Abstract
The demand for using parasympathetic activation for stroke therapy is unmet. In the current study, we investigated whether the neuroprotection provided by electroacupuncture (EA) in an experimental stroke model was associated with activation of the parasympathetic nervous system (PNS). The results showed that parasympathetic dysfunction (PD), performed as unilateral vagotomy combined with peripheral atropine, attenuated both the functional benefits of EA and its effects in improving cerebral perfusion, reducing infarct volume, and hindering apoptosis, neuronal and peripheral inflammation, and oxidative stress. Most importantly, EA rats showed a dramatically less reduction in the mRNA level of choline acetyltransferase, five subtypes of muscarinic receptors and α7nAChR, suggesting the inhibition of the impairment of the central cholinergic system; EA also activated dorsal motor nucleus of the vagus, the largest source of parasympathetic pre-ganglionic neurons in the lower brainstem (detected by c-fos immunohistochemistry), and PD suppressed these changes. These findings indicated EA may serve as an alternative modality of PNS activation for stroke therapy.
Keywords: Cerebral ischemia, cholinergic, electroacupuncture, muscarinic, parasympathetic nervous system
Introduction
Ischemic stroke is a debilitating disease with limited therapies. Optimistically, activation of the parasympathetic nervous system (PNS), including vagus nerve stimulation1 and sphenopalatine ganglion stimulation,2 has shown multiple salutary benefits in preclinical models of cerebral ischemia. However, the invasive characteristics may limit the routine clinical use of these activation techniques. Thus, a well-tolerated method of stimulating the PNS is needed to treat ischemic stroke.3
Acupuncture is a historical practice of Chinese traditional medicine, with accumulating evidence supporting its effects in stroke rehabilitation,4,5 but its theoretical foundation in the framework of western medicine is relatively weak. One form of acupuncture, electroacupuncture (EA), is derived from the combination of traditional medicine and modern electrotherapy. Applying EA during the acute phase of cerebral ischemic injury has been reported to significantly reduce the infarct volume and neurological deficit score while increasing cerebral perfusion, cortical acetylcholine (ACh) and M3 muscarinic receptor expression. In contrast, atropine almost abolished the perfusion changes. In light of the notion that both ACh and the muscarinic receptor are closely related to the PNS and the evidence demonstrated that acupuncture can modulate the activities of the autonomic nervous system (ANS), we hypothesized that the EA-induced neuroprotection against ischemic stroke requires an intact parasympathetic activity.
To test this hypothesis, we inhibited the function of the PNS via unilateral vagotomy combined with peripheral application of atropine and examined whether the protective effects of EA in ischemic stroke were associated with PNS activation.
Materials and methods
The study was approved by the Animal Care Committee of Harbin Medical University (China) and followed national guidelines (Guidelines on Administration of Laboratory Animals in China and Guidelines on the Humane Treatment of Laboratory Animals in China). The animal experiment was reported in compliance with the ARRIVE guidelines (Animal Research: Reporting in Vivo Experiments). Male Sprague-Dawley (SD) rats aged 8–9 weeks, weighing 220–250 g were obtained from the Animal Center of Harbin Medical University. All rats were fasted for 8 h prior to operation to prevent aspiration and regurgitation but permitted ad libitum access to water.
Experimental protocol
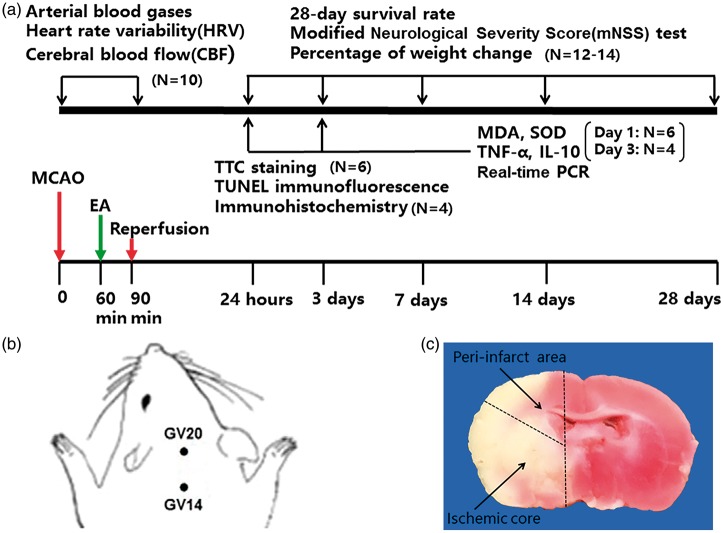
The rats were randomized into three groups: no electroacupuncture (NEA), electroacupuncture (EA), and parasympathetic dysfunction + electroacupuncture (PD + EA). The study was divided into two protocols according to the differences in monitoring indices: (1) Rats were studied for 28 days after surgery to measure neurological function, survival rate, percentage weight change and 28-day heart rate variability (HRV); (2) Rats were sacrificed on one or three days after injury to harvest samples for the subsequent measurements, including the infarct volume measurement, detection of inflammatory cytokines and oxidative stress, TUNEL staining or immunohistochemistry, and real-time PCR. The experimental protocol is shown in Figure 1.
Figure 1.
Summary of experimental protocols. (a) Schematic description of study design and experimental timeline. (b) The location of the acupoints in a schematic diagram. (c) Depiction of the peri-infarct area and the ischemic core of the coronal section.
Surgical procedure for cerebral ischemia
Anesthesia was achieved by face mask-delivered isoflurane (2% induction and 1.5% maintenance with spontaneous respiration, in 30% O2). The regional cerebral blood flow (rCBF) over the left middle cerebral artery territory was continuously monitored using a laser Doppler flowmetry (TF5000; PERIMED AB; Stockholm; Sweden). The baseline cerebral blood flow (CBF) values in the supine position before surgery were defined as 100% flow. Middle cerebral artery occlusion/reperfusion (MCAO/R) was conducted as described previously.6 The rats showed a sharp reduction in rCBF of at least 70% if the filament was appropriately inserted. The filament was withdrawn after 90 min to allow reperfusion. Additionally, the tail artery was cannulated to monitor arterial blood gases and mean arterial pressure (MAP) at three time points: 5 min before EA, 20 min after EA and 20 min after reperfusion.
Electroacupuncture technique
The EA treatment was performed 60 min after ischemia and involved two veterinary acupoints determined by the transpositional method7: Baihui (GV20), located at the right midpoint of the parietal bone, and Dazhui (GV14), located on the posterior midline and in the depression below the spinous process of the seventh cervical vertebra. Stainless steel acupuncture needles (outer diameter 0.3 mm) were inserted obliquely 2 mm into GV20 and vertically 5 mm into GV14. The acupoints were then stimulated using an EA instrument (HANS-200E, Nanjing, China) with “optimal” parameters:8,9 an intensity of 1 mA and a 2/15 Hz sparse-dense frequency for 30 min. A set of non-acupoints located on the ulna side of the metacarpus served as controls in the NEA group to exclude the effect of the electric current. Reperfusion started immediately when the EA treatment ended.
