A change of mean EOLD-total scores of 3 had been defined as a clinically relevant change.
Table 3 shows the testing of effects of the feedback strategies on the EOLD-scores compared to the control condition, with and without adjustment for patient and family caregivers’ characteristics. The EOLD-SWC total scores of the generic feedback intervention group were significantly lower during the intervention phase compared to the nursing homes of the control condition in unadjusted and adjusted analyses (B = −1.65, CI = −3.27; −0.21 and B = −2.41, CI = −4.07; −0.76, respectively). Furthermore, the EOLD-CAD total score of the patient-specific feedback intervention group was significantly higher compared to the control condition during the intervention phase in unadjusted analysis (B = 2.20, CI = 0.15; 4.39).
Table 3.
Intervention effects on end-of-life outcomes (B, 95% confidence interval).
| Effect generic feedback strategy compared to control condition | Effect of patient-specific feedback strategy compared to control condition | |
|---|---|---|
| EOLD-SWC | ||
| Change, unadjusteda | −1.65* (−3.27; −0.21) | −0.27 (−1.98; 1.45) |
| Change, adjusted | −2.41** (−4.07; −0.76) | −0.87 (−2.63; 0.89) |
| EOLD-CAD | ||
| Change, unadjusted | 1.03 (−1.04; 3.10) | 2.20* (0.15; 4.39) |
| Change, adjusted | 0.48 (−1.61; 2.58) | 1.88 (−0.34; 4.10) |
EOLD-SWC: End-of-Life in Dementia–Satisfaction With Care; EOLD-CAD: End-of-Life in Dementia–Comfort Assessment in Dying.
The adjusted results refer to models that not only included the group, period and interaction of group x period, but also, for residents: length of nursing home stay, age at death, gender and food/drink intake; and for family caregivers: age, gender, educational level and relationship with the nursing home resident.
p < 0.05; **p < 0.01.
A comparison of EOLD-CAD subscale scores between the patient-specific feedback intervention group and the control condition showed a significant difference at the subscale score “Dying symptoms” during the intervention phase in unadjusted and adjusted analyses (Table 4; B = 0.86, CI = 0.43; 1.68 and B = 1.18, CI = 0.38; 2.00, respectively).
Table 4.
Intervention effects of the patient-specific feedback strategy on quality of dying subscale scores (B, 95% confidence interval).
| Effect of patient-specific feedback strategy compared to control condition | |
|---|---|
| EOLD-CAD subscale Physical distress | |
| Change, unadjusted | 0.39 (−0.39; 1.18) |
| Change, adjusteda | 0.20 (−0.60; 1.00) |
| EOLD-CAD subscale Dying symptoms | |
| Change, unadjusted | 0.86* (0.43; 1.68) |
| Change, adjusted | 1.18* (0.38; 2.00) |
| EOLD-CAD subscale Emotional distress | |
| Change, unadjusted | 0.78 (−0.06; 1.62) |
| Change, adjusted | 0.68 (−1.80; 1.54) |
| EOLD-CAD subscale Well-being | |
| Change, unadjusted | 0.61 (−0.71; 0.83) |
| Change, adjusted | −0.19 (−0.99; 0.61) |
EOLD-CAD = End-of-Life in Dementia–Comfort Assessment in Dying.
The subscale Physical distress (range: 4–12) included the items: discomfort, pain, restlessness, and shortness of breath. The subscale Dying symptoms (range: 4–12) included the items: shortness of breath (therefore, included in two subscales), choking, gurgling, and difficulty swallowing. The subscale Emotional distress (range: 4–12) included the items: fear, anxiety, crying, and moaning. The subscale Well-being (range: 3–9) included the items: serenity, peace, and calm.
The adjusted results refer to models that not only included the group, period, and interaction of group × period but also for residents: length of nursing home stay, age at death, gender, and food/drink intake; and for family caregivers: age, gender, educational level, and relationship with the nursing home resident.
p < 0.05; **p < 0.01.
