Abstract
The establishment of a fully functional blood vascular system requires elaborate angiogenic and vascular maturation events in order to fulfill organ-specific anatomical and physiological needs. Although vascular mural cells, i.e. pericytes and vascular smooth muscle cells, are known to play fundamental roles during these processes, their characteristics during vascular development remain incompletely understood. In this report, we utilized transgenic reporter mice in which mural cells are genetically labeled to examine developing vascular mural cells in the central nervous system (CNS). We found platelet-derived growth factor receptor β gene (Pdgfrb)-driven EGFP reporter expression as a suitable marker for vascular mural cells at the earliest stages of mouse brain vascularization. Furthermore, the combination of Pdgfrb and NG2 gene (Cspg4) driven reporter expression increased the specificity of brain vascular mural cell labeling at later stages. The expression of other known pericyte markers revealed time-, region- and marker-specific patterns, suggesting heterogeneity in mural cell maturation. We conclude that transgenic reporter mice provide an important tool to explore the development of CNS pericytes in health and disease.
Keywords: CNS vasculature development, mural cells, pericytes, platelet-derived growth factor receptor β, transgenic reporter mice
Introduction
The delivery of oxygen and nutrients to tissues and organs relies on the blood vascular system. Blood vessel formation is initiated by vasculogenesis, de novo synthesis of vessels during embryogenesis.1 Specifically, mesodermal precursor cells (angioblasts) give rise to a primitive vascular plexus starting at approximately embryonic day (E) 6. Thereafter (∼E9), new vessels form from pre-existing ones. This process, termed angiogenesis, is coordinated by a variety of signaling cues.2,3 Primitive vascular networks undergo a series of morphogenetic changes to comply with local, tissue-specific demands, ultimately establishing functional blood vascular networks with characteristics that are precisely adapted to the specific needs of different organs and tissues.
Microvessels in the central nervous system (CNS) are structurally and functionally distinct from the vascular beds in other organs.4 Accumulating data support the idea that vascular development in the CNS depends on sprouting angiogenesis, in which endothelial cells penetrate and migrate into the neural tube following ventral-dorsal and caudal-rostral gradients.5 The CNS endothelium establishes a complex barrier function known as the blood–brain barrier (BBB) already during embryogenesis6 and eventually engages in a unique organization with surrounding cell types (neurons, astrocytes and pericytes) termed the neurovascular unit.7 Newly formed blood vessels concomitantly recruit supporting cells of the mural cell lineage around the endothelium by secreting attractants, including platelet-derived growth factor-B (PDGF-B).8,9
Mural cells are the collective term for vascular smooth muscle cells (vSMCs) and pericytes, two related cell types that differ based on morphology and location within the vascular beds: vSMCs form relatively continuous cell layers around arteries and veins, and regulate blood vascular tone and vessel diameter, blood pressure and flow.10 At the molecular level, myocardin and serum response factor/CArG-dependent transcriptional regulation is known to be critical for vSMC differentiation, leading to SMC marker gene expression such as αSMA, SM myosin heavy chains, SM myosin light chains, h1-calponin and SM22a which confers contractile function.11,12 Pericytes, on the other hand, are associated with microvessels, i.e. arterioles, venules and capillaries, and form a discontinuous layer around the endothelium.10,13 It has been demonstrated that pericyte recruitment coincides with expansion of the CNS vasculature during murine brain development.14 Indeed, pericyte coverage is shown to be crucial for vascular stabilization.8,9 In the CNS, pericytes are also indispensable for the maturation and maintenance of the BBB, the interaction between the microvessels and surrounding astrocytes within the neurovascular unit, and capillary blood flow, although the latter function remains debated.14,18
The discovery of the cells that are nowadays referred to as pericytes goes back to the late 19th century.19 Later, transmission electron microscopy showed that pericytes share basement membrane with endothelial cells and extend cytoplasmic processes along or around the endothelium.19 Despite significant advances in pericyte biology, precise mechanisms describing pericyte recruitment and regulation of pericyte gene expression levels are largely unanswered. Current immunohistochemical approaches to identify pericytes use antibodies against PDGFRβ, NG2 (Cspg4), CD13 (Anpep), desmin and alpha-smooth muscle actin (αSMA, Acta2), depending on organ, species, and microvessel types.4,13,20 Because these markers are also expressed by other cell types that may be found in close vicinity to blood vessels, including perivascular fibroblasts, vSMCs and macrophages,10,20 anatomical location is still a key criterion to define pericytes.20 The ontogenetic relationships between different perivascular mesenchymal cell types are also unclear. In this report, we examined two transgenic fluorescent reporter mice to determine whether these mice can be useful to recognize pericytes in the developing brain and subsequently to characterize the emergence and distribution of CNS pericytes, focusing on the embryonic mouse brain cortex. Utilizing these genetic markers, we describe the sequence and patterns of pericyte marker expression in the developing cerebral vasculature.
Materials and methods
Animals
Animal care and experiments were conducted in accordance with Swedish legislation and approved by Uppsala University animal ethics committee (ethical number: C224/12 and C115/15). Pdgfrb-EGFP transgenic reporter animals (Tg(Pdgfrb-EGFP)jn169Gsat/Mmucd, Stock number 031796-UCD) were obtained from Mutant Mouse Regional Resource Centers (GENSAT, Rockefeller university, New York).21 Reporter mouse strains mTmG (Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J, stock number 007576), Cspg4-DsRed (Tg(Cspg4-DsRed.T1)1Akik, stock number 008241, also called NG2-DsRed or NG2DsRedBAC)22 and constitutive Cspg4-Cre (B6;FVB-Tg(Cspg4-cre)1Akik/J, stock number 008533)22 were purchased from Jackson Laboratory. Pdgfrb-Cre23 mouse strain was a kind gift from Dr. Ralf Adams. Mice were genotyped by PCR. The following primers are used for PCR-based genotyping: Pdgfrb-EGFP FWD, 5′- CCTACGGCGTGCAGTGCTTCAGC-3′; Pdgfrb-EGFP REV, 5′- CGGCGAGCTGCACGCTGCCGTCCTC-3’ generates a 300 bp fragment. Cspg4-DsRed FWD, 5′-TTCCTTCGCCTTACAAGTCC-3′; Cspg4-DsRed REV, 5′-GAGCCGTACTGGAACTGG-3′ generating a 280 bp product. Generic Cre FWD, 5′- GATATCTCACGTACTGACGG-3′; generic Cre REV, 5′-TGACCAGAGTCATCCTTAGC-3′ generating a 300 bp fragment. Animal experiments were reported in compliance with the ARRIVE guidelines.
