Introduction
EPIICAL is a network of excellence concerned with a wide range of activities, including developmental research on novel disease modifying therapies (NDMTs), with the goal of achieving HIV remission. A central and timely theme of the network is the establishment of a predictive in vitro and in vivo platform to optimise the management of perinatally HIV-infected children and to inform treatment strategies (Figure 1). The EPIICAL project is a global collaboration of 27 international institutions, funded through an independent grant by ViiV Healthcare. Rome, Italy, hosted the first EPIICAL General Assembly (9–11 November 2017). Over 70 participants from 12 countries were brought together to discuss the updates of the first 18 months of the project, future strategies and perspectives. The meeting, chaired by Paolo Rossi (OPBG) and Carlo Giaquinto (Penta Foundation), included lectures by invited speakers, roundtable discussions and oral presentations by each work group.
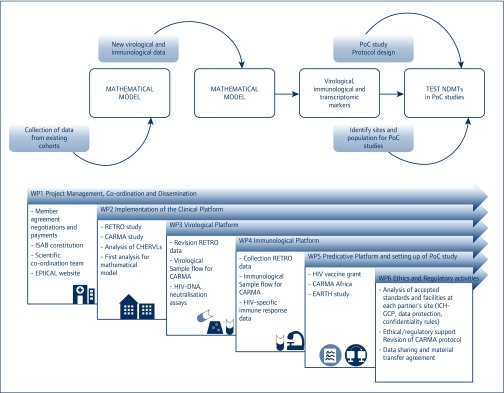
Figure 1.
The overall goals of the EPIICAL project (above) and the milestones achieved during the first 18 months of activity (below). PoC: proof of concept; NDMT: novel disease modifying therapies
This conference report captures much of the ongoing research and the major themes discussed during the three-day meeting.
Collection of retrospective data to provide an informative platform
The mathematical modelling of retrospective data from unique existing cohorts of early-treated, perinatally HIV-infected children will provide major novel insights in the EPIICAL project [1]. The work devoted to the implementation of the clinical platform is co-ordinated by Pablo Rojo (Hospital 12 de Octubre, Spain) and Louise Kuhn (Columbia University), who presented an overview on the work completed during the first 18 months of activity.
Man Chan (Medical Research Council, UK) illustrated the analysis of the RETRO study, which included data obtained from 51 perinatally HIV-infected children from five European sites, treated before 6 months of age. The analyses were focused on the main factors associated with low HIV-DNA. In multivariable analysis, lower total HIV-1 DNA was associated with younger age at antiretroviral treatment (ART) start, longer proportion of time spent virally suppressed and absence of viral failure/suboptimal response to ART. This report, on behalf of the EPIICAL Consortium, has been recently accepted for presentation at the Conference on Retroviruses and Opportunistic Infections (CROI) 2018 [2].
Juliane Schroeter (Utrecht University) presented a first analysis to identify determinants of different viral responses (rapid versus slow controllers) after early ART initiation through a mathematical modelling approach on a subgroup of HIV-infected children treated before 6 months of age from the EPPICC cohort. This exploratory study, albeit limited by the heterogeneity of data deriving from different cohorts, supports the utility of a mathematical approach to predict viral dynamics after ART initiation in HIV-infected infants. Through the ongoing partnership between the EPIICAL and the CHER (Children with HIV Early Antiretroviral Therapy) study groups, a new case of HIV viral remission has been identified.
Avy Violari (Perinatal HIV Research Unit, South Africa) presented the main new clinical and virological findings from the CHER cohort, highlighting the case of a perinatally HIV-infected child in viral remission for more than 9 years, previously reported at the 2017 International AIDS Society (IAS) conference in Paris [3]. The child started ART at age 61 days and treatment was interrupted 40 weeks later. During follow-up, virological tests confirmed the undetectability of HIV-RNA, with a very low level of HIV-DNA and no replication-competent virus. HIV serology by enzyme-linked immunosorbent assay was negative, but Western blot showed a weak reaction to Gag and p24. The unique availability of stored or newly collected samples from this cohort to the EPIICAL Consortium will supplement our knowledge on host viral control after ART interruption.
Additional retrospective data on early ART perinatally HIV-infected children from Johannesburg were illustrated by Louise Kuhn. Studies performed in the Nevirapine Resistance Study (NEVEREST) cohort confirmed the well-known relationship between HIV-DNA and timing of ART initiation. Notably, the relevance of the HIV antibody profile as a surrogate of reservoir size has previously been reported by Louise Kuhn and Paolo Palma in a different group of early-treated children [4]. Viral dynamics in very early-treated children (starting ART within 48 hours from birth) has been investigated in the Latency and Early Neonatal Provision of Antiretroviral Drugs Clinical Trial (LEOPARD) study.