Parasympathetic dysfunction
Rats in the PD + EA group received 2 mg/kg atropine sulfate intraperitoneally 15 min before EA and a left vagotomy 10 min before EA. The left vagus nerve trunks were identified at the mid-cervical level, gently separated from the carotid artery and sympathetic nerve trunk, ligated with 4–0 silk sutures and divided. In the other rats, the same volume of saline was injected, and the left cervical vagus nerve was isolated but not transected at the same time point.
Heart rate variability analysis
A standard lead II electrocardiogram was obtained and recorded for 5 min at the following moments: prior to the surgery, 10 min prior to EA (50 min after ischemia), during EA (20 min after EA), and 20 min after recanalization. Heart rate variability (HRV) was analyzed using frequency domain analysis (AcqKnowledge 4.0 software, BIOPAC Systems Inc., Santa Barbara, CA, USA). The LF (low frequency) component reflects both sympathetic and parasympathetic modulation of the heart rate; and the HF (high frequency) component reflects parasympathetic modulation of the heart rate. The LF to HF ratio can be used as an index of cardiac autonomic balance. An elevation in the ratio indicates sympathetic predominance, and a decrease accordingly suggests parasympathetic predominance. Additionally, HRV analysis was performed in rats studied for 28 days in the same manner before euthanasia with overdose 10% chloral hydrate (1 ml/100 g, i.p.).
Assessment of neurologic outcome, survival rate and percentage weight change
Neurological deficits were measured with the modified Neurological Severity Score (mNSS)10 on days 1, 3, 7, 14 and 28 depending on the length of survival, the 28-day survival rates were also recorded (n = 12–14/group). All the rats were trained twice for adaptation before surgery. The evaluations were performed by the same investigator who was blinded to the assignment.
The weight was measured daily for 28 days. The percentage weight change was determined relative to the baseline weight.
Infarct volume measurement
Rats were decapitated after deep anesthetized with 10% chloral hydrate (0.4 ml/100 g, i.p.) 24 h after reperfusion (n = 6/group). The brains were rapidly removed and sliced into 2-mm coronal sections, stained with 1% 2,3,5-triphenyltetrazolium chloride (TTC, Biosharp, Beijing, China) for 15 min at 37 ℃, followed by overnight immersion in 4% paraformaldehyde. The infarct and hemisphere area were measured using Image-Pro Plus software (Version 6.0, Media Cybernetics, Bethesda, MD, USA). To compensate for the effect of brain edema, the ratio of hemispheric infarct was calculated as: (contralateral hemisphere area – ipsilateral hemisphere without infarct) /contralateral hemisphere area.
Measurement of inflammatory cytokines
Rats were sacrificed on day 1 or 3 by cardiac puncture to collect blood and then decapitated to harvest the peri-infarct area and ischemic core.11 All the samples were stored at−80 ℃ until use. The levels of TNF-α and IL-10 in the blood and 10% peri-infarct area homogenate made in saline were determined using ELISA (Rat TNF-α and Rat IL-10 ELISA Kit, Boster, China) according to the manufacturer’s instructions.
Measurements of malondialdehyde (MDA) and superoxide dismutase (SOD)
The SOD and MDA levels in 10% peri-infarct area homogenate were quantified using the appropriate detection kits (Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions.
Brain tissue preparation for immunofluorescence and immunohistochemistry
At 24 h after reperfusion, rats (n = 4/group) were deeply anesthetized and transcardially perfused with 0.9% saline and 4% paraformaldehyde. The whole brains were dissected out, and coronal sections from +1.0 to −1.0 mm from bregma (covering the infarct area) were post-fixed in 4% paraformaldehyde overnight and subsequently soaked in 20% sucrose followed by 30% sucrose at 4 ℃ until they sank to the bottom for immunofluorescence. Sections from −1.0 to −3.0 mm from the bregma and the medullary regions from −13.0 to −14.5 mm from the bregma were fixed in 4% paraformaldehyde for immunohistochemistry.
Double immunofluorescence for TUNEL and NeuN
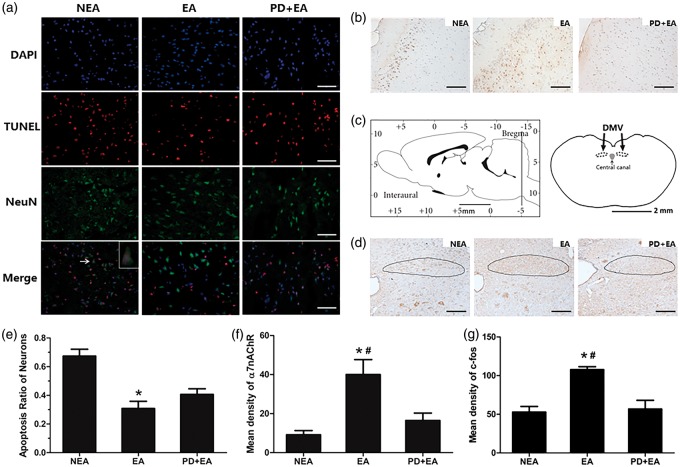
Infarct segments were cut into 10-µm transverse frozen sections −20 ℃. After 0.1 % Triton X-100 wash for 20 min, TUNEL staining was conducted using an in situ cell death detection kit (12156792910, Roche, Germany), then blocked with 3% BSA for 30 min. The sections were incubated with primary antibody against NeuN (1:200, ab 104224, Abcam) at 4 ℃ overnight and FITC-conjugated secondary antibody for 50 min sequentially. Finally, the sections were incubated with DAPI for 10 min prior to visualizing with an optic microscope (Nikon Eclipse Ti-SR, Japan) at 400 magnification. Images were merged using Image-Pro Plus software at the same contrast. The positive neurons were identified by red TUNEL dots located in green neurons with a blue nucleus. Quantitation was performed by calculating the apoptosis ratio of the neurons in three randomly chosen fields within each section. One section per animal was selected.
α7nAChR and c-fos immunohistochemistry
Immunohistochemistry was performed on 5-µm paraffin sections. For antigen retrieval, slides were boiled in a microwave oven for 8 min. Endogenous peroxidase activity was blocked using 0.3% H2O2 for 10 min. Sections were incubated with primary antibodies (1:400, ab 10096, Abcam; 1:200, sc-52, Santa Cruz Biotechnology) for 2 h at room temperature. After PBS wash, sections were incubated with biotinylated goat anti-rabbit IgG secondary antibodies for 30 min. Immunoreactivity was evaluated using DAB (Boster) with hematoxylin counterstaining. The location of the dorsal motor nucleus of the vagus (DMV) at −14.06 mm was determined according to the Rat Brain Stereotaxic Coordinates.12 The mean density of α7nAChR in three randomized fields per section from the infarct cortex and c-fos in DMV were calculated as the integrated optical density (IOD)/area of interest (AOI) of the stained area using Image-Pro Plus software in a blinded manner.
