Abstract
Obesity and diabetes associate with neurodegeneration. Brain glucose and BDNF are fundamental in perinatal development. BDNF is related to brain health, food intake and glucose metabolism. We characterized the relationship between glycemia and/or brain glucose utilization (by 18FDG-PET during fasting and glucose loading), obesity and BDNF in 4-weeks old (pre-obese) and 12-weeks old (obese) Zucker fa/fa rats, and their age-matched fa/+ controls. In 75 human infants, we assessed cord blood BDNF and glucose levels, appetite regulating hormones, body weight and maternal factors. Young and adult fa/fa rats showed glucose intolerance and brain hyper-utilization compared to controls. Glycemia and age were positively related to brain glucose utilization, and were negative predictors of BDNF levels. In humans, fetal glycemia was dependent on maternal glycemia at term, and negatively predicted BDNF levels. Leptin levels were associated with higher body weight and lower BDNF levels. Glucose intolerance and elevated brain glucose utilization already occur in young, pre-obese rats, suggesting that they precede obesity onset in Zucker fatty rats. Glycemic elevation and brain glucose overexposure predict circulating BDNF deficiency since perinatal and early life. Future studies should evaluate whether the control of maternal and fetal glycemia during late intrauterine development can prevent these unfavorable interactions.
Keywords: Glucose intolerance, brain glucose, positron emission tomography, childhood, BDNF
Introduction
Diabetes, obesity and cognitive decline tend to occur in association. Brain glucose hypermetabolism has been shown to precede and predict the development of cognitive decline in humans.1,2 It is also a feature of human obesity and diabetes, responding to weight loss.3 We have previously shown that adult, hyperglycemic obese and diabetic Zucker rats are characterized by an elevated utilization of glucose in the brain.4 These animals manifest a severe reduction in brain tissue volume,5 thereby recapitulating the association of metabolic and neurodegenerative features seen in humans, in which brain glucose overexposure seems to be a common underlying factor.2,3
Brain-derived neurotrophic factor (BDNF) is an important neurotrophin, widely expressed throughout the central nervous system, and released by the brain into the circulation. It is involved in normal brain development and plasticity, learning and memory, aging and neurodegenerative disorders.6–9 Recent studies indicate that a negative interaction occurs between impaired glucose tolerance and circulating BDNF levels,10 which may contribute to explain the frequent association observed between diabetes and neurodegeneration.11 In addition, a large body of evidence links diminished BDNF to the pathogenesis of obesity in humans and rodents.10,12–14 Though the infusion of BDNF improves glucose metabolism15 and ameliorates cognitive function and neurogenesis,16–18 the pharmacological targeting of this pathway requires caution. A safer approach may be to identify and optimize mediators that indirectly promote BDNF production. The maintenance of adequate BDNF levels may be particularly important during early life, i.e. a time in which BDNF levels and brain plasticity are elevated, and diseases have not yet established.
The current study was undertaken to examine the relationship between body weight, glucose tolerance and BDNF in the Zucker rat model studied during early life or adulthood, i.e. before or after the onset of obesity, in fa/fa compared to fa/+ control rats. We used brain imaging by positron emission tomography (PET) to test the relationship linking brain glucose utilization and circulating glucose and BDNF levels. In human neonates, we explored the association between umbilical cord blood BDNF and glucose levels, appetite related hormones, birth body weight, and maternal factors.
Methods
Study in rats
Study design
We studied fa/fa Zucker pups (n = 22, age 26.4 ± 1.0 days) before the onset of obesity, and adult fa/fa Zucker rats (n = 21, age 11.7 ± 0.3 weeks) after development of the obese phenotype. Age-matched fa/+ Zucker pups (n = 17, 28.6 ± 0.8 days) and adult rats (n = 18, age 11.6 ± 0.3 weeks) were studied as controls. Adult animals were purchased from Charles Rivers Laboratories. Pups were produced by mating obese (fa/fa) males and heterozygous lean (fa/+) females. Since pups were indistinguishable based on body weight, group allocation was achieved by genotyping and plasma leptin measurements. Male pups were studied. In the adult groups, there were 19/2 (fa/fa) and 15/3 males/females (fa/+). Brain glucose utilization was determined after an overnight fasting period, by using PET in combination with 18F-2-fluoro-2-deoxyglucose ([18F]FDG). Approximately half of the animals in each group were studied during fasting and the remaining during i.p. glucose loading. The experimental protocol was notified to the Ministry of Health (Dept. of Public Veterinary Health) in accordance with the D.L. 116/92, implementation of the directive EEC 609/86, regarding the protection of animals used for experimental and other scientific purposes, and is reported in compliance with the ARRIVE guidelines (Animal Research: Reporting in Vivo Experiments).
