Abstract
Background: The benefits of calcium and vitamin D supplementation for low bone mass remains controversial. This study assessed the changes in bone mineral density (BMD) during periods without and with calcium and vitamin D supplementation among HIV-infected adolescents with low BMD.
Method: Perinatally HIV-infected Thai adolescents aged 12–20 years were enrolled into Phase 1 (pre-supplementation) to evaluate longitudinal change of BMD. We provided education about appropriate dietary intake and exercise. Lumbar spine (L2–L4) BMD and vitamin D status (25-hydroxyvitamin D [25(OH)D]) were assessed at baseline and at 12–24 month intervals. Participants with a BMD Z-score≤−2 were enrolled into Phase 2 (supplementation) that provided calcium 600 mg plus cholecalciferol 200 IU twice daily for 6 months. BMD and 25(OH)D were re-assessed at the end of study.
Results: Ninety-four participants were enrolled into the Phase 1. Median age (IQR) was 14.3 (13.0–15.5) years, with 67% at Tanner stage 3–5, 89% with a plasma HIV-1 RNA<50 copies/mL. During Phase 1 and a 22.7-month follow-up, median L2–L4 BMD Z-scores remained unchanged (−1.06 vs −1.08, P=0.08), but 25(OH)D levels increased (24.7 vs 26.7 ng/mL, P=0.01). Twenty-six (28%) adolescents had low BMD and were enrolled into Phase 2, with 24 (92%) completing follow-up. The median L2–L4 BMD Z-scores (−2.59 vs −1.70; P<0.001) and calcium level (9.3 vs 9.5 mg/dL, P=0.04) significantly improved. There was an increase in BMD Z-scores during the 6-months post-supplementation as compared to the 21-month pre-supplementation period (0.65 vs −0.50, P=0.03).
Conclusion: HIV-infected adolescents with low BMD had improved bone health after calcium and vitamin D supplementation. A randomised controlled trial is warranted to confirm the benefits of these supplements.
Keywords: HIV-infected adolescents, osteoporosis, vitamin D, calcium supplementation
Introduction
In the past decade, antiretroviral therapy (ART) has led to a significant reduction in morbidity and mortality among infants and children perinatally infected with HIV. Many are now reaching adolescence or young adulthood and experiencing some of the long-term complications of HIV infection and therapy [1]. Low bone mineral density (BMD) is of concern since the peak bone mineral accrual usually occurs during adolescence [2]. Previous studies among HIV-infected Thai and Brazilian children demonstrated a high prevalence, in a range of 24–32%, of low BMD, defined as a BMD Z-score≤−2 [3,4]. In contrast, the prevalence in resource-rich countries is in a lower range, around 7–8% [5,6]. Deficits in bone mass accrual during adolescence may result in increased risk for osteoporosis and fractures in later life [7].
Vitamin D deficiency (VDD) is common among HIV-infected children and adolescents [8,9]. Our previous study found that about 25% of perinatally HIV-infected Thai adolescents had VDD [8]. Among healthy individuals, VDD causes negative biological consequences in terms of calcium homeostasis leading to decreased BMD through a mechanism of abnormal intestinal calcium absorption with alterations in parathyroid hormone (PTH) secretion [10–12]. However, the extent to which VDD and hypocalcaemia contribute to bone loss in the HIV-infected population is still controversial [3,13,14]. To date, there are limited data demonstrating the effect of calcium and vitamin D supplementation in improving bone mass among HIV-infected individuals, especially in resource-limited countries [15–17]. This study aimed to assess the changes in BMD during the periods without and with calcium and vitamin D supplementation among perinatally HIV-infected Thai adolescents.
Methods
Participants
We conducted a longitudinal study among a cohort of perinatally HIV-infected Thai adolescents aged 12–20 years at Siriraj and King Chulalongkorn Memorial hospitals from 2011 to 2014. The study consisted of two phases. In the Phase 1 (pre-supplementation) period, we evaluated baseline measurements and longitudinal changes of BMD and vitamin D status with health education but in the absence of calcium and vitamin D supplementation. During the Phase 2 (supplementation) period, studied the effect of calcium and vitamin D supplementation on BMD among individuals with low BMD, as defined by a BMD Z-score≤−2. The protocol was approved by the institutional review boards at both medical centres. Written informed consent was obtained from the participating adolescents and their caregivers. Baseline BMD and vitamin D levels have been previously reported [3,8].