Sample processing
Female mice were plugged, and monitored to time of conception. Embryonic day (E) 0.5 was defined as the morning of observed plug. Embryos were prepared as described.24 Briefly, after removing yolk sac and embryonic membranes, embryos were fixed in 4% paraformaldehyde in PBS (PFA, pH 7.4) overnight at 4℃, then transferred to PBS for storage at 4℃. Embryos were cryoprotected by immersion in 30% sucrose solution in PBS for two days at 4℃, embedded into OCT and kept at −80℃ until sectioning. Coronal cryosections were made at 18–20 µm. Alternatively, forebrains (telecephalon) and hindbrains were processed whole, then stained, flat-mounted and imaged.
Immunofluorescence staining and confocal microscopy
Forebrain sections and/ or post-fixed, whole-dissected forebrains and hindbrains were permeabilized in 0.3% Triton-X100-containing PBS for 30 min at room temperature (RT). Specimens were incubated in blocking buffer (1% BSA, 0.3% TritonX-100, 4% normal goat serum in PBS) for 1 h at RT, labeled with primary antibodies overnight at 4℃ followed by appropriate fluorophore-conjugated secondary antibodies for 2 h at RT. After three times washing in PBS, slides were mounted using anti-fade mounting medium with DAPI (Invitrogen). High-resolution images were collected using an SP8 confocal microscope (Leica). All images presented are projection of z-stacks.
Antibodies
Primary antibodies used are as follows: rabbit anti-NG2 chondroitin sulfate proteoglycan (AB5320, Millipore; 1:300), mouse anti-actin, α-smooth muscle (clone 1A4, Sigma Aldrich; 1:1000 or Santa Cruz Biotechnology; 1:500), rabbit anti-desmin (ab15200, Abcam; 1:500), rat anti-PDGFRβ (APB5, eBioscience; 1:300), rat anti-TER119 (14-5921, eBioscience; 1:300), rat anti-CD13 (clone R3-63, AbD Serotec; 1:300). Alexa-conjugated lectin from Griffonia simplificosa (Invitrogen, 1:300), rat anti-CD31 (553370, Pharmingen; 1:500), rabbit anti-collagen IV (2150-1470, AbD Serotec, 1:500) and rabbit anti-Glut1 (07-1401, Millipore; 1:500) were used to visualize endothelium. Rabbit anti-DsRed (632496, Clonetech; 1:250) and Chicken anti-EGFP (ab13970, Abcam; 1:250) were used to amplify endogenous fluorescent signal, with secondary antibody kept in equivalent channel (DsRed – Alexa 568; EGFP – A488). Secondary antibodies are Alexa 488-, 568-, or 647-conjugated donkey anti-rat-, anti-mouse- or anti-rabbit (Invitrogen).
Statistics
Three or more animals each group at various stages of development were used for all experiments (n ≥ 3), otherwise mentioned. To quantify number of vascular mural cells from double transgenic reporter mouse embryonic brain cortex, we defined a vascular mural cell as a singular cell with the distinct cell body (Pdgfrb-EGFP+ /Cspg4-DsRed−, Cspg4-DsRed+ /Pdgfrb-EGFP− or Pdgfrb-EGFP+ /Cspg4-DsRed+) and its processes, present in close vicinity to the endothelium (either IB4+, CD31+ or Glut1+ cells). Cspg4-DsRed-positve cells observed outside the endothelium were counted and considered as non-vascular Cspg4-DsRed+ cells. Image J (National Institutes of Health) and Prism 5 software are used for vascular mural cell counting and statistical analysis. P-values were determined using unpaired two-tailed Student’s t-test and ordinary ANOVA. Differences were considered significant with a P < 0.05. Data are presented as mean ± s.e.m.
Results
Appearance of Pdgfrb-EGFP and Cspg4-DsRed expression by the CNS vasculature during mouse embryogenesis
Whereas a relatively sharp distinction can be made between vSMCs and pericytes in the adult cerebral vasculature – remodeling of the CNS vasculature completes approximately 24 days after birth,4 it is unclear how these two cell types relate and whether they can be at all distinguished in a developing vasculature. Certainly, it is challenging to specify the type of mural cells by applying anatomical criteria for their identification due to the less defined arterio-venous hierarchy, ongoing vascular remodeling and the distribution of mural cells as single cells along the developing blood vessel branches. Furthermore, no markers that separate these cell types exist at early stages. Herein, we therefore refer to singular cells displaying a typical ‘pericyte-like’ morphology around brain microvessels as developing vascular mural cells.