PET scanning protocol
Anaesthesia was induced and maintained by isofluorane (2.5 and 1.5% in oxygen, respectively). Femoral veins were surgically accessed and catheterized for tracer administration. PET imaging was performed with a dedicated small animal scanner (YAP(S)PET. I.S.E. s.r.l., Vecchiano, Italy). Animals were positioned supine on the scanner bed, and received an i.v. injection of [18F]FDG (21 ± 1 MBq in pups, 40 ± 1 MBq in adults) in a dilution volume of 100 µl (pups) or 200 µl (adults), followed by 100 µl of saline. A 20-min PET scan of the brain region was performed 60 min after injection. In the glucose loading studies, [18F]FDG injection was preceded (t ≅ −60 min) by the i.p. administration of 2 g/kg dextrose solution, and blood was frequently sampled by strip measurements via the tail tip to monitor and correct (via small administrations) circulating glucose to maintain constant glycemic values for one hour preceding the [18F]FDG scan and during the imaging period. Animals were euthanized by anesthetic overdose at the end of the experiments and blood was withdrawn for the assessment of insulin, BDNF and leptin levels. A sample of tail tissue was collected in young rats for genotyping.
Image analysis
Images were reconstructed using an iterative OSEM algorithm, as previously described.4 Brain glucose utilization (reflecting transport and phosphorylation) was measured by drawing regions of interest (ROIs) on brain [18F]FDG PET images in areas involved in cognitive and metabolic control (i.e. frontal cortex, hippocampus, thalamus, striatum, putamen/caudate, pallidus, substantia nigra, hypothalamus, amygdala, olfactory bulb), as based on atlas guidance. Tissue [18F]FDG levels were divided by the dose of injected tracer per gram of animal to obtain standardized uptake values (SUV), representing an estimate of the regional brain glucose fractional extraction, and multiplied by blood glucose levels (SUVg) to reflect brain glucose utilization rates. Whole brain glucose utilization was estimated as average of the above regional values.
Genotyping
Genotyping was performed in genomic DNA, by identifying the single or double restriction segments specific to homozygote (fa/fa) and heterozygous (fa/+) genotypes, as reported by Phillips et al.19 and modified as described below.
Genomic DNA was purified from rat-tail (∼0.5 cm) using a commercially available kit (DNeasy blood and tissue kit, Qiagen s.r.l, Milan, Italy), according to manufacturer’s instructions. DNA integrity was assessed by electrophoresis in 1% agarose gel, and DNA purity and quantity were given by value of 1.7–2.1 in the 260/280 nm optical density ratio (Infinite® 200 PRO, Tecan s.r.l. Milan, Italy). Genomic DNA was amplified in 40 μl reactions containing ∼60 ng of genomic DNA, with reported primers (5′- GTT TGC GTA TGG AAG TCA CAG-3′ and 5′-ACC AGC AGA GAT GTA TCC GAG-3′),19 at a final concentration of 500 nM each, and 20 μl of GoTaq® Green Master Mix ready-to-use solution (Promega Italia s.r.l. Milan, Italy). The amplification protocol was 30 cycles at 95℃ for 30 s, 58℃ for 30 s and 72℃ for 2 min. PCR amplification resulted in a 1.8 kb product, whose integrity was assessed by electrophoresis in 1% agarose gel/TBE 0.5X (Sigma-Aldrich srl, Milan, Italy). PCR products were digested by incubating 17 µl of PCR product with 1.5 µl of restriction enzyme MspI (Promega Italia s.r.l. Milan, Italy) in a total volume of 20 µl at 37℃ overnight. MspI digestion of the 1.8 Kb fragment results in a single restriction site (950 bp fragment) or in two restriction sites (1100 bp and 950 bp fragments) for the obese homozygous and the lean heterozygous rat, respectively.
