Abstract
The purpose of this study was to develop and evaluate a scoring system for assessing reperfusion status based on arterial spin labeled (ASL) perfusion MRI in acute ischemic stroke (AIS) patients receiving thrombolysis and/or endovascular treatment. Pseudo-continuous ASL with background suppressed 3D GRASE was acquired along with DWI in 90 patients within 24 h post-treatment. An automatic reperfusion scoring system (auto-RPS) was devised based on the Alberta Stroke Program Early CT Score (ASPECTS) template, and compared with manual RPS and DWI-ASPECTS. TICI (thrombolysis in cerebral infarction) scores were graded in 48 patients who received endovascular treatment. Favorable outcomes were defined by a modified Rankin Scale score of 0–2 at three months. Auto-RPS was positively correlated with DWI-ASPECTS (ρ = 0.6, P < 0.001) and was on average 1 point lower than DWI-ASPECTS (P < 0.001). The area under the receiver operating characteristic curve for discriminating poor functional outcome (n = 90) was 0.75 (95% CI, 0.64–0.86) for manual RPS, 0.85 (95% CI, 0.76–0.94) for auto-RPS, and 0.81 (95% CI, 0.71–0.90) for DWI-ASPECTS. Multiple logistic regression analysis in the TICI-graded patients (n = 48) showed that auto-RPS is highly associated with functional outcome (OR = 25.2, 95% CI 4.02–496, P < 0.01). Post treatment auto-RPS within 24 h provides a useful tool to predict functional outcome in AIS patients.
Keywords: Acute ischemic stroke, arterial spin labelling, recanalization, reperfusion, reperfusion injury
Introduction
During the past decade, endovascular clot retrieval therapy has been introduced to provide more treatment options beyond the limited early time window for tPA1,2 in acute ischemic stroke (AIS) patients. Recently, a series of randomized clinical trials (MR CLEAN, ESCAPE, EXTEND-IA, SWIFT PRIME, and REVASCAT) have consistently shown the efficacy of adding endovascular therapy versus standard care in treating patients with acute anterior circulation ischemic stroke.3–7 These encouraging developments provide increased options for thrombolytic and revascularization therapy to improve the rate of reperfusion and ensuing clinical outcome of ischemic stroke patients.
Previous studies have shown that recanalization, if achieved early, can predict better clinical outcomes.8–11 However, despite an excellent recanalization rate, favorable clinical outcomes are not as satisfactory as expected.12 The hemodynamic status following successful recanalization is complex due to a variety of factors including heterogeneity of hyper and hypo-perfusion, no reflow phenomenon, and reperfusion injury;13 all of which contribute to clinical outcome despite successful recanalization.
It has been posited that reperfusion, rather than recanalization, is the key factor determining the clinical outcome of stroke patients.14 Indeed, recent research indicates that reperfusion outperforms recanalization in predicting patient clinical outcome.15,16 Nevertheless, these studies have largely overlooked the detrimental effect of reperfusion injury on patient clinical outcome. In animal models, reperfusion-induced injury may impair microcirculatory reflow and negatively affect survival despite recanalization of an occluded artery.17 Clinically, post-ischemic hyperperfusion is significantly related to hemorrhagic transformation (HT)18 which is a major risk and a potentially life-threatening complication in patients who receive clot-dissolving or retrieving therapies.19,20
Hyper- and hypo-perfusion can occur on post-treatment perfusion maps and may display as a heterogeneous reperfusion pattern. Also, a small stroke lesion in a critical location can be far more serious than a large stroke encompassing a less critical area.21 Based on these considerations, we devised an automatic reperfusion scoring system (auto-RPS) based on the Alberta Stroke Program Early CT Score (ASPECTS) system to individually predict patient clinical outcome post thrombolysis/endovascular treatment. For comparison, we also evaluated the predictive value of DWI (diffusion weighted imaging)-ASPECTS on post treatment MRI22 concurrent with ASL. We hypothesize that: (1) post treatment auto-RPS and DWI-ASPECTS have good predictive value of patients’ functional outcome; (2) auto-RPS score adds additional predictive value, compared to that of recanalization alone and the beneficial effects of recanalization are attributable to benign reperfusion.
Material and methods
Patient selection
The present study utilized data collected from July 2010 to June 2014 in an ongoing prospective registry of patients evaluated with diffusion–perfusion MRI at UCLA medical center. Patients with AIS were included in this study if: (1) they were adults (aged ≥18 years); (2) they had unilateral acute ischemic stroke with proximal vascular occlusion in anterior circulation (e.g., internal carotid artery, middle cerebral artery M1 and/or M2 segments) based on admission MR angiography (MRA) results; (3) intravenous tPA or endovascular therapy was performed; (4) ASL and DWI imaging were acquired within 24 h post-treatment, and (5) absence of previous intracranial hemorrhage, brain surgery, or large territorial lesion. The institutional review board of the University of California Los Angeles approved the study and waived requirement for informed consent. Analysis of patient data was performed in accordance with the Declaration of Helsinki.