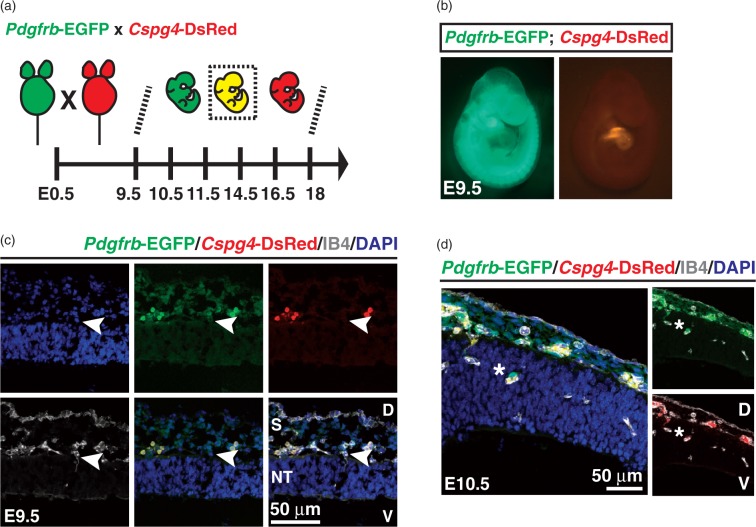
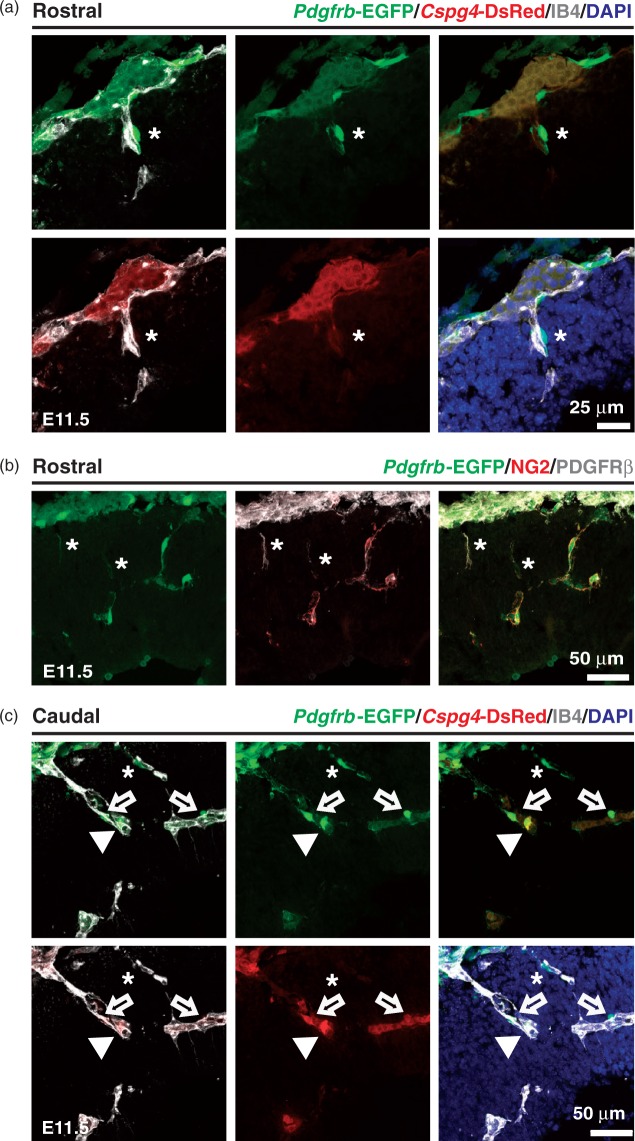
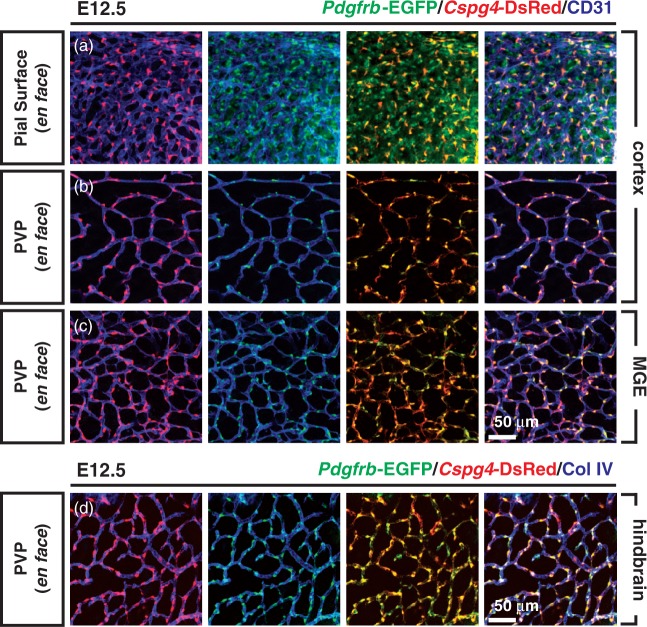
PDGFRβ and NG2 (Cspg4) are the most widely used pericyte markers in adult tissues.20,25 To address whether Pdgfrb and/or Cspg4-mediated genetic labeling can identify mural cells during mouse brain development, the Pdgfrb-EGFP reporter mouse was crossed to Cspg4-DsRed mouse to generate either single (Pdgfrb-EGFP or Cspg4-DsRed) or double (Pdgfrb-EGFP; Cspg4-DsRed) transgenic mural cell reporter mice (Figure 1(a)). E9.5 mouse embryos carrying both transgenes showed Pdgfrb-EGFP and Cspg4-DsRed signals with regional specificity (Figure 1(b)). The Pdgfrb-EGFP signal was observed throughout the body, including the head region, brachial arch, somites/skeleton, limb buds and skin. In contrast, Cspg4-DsRed expression was largely restricted to the cardiac region, with weaker expression in developing cartilage as previously reported.26 Endothelial cells (labeled with IB4) ingress into the neural tube starting at E9.5 (Figure 1(c)).27 Compared to vessels in the pia mater, these early CNS endothelial sprouts were devoid of apposed cells expressing Pdgfrb-EGFP or Cspg4-DsRed signals. One day later, at E10.5, we observed perivascular Pdgfrb-EGFP positive (Pdgfrb-EGFP+) and Cspg4-DsRed negative (Cspg4-DsRed−) cells surrounding early CNS vascular sprouts (Figure 1(d)). The frontal cortex at E11.5 displayed vascular protrusion and expansion following caudal-rostral gradients. The presence of perivascular Pdgfrb-EGFP+/ Cspg4-DsRed− cells was obvious in rostral regions (Figure 2(a)). Interestingly, these cells were also positive for PDGFRβ and NG2 immunostaining, although the distribution of the immunoreactivity varied, occasionally leaving the cellular processes PDGFRβ-positive but NG2-negative (Figure 2(b)). In caudal brain regions, we found that vessels were invested by both Pdgfrb-EGFP+/Cspg4-DsRed− and double Pdgfrb-EGFP+/Cspg4-DsRed+ cells (Figure 2(c)). These data suggest that, although Cspg4-DsRed transgene expression is weaker than endogenous protein expression at early stages of the CNS vascular development, vascular mural cells can be identified by both the Pdgfrb-EGFP transgene and PDGFRβ protein expression in rostral regions of the brain. By E12.5, all intra-parenchymal mural cells of the periventricular vascular plexus (PVP) were double-positive (Pdgfrb-EGFP+/Cspg4-DsRed+), as visualized in telencephalic (cortical and medial ganglionic eminence (MGE) regions, Figure 3(b) and (c)) and hindbrain flat mounts (Figure 3(d)). Additionally, we found vessel-enwrapping, Pdgfrb-EGFP+/Cspg4-DsRed+ cells in the pia mater of the head as well as many Pdgfrb-EGFP+/Cspg4-DsRed− cells not directly associated with pial vessels (Figure 3(a)). Together, these data indicate region-, and stage-specific marker expression in developing mural cells in the early embryonic mouse brain.