Discussion
The aim of the FOLlow-up study was to test and compare the effects of two strategies with feedback of bereaved family caregivers (i.e. a generic feedback strategy and a patient-specific feedback strategy) on perceived quality of end-of-life care and quality of dying (comfort) of nursing home residents dying with dementia. In sum, our cluster-randomized RCT showed little effects. The generic feedback intervention slightly lowered family perceptions of the quality of end-of-life care (satisfaction with care). The effect was statistically significant in adjusted and unadjusted analyses, and in adjusted analyses (−2.4), it was close to what we had considered clinically relevant when calculating power.3 Quality of dying was unchanged with generic feedback compared with any change over time in the control group. With the patient-specific feedback intervention, quality of dying improved, but the effect was not significant after adjustment for differences in patients’ and family caregivers’ characteristics compared to the control group. The quality of care was unchanged with the patient-specific intervention. The significant changes referred to coefficients of about 2 points. This represents a modest change. Relative to this, the improvement in the subscale score for “Dying symptoms” of about 1 point was larger.
Several explanations for these results could be provided. First, the effect of feedback on performance of professionals is more effective when baseline results are low.10 Only the nursing homes in the generic feedback group received a feedback report including baseline item and total scores that were flagged (green or bold and underscored) if the nursing home scored higher or lower than the norm.1 The baseline EOLD-total mean scores of both end-of-life outcomes applied in our study were relatively high compared to the norm we used, which was based on data from three earlier studies.1 These high baseline scores could be explained by an ongoing trend of more satisfied families as noted in the 2005–2010 dataset that was used to set the norm. The norm was not adjusted for a trend, but the distribution was skewed toward more recent evaluations (the median month of death was January 2009). Indeed, most items in these feedback reports were flagged green resulting in no improvement suggestions for these items. In total, 31 EOLD-SWC item mean scores and 8 EOLD-CAD item mean scores were flagged green in the generic feedback and only 1 EOLD-SWC item mean score and 9 EOLD-CAD item mean scores were flagged bold and underscored. Furthermore, 5 (out of 6) nursing homes actually discussed the feedback reports (each nursing home generated one feedback report after the pre-intervention phase), and 4 nursing homes formulated in total 10 improvement actions; a smaller number than the number of improvement actions formulated in the patient-specific feedback strategy (33 improvement actions). According to the control theory of Carver and Scheier,25 professionals are prompted to change behavior when they observe a discrepancy between their current performance and a goal. Feedback reports may be more effective when performance is compared to a clear performance goal. Perhaps, a feedback report that informed nursing home professionals that for the majority of the items the performance goal was accomplished, without clear instructions to further improve or motivation to excel (the result were not made public), may have resulted in the health care team to neglect those items or focus on other issues at the expense of sustained effort in areas relevant to these items. This may have served as a reassurance which may not have motivated nursing home professionals to further improve the quality of care at the end of life. In addition, since nursing homes were not asked to record actual implementation of the improvement actions, it may be that nursing homes, despite their intentions, did not, or were not successful in implementing improvement actions.
A second explanation for our results may relate to the nursing home context. Even though we targeted the behavior of the whole team,13 we only actively instructed physicians and in some cases the quality coordinator, potentially resulting in suboptimal implementation of the feedback strategies among the whole team. Indeed, according to Grol and Grimshaw,26 achieving behavioral change in care professionals requires the involvement of different levels, such as the individual caregiver and the care team, and Ivers et al.10 found that interventions directed at physicians are more likely to be successful if the feedback is given by a supervisor or colleague and when there are specific targets for improvement. Also organizational characteristics, such as staff composition, staff–patient ratios, and high staff turnover, were reported to function as barriers for the implementation of nursing home interventions.27
Third, we found that quality of dying decreased in the control group (change in mean pre-intervention and intervention phase: −1.5 points), while earlier studies suggested a possible increase in the quality of dying over time.1 It is possible that lower quality of dying is due to nursing home residents being sicker as a result of policies encouraging people to stay at home as long as possible;28 although mean length of stay in our study was not longer than in the previous study in 2007–2010.14 Last, improving family perceptions of the quality of care may be more difficult for nursing homes professionals than improving quality of dying, especially for the physicians who were mostly in charge of the interventions to improve the end-of-life outcomes. The physicians may also have focused on alleviating symptoms typical for the brief dying phase.