Study in humans
Study design
The study included 75 neonates and their mothers. Mothers were recruited either at the beginning or at the end of pregnancy. Maternal body mass index (BMI) and fasting plasma glucose and insulin measurements were available at the beginning of pregnancy, and/or at the time of glucose tolerance testing, and/or at delivery, according to sample sizes shown in Table 1. Body weight and height in neonates at the time of birth were used to estimate the ponderal index (PI), computed as ratio of weight to the third power of height (kg/m3). Umbilical cord blood was drawn after cord dissection to assess neonatal BDNF, glucose, insulin, leptin, and PYY levels. The human study was approved by the Local Ethical Committee (Comitato Etico per la sperimentazione clinica dei medicinali dell’Azienda USL1 di Massa e Carrara, CESM, project number 309 and 394), which complies with the Helsinki Declaration 1964 (and subsequent amendments), and the Oviedo Convention (1997, ratified by Law 28/03/2001, n. 145). Both parents gave written informed consent before study initiation.
Table 1.
Maternal and neonatal characteristics in the human study.
| N | ||
|---|---|---|
| Mother | ||
| Age (years) | 75 | 33.2 ± 0.6 |
| BMI (kg/m2) 1st trim | 75 | 24.3 ± 0.6 |
| BMI (kg/m2) 2nd trim | 58 | 28.0 ± 0.6 |
| BMI (kg/m2) 3rd trim | 69 | 29.0 ± 0.6 |
| Fasting glucose levels (mmol/l) 1st trim | 64 | 4.5 ± 0.1 |
| Fasting glucose levels (mmol/l) 2nd trim | 65 | 4.6 ± 0.1 |
| Fasting glucose levels (mmol/l) 3rd trim | 40 | 5.1 ± 0.2 |
| Fasting insulin levels (mU/l) 2nd trim | 29 | 9.0 ± 0.7 |
| Fasting insulin levels (mU/l) 3rd trim | 44 | 12.1 ± 1.5 |
| Parity (1, 2, >2, including current child) | 75 | 40/31/4 |
| Gestational diabetes (no/yes) | 75 | 57/18 |
| Mode of delivery (spontaneous/C-section) | 75 | 48/27 |
| Neonate | ||
| Gestational age (days) | 75 | 277 ± 1 |
| Ponderal index (kg/m3) | 75 | 26.8 ± 0.3 |
| Cord glucose levels (mmol/l) | 70 | 3.8 ± 0.2 |
| Glycemia 2 h after birth (mmol/l) | 67 | 3.4 ± 0.1 |
| Cord insulin levels (mU/l) | 72 | 4.8 ± 0.4 |
| Cord leptin levels (ng/ml) | 75 | 20.9 ± 2.2 |
| Cord PYY levels (ng/ml) | 75 | 0.29 ± 0.02 |
| Cord BDNF (ng/ml) | 75 | 12.9 ± 0.09 |
Measurement of circulating biomarkers
Human and animal blood samples were centrifuged for 10 min at 3000 rpm, and plasma or serum was stored at −80℃. In human samples, plasma glucose and insulin levels were analysed by routine enzymatic method (Synchron CX9 Pro Beckman Coulter, Inc, Brea, CA, USA and Abbott s.r.l. Italy, respectively); BDNF was measured by sandwich enzyme immunoassay (ChemiKine™ Chemicon International Inc, MA, USA), and circulating levels of leptin, and PYY were measured by multi-analyte panels based on Luminex® xMAP® technology (Milliplex map kit, CAT N# HMHMAG-34K and HPTP2MAG-66K, EMD Millipore Corp., MA, USA). In rats, glycemia was monitored by glucometer (LifeScan OneTouch®, Johnson and Johnson Medical SpA, Rome, Italy), throughout the study session, and values above the measurement range were set at the maximum of that range, circulating levels of leptin, insulin, and BDNF were measured by multi-analyte panels based on Luminex® xMAP® technology (Milliplex map kit, CAT N# RMHMAG-84K and RPTMAG-86K, EMD Millipore Corp., MA, USA). All procedures were carried out according to manufacturer’s instruction.
Statistical analysis
Data are presented as mean ± SEM. Image and biochemical analyses were done blindly with respect to genotype (animal study) or neonatal characteristics (human study). Age-matched groups were compared by using two-tailed analysis of variance. The Pearson correlation coefficient was used to evaluate the strength of linear correlations. Differences between groups were regarded as statistically significant when the p value was ≤0.05.