Figure 1.
Genetically labeled Pdgfrb-, and Cspg4 expression patterns in embryonic mouse brain. (a) Breeding scheme to generate single- (either Pdgfrb-EGFP or Cspg4-DsRed) and double (Pdgfrb-EGFP; Cspg4-DsRed) transgenic reporter mouse embryos used in this study. (b) General view of the double reporter mouse embryo under dissection microscope at E9.5. (c) The head region of Pdgfrb-EGFP; Cspg4-DsRed mouse embryos at E9.5 was stained with IB4 and DAPI to visualize endothelium and nucleus, respectively. An endothelial cell that protrudes toward ventricle is shown (arrowheads). (d) Appearance of a vascular mural cell in the brain cortex at E10.5. A vascular mural cell sitting on the protruding endothelium is shown (asterisks) S, skin; NT, neural tube; D, dorsal; V, ventral cortex.
Figure 2.
Pdgfrb-EGFP-, and Cspg4-DsRed expression in cerebral cortex vasculature. Rostral (a and b) and caudal (c) regions of the forebrain cortex from Pdgfrb-EGFP; Cspg4-DsRed (a and c) and Pdgfrb-EGFP (b) mice at E11.5. Pdgfrb-EGFP (b) mouse brain sections were stained with PDGFRβ (white) and NG2 (red). A vascular mural cell (Pdgfrb-EGFP+ /Cspg4-DsRed−) detected in vicinity to the IB4+ vessel (asterisks, a and c), cellular processes displaying Pdgfrb-EGFP with PDGFRβ+ and NG2- immunoactivity (asterisks, b), a cell showing strong Pdgfrb-EGFP and weak Cspg4-DsRed signals (open arrows, c), and a cell with both Pdgfrb-EGFP+ and Cspg4-DsRed+ signals (arrowheads, c) are shown.
Figure 3.
Pdgfrb-EGFP-, and Cspg4-DsRed expression in flat-mounted embryonic brain. Flat-mounted whole brains at E12.5 were immunostained to label vessels (CD31 or ColIV) and to amplify endogenous Cspg4-DsRed and Pdgfrb-EGFP fluourescence signals. Vessels were imaged en face at two depths: the pial surface (a) or the underlying periventricular vascular plexus (PVP, b–d), and in three locations: cortex (a and b), medial ganglionic eminence (MGE, c) or hindbrain (d). Note that localization of Cspg4-DsRed signal (red) and Pdgfrb-EGFP signal (green) in developing vascular mural cells is closely apposed to blood vessels labeled with CD31 (blue, a–c) or with ColIV (blue, d).
We occasionally detected nucleated, double Pdgfrb-EGFP+/Cspg4-DsRed+ cells residing within the vascular lumen in the early developing mouse brain cortex. We first hypothesized that these cells might be circulating progenitors that may give rise to vascular mural cells after transmigration through the vascular wall. However, immunofluorescence staining for the erythrocyte marker TER119 identified these cells as TER119+ nucleated erythrocytes, morphologically distinct from the periendothelial Pdgfrb-EGFP+/Cspg4-DsRed− or Pdgfrb-EGFP+/Cspg4-DsRed+ mural cells (Supplementary Figure 1(a)). Subtraction of the TER119+ erythrocyte-associated signal by lambda scan improved the vascular mural cell-specific signal procurement (Supplementary Figure 1(b)), suggesting that TER119+ erythrocytes located within the vessel lumens are responsible for autofluorescence in the early developing brain vasculature. Consistent with this hypothesis, amplification of Pdgfrb-EGFP and Cspg4-DsRed fluorescent signals using isostype-specific antibodies on flat-mounted embryonic brain (Figure 3) substantially improved the signal-to-noise ratio, allowing subtraction of the auto-fluorescence signals contributed by immature erythrocytes.