Quantitative real-time polymerase chain reaction
The expression of choline acetyltransferase (ChAT), five subtypes of muscarinic receptors (M1, M2, M3, M4, and M5) and α7nAChR were determined by quantitative real-time polymerase chain reaction (qRT-PCR) (primer sequences are shown in Supplementary Materials 1). Total RNA in the ischemic core was extracted using a Multisource Total RNA Miniprep Kit (AP-MN-MS-RNA-50, Axygen, USA) and then reverse transcribed to cDNA using HiScriptII Reverse Transcriptase (R223-01, Vazyme, China). The reverse transcription reaction product was amplified using a Bio-Rad CFX96 Detection System (Bio-Rad, CA, USA) with FastStart Universal SYBR Green Master (04913850001, Roche) in a 10-µl reaction volume. The expression of each gene was calculated in normal individuals (n = 3) as the standard before the experiment. Each sample was run in triplicate, and the relative gene expression was determined with β-actin as the reference and analyzed with 2−ΔΔCT method. The results are expressed as fold increases relative to the normal level.
Statistical analysis
Statistical analyses were performed using SPSS (version 20.0, Chicago, IL, USA). Categorical data of neurological scores are presented as median with interquartile range, and analyzed with Kruskal–Wallis test followed by post hoc Bonferroni correction. Other data are expressed as the mean ± standard error of mean (SEM). Arterial blood gases, MAP and CBF were analyzed using repeated measures analysis of variance (ANOVA) followed by the LSD test. Percentage weight changes were assessed using a generalized linear model. Kaplan–Meier survival curves were compared using log-rank testing. Other data were assessed with one-way ANOVA followed by LSD test. Values of P < 0.05 were considered significant.
Results
Physiological parameters
There were no among-group differences in arterial oxygen tension, arterial carbon dioxide tension, pH or MAP during the surgery. The rapid and shallow breathing induced by the surgical procedures took great responsibility for hypocapnia (Supplementary Materials 2).
Atropine combined with vagotomy enhanced cardiac sympathetic activity
The LF: HF ratio was significantly higher in the PD + EA group than the EA group after parasympathetic dysfunction, both during the surgery and at the moment prior to sacrifice on the 28th day (Table 1). EA treatment decreased the HRV but not significantly compared with that in the NEA group.
Table 1.
The LF/HF ratio in the experimental groups.
| Groups | A |
B |
C |
D |
E |
|---|---|---|---|---|---|
| prior to MCAO | during MCAO | during EA | 20 min after reperfusion | 28 days after reperfusion | |
| NEA | 0.73 ± 0.04 | 1.18 ± 0.14 | 1.45 ± 0.19 | 1.18 ± 0.15 | 1.30 ± 0.15 |
| EA | 0.72 ± 0.04 | 1.40 ± 0.17 | 0.98 ± 0.10 | 0.87 ± 0.05 | 0.98 ± 0.12 |
| PD + EA | 0.71 ± 0.07 | 1.15 ± 0.11 | 1.60 ± 0.28$ | 2.13 ± 0.43$ | 1.75 ± 0.35$ |
Data are presented as mean ± SEM; n = 10/group. $P < 0.05 vs. EA group.
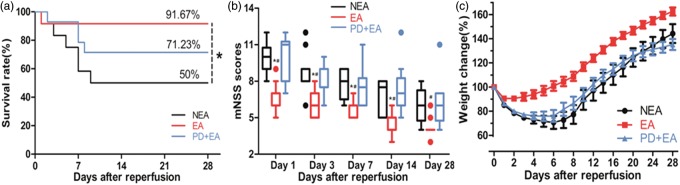
Effect of EA on neurological outcome, percentage weight change and survival rate
A significant survival benefit was found for the EA treatment (Figure 2(a)). Six rats (50%, 6/12) in the NEA group compared with 11 rats (91.67%, 11/12) in the EA group survived until day 28 (P < 0.05), but not significantly compared with that in the PD + EA group (71.23%, 10/14, P > 0.05); this difference was accompanied by lower neurological deficit scores (Figure 2(b)), except for the insignificance between the NEA and the EA groups on day 28. With respect to the postoperative weight change (Figure 2(c)), the EA group showed a small degree of loss with faster recovery (reached the lowest value, 90.46%, on the 3rd day and returned to the preoperative level on the 7th day), the difference was significant compared with the other two groups on each day during evaluation. The weight in the NEA group was at the lowest value, 70.64%, on day 7 and recovered to the baseline on day 14, while was at the lowest value, 75.78%, on day 6 and recovered to baseline on day 13 in the PD + EA group.
Figure 2.
Long-term functional benefit of EA. (a) EA increased the 28-day survival rate. (b) Results of the mNSS test. Data are presented as median with interquartile range. (c) Comparison of the weight change. Data are presented as the mean ± SEM. n = 12-14/group. *P < 0.05 vs. NEA group, #P < 0.05 vs. PD + EA group.
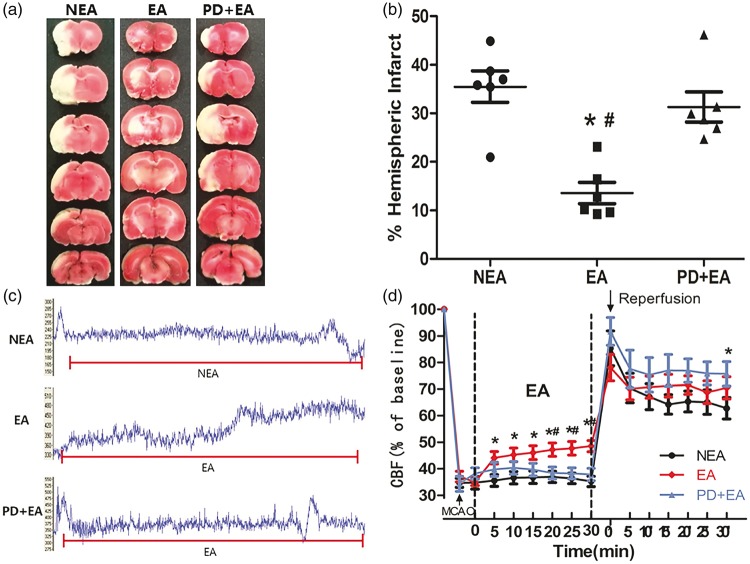
EA reduced infarct volume
As shown in Figure 3(a), infarcts were mainly concentrated in the subcortical area in the EA group, whereas infarcts were detected in both the cortical and subcortical regions in the other two groups. The ratio of hemispheric infarct was significantly lowered by EA (13.60 ± 2.20%, P < 0.05), and there was no significant difference between the NEA (35.48 ± 3.23%) and PD + EA (31.32 ± 3.13%) groups.