The issue of implementing HIV diagnosis at birth in order to provide very early treatment in HIV-infected children in low-income countries was presented by Lynne Mofenson (Elizabeth Glaser Pediatric AIDS Foundation) who gave an in-depth overview of the recent literature. Dr Mofenson gave a state-of-the-art review of the virological and clinical benefits of early treatment. Despite considerable progress towards prevention of new paediatric HIV infections, there still is much more to be done before mother-to-child transmission is eliminated. Major challenges include the timing and modality of HIV test performance and limited availability of paediatric drug formulations. Use of diagnostic HIV nucleic acid testing (NAT) at birth could be beneficial to enable early identification of, and early treatment for, in utero infections. This approach requires linkage to a healthcare system, effective longitudinal follow-up and treatment with age-appropriate antiretroviral (ARV) drugs. An additional major issue raised is the pressing need to develop formulations, dosing and more safe ARV drugs for newborns.
Development of an experimental platform to produce new virological and immunological data
Caroline Foster (Imperial College London, UK) introduced the CARMA study design (Child and Adolescent Reservoir Measurements on early suppressive ART), a cross-sectional multicentre EPIICAL sub-study, which aims to identify the factors that influence the establishment of a low total HIV-DNA in perinatally HIV-infected children and adolescents on suppressive ART. The recruitment at different European sites has already started and Louise Kuhn proposed to enlarge the study population by including new cohorts from ‘CARMA Africa’. The virological work group is led by Eleni Nastouli (University College London, UK) who presented the virological questions that will be the focus in current and future EPIICAL studies. Sarah Watters (University College London, UK) reported on the operational programme from the laboratory perspective. Working with the immunological team, standard operating procedures (SOPs) on sample flow, sample processing and international shipping have been developed. Moreover, a database for data storage and sample tracking has been created with the help of the PENTA (Paediatric European Network for Treatment of AIDS) Foundation. This successful operational strategy based on communication between working groups will be extended to other EPIICAL studies.
Anne Genevieve Marcelin (Université Pierre et Marie Curie, France) presented data on HIV-DNA drug resistance and defectivity in vertically HIV-infected children with virological suppression from Mali.
Laura McCoy (University College London, UK) presented preliminary tests and data on neutralising antibodies that will be extended to the larger cohort of the CARMA study. A sub-study of CARMA, with the aim of investigating anatomical reservoirs was also proposed.
The immunological work group, led by Paolo Palma (OPBG) and Savita Pahwa (University of Miami, USA) gave an overview of the cellular HIV-specific immune response in early-treated children. Paolo Palma focused on HIV-specific memory responses in early ART-treates subjects. Interestingly, preliminary data from the Rome cohort suggest that in early ART-treated children there is a detectable frequency of HIV-specific memory B and T cells, even though these children are seronegative as determined by standard HIV EIA (enzyme immunoassay). However, in seronegative children, the HIV-specific B memory response resides in IgM memory B cells, rather than in IgG B cells as is seen in seropositive patients. Transcriptional data deriving from sorted HIV-specific IgM memory B cells, presented by Nicola Cotugno (OPBG) showed distinct pathways of genes that are activated upon in vitro stimulation with HIV peptides in seronegative patients.
Stefano Rinaldi (University of Miami, USA) integrated these data to show the association with polyfunctional HIV-specific CD4 T cells in the early ART population. Mark Cameron (Case Western Reserve University) discussed preliminary analyses performed on distinct signatures detected by RNASeq in total peripheral blood mononuclear cells, and after deconvolution analysis in memory T cells between early- and late-treated children (two abstracts with these data have been accepted at CROI 2018) [5,6]. In concluding this section, Nigel Klein (University College London, UK) presented a next-generation sequencing approach for studying the T cell repertoire in children and the applicability of this methodology in the EPIICAL studies.
The selection of populations for proof-of-concept (PoC) studies: testing NDMTs in the most appropriate candidates
The work group led by Carlo Giaquinto (University of Padova and PENTA Foundation) and Jintanat Ananworanich (Henry M Jackson Foundation for the Advancement of Military Medicine, USA) presented ongoing work to establish a predictive platform based on PoC studies. Many potential clinical trials were discussed. Alfredo Tagarro (Hospital 12 de Octubre, Spain) illustrated the details of EARTH (Early Anti-Retroviral Treatment in HIV-infected Children), a prospective, cohort study where early-treated children from Mozambique and South Africa will be enrolled at birth and followed for 2 years. The main aim of this study is to identify participants more likely to benefit from NDMTs. A closer international collaboration between countries with high HIV prevalence and high-income country HIV-research organisations was suggested to be crucial to build a clinical platform and perform large clinical trials in HIV-positive children. The selection of promising immunotherapeutic strategies through PoC studies in early-treated population models is one of the milestones of the project. In line with this, Jintanat Ananworanich presented a study proposal, currently under development, on the impact of an adjuvanted HIV-DNA vaccine with modified vaccinia Ankara (MVA) boosting on the viral reservoir in early-treated children. The central hypothesis of the trial is that HIVIS DNA priming and MVA boosting will lead to a reduction in HIV reservoir markers as a result of vaccine-induced immune responses. The regulatory WP led by Adriana Ceci (Consorzio per Valutazioni Biologiche e Farmacologiche, CVBF, Italy) and Maria Grazia Lain (Ariel Glaser Foundation, Mozambique) provided support on ethical and regulatory issues for all the ongoing studies. The work performed, with particular attention to data sharing and material transfer agreement, was presented in a dedicated session by Viviana Giannuzzi (CVBF) and Francesca Rocchi (OPBG).