MRI protocols and analysis
All patients underwent MRI on Siemens 1.5 T Avanto or 3.0 T TIM Trio systems (Erlangen, Germany), using 12 channel head coils. ASL was acquired along with other MR sequences within 24 h after treatment as a routine clinical protocol. A pseudo-continuous ASL (pCASL) pulse sequence with background suppressed 3D GRASE (gradient and spin echo) readout was applied with the following parameters: TR/TE/label time/post-labeling delay (PLD), 4000/22/1500/2000 ms; field of view, 22 cm; matrix size, 64 × 64, 26 × 5 mm slices; GRAPPA factor of 2, 4/8 partial k-space, 30 pairs of label and control images with a scan time of 4 min. In Dec 2011, a pCASL GRASE protocol with four PLDs (1500, 2000, 2500, 3000 ms) was implemented and the present analysis included CBF data obtained with the PLD of 2000 ms. The imaging parameters of the four-PLD ASL protocol were the same as those of the single PLD protocol except that eight pairs of label and control images were acquired for each PLD resulting in a scan time of 4 min 30 s. Two global inversion pulses were applied during the PLD for background suppression (BS).23,24
Data analysis was performed with interactive data language (IDL (Boulder, CO, USA)) software programs developed in-house. Motion correction was performed on ASL images of each PLD, respectively. Pairwise subtraction between label and control images was performed followed by averaging to generate the mean difference image. Quantitative CBF maps were calculated based on a previously published model.23 CBF maps were co-registered with DWI and normalized into the Montreal Neurological Institute template space using SPM8 (Wellcome Department of Cognitive Neurology, UCL, UK).
A 1-3 Likert-type scale was used by two reviewers respectively to evaluate the ASL image quality with scoring as follows: (1) severe image artifact (e.g. head motion), not interpretable; (2) fair diagnostic image quality, some distortion and noise, acceptable delineation of major structures; and (3) good image quality, no to minimal distortion with detailed delineation of all structures. ASL images with an image quality score of 1 by both reviewers were discarded.
Development of automatic reperfusion scoring (auto-RPS) system
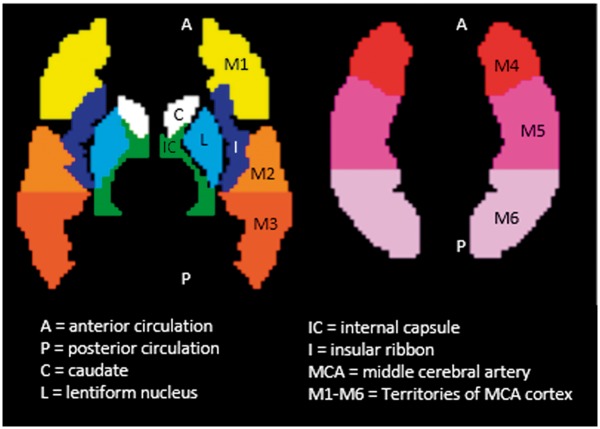
According to our preliminary data, patients with extremely low whole brain perfusion generally had bad outcome because very low CBF can lead to complete electrical failure of neurons based on animal literature.25,26 Due to the difficulty in differentiating low CBF due to physiological or technical reasons (e.g., poor labeling effect), we excluded patients with extremely low whole brain perfusion (less than 16 ml/100 g/min) in analyses. For the rest of the patients, auto-RPS was automatically calculated based on the ASPECTS template,21 which is a 10-point scale used clinically to assess early ischemic changes after stroke and is now extended to DWI due to excellent intermodality agreement.27,28 This ASPECTS template (Figure 1) was developed based on the PickAtlas anatomical template (http://fmri.wfubmc.edu/software/pickatlas), and a published reference of anatomic regions and vascular territories.29 The ASPECTS regions studied included the insula, caudate, lentiform, internal capsule, and six cortical distributions of the MCA vascular territory.21 Regional perfusion parameters in the 10 ASPECTS brain regions and their contralateral mirror regions were extracted automatically from the calculated CBF in each patient using in-house developed Matlab programs (The MathWorks, Inc., Natick, MA, USA). The relative ratio of CBF in the lesion region to that in its unaffected contralateral mirror region was calculated to describe reperfusion in the lesion hemisphere relative to the unaffected contralateral hemisphere. Because either hyper- or hypo-perfusion is related to bad outcome as seen in the result of our preliminary study,18 points are deducted from 10 where hyper- or hypo-perfusion of a scored region warrants a point deduction.
Figure 1.
ASPECTS template with 10 regions for scoring. Subcortical structures (C, L, and IC) account for three points. Middle cerebral artery cortex accounts for seven points (I, M1 to M6). A published reference of anatomic regions and vascular supply territories was used in the three-dimensional region-drawing process.
The flow chart of the proposed scoring system is shown in Figure 2. Based on our database of CBF measurements, hypo- and hyper-perfusion was tentatively defined as the 12.5 and 87.5th percentiles, 25 and 75th percentiles, 37.5 and 63.5th percentiles using box plot construction analysis, respectively, in each ASPECTS region to identify the optimal range that provided the best performance for association with outcome. Furthermore, to test whether hyperperfusion is associated with good or bad functional outcome, points were deducted from 10 where only hypoperfusion of a scored region warrants a point deduction. The association of hypoperfusion- only auto-RPS with functional outcome was compared with that including both hypo- and hyperperfusion.
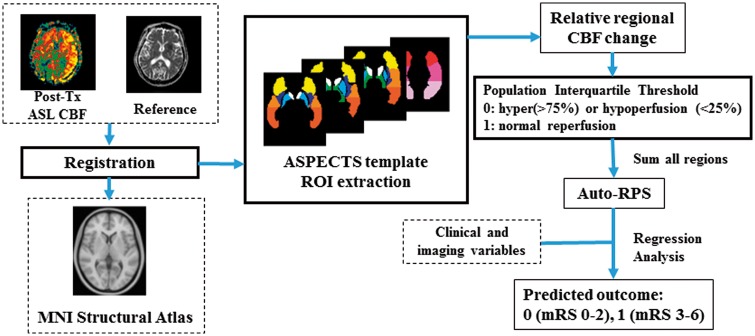
Figure 2.
The flow chart of the proposed reperfusion scoring (auto-RPS) system. Post treatment ASL-CBF maps were registered to the MNI Structural Atlas, and relative regional CBF ratio was calculated based on the devised ASPECTS template. Points are deducted from 10 where hyper- or hypo-perfusion of a scored region warrants a point deduction. Auto-RPS is finally acquired by taking the sum of all regions.