Figure 3.
Effect of electroacupuncture on infarct volume and cerebral perfusion. (a) Representative TTC staining in comparable sections from the three groups. (b) Quantification of the ratio of hemispheric infarct (n = 6/group). (c) Representative trace recordings of the blood perfusion during EA in the three groups. (d) Quantification of rCBF compared with baseline (n = 10/group). Data are presented as the mean ± SEM. *P < 0.05 vs. NEA group, #P < 0.05 vs. PD + EA group.
EA improved cerebral perfusion during ischemia and moderated the sharp increase in blood flow after reperfusion
The CBF decreased immediately upon the insertion of the filament and remained at a low level before EA. As shown in Figure 3(c), EA induced a constant and stable increase in the CBF to the ischemic area, with a significant difference compared with the other two groups at 20, 25, 30 min (P < 0.05). After reperfusion, the cerebral perfusion increased slightly and then remained stable in the EA group, tended to decrease in the NEA group, and remained high in the PD + EA group (Figure 3(d)). These results indicated that EA improved cerebrovascular compliance.
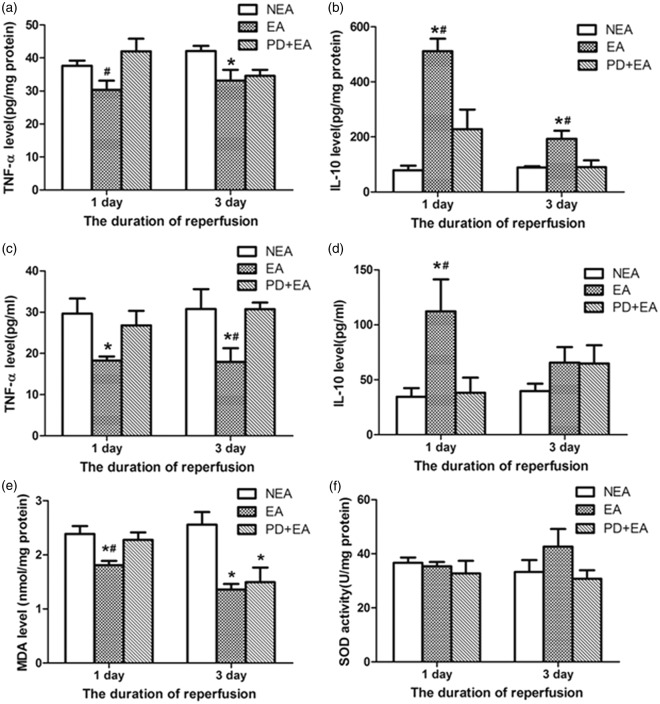
Effects of EA on inflammation
Cytokines in peri-infarct area
The TNF-α level in the EA group significantly decreased, differed from that in the PD + EA group at 24 h and that in the NEA group at three days (Figure 4(a)). Additionally, the IL-10 level in the peri-infarct area was much higher in the EA rats than in the other two groups on both day 1 and 3 after reperfusion (Figure 4(b)).
Figure 4.
Effect of electroacupuncture on inflammation and oxidative stress. (a) TNF-α level in the peri-infarct area. (b) IL-10 level in the peri-infarct area. (c) Serum TNF-α level. (d) Serum IL-10 level. (e) MDA level. (f) SOD activity. Data are presented as the mean ± SEM (n = 6 per group on day 1, n = 4 per group on day 3). *P < 0.05 vs. NEA group, #P < 0.05 vs. PD + EA group.
Cytokines in serum
The TNF-α levels in the serum of the EA rats were lowest at the indicated time points (Figure 4(c)). EA led to a significant elevation of serum IL-10 at 24 h but not on day 3 (P > 0.05, Figure 4(d)).
EA alleviated generated oxidative stress
The level of the lipid peroxidation by-product MDA in the peri-infarct area was reduced prominently by EA (1.81 ± 0.08%, P < 0.05) on day 1 compared with the others, and there was no significant difference between the NEA (2.38 ± 0.14%) and PD + EA (2.28 ± 0.14%) groups. On day 3, the MDA level remained rather high in the NEA group, while the other two groups showed a relative decrease (Figure 4(e)). In contrast, no noticeable changes in SOD activity were detected (Figure 4(f)).
EA treatment inhibited neuronal apoptosis
To locate the apoptotic neurons, we used TUNEL/NeuN/DAPI double labeling. The neuronal apoptosis ratio was markedly decreased in the EA group (0.31 ±0.05) compared with the NEA group (0.67 ± 0.05, P < 0.05), and the difference was not significant compared with the PD + EA group (0.47 ± 0.05, P > 0.05).
EA activated neurons in medullary parasympathetic nuclei
The dorsal motor nucleus of the vagus (DMV) has been acknowledged as the largest source of parasympathetic pre-ganglionic neurons within the lower brainstem. We observed a trend increased c-fos expression in the DMV induced by EA (107.68 ± 3.92) 24 h after reperfusion, and diminished substantially with PD therapy (P < 0.05; Figure 5(d) and (g)).
Figure 5.
Results of dual immunofluorescence and immunohistochemistry. (a) Representative double immunofluorescence, scale bar = 25 µm. (b) Representative α7nAChR immunohistochemistry, scale bar = 50 µm. (c) Schematic showing location of the dorsal motor nucleus of the vagus (DMV) in the rat brain. (d) Representative c-fos immunohistochemistry, scale bar = 50 µm. (e) Statistical analysis of the apoptosis ratio of the neurons (n = 4/group). (f) Statistical analysis of the mean density of α7nAChR (n = 4/group). (g) Statistical analysis of the mean density of c-fos (n = 4/group). Data are presented as the mean ± SEM. *P < 0.05 vs. NEA group, #P < 0.05 vs. PD + EA group.