Results
Animal study
No differences in body weight were observed between fa/+ (68 ± 5 g) and fa/fa rats (61 ± 5 g) at the age of four weeks. Instead, adult (12 week old) fa/fa animals were 39% heavier than age-matched controls (388 ± 9 vs. 280 ± 10 g, p < 0.0001). As expected, leptin values were higher in fa/fa compared to respective controls (pups: 10031 ± 1135 vs. 2283 ± 287 pg/ml, p < 0.0001; adults: 21999 ± 2967 vs. 948 ± 156 pg/ml, p < 0.0001). Leptin levels declined by 59% in adults compared to pups in fa/+ rats (p = 0.0004), whereas they rose by 119% in fa/fa rats (p = 0.002).
In both age groups, fa/fa Zucker rats were glucose intolerant, as compared with age-matched fa/+ animals (Figure 1). Hyperglycemia was present during the glucose load in young fa/fa animals, in spite of normal body weight, and during both fasting and glucose loading in adult obese fa/fa animals compared to respective control groups. Consequently, glucose levels were higher in glucose loaded than fasted rats, except for the adult fa/fa group in which no difference occurred. In fa/+ animals, insulin levels were lower in adults than pups, due to physiological aging, but this age-related pattern was not observed in fa/fa rats (Figure 1), as adult obese rats showed fasting hyperinsulinemia. Compared to respective control rats, insulin levels were four times higher in fa/fa adults during fasting, and 70% lower in fa/fa pups during glucose loading. In glucose loading studies, insulin levels were 2–3 folds higher than in fasting studies in fa/+ groups, whereas no such β-cell response was seen in fa/fa groups.
Figure 1.
Glucose levels are shown in the left panel, documenting post-glucose load hyperglycemia in 4-week-old (non-obese) fa/fa rats, and fasting and post-glucose load hyperglycemia in adult 12-week-old (obese) fa/fa rats compared to respective controls. Insulin levels (right panel) demonstrate fasting hyperinsulinemia in adult fa/fa rats, and the lack of β-cell response to hyperglycemia in all fa/fa groups. ***p ≤ 0.003, **p = 0.02 and *p = 0.04 vs. age-matched fa/+ controls; #p < 0.03, §p ≤ 0.0005 vs. fasting state.
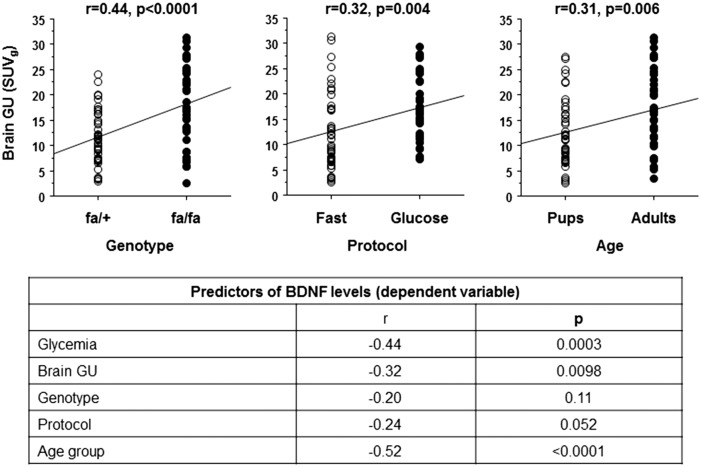
Brain glucose utilization (Figure 2) during glucose loading was ∼50% higher in 4-week and 12-week-old fa/fa versus age-matched fa/+ animals. In adult fa/fa animals, brain glucose utilization was severely increased also during fasting (+100%) compared to adult fa/+ rats. Thus, the physiological difference in brain glucose utilization occurring during glucose loading versus fasting conditions was absent in this group. Brain glucose utilization was positively predicted by adult age, fa/fa genotype and glucose loading (Figure 3). In turn, brain glucose utilization was a strong negative predictor of circulating BDNF levels together with concurrent glycemia, and age (Figure 3). Relationships between BDNF and genotype per se (p = 0.11), or leptin levels (p = 0.51) or body weight (age-adjusted, p = 0.75) were not significant.
Figure 2.