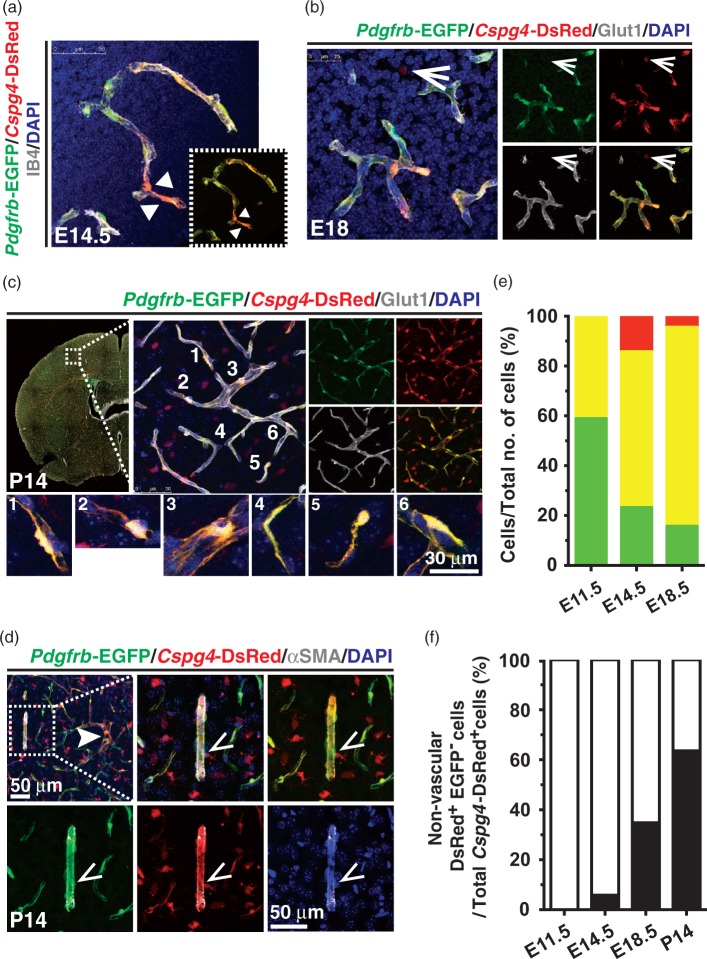
At E14.5 to E18.5, the frontal cortex displayed a massive expansion of the vasculature as previously described.5 During this developmental period, blood vessels in the frontal cortex of double Pdgfrb-EGFP/Cspg4-DsRed transgenic mice exhibited complex capillary networks surrounded by double Pdgfrb-EGFP+/Cspg4-DsRed+ mural cells. Notably, the distribution and fluorescent intensity of each marker varied, even within the same vascular region in embryonic- (Figure 4(a) and (b)), and postnatal brain cortex (Figure 4(c) and (d)). We quantified the numbers of developing vascular mural cells in the cortex by counting vessel-associated cells with strong perinuclear (cell body) expression of EGFP and/or DsRed. This analysis revealed a relative reduction in the number of Pdgfrb-EGFP+/Cspg4-DsRed− mural cells over time (48.94 ± 10.71 at E11.5, 23.23 ± 5.37 at E14.5 and 15.78 ± 6.259 at E18.5), whereas the number of double Pdgfrb-EGFP+/Cspg4-DsRed+ cells increased from 41% to 80% during this same period (41.06 ± 10.71 at E11.5, 62.57 ± 7.488 at E14.5 and 79.82 ± 6.649 at E18.5) (Figure 4(e)). We also observed distinct populations of vessel-associated or parenchymal Cspg4-DsRed+/Pdgfrb-EGFP- cells present at later stages of the embryonic brain cortex development (14.2 ± 3.559 at E14.5 versus 5.837 ± 3.018 at E18.5). These cells expanded in number from E14.5 onward (5.833 ± 3.056 at E14.5, 34.77 ± 2.897at E18.5, and 63.59 ± 4.1 at P14) (Figure 4(f)). A recent report documenting the association of NG2+ oligodendrocyte precursor cells (OPCs) with developing cerebral vasculature28 indicates that these Cspg4-DsRed+/Pdgfrb-EGFP− cells are likely OPCs. Our results are consistent with a model14 in which developing Pdgfrb-EGFP+/Cspg4-DsRed− vascular mural cells might be recruited from the pial surface of the CNS to newly formed endothelial sprouts, and later acquire NG2 expression as vascular growth and maturation proceed.
Figure 4.
Heterogeneity of vascular mural cells in developing mouse brain cortex. (a–d) Frontal brain cortex from Pdgfrb-EGFP; Cspg4-DsRed mice was examined at E14.5 (a), E18 (b) and P14 (c and d). The CNS microvasculature was visualized by either IB4 (a) or Glut1 (b and c) immunostaining over DAPI. Dotted box in (a) shows Pdgfrb-EGFP+ and Cspg4-DsRed+ signals only. Various shapes of mural cells in vicinity to the CNS microvessels at P14 are shown (c, 1–6). (d) Arteriole was depicted by αSMA staining (dotted box, d). Perivascular Cspg4-DsRedhi Pdgfrb-EGFPlo mural cells (arrowheads, a), a non-vascular Cspg4-DsRed+ Pdgfrb-EGFP− cell (open arrows, b) and a perivascular Cspg4-DsRed+ Pdgfrb-EGFP− cell (open arrowheads, d) are indicated. (e) Quantification of perivascular Pdgfrb-EGFP+ / Cspg4-DsRed− (green), Cspg4-DsRed+ /Pdgfrb-EGFP- (red) and Pdgfrb-EGFP+ / Cspg4-DsRed+ (yellow) cells over total cell number counted per field. (f) Quantification of parenchymal Cspg4-DsRed+ /Pdgfrb-EGFP- cells over total Cspg4-DsRed+ cells. (n ≥ 3 per each time point except P14 (N = 2), one-way ANOVA).