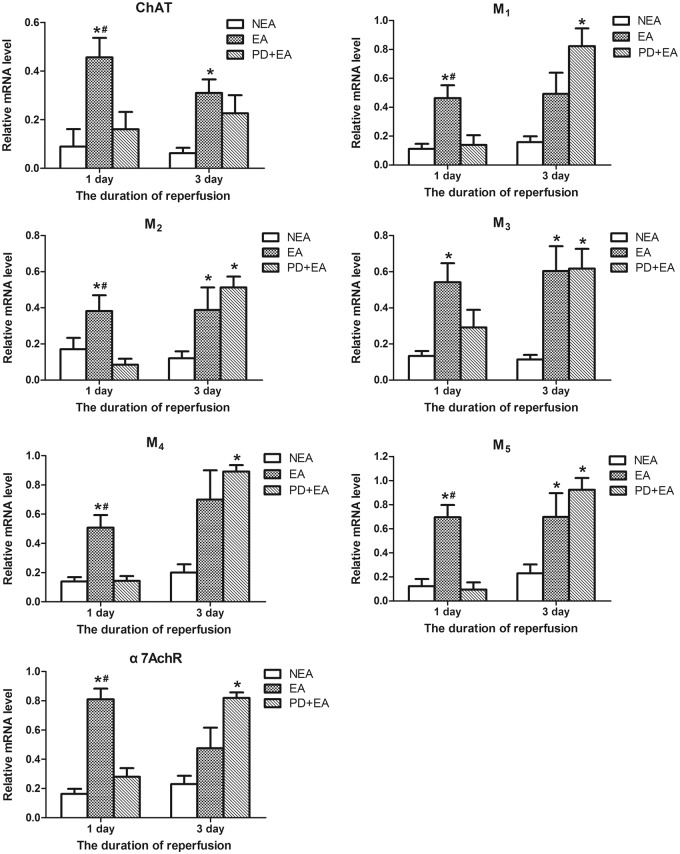
EA hindered the impairment of the central cholinergic system
As shown in Figure 6, the mRNA levels of ChAT, 5 subtypes of muscarinic receptors and α7nAChR dramatically dropped in the NEA and PD + EA groups at 24 h after ischemia. EA rats exhibited dramatically less shrinkage in the expression of the aforementioned genes, suggesting that EA hindered the impairment of the central cholinergic system. Accordingly, the immunohistochemistry and PCR results for α7nAChR expression in the ischemic core were consistent (Figure 5(b) and (f)). Two days later, the mRNA levels for all receptors were similar to those on the first day in the NEA and EA groups but upregulated prominently in PD + EA rats, approaching or even surpassing the levels of the EA rats.
Figure 6.
EA inhibits the impairment of central cholinergic system caused by ischemia. Data are presented as the mean ± SEM (n = 6 per group on day 1, n = 4 per group on day 3). *P < 0.05 vs. NEA group, #P < 0.05 vs. PD + EA group.
Discussion
Consistent with previous studies, we confirmed that EA was an effective approach for neuroprotection that could reduce infarct volume, improve cerebral perfusion and neurological deficits, and suppress apoptosis, oxidative stress and inflammation.13–15 The key finding is that EA attenuated the impairment of the central cholinergic system caused by ischemia and activated the parasympathetic nuclei in the medulla oblongata. Parasympathetic dysfunction reversed the protection to some degree.
The list of acupoints affecting the diverse meridians of the body is quite long. Traditional Chinese medicine holds the theory that Baihui (GV20) and Dazhui (GV14) both belong to the “Du meridian,” may function to govern vessel, collect the yang from peripheral regions and transport it to the brain, thus energizing the entire body. The therapeutic effect of Baihui (GV20)-based scalp acupuncture in animal models of focal cerebral ischemia has been validated in a recent systemic review.16 In clinical practice, different acupoints can be used to treat the same disorder, whereas one acupoint can be used to treat various disorders.17 Our goal here was to verify the mechanism of EA, not to find the most effective acupoints combination. Therefore, despite many acupoints are chosen for stroke in the literature, including Renzhong (GV6), Shuigou (GV26) and Zusanli (ST36), Baihui (GV20) and Dazhui (GV14) were stimulated in our study to exert synergistically beneficial effects against ischemic stroke.
There is no standard method for parasympathetic dysfunction in the literature. Given that vagus nerve is both the main component of the PNS and the major output of the central cholinergic system, and atropine is a non-selective muscarinic antagonist that can pass the blood–brain barrier via peripheral application without an obvious hemodynamic influence. Therefore, we chose intraperitoneal atropine instead of mecamylamine combined with unilateral vagotomy for parasympathetic dysfunction to avoid the hypoperfusion secondary to the severe hemodynamic changes and the potential deleterious effects associated with bilateral cervical vagotomy. We also performed a sub-experiment of PD group besides the aforementioned allocation to investigate the effects of parasympathetic dysfunction on ischemic stroke. Considering the results that there were no noticeable differences in the ratio of hemispheric infarct and CBF between the NEA and the PD groups (data not shown), we considered that PD alone had insignificant effects on the experimental model employed in this study, thus the subsequent measurements were not conducted.
We delivered EA 60 min after the onset of cerebral ischemia. Our data showed that the therapeutic window of EA extends to the acute phase and verified that EA had therapeutic benefits beyond its preventive14,15,18 effects and against disease recurrence.5 Recovery from stroke in humans is usually evaluated after weeks and months; thus, the clinical improvement after an intervention should also be assessed for a relatively longer observational period. As shown in Figure 2, the long functional benefit of EA is prominent, demonstrating its therapeutic effect more comprehensively. We assume that both the self-healing characteristics of sensorimotor dysfunction and the death of the subjects with severe insult in the first few days contributed to the insignificance on the 28th day. A recent study reported that nutritional support (dry pellets on the cage floor, oral feeding with jelly food, and jelly food in Petri dishes) beginning on the second day after filament MCAO reduced the 14-day mortality rate from 59% to 15% in mice.19 What’s more, the infarct size was similar among all the groups in that study, suggesting that post-stroke care was not neuroprotective. In our experiment, the rats had access to food and water ad libitum without additional nutritional support after surgery. Therefore, we suppose that the low survival rate in the NEA group is the consequence of ischemic insult and inadequate food and/or water intake. The difference in 28-day survival rates between the EA and PD + EA groups was not significant primarily because of the small sample. Additionally, the vagus nerve provides vital innervation of the gastrointestinal tract, and changes in digestive function may also be responsible for the slower weight gain.
Cerebral circulation is innervated by cholinergic nerves from intrinsic and extrinsic systems.20 The intrinsic systems originate within the central nervous system (CNS) and do not exit the brain (for instance, the innervation of cerebral parenchymal vessels from the basal forebrain).21 The extrinsic systems commence within the CNS but exit and have an extra-axial synapse before dominating the cerebral vessels (i.e., the parasympathetic innervation of the cerebral circulation arising from the superior salivatory nucleus, exiting the brain at the facial (VIIth cranial) nerve, and distributing fibers through the sphenopalatine to improve perfusion).20 The ACh released from excited cholinergic nerves from the basal forebrain and sphenopalatine ganglion dilates vessels through muscarinic activation of endothelial NO production, which correlates best with the M5 receptor subtype.22,23 The increased perfusion elicited by EA was completely abrogated by atropine and by endothelial nitric oxide synthase knockout in mice,13 further corroborating the proposal that EA enhances CBF via the muscarinic receptor-endothelial NO mechanism. However, whether the perfusion improvement induced by EA depends on the stimulation of the basal forebrain or the superior salivatory nucleus remains uncertain. Furthermore, the CBF after reperfusion in the EA group increased slightly and then remained at a stable level in contrast to that of the other two groups; this pattern may also relieve the damage resulting from the “explosive generation of oxygen free radicals” caused by rapid oxygen recovery.