Regional brain glucose utilization (GU) rates (top panel) show hypermetabolic regions during glucose loading in fa/fa pups (top panel), and during both fasting and glucose loading in fa/fa adults (bottom panel) compared to respective controls. A significant increase in GU occurs in all brain regions during glucose loading compared to fasting in all groups, except for adult fa/fa rats. Representative examples of 4-week and a 12-week-old rats are shown on the right; the color scale ranges from red (higher activity) to orange, yellow, green and blue (lower activity). **p ≤ 0.002, *p < 0.05 and #p = 0.052 vs. age-matched fa/+ rats; in pups, the remaining regions were close to significance (p = 0.6–0.9); ^p < 0.005 and §p ≤ 0.05 vs. fasting state.
Figure 3.
Across groups, brain glucose utilization (GU, top panels) shows positive dependence on fa/fa genotype, glucose loading protocol and adult age. Data in the bottom panel indicate that brain GU, glycemia (primary regulator of GU) and age group, rather than genotype per se, were strong negative predictors of BDNF levels in rats.
Human study
Metabolic characteristics of mothers and neonates are given in Table 1. Maternal glycemia at term was significantly and positively correlated with neonatal glycemia (r = +0.66, p < 0.0001), whereas maternal glycemia in the first and second trimesters was not (p = 0.24–0.25). In turn, umbilical cord glycemia was a strong negative predictor of cord BDNF levels (Table 2). Cord leptin was more weakly associated, whereas neonatal body weight (p = 0.96) or ponderal index (p = 0.39) were not correlated with BDNF levels at birth. Body weight was significantly associated with leptin levels (r = 0.27, p = 0.02). Maternal factors, including BMI, frequency of gestational diabetes, parity, age, mode of delivery (C-section vs. spontaneous delivery), and gestational age were not correlated with fetal BDNF levels.
Table 2.
Predictors of cord BDNF levels (dependent variable).
| r | p | |
|---|---|---|
| Cord glycemia | −0.42 | 0.0003 |
| Cord leptinemia | −0.28 | 0.01 |
| Cord PYY | +0.21 | 0.08 |
| Ponderal index | −0.10 | 0.39 |
| Maternal glycemia | −0.29 | 0.08 |
Discussion
Our data in rats suggest that glucose intolerance and cerebral glucose utilization are strong negative predictors of BDNF levels. In human neonates, we found a similar negative relationship between cord blood BDNF and glucose levels, and the latter seemed determined by maternal glucose levels at term.
The animal study was conducted in young and adult rodents to examine the relationship linking BDNF to glucose tolerance or obesity. Young fa/fa animals were normal weight compared to age-matched controls, although genetically committed to develop obesity. The pre-obesity phase was characterized by glucose intolerance, apparently due to insufficient insulin release in response to glucose loading. Compared to young non-obese fa/fa rats, the occurrence of obesity in adult fa/fa animals did not further affect insulinemia after glucose loading. This implicates β-cell glucose insensitivity in the development of glucose intolerance in this animal model, in keeping with data obtained in isolated perfused pancreas, showing that no insulin secretory response to incremental glucose stimulation in the upper glycemic range occurs in fatty Zucker rats.20 Instead, the onset of obesity in our adult rats resulted in fasting hyperglycemia and fasting hyperinsulinemia, which reflect hepatic insulin resistance.
Our data support the notion that age is one main negative regulator of central BDNF production.21 As seen in relation to glucose intolerance, obesity per se did not explain BDNF concentrations, whereas our results suggest that circulating levels of BDNF strongly relate to plasma glucose concentrations. We reported an inverse association linking chronic glycemia and BDNF. In addition, we showed that BDNF levels were lower during the glucose loading than fasting protocol, supporting a suppressive effect of acute glucose loading on circulating BDNF concentrations. The current fa/fa model is characterized by the genetic lack of leptin receptors, and leptin infusion has been shown to stimulate BDNF expression.13,14,22 However, different from an exogenous infusion, leptin secreted by adipose tissue in this animal model reflects the combined degrees of adiposity and leptin resistance due to the lack of leptin receptors, and the balance between these factors did not result in a significant correlation between leptin and BDNF levels, nor was BDNF dependent on genotype per se.