Lineage analysis of developing vascular mural cells in the CNS
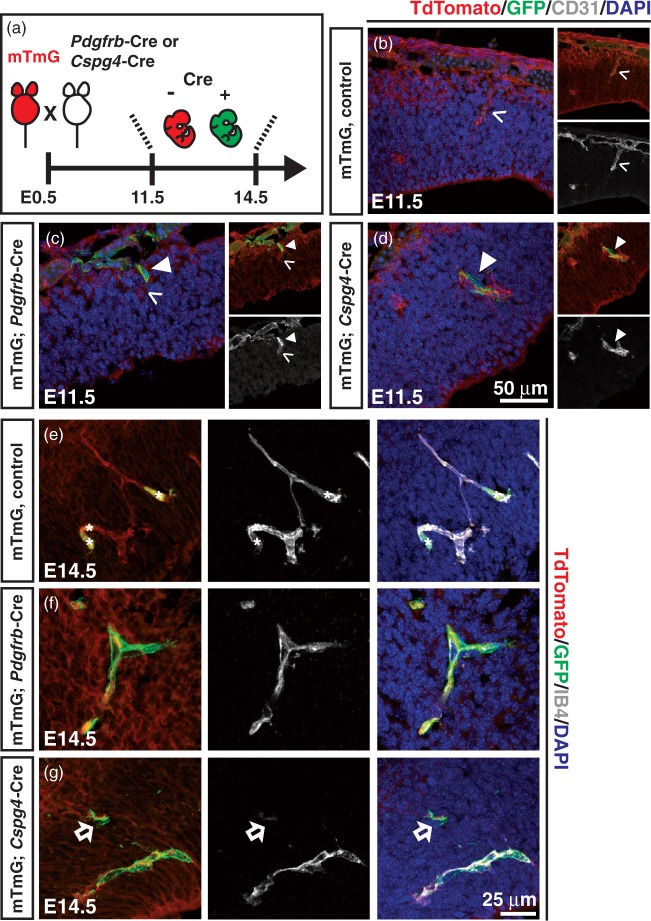
To determine whether PDGFRβ and/or NG2-expressing vascular mural cells can differentiate into other cell types during mouse embryogenesis, Cre recombinase-based lineage analysis was employed. We generated mouse crosses in which expression of Cre, driven by the Pdgfrb promoter (Pdgfrb-Cre23) or the Cspg4-promoter (Cspg4-Cre22), permanently switches reporter expression from tdTomato to GFP (mTmG reporter) thereby marking the cells and their progeny (Figure 5(a)). Similar to the aforementioned observations in Pdgfrb-EGFP mice, vessel-associated Pdgfrb-Cre, mGFP-positive (mGFP+) cells were seen in the cerebral vasculature and the pia mater (Figure 5(c) and (f)). Cspg4-Cre; mGFP+ cells were also detected around protruding endothelial cells in the E11.5 brain cortex (Figure 5(d)), which was comparable to the Pdgfrb-Cre, mGFP signal at E14.5 (Figure 5(f) and (g)). Additionally, Cspg4-Cre, mGFP+ cells were observed in both blood vessels and parenchyma at E14.5 in the ganglionic eminence (Figure 5(g) and Supplementary Figure 2). Non-vascular parenchymal Cspg4-Cre, mGFP+ cells of presumably oligodendrocyte lineage29 were also observed. These data are consistent with those obtained using the Pdgfrb-EGFP and Cspg4-DsRed reporter mice.
Figure 5.
Pdgfrb-Cre-, and Cspg4-Cre-mediated recombination in embryonic CNS vasculature. (a) Breeding strategy to obtain mTmG; Pdgfrb-Cre or mTmG; Cspg4-Cre mouse embryos at indicated time points. Note that the recombination occurs upon Cre action, which can be detected by tracing GFP (green). (b–d) Embryonic mouse brain from littermate control (mTmG alone, Cre−) (b), Pdgfrb-Cre (c) or Cspg4-Cre (d) was stained for endothelium (CD31, grey) and nucleus (DAPI, blue) at E11.5. See Materials and Methods for further detail. (e-g) Forebrain cortex vasculature from mTmG littermate control (e), mTmG; Pdgfrb-Cre (f) and mTmG; Cspg4-Cre (g) mice at E14.5. Red blood cells (asterisks, e), GFP+ cells (arrowheads, c and d), a sprouting endothelial cell (open arrowheads, b and c), and a parenchymal, Cspg4-driven GFP+ cell (open arrows, g) are indicated.
Transgenic and antibody marker expression patterns of cerebral vascular mural cells during mouse brain development
Next, we compared immunostaining for various pericyte markers with the expression of Pdgfrb-EGFP and Cspg4-DsRed in the developing mouse brain cortex. Immunofluorescent staining of the brain tissue sections from Pdgfrb-EGFP mice with antibodies against PDGFRβ, NG2 and desmin showed co-localization between Pdgfrb-EGFP and PDGFRβ, NG2 and desmin immunostaining in cells surrounding cerebral microcapillaries (Figure 6(a) to (c)). Similarly, these markers co-localized with Cspg4-DsRed+ vascular mural cells in brain tissue sections from Cspg4-DsRed mice (Figure 6(e) to (g)). As expected, we found non-vascular Cspg4-DsRed+ cells that were NG2 immunostaining-positive (Figure 6(f)). Moreover, PDGFRβ and NG2 immunostaining-positive vascular mural cells observed in the littermate control brain cortex were completely absent in Pdgfrb-null mice, as previously described,8,9 supporting the mural cell-specific expression patterns of these markers in the cerebral vasculature (Supplementary Figure 3). On the other hand, CD13 staining was observed only in the pia mater (Figure 6(d)), but not in CNS vasculature (Figure 6(d) and 6(h)) before P6 (Figure 6(i) and 6(j)). Taken together, these results indicate that the staining pattern of known pericyte markers in the developing brain is age-dependent and cell type-specific.
Figure 6.
Validation of genetically labeled developing vascular mural cells by immunofluorescence staining. Either Pdgfrb-EGFP (a–d)- or Cspg4-DsRed (e–h)-expressing mouse brain was prepared at E14.5 (a–c) and E16.5 (d–h), and immunofluorescence staining for PDGFRβ (a and e), NG2 (b and f), desmin (c and g) and CD13 (d and h) in combination with IB4 and DAPI was performed. Pdgfrb-EGFP+ cells lacking other marker staining (arrowheads, shown in c, d and h) and parenchymal Cspg4-DsRed+ cells (open arrowheads, e–g) are indicated. Note that CD13 expression was depicted only in the pial surface (asterisk, d). Postnatal cerebral cortex from the double Pdgfrb-EGFP; Cspg4-DsRed reporter mouse was stained for CD13 at P6 (i) and P14 (j).
Discussion
Although the involvement of a number of signaling pathways have been implicated in mural cell development, specification and recruitment,10,20 the identification of pericytes remains a major challenge in pericyte biology due to the lack of definitive pericyte markers. Therefore, pericyte identification in fixed tissues has hitherto relied on immunostaining techniques employing a few markers that are shared with other cell types such as fibroblasts and vSMCs. Here, we explored transgenic reporter mouse models to visualize pericyte recruitment during development of the mouse brain. Of note, our attempt to distinguish vSMCs and pericytes using the double transgenic mice during embryonic brain development was unsuccessful as the expression of αSMA, a pan-vSMCs marker, by immunofluorostaining was absent in embryonic brain microvessels (data not shown). Because pericyte and vSMC progenitors could not be distinguished, and because it is likely that they are shared at least in some organs,30 we refer to embryonic pericytes as developing vascular mural cells. Our data documents developmental expression patterns of known pericyte markers in the mouse brain and demonstrate the utility of the double Pdgfrb-EGFP; Cspg4-DsRed mice in the characterization of this intriguing, yet poorly defined cell type.