Cardiac abnormalities and infections are the two leading causes of non-neurogenic post-stroke death.24 ANS alterations, particularly parasympathetic withdrawal and sympathetic overactivity, are involved in both conditions. Stroke patients may exhibit impaired HRV, blunted baroreceptor sensitivity, increased risk of cardiac arrhythmias and myocardial injury.24 HRV can serve as a noninvasive marker of autonomic input to the heart, the value of a long-term abnormal HRV in predicting poor post-stroke outcome is well documented.25,26 As indicated in Table 1, HRV was lower in the EA group than in the NEA group, but the difference was not significant. However, the HRV was higher in the PD group after reperfusion than the NEA group (data not shown). Therefore, the elevation in the PD + EA group is probably due to both the loss of vagal control caused by vagotomy and stroke-induced autonomic disturbance. Consequently, its prognostic effectiveness cannot be generalized to normal pathological conditions. A highlighted characteristic of EA that differs from other treatments is the acupoint specificity. EA at Neiguan acupoints (PC6) led to an apparently higher HF component of HRV than sham acupuncture in healthy subjects.27 Baihui (GV20) and Dazhui (GV14) were chosen because the theory of meridians in traditional Chinese medicine indicates that they are intimately associated with the brain and spinal cord, whose cardiovascular effects that may not be as prominent as the brain effects. Besides, a previous study from our team showed that EA could reduce LF/HF ratio of myocardial ischemia-reperfusion model in aged rats.28 It is generally accepted that the ANS function changes dramatically with age and that PNS activity decreases with age.29 The ANS of the 2-month-old immature rats used for this investigation was not completely developed, also complicating the interpretation of the results. Overall, we tend to believe that EA is capable of regulating anatomic balance; the acupoint specificity, the cerebral ischemia model we employed and the PNS activity all might be attributable to the insignificance.
Generally, the post-stroke immune response occurs in two phases. The acute response is proinflammatory and is driven by increased sympathetic activity. Later, when the spleen has exhausted its reserves of immune cells, immunodepression begins.30 In contrast with the attenuated production of TNF-α, the IL-10 level was elevated in the EA group. We can explain the findings via the regulation of the immune response by the CNS: EA serves as an alternative strategy for vagal stimulation31 to activate the cholinergic anti-inflammatory pathway, then enhanced endogenous ACh acts on the α7nAChR32 expressed by immune cells to inhibit proinflammatory cytokine release. Consequently, it is not surprising that vagotomy suppressed the anti-inflammatory effect of EA.
In in vitro studies, TNF-α was decreased but IL-10 was not affected by the activation of nicotinic receptors in lipopolysaccharide-stimulated human macrophages33 and rat microglia.34 Previous studies also showed that EA and direct stimulation of the efferent vagus nerve did not stimulate an increase in either the serum corticosteroid or IL-10; specifically, EA and direct stimulation had no modulatory effect on humoral anti-inflammatory mediators.33,35 The following might partly account for the paradoxical effects. First, different models were used. The inflammation of the endotoxemia models used in the former research is characterized by a very rapid and overwhelming innate immune response that is very difficult to stop once it is in motion and is far more severe than that in cerebral ischemia. Whether the reactiveness of the humoral response in the two models are the same is unknown. Another possible reason could be their earlier measurement time (several hours after endotoxemia) relative to ours (24 h and 3 days after reperfusion). The cholinergic anti-inflammatory pathway responses occur more rapidly,33 whereas the amount of IL-10 may not increase to significantly different levels at such an early measurement time. Given that a negative association between serum IL-10 levels and cerebral infarction was identified in a meta-analysis,36 we presume that the increased IL-10 may be a sub-phenomenon of the reduction in infarct volume other than EAs effect on the cholinergic anti-inflammatory pathway.
The central cholinergic system has been proven to be vulnerable to ischemic injury, and even mild hypoxia can inhibit Ach synthesis.37 Application of choline analogs can reduce the ischemic injury in organs to some extent.34,35 The rate of ACh synthesis is controlled by choline acetyltransferase (ChAT), which can serve as an indicator of the functional stage of cholinergic neurons in the CNS; muscarinic receptors are innervated by cholinergic neurons and are present in various areas of the brain. M1 receptors are the prevalent muscarinic receptors in the brain cortex and contribute to learning, memory and other advanced cognitive behaviors via interaction between the hippocampus and cortex. M2 receptors perform multiple functions: inhibition of high voltage-operated calcium channels and regulation of transmitters, including glutamate and ACh. M4 receptors co-localize with dopamine receptors in the striatum and modulate ACh-mediated athletic ability. M5 receptors mediate the NO-dependent cholinergic vasodilation.19,20 α7nAChR is the target of the cholinergic anti-inflammatory pathway. Furthermore, the cardioprotective actions of M2 and M3 receptors in the regulation of the redox state38 and the alleviation of endoplasmic reticulum stress39 and anti-apoptotic effect40 have been confirmed. Few reports have focused on the function of muscarinic receptors in the cortex, and further studies are required to demonstrate whether the muscarinic-mediated mechanisms that facilitate anti-oxidative stress and the anti-apoptotic effect are involved in EA-related neuroprotection.
The mRNA levels of ChAT, five subtypes of muscarinic receptors and α7nAChR significantly decreased in the NEA and PD + EA groups, and EA attenuated the impairment of the central cholinergic system induced by ischemia. Although EA relieved the reduction of M5, the CBF started to increase approximately 30 s after the onset of EA treatment (Figure 3(c)), and the amount of receptors changed minimally within such a short time. Therefore, we assume that the EA-evoked cerebral vasodilation was mediated by muscarinic activation of endothelial NO production. Unlike the other groups, the PD + EA group exhibited significantly higher levels of all the tested receptors on day 3 after ischemia compared with those at 24 h. This finding may be due to the rebound phenomenon resulting from decreased central vagal activity and the opposing agonist activity of the muscarinic antagonist, which increases the proportion of inactive receptors at the expense of the active state,41 thereby attenuating receptor downregulation.