Manual RPS, DWI-ASPECTS, and TICI (thrombolysis in cerebral infarction) scores
Two stroke fellows with ASPECTS training who were blinded to clinical information except the affected hemisphere calculated manual RPS on post treatment ASL perfusion maps by manually detecting hyper- and hypo-perfusion in each ASPECTS region. One week later, they were required to calculate DWI-ASPECTS based on post treatment DWI imaging where visible lesion of a scored region warrants a point deduction from 10. The average score from the two raters was regarded as the final result for manual RPS and DWI-ASPECTS.
For patients who underwent endovascular treatment, the thrombolysis in cerebral infarction (TICI) score30 was rated by two experienced neurologists to evaluate the degree of recanalization on digital subtraction angiography (DSA). For the purposes of dividing the cohort, TICI 2B/3 was considered adequate recanalization.
Clinical variables and outcomes
Patients’ medical charts were reviewed for demographics, comorbidities (hypertension, diabetes and hyperlipidemia), and neurological deficits using the National Institutes of Health Stroke Scale (NIHSS) score. Patient functional outcomes (modified Rankin Scale (mRS) score) were routinely collected three months after the occurrence of stroke and assessed by phone interview at our institution.
Statistical analysis
Statistical analysis was performed using SPSS (IBM SPSS Statistics, Armonk, NY) and R (R Core Team (2012) Vienna, Austria). The linearly weighted kappa test was used to evaluate the inter-rater agreement in terms of ASL imaging quality. Inter-rater agreement of manual RPS (for each ASPECTS region and total scores) and DWI-ASPECTS was calculated using the intraclass correlation coefficient (ICC). Interpretation of ICC was as follows: poor, <0.40; fair-to-good, 0.40 to 0.75; excellent, >0.75. A Bland–Altman plot was used to illustrate the magnitude of differences between auto-RPS and DWI-ASPECTS. Paired-Samples T test was used to investigate if there was significant difference between auto-RPS and DWI-ASPECTS. The correlation between different scoring methods (auto-RPS vs. DWI-ASPECTS and auto-RPS vs. TICI) was assessed by Spearman rank test. To evaluate the predictive value of auto-RPS, manual RPS, and DWI-ASPECTS, receiver operating characteristic (ROC) curves was constructed. The area under the ROC curve (AUC) was used as a scalar measure to assess the performance of prognostic risk scores. The AUC between groups was compared by using the Delong et al.31 method implemented in R.
In patients who received endovascular treatments and thus had TICI results, univariate analysis was performed to evaluate the predictive value of the imaging and clinical variables. Subsequently, multiple logistic regression model fitting (forward, backward, and bi-directional stepwise analysis) was applied to identify predictors for favorable clinical outcome from the variables that had a univariate probability value <0.1. Good functional outcome was defined as a 90-day mRS score of 0 to 2. The stepwise analyses chose the best multivariate model fit by selecting variables that minimized the Akaike Information Criterion (AIC). A p value of less than 0.05 (2-sided) was considered to be statistically significant.
Results
A total of 99 patients (age = 72.4 ± 14.4 (mean ± SD) years; 43 males) were collected from July 2010 to June 2014, excluding two patients whose ASL scans were rated as non-diagnostic (see Supplement Table 1). Nine patients had extremely low whole brain perfusion and were excluded from the study. In the remaining 90 patients, the median NIHSS score was 16 (interquartile range, 9–20). All the patients had ASL and concurrent DWI examinations within 24 h post thrombolysis or endovascular treatments. The median (interquartile range) time from treatment to ASL/DWI imaging was 6.4 (4.6–17.1) h. All 90 patients had mRS score evaluation at three months, and 29 patients had favorable outcome (mRS = 0–2).
Auto-RPS is slightly more associated with patient functional outcome when hyperperfusion is regarded as detrimental rather than beneficial; however, the difference is not statistically significant. Among the three threshold range groups, 25th and 75th percentile defined hypo-perfusion and hyper-perfusion best and provided the best performance for association with outcome as observed by the area under curve (AUC) calculations in Supplement Table 2.
The quartile cut-off value (relative CBF ratio) ranged from 0.71 to 0.89 and 1.10 to 1.26, respectively, to define hypoperfusion and hyperperfusion in the ASPECTS regions (Supplement Figure 1). The absolute mean lesion CBF ranged from 21.13 ± 11.40 (mean ± SD) to 29.23 ± 11.71 ml/100 g/min and from 35.16 ± 15.86 to 54.49 ± 21.24 ml/100 g/min in the hypoperfusion and hyperperfusion regions, respectively (Supplement Figure 2).
Inter-reader and inter-modality agreement
The average score of image quality of included ASL maps was 2.60 ± 0.50. The κ coefficient was 0.72 for ASL imaging quality between the two raters. For manual RPS, the agreement between two raters for each ASPECTS region ranged from poor to excellent (see Supplement Table 3). Intra-reader ICCs for manual RPS were 0.76 (95% CI: 0.60–0.85) and 0.94 (95% CI: 0.91–0.96) for DWI-ASPECTS, respectively. Altogether, there were 36 patients scanned at 3.0 T and 54 patients scanned with 1.5 T. The average ASL image quality was 2.64 ± 0.49 (mean ± SD) and 2.57 ± 0.50 at 3 and 1.5 T, respectively, without significant difference (P = 0.22). Auto-RPS was lower than DWI-ASPECT in patients scanned at either 1.5 or 3.0 T. The average difference between auto-RPS and DWI-ASPECT was −0.96 and −0.81 in patients scanned at 3.0 and 1.5 T respectively without significant difference (P = 0.72).