Vascular mural cells: Appearance, distribution and heterogeneity
Using Pdgfrb-EGFP, Cspg4-DsRed double reporter mice, we observed vascular mural cells around the CNS vessels in the frontal cortex, as early as around E10. Pdgfrb-EGFP+ cells surrounding the nascent vasculature were first detected in the rostral cortex region (Figure 7(a)), suggesting that Pdgfrb gene expression precedes Cspg4 expression in cortical vasculature. At E12.5, the pia mater of the developing head showed a sizable population of non-vessel associated Pdgfrb-EGFP+/Cspg4-DsRed− cells. It is possible that these cells represent mesenchymal progenitors that may give rise to mural cells, but perhaps also to other cell types, such as meningeal fibroblasts.19,20
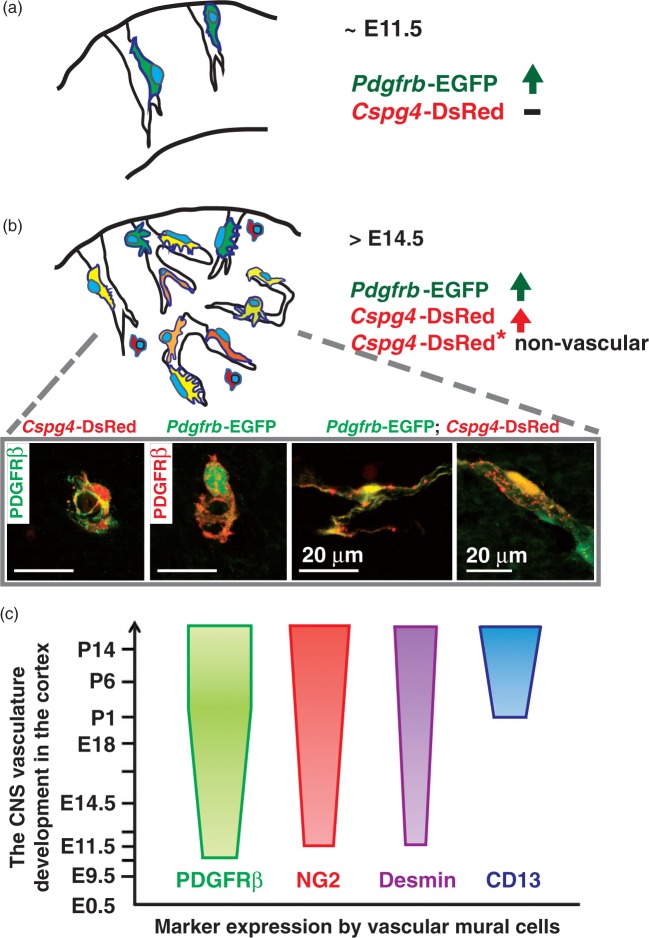
Figure 7.
Distribution and marker expression patterns of vascular mural cells during mouse CNS vasculature development. (a) Vascular mural cells are first recruited to surround the nascent CNS vessels express Pdgfrb-EGFP (∼E11.5). (b) Cspg4-DsRed+ cells exhibit both perivascular- and parenchymal expression patterns in relatively mature cerebral capillaries (>E14.5). Various cell shapes and degree of these marker expression patterns among developing CNS mural cells can be observed in single- and/or double reporter mouse embryonic brain. Note a strong expression of these markers in the cell soma. (c) A summary of expression patterns of pericyte markers over the course of CNS vasculature development based on the observation from Pdgfrb-EGFP; Cspg4-DsRed reporter mouse brain in combination with immunostaining against widely-used pericyte markers. Note that each marker shows unique expression patterns.
At E14.5 and onwards, developing mural cells in the cerebral microvessels exhibited heterogeneous marker expression patterns (Figure 7(b)). Moreover, these cells displayed certain differences in the expression of the pericyte markers (Figure 7(c)). Early work from avian chimeras and tracer injection studies suggest that both neural crest progenitors and mesodermal stem cells contribute to the formation of brain vascular mural cells.27,31,32 However, it is unclear whether this difference in origin leads to any differences in gene expression, cell function, or morphology.
Recently, Hartmann et al.25 used Cre/LoxP based genetic labeling of pericytes in combination with high-resolution imaging to show that Pdgfrβ-Cre-driven cell recombination in the adult mouse brain comprised 99% of the CD13 staining positive pericytes, supporting CD13 as a broad and reliable marker for pericyte visualization in adult murine brain.20,33 We found that CD13 expression was rather weak and almost absent in the embryonic mouse brain cortex with exception of the dura/pia mater (Figure 7(c)). In contrast, the presence of CD13 immunostaining-positive vascular mural cells was apparent in postnatal mouse cerebral microvasculature, indicating that CD13 is a good marker for mature CNS pericytes, yet an ambiguous marker for developing cerebral vascular mural cells.
Transgenic pericyte reporter mice as a powerful genetic tool
Although it is assumed that pericytes are critical for a range of physiological and pathological processes,10,13,16,31 it is still largely unclear how pericytes exert their functions. In this regard, the double pericyte reporter mouse might be useful, as vascular mural cells can be easily identified by the accumulation of reporter protein in the cell cytoplasm, highlighting the cell soma and its major processes. Live imaging, for instance, would provide valuable information concerning the engagement of vascular mural cells in regulation of vascular remodeling processes. Additionally, pericytes from double Pdgfrb-EGFP; Cspg4-DsRed animals may be isolated (e.g. by FACS) for molecular profiling, cell culture studies and transplantation. Indeed, the ability to image, isolate and characterize brain pericytes from pathological states, such as stroke, cancer and neurodegenerative disease would promote a better understanding of pericyte contribution to vascular dysfunction in these diseases.