Recommendations for practice and future research
First, when feedback is provided for internal use with generic scores that are compared to a norm, flagging scores as higher than the norm should be considered cautiously for possible effects. Nursing homes should be encouraged to formulate specific performance targets, to record these and ensure follow-up, and to continue quality improvement even if they performed better than the performance norm. Second, the full multi-disciplinary team should be actively involved in all stages of implementing the intervention. Third, in general, feedback effects were found to be larger when feedback is provided more than once using verbal and non-verbal formats.10 Therefore, feedback intervention studies should use short and frequent follow-up timeframes (such as with the patient-specific intervention in our study) and organize more frequent team meetings, both multi-disciplinary and mono-disciplinary, to discuss feedback and to reach consensus about the appropriate actions that need to be taken for quality improvements. The FOLlow-up study results provide insufficient basis to encourage the providing of generic or recommend the use of patient-specific feedback. Future studies, also replication studies in other cultures, are needed to increase the evidence base on audit and feedback with family caregiver evaluations of the end of life with dementia in nursing homes.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by the Fonds NutsOhra, The Netherlands (project number 0904-020) and The Netherlands Organisation for Health Research and Development (ZonMw, project number 11150.0003.1).
References
- 1. Van der Steen JT, van Soest-Poortvliet MC, Gijsberts MJ, et al. [Improved end-of-life care for patients with dementia: greater family satisfaction and possibly greater end-of-life comfort]. Ned Tijdschr Geneeskd 2013; 157(17): A5324 (article in Dutch). [PubMed] [Google Scholar]
- 2. Mitchell SL, Mor V, Gozalo PL, et al. Tube feeding in US nursing home residents with advanced dementia, 2000–2014. JAMA 2016; 316(7): 769–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Van der Maaden T, de Vet HC, Achterberg WP, et al. Improving comfort in people with dementia and pneumonia: a cluster randomized trial. BMC Med 2016; 14(1): 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Estabrooks CA, Hoben M, Poss JW, et al. Dying in a nursing home: treatable symptom burden and its link to modifiable features of work context. J Am Med Dir Assoc 2015; 16(6): 515–520. [DOI] [PubMed] [Google Scholar]
- 5. Hendriks SA, Smalbrugge M, Galindo-Garre F, et al. From admission to death: prevalence and course of pain, agitation, and shortness of breath, and treatment of these symptoms in nursing home residents with dementia. J Am Med Dir Assoc 2015; 16(6): 475–481. [DOI] [PubMed] [Google Scholar]
- 6. Mitchell SL, Teno JM, Kiely DK, et al. The clinical course of advanced dementia. N Engl J Med 2009; 361(16): 1529–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sampson EL, Candy B, Davis S, et al. Living and dying with advanced dementia: a prospective cohort study of symptoms, service use and care at the end of life. Palliat Med. Epub ahead of print 1 August 2017. DOI: 10.1177/0269216317726443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Givens JL, Lopez RP, Mazor KM, et al. Sources of stress for family members of nursing home residents with advanced dementia. Alzheimer Dis Assoc Disord 2012; 26(3): 254–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Etkind SN, Daveson BA, Kwok W, et al. Capture, transfer, and feedback of patient-centered outcomes data in palliative care populations: does it make a difference? A systematic review. J Pain Symptom Manage 2015; 49(3): 611–624. [DOI] [PubMed] [Google Scholar]