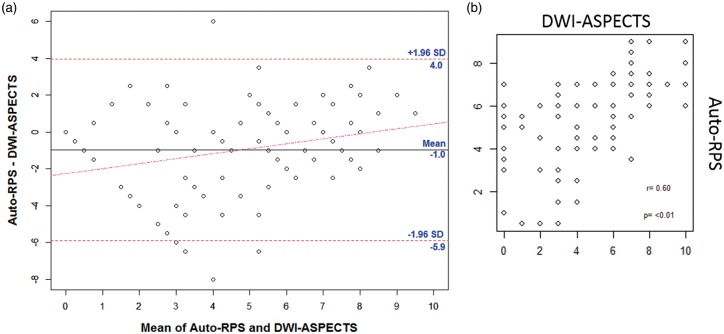
Auto-RPS was positively correlated with manual RPS (ρ = 0.65, P < 0.001, Spearman correlation coefficient) and with post-treatment DWI-ASPECTS (ρ = 0.6, P < 0.01, Spearman test) (Supplement Figure 3). Auto-RPS (mean ± SD, 4.69 ± 2.89) was significantly lower than DWI-ASPECTS (5.43 ± 2.10; P < 0.001). Figure 3(a) shows the Bland and Altman plot, and Figure 3(b) shows the correlation plot. The mean difference between auto-RPS and DWI-ASPECTS was −0.74 (95% CI, −0.25 to −1.24) indicating that slightly more abnormal perfusion regions were observed using auto-RPS compared to the observation of regions of ischemic injury using DWI. Figure 4 includes examples with discordance of auto-RPS and DWI-ASPECTS which showed tissues with abnormal perfusion but normal diffusion were still at risk of dying (Figure 4(a) and (b)) and tissues with normal perfusion but abnormal diffusion may indicate good outcome or tiny lesion territory (Figure 4(c) and (d)).
Figure 3.
Bland–Altman plot (a) of post treatment auto-RPS and DWI-ASPECTS. The mean difference between the two modalities was −0.7 indicating that slightly more abnormal perfusion regions is observed on auto-RPS relative to regions of ischemic injury observed using DWI. The horizontal lines represent the mean difference in scores and ±1.96 SD. The red dashed line represents the regression line of differences. Correlation plot (b) showing positive correlation of Auto-RPS with DWI-ASPECTS, ρ = 0.6, p < 0.01.
Figure 4.
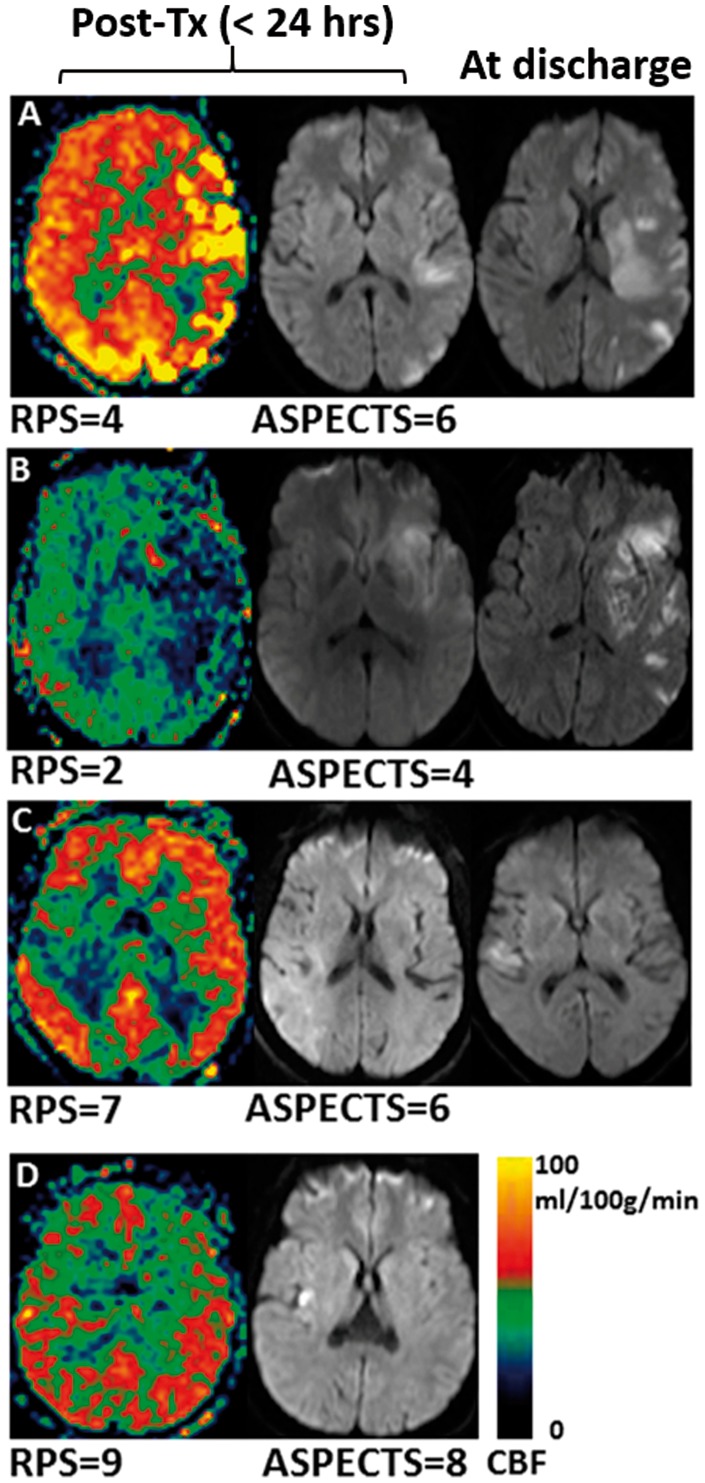
Cases with discordance between post treatment auto-RPS and DWI-ASPECTS. Lower auto-RPS due to reperfusion injury (a) or ischemia (b) explained tissue damages on discharge. Alternately, higher auto-RPS can lead to lesion reversal on DWI (c) or indicates the lesion is not large enough to cause abnormal region perfusion (d).