Based on the conclusion that the vagus nerve and vagal pre-ganglionic neurons in the brainstem are essential for remote ischemic preconditioning,42,43 we assume that autonomic nuclei, as well as the functional integrity of the central nervous parasympathetic circuitry, are also required for the neuroprotection against cerebral ischemia. C-fos is the product of an immediate early gene and is widely used to evaluate neuronal activation; it responds transiently to acute stimuli, with the peak c-fos protein level occurring at approximately several hours.44 Prolonged (up to 24 h) differential expression in the DMV, the largest source of parasympathetic pre-ganglionic neurons in the lower brainstem, was still detected by immunohistochemistry in the EA group, possibly confirming that EA directly activates the PNS. Neuronal tracing experiments have identified DMV as one source of neurons with long-latency C-fiber axons converging on cardiac plexuses,45 the activity of the DMV vagal preganglionic neurons is also responsible for tonic parasympathetic control of ventricular excitability.46 Hence, the decreased trend of HRV in EA group might be partly attributable to the activation of DMV. Since the vagus nerve is composed of predominantly afferent fibers (80%), cervical vagotomy interrupts both the transmission of sensory information from the heart (and other internal organs) to the CNS and descending efferent pathways, the conclusion that vagal afferent fibers are involved in mediating EAs effect is premature in our study. Selectively block vagal afferent fibers by capsaicin and silencing the vagal pre-ganglionic neurons by cell-specific targeting technique might give a further exploration of such doubt.
Accordingly, we speculatively suggest the following framework for the role of the PNS in EA-induced neuroprotection. EA activates a brain muscarinic receptor-mediated network and medullary autonomic nuclei possibly through somato-autonomic reflexes,47 subsequently increasing vagus nerve activity. Via the cholinergic anti-inflammatory pathway and central cholinergic system, EA confers pleiotropic neuroprotective effects, and some identified targets as inflammation, cerebral perfusion, oxidative stress and apoptosis may be included.
Despite the advantages mentioned above, some inevitable limitations should be acknowledged. Stroke is a disease that mainly affects the elderly, but the present study was performed exclusively in young male rats, so age differences in stroke must be considered in further trials. Second, we focused on the function of the PNS, with minimal attention to the status of the sympathetic nervous system, which could also be of importance in this model. Central autonomic nuclei in the brainstem have been difficult to evaluate non-invasively, the employment of electrophysiology or neuroimaging modalities, such as functional magnetic resonance imaging (fMRI), might detect the real-time activities of the nuclei during stimulation.
Conclusion
The mechanisms of EA-related neuroprotection include the regulation of multiple signaling pathways. Our results do not exclude the involvement of these biochemical mechanisms but illustrate the EA effect on autonomic balance, thus providing a more macroscopic perspective. The PNS plays a critical role in the EA-induced neuroprotection against ischemic stroke, suggesting that EA could be an alternative modality of PNS activation for treating stroke. Moreover, autonomic disturbance is correlated with various clinical diseases, promising strategies to cope with sympathetic-parasympathetic dysfunction, thereby regulating the physiological function of various organs may be more profound.
Supplementary Material
Acknowledgements
The authors greatly appreciate the technical assistance of the Department of Physiology of Harbin Medical University. This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
Study design and manuscript preparation: LTC. Experimental studies and data acquisition: LTC and KRD. Data analysis/interpretation and statistical analysis: DDL and YLB. Manuscript editing/review: WZL.
Supplementary material
Supplementary material for this paper can be found at the journal website: http://journals.sagepub.com/home/jcb
References
- 1.Ay I, Lu J, Ay H, et al. Vagus nerve stimulation reduces infarct size in rat focal cerebral ischemia. Neurosci Lett 2009; 459: 147–151. [DOI] [PubMed] [Google Scholar]
- 2.Henninger N, Fisher M. Stimulating circle of Willis nerve fibers preserves the diffusion-perfusion mismatch in experimental stroke. Stroke 2007; 38: 2779–2786. [DOI] [PubMed] [Google Scholar]
- 3.Cheyuo C, Jacob A, Wu R, et al. The parasympathetic nervous system in the quest for stroke therapeutics. J Cereb Blood Flow Metab 2011; 31: 1187–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang S, Wu B, Liu M, et al. Acupuncture efficacy on ischemic stroke recovery: Multicenter randomized controlled trial in China. Stroke 2015; 46: 1301–1306. [DOI] [PubMed] [Google Scholar]
- 5.Shih CC, Liao CC, Sun MF, et al. A retrospective cohort study comparing stroke recurrence rate in ischemic stroke patients with and without acupuncture treatment. Medicine 2015; 94: e1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belayev L, Alonso OF, Busto R, et al. Middle cerebral artery occlusion in the rat by intraluminal suture. Neurological and pathological evaluation of an improved model. Stroke 1996; 27: 1616–1622; discussion 23. [DOI] [PubMed] [Google Scholar]
- 7.Yin CS, Jeong HS, Park HJ, et al. A proposed transpositional acupoint system in a mouse and rat model. Res Vet Sci 2008; 84: 159–165. [DOI] [PubMed] [Google Scholar]
- 8.Zhou F, Guo J, Cheng J, et al. Electroacupuncture increased cerebral blood flow and reduced ischemic brain injury: Dependence on stimulation intensity and frequency. J Appl Physiol 2011; 111: 1877–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou F, Guo J, Cheng J, et al. Effect of electroacupuncture on rat ischemic brain injury: importance of stimulation duration. Evid Based Complement Alternat Med 2013; 2013: 878521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J, Sanberg PR, Li Y, et al. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke 2001; 32: 2682–2688. [DOI] [PubMed] [Google Scholar]
- 11.Ashwal S, Tone B, Tian HR, et al. Core and penumbral nitric oxide synthase activity during cerebral ischemia and reperfusion. Stroke 1998; 29: 1037–1046; discussion 47. [PubMed] [Google Scholar]
- 12.Paxinos G, Watson C. The rat brain in stereotaxic coordinates, 6th ed San Diego: Academic Press, 2007. [Google Scholar]