An elevated brain glucose utilization in response to insulin has been observed in human subjects with obesity and with impaired glucose tolerance.3,23 Extending this observation to daily-life conditions, we previously reported a chronic (fasting and post-glucose load) state of brain glucose hyper-utilization in adult obese fa/fa rats, which was reinforced in Zucker diabetic fatty (ZDF) rats.4 The present study is consistent with the above evidence in adult models. However, studies in adult humans and rats with the metabolic syndrome do not allow disentangle the interdependency of chronic hyperglycemia and obesity in regulating brain glucose utilization. One novelty of the present study was to demonstrate that brain glucose utilization was 50% higher during glucose loading before the onset of weight gain in glucose intolerant young fa/fa rats. Brain glucose hypermetabolism has been recently recognized as a pre-eminent feature of neurodegenerative diseases.2 BDNF is implicated in the pathogenesis of these diseases, and its deficiency is proportional to the severity of cognitive impairment in humans.7 BDNF levels are also reduced in the brain of diabetic mice and in the circulation of diabetic humans.10,11,24 In our study, brain glucose utilization was a strong negative predictor of BDNF levels, independent of body weight. In humans, hyperglycemic, and not euglycemic hyperinsulinemia leads to a decline in BDNF release by the brain, as directly measured by arterial-venous catheterization.10 We observed a correlation between glucose levels and brain glucose utilization. Though we may speculate that brain glucose utilization mediates the effects of hyperglycemia on BDNF levels, correlations do not imply causality, and studies addressing the effects of hyperglycemia on cerebral glucose phosphorylation and subsequent signaling in vivo are needed to support this interpretation.
Brain metabolism and BDNF are also implicated in the control of eating behavior and body weight. Meal or glucose ingestion inhibit, whereas fasting stimulates appetite. The expression of BDNF in the brain is suppressed by fasting and stimulated by refeeding.13 Glycemic excursions are also important in the cycling between fasting and food seeking behavior,25 and the hypothalamic unresponsiveness to a glucose load has been suggested to explain continuing appetite and overeating in obese people.26 One interesting difference between our young pre-obese and adult obese fa/fa animals was that the former maintained an up-regulatory excursion in brain utilization during glucose loading compared to fasting, whereas brain glucose utilization was constantly high and did not change in fasting versus glucose loaded conditions in obese adult rats. It is tempting to speculate that the preservation of metabolic flexibility in the brain of our young fa/fa rats may contribute to maintain their normal weight, as opposed to the loss of metabolic excursion seen in the brain of adult fa/fa rats, which may stimulate hyperphagia. Higher glycemic and brain utilization levels achieved post-glucose loading in young fa/fa than control rats, suppressing BDNF, seemed to precede and may contribute to the establishment of weight gain, in agreement with the suggested involvement of BDNF in the inhibition of appetite.13,14
We conducted an explorative analysis in human neonates. Human findings from the current study provided support to the concepts derived in the animal model. First, BDNF values observed in our neonates were higher than reported in healthy adults27 and elderly people,9 in agreement with the strong age-dependency seen in our rats. This is also consistent with post-mortem human lifespan brain findings on BDNF expression,28 showing peak values in the neonatal period, underlying the importance of BDNF in early cognitive development. Second, the inverse association between glucose and BDNF levels in neonatal plasma was strong. Notably, both the tightness (r = −0.44 vs. −0.42) and the level of significance of this correlation (p = 0.0003 vs. 0.0003) were identical in the animal and human model. Glycemia in newborns was predicted by maternal glycemia occurring at the end of pregnancy, not in the first or second trimesters, indicating a critical time window in which the control of maternal glycemia may be protective of brain health in the upcoming child. In fact, the last weeks of pregnancy witness a many-fold increase in brain size, and the delineation of brain structures and functions. Also, occurring during this period is most of body growth and adipose tissue hypertrophy. Interestingly, we found that cord leptin, reflecting adiposity, was negatively correlated with BDNF levels, and positively related to body weight in infants. Again similar to the animal model, we did not observe a direct relationship between BDNF levels in cord blood and ponderal index in newborns. Though associations do not demonstrate causality, it is important to underline that BDNF is a limiting factor for the action of leptin in the brain.14 Thus, one possibility to explain our finding is that lower BDNF values may result in brain leptin resistance, leading to a compensatory expansion in adipose tissue to release more leptin. From a clinical standpoint, it is of note that our human findings mostly referred to physiological maternal and neonatal conditions, and were not dependent on gestational diabetes. Thus, our data prompt for a reappraisal of the optimal maternal glycemic range, specific to late gestation, to protect neonatal brain development from BDNF lowering.