Association of manual RPS, auto-RPS and DWI-ASPECTS (n = 90) with patient functional outcome
The AUC for discriminating favorable functional outcome (n = 90) was 0.75 (95% CI, 0.64–0.86) for manual RPS, 0.85 (95% CI, 0.76–0.94) for auto-RPS, and 0.81 (95% CI, 0.71–0.90) for DWI-ASPECTS (Figure 5). There was no significant difference of AUCs between manual RPS vs. auto-RPS (P = 0.1), manual RPS vs. DWI-ASPECTS (P = 0.4), and auto-RPS vs. DWI-ASPECTS (P = 0.3) (DeLong method). The best cut-off value for identifying favorable functional outcome was auto-RPS ≥ 6 with resultant sensitivity of 0.86, specificity of 0.77, positive predictive value of 0.64, negative predictive value of 0.92; and DWI-ASPECTS ≥ 7 with resultant sensitivity of 0.66, specificity of 0.77, positive predictive value of 0.58, negative predictive value of 0.83. Table 1 shows the univariate logistic regression analysis results for each independent variable’s ability to discriminate good functional outcome for the 90 patients.
Figure 5.
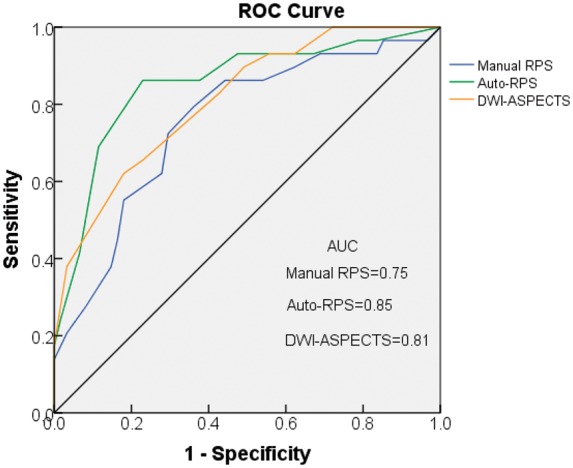
Receiver operating characteristic (ROC) curves of manual RPS, auto-RPS, and DWI-ASPECTS for discriminating functional outcome. Auto-RPS performed the best in identifying good functional outcome, although the differences in AUC were not statistically different. Auto-RPS indicates automatic reperfusion scoring system; DWI-ASPECTS indicates diffusion weighted imaging-Alberta Stroke Program Early CT Score; AUC, area under the curve.
Table 1.
Univariate Logistic Regression results for discriminating good functional outcome (Modified Rankin Scale, 0–2) in total cohort (N = 90) and TICI-scored Subgroup (N = 48).
| Variable, N = 90 | mRS ≤ 2 (N = 29) | mRS > 2 (N = 61) | P | OR† | CI† |
|---|---|---|---|---|---|
| Female Gender, n (%) | 14 (48) | 37 (61) | 0.26 | ||
| Hypertension, n (%) | 17 (59) | 34 (56) | 0.79 | ||
| Diabetes, n (%) | 5 (17) | 16 (26) | 0.35 | ||
| Hyperlipidemia, n (%) | 6 (21) | 23 (38) | 0.11 | ||
| Time to MR exam (mean ± std), hours | 4 ± 8 | 6 ± 9 | 0.23 | ||
| Age (mean ± std), y | 67 ± 11 | 73 ± 15 | 0.07* | ||
| NIHSS (med, IQR) | 9, 6–19 | 17, 11–21 | 0.02* | ||
| Auto-RPS ≥ 6, n (%) | 20 (69) | 7 (11) | <0.01* | 21 | 6.8–80.9 |
| Manual-RPS ≥ 6, n (%) | 18 (62) | 17 (28) | <0.01* | 6.27 | 2.4–17.6 |
| DWI-ASPECTS ≥ 7, n (%) | 18 (62) | 11 (18) | <0.01* | 2.35 | 1.6–3.8 |
| Variable, N = 48 |
mRS ≤ 2 (N = 10) |
mRS > 2 (N = 38) |
P
|
OR† |
CI† |
| Age (mean ± std), y | 64 ± 11 | 71 ± 14 | 0.23 | ||
| Female Gender, n (%) | 4 (40) | 22 (56) | 0.32 | ||
| Hypertension, n (%) | 6 (60) | 22 (56) | 0.90 | ||
| Diabetes, n (%) | 1 (10) | 12 (31) | 0.16 | ||
| Hyperlipidemia, n (%) | 2 (20) | 15 (38) | 0.26 | ||
| Atrial Fibrillation, n (%) | 4 (40) | 11 (28) | 0.62 | ||
| NIHSS (med, IQR) | 15.5, 8.25–19 | 18, 14–23 | 0.08* | ||
| TICI ≥ 2b, n (%) | 8 (80) | 16 (41) | 0.03* | 6.13 | 1.32–44.5 |
| Auto-RPS ≥ 6, n (%) | 9 (90) | 10 (26) | <0.01* | 25.2 | 4.02–496 |
| DWI-ASPECTS ≥ 7, n (%) | 7 (70) | 5 (13) | <0.01* | 15.4 | 3.23–93.5 |
Odds ratios and 95% confidence intervals reported only for binary significant variables. *Indicates significant independent variable for consideration in multivariate logistic regression. NIHSS: National Institutes of Health Stroke Scale; TICI: thrombolysis in cerebral infarction; RPS: reperfusion scores; DWI: diffusion-weighted imaging; ASPECTS: Alberta Stroke Program Early CT Score med, median; IQR: interquartile range; OR: odds ratio; CI: 95% confidence limits.