- 13.Kim JH, Choi KH, Jang YJ, et al. Electroacupuncture acutely improves cerebral blood flow and attenuates moderate ischemic injury via an endothelial mechanism in mice. PLoS One 2013; 8: e56736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin Z, Liang J, Wang J, et al. Delayed brain ischemia tolerance induced by electroacupuncture pretreatment is mediated via MCP-induced protein 1. J Neuroinflammation 2013; 10: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun S, Chen X, Gao Y, et al. Mn-SOD Upregulation by electroacupuncture attenuates ischemic oxidative damage via CB1R-mediated STAT3 phosphorylation. Mol Neurobiol 2016; 53: 331–343. [DOI] [PubMed] [Google Scholar]
- 16.Wang WW, Xie CL, Lu L, et al. A systematic review and meta-analysis of Baihui (GV20)-based scalp acupuncture in experimental ischemic stroke. Sci Rep 2014; 4: 39–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stux G HR. Clinical acupuncture: Scientific basis, Heidelberg: Springers, 2001. [Google Scholar]
- 18.Wang Q, Wang F, Li X, et al. Electroacupuncture pretreatment attenuates cerebral ischemic injury through alpha7 nicotinic acetylcholine receptor-mediated inhibition of high-mobility group box 1 release in rats. J Neuroinflammation 2012; 9: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lourbopoulos A, Mamrak U, Roth S, et al. Inadequate food and water intake determine mortality following stroke in mice. J Cereb Blood Flow Metab Epub ahead of print 22 July 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goadsby PJ. Autonomic nervous system control of the cerebral circulation. Handb Clin Neurol 2013; 117: 193–201. [DOI] [PubMed] [Google Scholar]
- 21.Hamel E. Cholinergic modulation of the cortical microvascular bed. Prog Brain Res 2004; 145: 171–178. [DOI] [PubMed] [Google Scholar]
- 22.Elhusseiny A, Hamel E. Muscarinic–but not nicotinic–acetylcholine receptors mediate a nitric oxide-dependent dilation in brain cortical arterioles: a possible role for the M5 receptor subtype. J Cereb Blood Flow Metab 2000; 20: 298–305. [DOI] [PubMed] [Google Scholar]
- 23.Yamada M, Lamping KG, Duttaroy A, et al. Cholinergic dilation of cerebral blood vessels is abolished in M(5) muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci USA 2001; 98: 14096–14101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dorrance AM, Fink G. Effects of stroke on the autonomic nervous system. Compr Physiol 2015; 5: 1241–1263. [DOI] [PubMed] [Google Scholar]
- 25.Makikallio AM, Makikallio TH, Korpelainen JT, et al. Heart rate dynamics predict poststroke mortality. Neurology 2004; 62: 1822–1826. [DOI] [PubMed] [Google Scholar]
- 26.Chen CH, Huang PW, Tang SC, et al. Complexity of heart rate variability can predict stroke-in-evolution in acute ischemic stroke patients. Sci Rep 2015; 5: 17552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang ST, Chen GY, Lo HM, et al. Increase in the vagal modulation by acupuncture at neiguan point in the healthy subjects. Am J Chin Med 2005; 33: 157–164. [DOI] [PubMed] [Google Scholar]
- 28.Yuan S, Zhang X, Bo Y, et al. The effects of electroacupuncture treatment on the postoperative cognitive function in aged rats with acute myocardial ischemia-reperfusion. Brain Res 2014; 1593: 19–29. [DOI] [PubMed] [Google Scholar]
- 29.Pfeifer MA, Weinberg CR, Cook D, et al. Differential changes of autonomic nervous system function with age in man. Am J Med 1983; 75: 249–258. [DOI] [PubMed] [Google Scholar]
- 30.Dirnagl U, Klehmet J, Braun JS, et al. Stroke-induced immunodepression: Experimental evidence and clinical relevance. Stroke 2007; 38: 770–773. [DOI] [PubMed] [Google Scholar]
- 31.Torres-Rosas R, Yehia G, Pena G, et al. Dopamine mediates vagal modulation of the immune system by electroacupuncture. Nat Med 2014; 20: 291–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang Y, Li L, Liu B, et al. Vagus nerve stimulation attenuates cerebral ischemia and reperfusion injury via endogenous cholinergic pathway in rat. PLoS One 2014; 9: e102342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000; 405: 458–462. [DOI] [PubMed] [Google Scholar]
- 34.De Simone R, Ajmone-Cat MA, Carnevale D, et al. Activation of alpha7 nicotinic acetylcholine receptor by nicotine selectively up-regulates cyclooxygenase-2 and prostaglandin E2 in rat microglial cultures. J Neuroinflammation 2005; 2: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song JG, Li HH, Cao YF, et al. Electroacupuncture improves survival in rats with lethal endotoxemia via the autonomic nervous system. Anesthesiology 2012; 116: 406–414. [DOI] [PubMed] [Google Scholar]
- 36.Diao ZY, Wang CL, Qi HS, et al. Significance of decreased serum interleukin-10 levels in the progression of cerebral infarction. Clin Exp Med 2015; 16: 203–211. [DOI] [PubMed] [Google Scholar]
- 37.Hartig W, Bauer A, Brauer K, et al. Functional recovery of cholinergic basal forebrain neurons under disease conditions: Old problems, new solutions? Rev Neurosci 2002; 13: 95–165. [DOI] [PubMed] [Google Scholar]
- 38.Miao Y, Zhou J, Zhao M, et al. Acetylcholine attenuates hypoxia/ reoxygenation-induced mitochondrial and cytosolic ROS formation in H9c2 cells via M2 acetylcholine receptor. Cell Physiol Biochem 2013; 31: 189–198. [DOI] [PubMed] [Google Scholar]
- 39.Bi X, He X, Xu M, et al. Acetylcholine ameliorates endoplasmic reticulum stress in endothelial cells after hypoxia/reoxygenation via M3 AChR-AMPK signaling. Cell Cycle 2015; 14: 2461–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patane S. M3 muscarinic acetylcholine receptor in cardiology and oncology. Int J Cardiol 2014; 177: 646–649. [DOI] [PubMed] [Google Scholar]
- 41.Hilf G, Jakobs KH. Agonist-independent inhibition of G protein activation by muscarinic acetylcholine receptor antagonists in cardiac membranes. Eur J Pharmacol 1992; 225: 245–252. [DOI] [PubMed] [Google Scholar]
- 42.Donato M, Buchholz B, Rodriguez M, et al. Role of the parasympathetic nervous system in cardioprotection by remote hindlimb ischaemic preconditioning. Exp Physiol 2013; 98: 425–434. [DOI] [PubMed] [Google Scholar]
- 43.Mastitskaya S, Marina N, Gourine A, et al. Cardioprotection evoked by remote ischaemic preconditioning is critically dependent on the activity of vagal pre-ganglionic neurones. Cardiovasc Res 2012; 95: 487–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kovacs KJ. Measurement of immediate-early gene activation- c-fos and beyond. J Neuroendocrinol 2008; 20: 665–672. [DOI] [PubMed] [Google Scholar]
- 45.Jones JF, Wang Y, Jordan D. Activity of C fibre cardiac vagal efferents in anaesthetized cats and rats. J Physiol 1998; 507(Pt 3): 869–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Machhada A, Ang R, Ackland GL, et al. Control of ventricular excitability by neurons of the dorsal motor nucleus of the vagus nerve. Heart Rhythm 2015; 12: 2285–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Budgell B, Sato A. Modulations of autonomic functions by somatic nociceptive inputs. Prog Brain Res 1996; 113: 525–539. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.