In conclusion, we show that Pdgfrb-EGFP is a useful mural cell marker at the earliest stages of brain vascular development before Cspg4-DsRed expression becomes prevalent. Immunostaining results for PDGFRβ protein are consistent with the Pdgfrb-EGFP expression, validating the reporter. Double Pdgfrb-EGFP and Cspg4-DsRed expression patterns are valuable for mural cell identification at later embryonic-, and early postnatal stages of CNS development. Taken together, our findings illustrate a broad utility for Pdgfrb-EGFP; Cspg4-DsRed mice in the study of CNS vascular mural cells, and presumably of mural cells in other organs, which warrants additional study.
Supplementary Material
Acknowledgements
We thank Cecilia Olsson, Helene Leksell, Pia Peterson, Jana Chmielniakova and Verinica Sundell for technical assistance.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by the European Research Council (AdG 294556 BBBARRIER), the Swedish Research Council, the Swedish Cancer Foundation, Knut and Alice Wallenberg Foundation, the Leducq Foundation transatlantic network grant Sphingonet, and Uppsala University.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
BJ conceived the ideas, designed/ performed experiments, analyzed data and wrote the paper. TA performed experiments, analyzed data and wrote the paper. ER provided initial genotyping PCR materials and mice. KG analyzed erythrocytes data. CB conceived the ideas, analyzed data and wrote the paper.
Supplementary material
Supplementary material for this paper can be found at the journal website: http://journals.sagepub.com/home/jcb
References
- 1.Carmeliet P. Angiogenesis in life, disease and medicine. Nature 2005; 438: 932–936. [DOI] [PubMed] [Google Scholar]
- 2.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Bio 2007; 8: 464–478. [DOI] [PubMed] [Google Scholar]
- 3.Herbert SP, Stainier DYR. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat Rev Mol Cell Bio 2011; 12: 551–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walchli T, Wacker A, Frei K, et al. Wiring the vascular network with neural cues: A CNS perspective. Neuron 2015; 87: 271–296. [DOI] [PubMed] [Google Scholar]
- 5.Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med 2013; 19: 1584–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ben-Zvi A, Lacoste B, Kur E, et al. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature 2014; 509: 507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 2006; 7: 41–53. [DOI] [PubMed] [Google Scholar]
- 8.Lindahl P, Johansson BR, Leveen P, et al. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science 1997; 277: 242–245. [DOI] [PubMed] [Google Scholar]
- 9.Hellstrom M, Kalen M, Lindahl P, et al. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 1999; 126: 3047–3055. [DOI] [PubMed] [Google Scholar]
- 10.Gaengel K, Genove G, Armulik A, et al. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscl Throm Vas 2009; 29: 630–638. [DOI] [PubMed] [Google Scholar]
- 11.Alexander MR, Owens GK. Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease. Annu Rev Physiol 2012; 74: 13–40. [DOI] [PubMed] [Google Scholar]
- 12.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 2004; 84: 767–801. [DOI] [PubMed] [Google Scholar]
- 13.Attwell D, Mishra A, Hall CN, et al. What is a pericyte? J Cereb Blood Flow Metab 2016; 36: 451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daneman R, Zhou L, Kebede AA, et al. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 2010; 468: 562–U238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armulik A, Genove G, Mae M, et al. Pericytes regulate the blood-brain barrier. Nature 2010; 468: 557–U231. [DOI] [PubMed] [Google Scholar]
- 16.Hall CN, Reynell C, Gesslein B, et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 2014; 508: 5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bell RD, Winkler EA, Sagare AP, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 2010; 68: 409–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill RA, Tong L, Yuan P, et al. Regional blood flow in the normal and ischemic brain is controlled by arteriolar smooth muscle cell contractility and not by capillary pericytes. Neuron 2015; 87: 95–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sims DE. The pericyte – A review. Tissue Cell 1986; 18: 153–174. [DOI] [PubMed] [Google Scholar]
- 20.Armulik A, Genove G, Betsholtz C. Pericytes: Developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 2011; 21: 193–215. [DOI] [PubMed] [Google Scholar]
- 21.He LQ, Vanlandewijck M, Raschperger E, et al. Analysis of the brain mural cell transcriptome. Sci Rep 2016; 6: 35108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu XQ, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development 2008; 135: 145–157. [DOI] [PubMed] [Google Scholar]
- 23.Foo SS, Turner CJ, Adams S, et al. Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell 2006; 124: 161–173. [DOI] [PubMed] [Google Scholar]
- 24.Arnold TD, Niaudet C, Pang MF, et al. Excessive vascular sprouting underlies cerebral hemorrhage in mice lacking alpha V beta 8-TGF beta signaling in the brain. Development 2014; 141: 4489–4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hartmann DA, Underly RG, Grant RI, et al. Pericyte structure and distribution in the cerebral cortex revealed by high-resolution imaging of transgenic mice. Neurophotonics 2015; 2: 041402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozerdem U, Grako KA, Dahlin-Huppe K, et al. NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev Dynam 2001; 222: 218–227. [DOI] [PubMed] [Google Scholar]
- 27.Kurz H. Cell lineages and early patterns of embryonic CNS vascularization. Cell adhesion & migration 2009; 3: 205–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsai HH, Niu J, Munji R, et al. Oligodendrocyte precursors migrate along vasculature in the developing nervous system. Science 2016; 351: 379–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishiyama A, Komitova M, Suzuki R, et al. Polydendrocytes (NG2 cells): Multifunctional cells with lineage plasticity. Nat Rev Neurosci 2009; 10: 9–22. [DOI] [PubMed] [Google Scholar]
- 30.Volz KS, Jacobs AH, Chen HI, et al. Pericytes are progenitors for coronary artery smooth muscle. eLife 2015; 4: e10036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat Neurosci 2011; 14: 1398–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trost A, Lange S, Schroedl F, et al. Brain and retinal pericytes: Origin, function and role. Front Cell Neurosci 2016; 10: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winkler EA, Sengillo JD, Bell RD, et al. Blood-spinal cord barrier pericyte reductions contribute to increased capillary permeability. J Cereb Blood Flow Metab 2012; 32: 1841–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.