Since DWI can exhibit the phenomenon of transient reversal at early time points after therapy is administered, we also evaluated the performance of DWI-ASPECTS and auto-RPS for subjects with scans acquired more than 12 h after therapy. For these 13 subjects, both DWI-ASPECTS (P = 0.26) and auto-RPS (P = 0.31) were not significant regressors for functional outcome.
Association of auto-RPS, DWI-ASPECTS and TICI (n = 48) with patient functional outcome
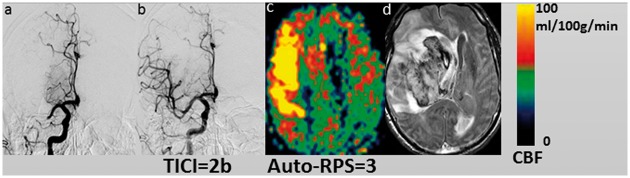
Recanalization status was known in 48 cases. Twenty-three patients had successful recanalization (TICI = 2b or 3) of whom only 12 patients had good reperfusion score (auto-RPS ≥ 6) and 8 patients had good functional outcome. Auto-RPS ≥ 6 and TICI ≥ 2b did not correlate well (ρ = 0.27, P > 0.05, Spearman test). Figure 6 shows one case with adequate recanalization that led to hyperperfusion and resulted in a low auto-RPS score.
Figure 6.
One case (male, 75 years old) with MCA occlusion (a) and presented with sudden-onset left-sided weakness with a baseline NIHSS of 17. Adequate recanalization was performed with final TICI = 2b (b). Hyperperfusion was detected in the right MCA region on ASL (auto-RPS = 3) performed 15 h post recanalization (c) and a hematoma (PH-2) developed 13 days after treatment (d). The mRS scores at three months of the patient was 6.
In univariate logistic regression analysis, auto-RPS ≥ 6 (P < 0.01), DWI-ASPECTS ≥ 7 (P < 0.01), TICI ≥ 2b (P = 0.03), and baseline NIHSS (P = 0.08) were associated with favorable functional outcome at three months for the 48 patients with known recanalization status (Table 1). Age, gender, history of diabetes, hypertension, atrial fibrillation, and hyperlipidemia were not significantly associated with functional outcome in the univariate analysis, and were not considered in the multiple logistic regression model fitting.
In stepwise multivariate logistic regression analysis, auto-RPS ≥ 6 and TICI ≥ 2b were found to form the best logistic regression model fit (AUC = 0.875). However, only auto-RPS ≥ 6 emerged as a significant explanatory variable for discriminating functional outcome (OR = 25.2, 95% CI 4.02–496, P < 0.01) in this multivariate model. Since auto-RPS and DWI-ASPECTS are so well-correlated, in principle they could be interchangeable. However, when placed in the full model together prior to the stepwise selection, we observed that forward, backward, and bi-directional stepwise regression all selected auto-RPS over DWI-ASPECTS. We included age, history of diabetes, hypertension, atrial fibrillation, and hyperlipidemia in an additional multiple logistic regression model to adjust for possible confounders, and we still found that only auto-RPS emerged as a significant regressor, although the AUC improved to 0.93.
Discussion
In this study, we devised an automatic reperfusion scoring system (auto-RPS) based on the ASPECTS template which can produce a quick evaluation of reperfusion status in AIS patients post thrombolysis or endovascular treatments and the results showed that auto-RPS is highly associated with favorable functional outcome. Auto-RPS is devised based on our empirical observation that both extremely low and high brain perfusion can be detrimental. In this study, when hyperperfusion is regarded as detrimental, auto-RPS has a slightly stronger association with patient functional outcome compared to standard consideration of hypoperfusion alone, though this difference is not statistically significant. Further work is needed to validate this classification of hyperperfusion in a larger cohort.
Auto-RPS is on average 1 point lower than DWI-ASPECTS. In this study, 58 patients had equal or lower auto-RPS scores than DWI-ASPECTS. One reason is that auto-RPS is more sensitive than DWI-ASPECTS at detecting ischemic tissue changes, especially brain tissues presenting with hypoperfusion that are still viable (Figure 4(b)). Another reason is that auto-RPS can also detect brain tissues with hyperperfusion which can lead to reperfusion injury13,32 but cannot be detected on DWI (Figure 4(a)). Our study also indicates that hyperperfusion is associated with bad functional outcome. There were 32 patients with auto-RPS scores higher than DWI-ASPECTS. Regions with normal perfusion but with presence of lesions on DWI might indicate future reversal of the lesion (Figure 4(c)) or simply that the lesion is not large enough to cause regional perfusion abnormality (Figure 4(d)). Another unique finding in this study was the comparison of auto-RPS and DWI-ASPECTS as an outcome predictor. The area under the receiver operating characteristic curve for predicting outcomes with auto-RPS was higher than that of DWI-ASPECTS, although the difference was not statistically significant. Auto-RPS appears to be at least equivalent to DWI-ASPECTS in predicting stroke outcomes.
In addition, we observed that auto-RPS scores ≥6 and <6 tend to differentiate between favorable and non-favorable functional outcome at three months. This finding may suggest, as has been proposed previously,33,34 that there is a threshold for ischemic or reperfusion injury at which damage beyond a certain extent is generally associated with poor recovery from stroke. The auto-RPS may provide assistance for physicians to predict functional outcome and plan treatment strategies accordingly.
Auto-RPS outperformed manual RPS which is most likely attributed to the relatively low spatial resolution on ASL maps, which made it difficult to distinguish brain anatomic structures, especially those in deep brain areas. This explains why ICC between two raters is poor in deep ASPECTS regions (Supplement Table 3). Also, manual scoring depended largely on clinical experience to define hypo- or hyper-perfusion. The inter-rater agreement for manual RPS in individual ASPECTS region ranged from poor to excellent even though the two raters were experienced neurologists and received training before scoring. Auto-RPS is more accurate because it defines the abnormal perfusion quantitatively rather than qualitatively, thus requiring negligible training.