The lecture by Philip Goulder (University of Oxford) focused on paediatric non-progressors and their underlying genetic, immune and virological mechanisms. He described the role of cytotoxic T lymphocyte (CTL) escape in immune control of HIV and the impact of escape mutations on viral replicative capacity, illustrating the potential of immunotherapeutic strategies for HIV remission. Anastasios Karadimitris (Imperial College London, UK) presented the fascinating field of chimeric antigen receptor T cell (CART) therapy that has dramatically changed the therapeutic prospects for B cell malignancies, illustrating potential applications in HIV research.
Conclusions
The first EPIICAL General Assembly meeting was held in an atmosphere of growing optimism. Many novel and exciting proposals for HIV research studies were discussed and are described above. The consortium aims to maintain this integrated developmental research on NDMTs, from predictive platforms to proof-of-concept studies, through the excellent collaborative effort made during the first 18 months of EPIICAL, some of which is described in this report. The emphasis on an innovative research platform is unique and may lead to optimisation of the management of perinatally HIV-infected children. Collectively, the updates and the discussions from the General Assembly attest to the benefit of nurturing an international collaborative effort on paediatric HIV research and confirm that EPIICAL is successfully on track.
Acknowledgements
The EPIICAL Consortium study team:
Nigel Klein, Diana Gibb: University College London (UCL) UK; Vincent Calvez: Université Pierre et Marie Curie (UPMC) France; Maria Angeles Munoz: Servicio Madrileño de Salud – Hospital General Universitario Gregorio Marañon (SERMAS-HGUGM) Spain; Britta Wahren: Karolinska Institutet (KI) Sweden; Mark Cotton: Stellenbosch University Faculty of Medicine and Health Sciences (SU) South Africa; Merlin Robb: The Henry M. Jackson Foundation for the Advancement of Military Medicine, Maryland, USA; Polly Claiden: HIV i-Base (HIViBase) UK; Deenan Pillay: University of KwaZulu-Natal Africa Center (UKZN/AC) South Africa; Rob J De Boer, Juliane Schroeter: University of Utrecht (UU) Netherlands; Thanyawee Puthanakit: Chulalongkorn University, Bangkok, Thailand; Adriana Ceci, Viviana Giannuzzi: Consorzio per Valutazioni Biologiche e Farmacologiche (CVBF) Italy; Kathrine Luzuriaga: University of Massachusetts Medical School (UMMS), Worcester, Massachusetts, USA; Nicolas Chomont: Centre de Recherche du Centre Hospitalier de l’Universitè de Montreal – University of Montreal (CRCHUM), Canada; Caterina Cancrini: Università degli Studi di Roma Tor Vergata (UNITOV), Italy; Andrew Yates: Columbia University, New York, USA; Kennedy Otwombe: University of the Witwatersrand, Johannesburg (PHRU), South Africa; Francesca Rocchi: Children's Hospital ‘Bambino Gesù’ (OPBG) Italy; Stefano Rinaldi: University of Miami, Miller School of Medicine (UM), Florida, USA. Maria Grazia Lain, Paula Vaz: Fundação Ariel Glaser contra o SIDA Pediátrico (ARIEL), Mozambique; Elisa Lopez, Tacilta Nhampossa: Fundação Manhiça, Mozambique.
Conflicts of interest and source of funding
The authors declare no conflict of interest directly related to the topic of this work. JA has received research funding and honoraria from ViiV Healthcare. This work was supported by the EPIICAL project, funded through an independent grant by ViiV Healthcare UK.
References
- 1. Palma P, Foster C, Rojo P et al. The EPIICAL project: an emerging global collaboration to investigate immunotherapeutic strategies in HIV-infected children. J Virus Erad 2015; 1: 134– 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chan M, Tagarro A, Zangari P et al. Factors associated with HIV-DNA levels in children starting ART early in infancy. Conference on Retroviruses and Opportunistic Infections. March 2018. Boston, MA, USA.
- 3. Violari A, Cotton M, Kuhn L et al. Viral and host characteristics of a child with perinatal HIV-1 following a prolonged period after ART cessation in the CHER trial. International AIDS Society Conference. July 2017. Paris, France. Abstract TUPDB0106LB.
- 4. Kuhn L, Schramm DB, Shiau S et al. Young age at start of antiretroviral therapy and negative HIV antibody results in HIV-infected children when suppressed. AIDS 2015; 29: 1053– 1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cotugno N, Morrocchi E, Pepponi I et al. HIV specific IgM memory B cells dominate in seronegative early-treated children. Conference on Retroviruses and Opportunistic Infections. March 2018. Boston, MA, USA.
- 6. Cameron M, Rinaldi S, Richardson B et al. Lasting immune impacts of age at start of ART in vertically HIV-infected adolescents. Conference on Retroviruses and Opportunistic Infections. March 2018. Boston, MA, USA.