- 10. Ivers N, Jamtvedt G, Flottorp S, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev 2012; 6: CD000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Castle NG. Providing outcomes information to nursing homes: can it improve quality of care? Gerontologist 2003; 43(4): 483–492. [DOI] [PubMed] [Google Scholar]
- 12. Vandenberg EV, Tvrdik A, Keller BK. Use of the quality improvement process in assessing end-of-life care in the nursing home. J Am Med Dir Assoc 2006; 7(3 Suppl.): S82–S87. [DOI] [PubMed] [Google Scholar]
- 13. Boogaard JA, van Soest-Poortvliet MC, Anema JR, et al. Feedback on end-of-life care in dementia: the study protocol of the FOLlow-up project. BMC Palliat Care 2013; 12(1): 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van der Steen JT, Ribbe MW, Deliens L, et al. Retrospective and prospective data collection compared in the Dutch End Of Life in Dementia (DEOLD) study. Alzheimer Dis Assoc Disord 2014; 28(1): 88–94. [DOI] [PubMed] [Google Scholar]
- 15. Volicer L, Hurley AC, Blasi ZV. Scales for evaluation of end-of-life care in dementia. Alzheimer Dis Assoc Disord 2001; 15(4): 194–200. [DOI] [PubMed] [Google Scholar]
- 16. Van Soest-Poortvliet MC, van der Steen JT, Zimmerman S, et al. Psychometric properties of instruments to measure the quality of end-of-life care and dying for long-term care residents with dementia. Qual Life Res 2012; 21(4): 671–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Van Soest-Poortvliet MC, van der Steen JT, Zimmerman S, et al. Measuring the quality of dying and quality of care when dying in long-term care settings: a qualitative content analysis of available instruments. J Pain Symptom Manage 2011; 42(6): 852–863. [DOI] [PubMed] [Google Scholar]
- 18. Van Soest-Poortvliet MC, van der Steen JT, Zimmerman S, et al. Selecting the best instruments to measure quality of end-of-life care and quality of dying in long term care. J Am Med Dir Assoc 2013; 14: 179–186. [DOI] [PubMed] [Google Scholar]
- 19. Van der Steen JT. Dying with dementia: what we know after more than a decade of research. J Alzheimers Dis 2010; 22(1): 37–55. [DOI] [PubMed] [Google Scholar]
- 20. Hennings J, Froggatt K, Keady J. Approaching the end of life and dying with dementia in care homes: the accounts of family carers. Rev Clin Gerontol 2010; 20: 114–127. [Google Scholar]
- 21. Birch D, Draper J. A critical literature review exploring the challenges of delivering effective palliative care to older people with dementia. J Clin Nurs 2008; 17(9): 1144–1163. [DOI] [PubMed] [Google Scholar]
- 22. Davidson KM. Family preparedness and end-of-life support before the death of a nursing home resident. J Gerontol Nurs 2011; 37(2): 11–16. [DOI] [PubMed] [Google Scholar]
- 23. Centers for Medicare & Medicaid Services. Long-term care facility resident assessment instrument 2.0. Washington, DC: Department of Health & Human Services, 2016. [Google Scholar]
- 24. Morris JN, Fries BE, Mehr DR, et al. MDS cognitive performance scale. J Gerontol 1994; 49(4): M174–M182. [DOI] [PubMed] [Google Scholar]
- 25. Roos-Blom MJ, Gude WT, de Jonge E, et al. Development of a web-based quality dashboard including a toolbox to improve pain management in Dutch Intensive Care. Stud Health Technol Inform 2017; 235: 584–588. [PubMed] [Google Scholar]
- 26. Grol R, Grimshaw J. From best evidence to best practice: effective implementation of change in patients’ care. Lancet 2003; 362(9391): 1225–1230. [DOI] [PubMed] [Google Scholar]
- 27. Mentes JC, Tripp-Reimer T. Barriers and facilitators in nursing home intervention research. West J Nurs Res 2002; 24(8): 918–936. [DOI] [PubMed] [Google Scholar]
- 28. Pavolini E, Ranci C. Restructuring the welfare state: reforms in long-term care in Western European countries. J Eur Soc Policy 2008; 18: 246–259. [Google Scholar]