Our study also demonstrates that auto-RPS is highly associated with outcome independent of the recanalization result, and the beneficial effects of recanalization are attributable to benign reperfusion. Several studies have directly compared the accuracy of reperfusion versus recanalization to explain imaging or clinical outcomes. These studies demonstrated that reperfusion outperforms recanalization and can be a surrogate marker for clinical outcome.14–16,35 Recanalization specifies the patency of the primary arterial occlusive lesion; however, adequate recanalization may fail to induce reperfusion because of distal microemboli or extensive damage to the microvascular circulation (no-reflow phenomenon).36 In some cases, sudden recanalization to badly damaged tissue can cause hyperperfusion and subsequent hemorrhagic transformation. Significant reperfusion indicating the restoration of blood flow at the distal capillary level can be observed despite inadequate recanalization, presumably from collateral sources.15,37 It is imperative to pay more attention to the discordance between recanalization and auto-RPS results, especially in those with adequate recanalization but poor auto-RPS scores, which might indicate no flow phenomenon or reperfusion injury. The difference between our study and previous literature is that reperfusion injury in our study is considered to have the same detrimental effects as non-reperfusion (both warrant a point deduction), and we applied the ASPECTS model to address the existence of heterogeneity of hypo- and hyper-perfusion.
This study has several limitations. First, although post treatment DWI-ASPECTS demonstrated its association with patient clinical outcome in this article, we did not compare it with baseline DWI-ASPECTS because MR examinations are challenging in real time in the emergency department. Also, DWI-ASPECTS in the early post-therapy period are not always reliable due to the transient reversal phenomenon of DWI at early time points. Only 13 subjects had ASL and DWI scans acquired 12 h after treatment, thus the sample size is likely too small to fully characterize the ability of DWI-ASPECTS to compete with auto-RPS at earlier time points after therapy. Secondly, auto-RPS was devised based on our 90 AIS patients. In the future, a larger database is needed to improve the accuracy of our scoring system and to validate our findings.
The future of acute stroke therapy lies in the advancement of our understanding of reperfusion beyond the concept of recanalization alone. As an entirely noninvasive and quantitative technique, ASL perfusion imaging offers unique advantages to trace the evolution of the pathophysiology in ischemic stroke and allows for the specific quantification of tissue-based changes. This may lend itself well to the development of protective treatments for ischemia and reperfusion injury in AIS patients after recanalization therapy.
Conclusion
A reperfusion scoring system was devised and was found to be highly associated with patient functional outcome. This reperfusion scoring system may provide a useful tool to complement existing clinical methods for improved management strategies of AIS patients after treatment.
Supplementary Material
Funding
This study was supported by the US National Institutes of Health grants UH2NS100614, R01-NS081077, R01-EB014922, R01-NS077706, K23-NS054084, P50-NS044378 and UL1TR000124, K24-NS072272 and American Heart Association grant 16POST26430064.
Declaration of conflicting interests
Dr. Gary R Duckwiler is scientific advisor for Medtronic and Sequent medical. The other authors declare no financial conflict of interest.
Authors’ contributions
SY and SJM involved in development of template, analysis and interpretation of data, and drafting the article. DY, NL, XS and LY involved in data analysis/interpretation. DSL, XQ, BY, FS, JDH, LA, NR, RJ, ST, GRD, JLS and NS contributed to the study via the collection of data. DJJW took part in conception and design. All the authors involved in revising the article critically for important intellectual content and final approval of the version to be published.
Supplementary material
Supplementary material for this paper can be found at the journal website: http://journals.sagepub.com/home/jcb
References
- 1.Kim D, Jahan R, Starkman S, et al. Endovascular mechanical clot retrieval in a broad ischemic stroke cohort. Am J Neuroradiol 2006; 27: 2048–2052. [PMC free article] [PubMed] [Google Scholar]
- 2.Nesbit GM, Luh G, Tien R, et al. New and future endovascular treatment strategies for acute ischemic stroke. J Vasc Intervent Radiol 2004; 15(1 Pt 2): S103–S110. [DOI] [PubMed] [Google Scholar]
- 3.Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015; 372: 2296–2306. [DOI] [PubMed] [Google Scholar]
- 4.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015; 372: 1019–1030. [DOI] [PubMed] [Google Scholar]
- 5.Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015; 372: 1009–1018. [DOI] [PubMed] [Google Scholar]
- 6.Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015; 372: 11–20. [DOI] [PubMed] [Google Scholar]
- 7.Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015; 372: 2285–2295. [DOI] [PubMed] [Google Scholar]
- 8.Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med 1995; 333: 1581–1587. [DOI] [PubMed] [Google Scholar]
- 9.del Zoppo GJ, Koziol JA. Recanalization and stroke outcome. Circulation 2007; 115: 2602–2605. [DOI] [PubMed] [Google Scholar]
- 10.Zaidat OO, Suarez JI, Sunshine JL, et al. Thrombolytic therapy of acute ischemic stroke: Correlation of angiographic recanalization with clinical outcome. Am J Neuroradiol 2005; 26: 880–884. [PMC free article] [PubMed] [Google Scholar]