This study had some limitations. Our comparisons were cross-sectional, as in the animal study we opted to examine two very distinct conditions (pre-obesity and overt obesity), and the current sample collection precluded the possibility to study the same animals repeatedly. Cross-sectional comparisons and correlations only allowed us to postulate causation, and speculate on disease progression. Though young and adult rats had the same genetic background, we recognize that longitudinal observations and the examination of intermediate age-points would be required to provide time-dependent trajectories. Similarly, follow-up studies in our human neonates will evaluate whether cord BDNF and glycemia may predict subsequent body growth, eating behavior and cognitive development.
In conclusion, our data indicate that glucose intolerance and elevated brain glucose utilization are already present in young, pre-obese rats, suggesting that they precede the onset of obesity in Zucker fatty rats, and are negatively related to BDNF levels. This interaction may be most incisive during early brain development, when BDNF levels were high. In line with animal data, neonatal glycemia was a strong negative predictor of BDNF levels in cord blood, and it was dependent on maternal glucose levels at term. Lower BDNF was also accompanied by higher cord leptin, relating to body weight. Overall, these findings call for action to evaluate whether the control of maternal and fetal glycemia during late intrauterine development can prevent its unfavorable interaction with BDNF.
Acknowledgements
We thank the technical staff and collaborators for support in data collection and genotyping.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Study data in rats were collected within the context of the project entitled “Early diagnosis of organ metabolic and inflammatory damage related with cancer and cardio-metabolic risk in childhood obesity. Validation of panel-oriented biomarkers in obese animals and implementation in children and adolescents”, supported by the Tuscany Region under the Research Call “Innovation in Medicine 2009”. Study data in humans were collected within the EU-FP7-HEALTH project entitled ‘Developmental Origin of Healthy and Unhealthy Ageing: the Role of Maternal Obesity (DORIAN)’ (grant agreement no. 278603).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
Guzzardi MA: coordination, data collection, genotyping, and blood assays in the animal and human studies; Sanguinetti E: co-coordination and data collection in the human study; Burchielli S: responsibility for animal safety and preparation; Bartoli A and Panetta D: image acquisition and reconstruction; Kemeny A: data collection in the human study; Salvadori PA: coordination and financing of imaging facilities and radiopharmacy, synthesis and provision of PET tracers; Iozzo P: project design and supervision, recipient of the Tuscany Region grant (Innovation in Medicine 2009) financing the study, analyses of images and data, and manuscript writing. All authors revised and accepted the manuscript in its current form.
References
- 1.Jack CR, Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer’s disease: An updated hypothetical model of dynamic biomarkers. Lancet Neurol 2013; 12: 207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashraf A, Fan Z, Brooks DJ, et al. Cortical hypermetabolism in MCI subjects: A compensatory mechanism? Eur J Nucl Med Mol Imaging 2015; 42: 447–458. [DOI] [PubMed] [Google Scholar]
- 3.Tuulari JJ, Karlsson HK, Hirvonen J, et al. Weight loss after bariatric surgery reverses insulin-induced increases in brain glucose metabolism of the morbidly obese. Diabetes 2013; 62: 2747–2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liistro T, Guiducci L, Burchielli S, et al. Brain glucose overexposure and lack of acute metabolic flexibility in obesity and type 2 diabetes: A PET-[18F]FDG study in Zucker and ZDF rats. J Cereb Blood Flow Metab 2010; 30: 895–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devisser A, Yang C, Herring A, et al. Differential impact of diabetes and hypertension in the brain: Adverse effects in grey matter. Neurobiol Dis 2011; 44: 161–173. [DOI] [PubMed] [Google Scholar]
- 6.Diniz BS, Teixeira AL. Brain-derived neurotrophic factor and Alzheimer’s disease: Physiopathology and beyond. Neuromolecular Med 2011; 13: 217–222. [DOI] [PubMed] [Google Scholar]