- 11.Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: A meta-analysis. Stroke 2007; 38: 967–973. [DOI] [PubMed] [Google Scholar]
- 12.Goyal M. Poor clinical outcome despite successful arterial recanalization. What went wrong? How can we do better? Neuroradiology 2010; 52: 341–343. [DOI] [PubMed] [Google Scholar]
- 13.Nour M, Scalzo F, Liebeskind DS. Ischemia-reperfusion injury in stroke. Intervent Neurol 2013; 1: 185–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Silva DA, Fink JN, Christensen S, et al. Assessing reperfusion and recanalization as markers of clinical outcomes after intravenous thrombolysis in the echoplanar imaging thrombolytic evaluation trial (EPITHET). Stroke 2009; 40: 2872–2874. [DOI] [PubMed] [Google Scholar]
- 15.Cho TH, Nighoghossian N, Mikkelsen IK, et al. Reperfusion within 6 hours outperforms recanalization in predicting penumbra salvage, lesion growth, final infarct, and clinical outcome. Stroke 2015; 46: 1582–1589. [DOI] [PubMed] [Google Scholar]
- 16.Eilaghi A, Brooks J, d'Esterre C, et al. Reperfusion is a stronger predictor of good clinical outcome than recanalization in ischemic stroke. Radiology 2013; 269: 240–248. [DOI] [PubMed] [Google Scholar]
- 17.Yemisci M, Gursoy-Ozdemir Y, Vural A, et al. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat Med 2009; 15: 1031–1037. [DOI] [PubMed] [Google Scholar]
- 18.Yu S, Liebeskind DS, Dua S, et al. Postischemic hyperperfusion on arterial spin labeled perfusion MRI is linked to hemorrhagic transformation in stroke. J Cereb Blood Flow Metab 2015; 35: 630–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fiorelli M, Bastianello S, von Kummer R, et al. Hemorrhagic transformation within 36 hours of a cerebral infarct: Relationships with early clinical deterioration and 3-month outcome in the European Cooperative Acute Stroke Study I (ECASS I) cohort. Stroke 1999; 30: 2280–2284. [DOI] [PubMed] [Google Scholar]
- 20.Berger C, Fiorelli M, Steiner T, et al. Hemorrhagic transformation of ischemic brain tissue: Asymptomatic or symptomatic? Stroke 2001; 32: 1330–1335. [DOI] [PubMed] [Google Scholar]
- 21.Barber PA, Demchuk AM, Zhang J, et al. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet 2000; 355: 1670–1674. [DOI] [PubMed] [Google Scholar]
- 22.Labeyrie MA, Turc G, Hess A, et al. Diffusion lesion reversal after thrombolysis: A MR correlate of early neurological improvement. Stroke 2012; 43: 2986–2991. [DOI] [PubMed] [Google Scholar]
- 23.Wang DJ, Alger JR, Qiao JX, et al. The value of arterial spin-labeled perfusion imaging in acute ischemic stroke: Comparison with dynamic susceptibility contrast-enhanced MRI. Stroke 2012; 43: 1018–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang DJ, Alger JR, Qiao JX, et al. Multi-delay multi-parametric arterial spin-labeled perfusion MRI in acute ischemic stroke – Comparison with dynamic susceptibility contrast enhanced perfusion imaging. Neuroimage Clin 2013; 3: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Branston NM, Symon L, Crockard HA, et al. Relationship between the cortical evoked potential and local cortical blood flow following acute middle cerebral artery occlusion in the baboon. Exp Neurol 1974; 45: 195–208. [DOI] [PubMed] [Google Scholar]
- 26.del Zoppo GJ, Sharp FR, Heiss WD, et al. Heterogeneity in the penumbra. J Cereb Blood Flow Metab 2011; 31: 1836–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barber PA, Hill MD, Eliasziw M, et al. Imaging of the brain in acute ischaemic stroke: Comparison of computed tomography and magnetic resonance diffusion-weighted imaging. J Neurol Neurosurg Psychiatr 2005; 76: 1528–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kosior RK, Lauzon ML, Steffenhagen N, et al. Atlas-based topographical scoring for magnetic resonance imaging of acute stroke. Stroke 2010; 41: 455–460. [DOI] [PubMed] [Google Scholar]
- 29.Kretschmann H-J, Weinrich W, Kretschmann H-J. Cranial neuroimaging and clinical neuroanatomy: Atlas of MIR imaging and computed tomography, 3rd ed Stuttgart, New York: Georg Thieme Verlag, Thieme Medical Publishers, 2003. [Google Scholar]
- 30.Higashida RT, Furlan AJ, Roberts H, et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke 2003; 34: e109–e137. [DOI] [PubMed] [Google Scholar]
- 31.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988; 44: 837–845. [PubMed] [Google Scholar]
- 32.Shen Q, Du F, Huang S, et al. Spatiotemporal characteristics of postischemic hyperperfusion with respect to changes in T1, T2, diffusion, angiography, and blood-brain barrier permeability. J Cereb Blood Flow Metab 2011; 31: 2076–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baird AE, Dambrosia J, Janket S, et al. A three-item scale for the early prediction of stroke recovery. Lancet 2001; 357: 2095–2099. [DOI] [PubMed] [Google Scholar]
- 34.Alexander LD, Pettersen JA, Hopyan JJ, et al. Long-term prediction of functional outcome after stroke using the Alberta Stroke Program Early Computed Tomography Score in the subacute stage. J Stroke Cerebrovasc Dis 2012; 21: 737–744. [DOI] [PubMed] [Google Scholar]
- 35.Soares BP, Tong E, Hom J, et al. Reperfusion is a more accurate predictor of follow-up infarct volume than recanalization: A proof of concept using CT in acute ischemic stroke patients. Stroke 2010; 41: e34–e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ames A, 3rd, Wright RL, Kowada M, et al. Cerebral ischemia. II. The no-reflow phenomenon. Am J Pathol 1968; 52: 437–453. [PMC free article] [PubMed] [Google Scholar]
- 37.Lou X, Yu S, Scalzo F, et al. Multi-delay ASL can identify leptomeningeal collateral perfusion in endovascular therapy of ischemic stroke. Oncotarget 2017; 8: 2437–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.