- 7.Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A 2011; 108: 3017–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gunstad J, Benitez A, Smith J, et al. Serum brain-derived neurotrophic factor is associated with cognitive function in healthy older adults. J Geriatr Psychiatry Neurol 2008; 21: 166–170. [DOI] [PubMed] [Google Scholar]
- 9.Gezen-Ak D, Dursun E, Hanağası H, et al. BDNF, TNFα, HSP90, CFH, and IL-10 serum levels in patients with early or late onset Alzheimer’s disease or mild cognitive impairment. J Alzheimers Dis 2013; 37: 185–195. [DOI] [PubMed] [Google Scholar]
- 10.Krabbe KS, Nielsen AR, Krogh-Madsen R, et al. Diabetologia 2007; 50: 431–438. [DOI] [PubMed]
- 11.Zhen YF, Zhang J, Liu XY, et al. Low BDNF is associated with cognitive deficits in patients with type 2 diabetes. Psychopharmacology 2013; 227: 93–100. [DOI] [PubMed] [Google Scholar]
- 12.Rios M. BDNF and the central control of feeding: Accidental bystander or essential player? Trends Neurosci 2013; 36: 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bariohay B, Lebrun B, Moyse E, et al. Brain-derived neurotrophic factor plays a role as an anorexigenic factor in the dorsal vagal complex. Endocrinology 2005; 146: 5612–5120. [DOI] [PubMed] [Google Scholar]
- 14.Liao G-Y, An JJ, Gharami K, et al. Dendritically targeted Bdnf mRNA is essential for energy balance and response to leptin. Nat Med 2012; 18: 564–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuroda A, Yamasaki Y, Matsuhisa M, et al. Brain-derived neurotrophic factor ameliorates hepatic insulin resistance in Zucker fatty rats. Metabolism 2003; 52: 203–208. [DOI] [PubMed] [Google Scholar]
- 16.Kuipers SD, Trentani A, Tiron A, et al. BDNF-induced LTP is associated with rapid Arc/Arg3.1-dependent enhancement in adult hippocampal neurogenesis. Sci Rep 2016; 6: 212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li M, Dai F-R, Du X-P, et al. Infusion of BDNF into the nucleus accumbens of aged rats improves cognition and structural synaptic plasticity through PI3K-ILK-Akt signaling. Behav Brain Res 2012; 231: 146–153. [DOI] [PubMed] [Google Scholar]
- 18.Kiprianova I, Sandkühler J, Schwab S, et al. Brain-derived neurotrophic factor improves long-term potentiation and cognitive functions after transient forebrain ischemia in the rat. Exp Neurol 1999; 159: 511–519. [DOI] [PubMed] [Google Scholar]
- 19.Phillips MS, Liu Q, Hammond HA, et al. Leptin receptor missense mutation in the fatty Zucker rat. Nat Genet 1996; 13: 18–19. [DOI] [PubMed] [Google Scholar]
- 20.Hirose H, Maruyama H, Kido K, et al. Alpha- and beta-cell function in obese Zucker (fa/fa) rats: A study with the isolated perfused pancreas. Clin Sci 1994; 86: 311–316. [DOI] [PubMed] [Google Scholar]
- 21.Silhol M, Bonnichon V, Rage F, et al. Age-related changes in brain-derived neurotrophic factor and tyrosine kinase receptor isoforms in the hippocampus and hypothalamus in male rats. Neuroscience 2005; 132: 613–624. [DOI] [PubMed] [Google Scholar]
- 22.Komori T, Morikawa Y, Nanjo K, et al. Induction of brain-derived neurotrophic factor by leptin in the ventromedial hypothalamus. Neuroscience 2006; 139: 1107–1115. [DOI] [PubMed] [Google Scholar]
- 23.Hirvonen J, Virtanen KA, Nummenmaa L, et al. Effects of insulin on brain glucose metabolism in impaired glucose tolerance. Diabetes 2011; 60: 443–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stranahan AM, Lee K, Martin B, et al. Voluntary exercise and caloric restriction enhance hippocampal dendritic spine density and BDNF levels in diabetic mice. Hippocampus 2009; 19: 951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campfield LA, Smith FJ. Blood glucose dynamics and control of meal initiation: A pattern detection and recognition theory. Physiol Rev 2003; 83: 25–58. [DOI] [PubMed] [Google Scholar]
- 26.Matsuda M, Liu Y, Mahankali S, et al. Altered hypothalamic function in response to glucose ingestion in obese humans. Diabetes 1999; 48: 1801–1806. [DOI] [PubMed] [Google Scholar]
- 27.Hadjighassem M, Kamalidehghan B, Shekarriz N, et al. Oral consumption of α-linolenic acid increases serum BDNF levels in healthy adult humans. Nutr J 2015; 14: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Webster MJ, Herman MM, Kleinman JE, et al. BDNF and trkB mRNA expression in the hippocampus and temporal cortex during the human lifespan. Gene Expr Patterns 2006; 6: 941–51. [DOI] [PubMed] [Google Scholar]