During the Phase 1 study period, all participants were educated about appropriate dietary intake, outdoor exercise and a healthy lifestyle. Then, we re-evaluated their BMD and vitamin D status at 12–24 month intervals. Participants who had low BMD, either persistent since the baseline measurement or diagnosed at the second measurement were enrolled into the Phase 2 study period. During Phase 2, participants were prescribed a 6-month supplement of a fixed-dose combination (FDC) of 1500 mg of calcium carbonate (equivalence to 600 mg of elemental calcium) and 200 IU of cholecalciferol (Oskept, Charoon Bhesaj, Thailand), both twice daily. Individuals who also had vitamin D deficiency (VDD), defined as 25-hydroxyvitamin D [25(OH)D]<20 ng/ml, received ergocalciferol 60,000 IU weekly for 8 weeks, followed by monthly doses until their 25(OH)D level was above 30 ng/mL. BMD and vitamin D measurements were repeated for each participant at the end of the 6-month supplementation. Additionally, participants had routine clinical evaluations at each visit, including medical history, physical examination, anthropometric measurements, Tanner stage assessment, and laboratory testing, including CD4 T cell count and percentage, plasma HIV RNA, serum calcium, intact parathyroid hormone (iPTH) and alkaline phosphatase (ALK). Clinical data extracted from medical records showed the WHO clinical stage before ART, nadir CD4 T cell count, peak plasma HIV-1 RNA level, ART history and its duration.
Bone mineral density assessment and interpretation
Each participant was evaluated for lumbar spine (L2–L4) BMD by dual energy X-ray absorptiometry (DEXA) scanners, Lunar Prodigy Bone Densitometer (General Electric Healthcare, Madison, WI, USA) at Siriraj Hospital, or Hologic Discovery A Bone Densitometer (Hologic Inc, Bedford, MA, USA) at King Chulalongkorn Memorial Hospital. These machines were calibrated daily according to the manufacturer's instructions. Because the results and interpretation differ between these two machines, and BMD normative references are only available for the Lunar Prodigy Densitometer [18], BMD results from the Hologic A Bone Densitometer were converted to comparable values with the Lunar Bone Densitometer, using the previously described equation (GE-Lunar=1.195×Hologic−0.023) [19]. The BMD Z-score was then calculated using age- and sex-matched healthy Thai adolescent BMD as a reference [18] with the equation: BMD Z-score=(BMDmeasured−BMDreference for age and sex)/SEreference for age and sex (where SE=standard error of mean). Low BMD was defined as a BMD Z-score equal to or lower than −2, according to the International Society for Clinical Densitometry [20].
Laboratory measurements
Serum 25(OH)D and iPTH concentrations were measured by chemiluminescent microparticle immunoassay (ARCHITECT 25(OH) vitamin D assay and ARCHITECT intact parathyroid hormone assay, Abbott Diagnostics Division, Wiesbaden, Germany). A 25(OH)D level<20 ng/mL was defined as VDD [21]. The upper normal limit of serum iPTH was 65 pg/mL [22]. Serum calcium, phosphorus and ALK were measured by the VITROS Ca Slide method, VITROS PHOS Slide method, and VITROS ALKP Slide method (Ortho-Clinical Diagnostics Inc, Rochester, NY, USA), respectively. Normal ranges for calcium and phosphorus were 8.6–10.0 mg/dL and 4.0–7.0 mg/dL, respectively. The normal range of ALK level for males and females were 100–390 U/L and 100–320 U/L, respectively.
Statistical analysis
Data distribution was checked by the Shapiro–Wilk test. Demographic characteristics were reported as median (interquartile range [IQR]) or proportion, as appropriate. The comparison of BMD, BMD Z-scores, and 25(OH)D, calcium, and iPTH levels between baseline measurements and the following ones was carried out using signed-rank test for continuous variables and McNemar's test for categorical variables. A P-value<0.05 was considered statistically significant. All statistical analyses were performed using Stata software, version 11.2 (StataCorpLP, College Station, TX, USA).
Results
Patient characteristics
Among the 94 adolescents participating in Phase 1, baseline median age (IQR) was 14.3 (13.0–15.5) years, and 46 (49%) were male. Thirty-one (33%) were WHO stage 1–2 and 63 in WHO stage 3–4. Sixty-three (67%) had Tanner stage 3–5. Eighty-four participants (89%) had a plasma HIV-1 RNA level<50 copies/mL. The median interval between assessments was 22.7 (20.5–26.7) months.
Changes in BMD during Phase 1 (pre-supplementation)
During the follow-up period, participants showed improved growth, as demonstrated by an increased body mass index (18.7 vs 17.5 kg/m2, P<0.001), height-for-age Z-scores (−0.74 vs −1.01, P=<0.001), and proportions of Tanner stage 3–5 (97.8 vs 67.0%, P=<0.001). Additionally, these participants were more frequently exposed to tenofovir disoproxil fumarate (TDF) (42.6 vs 13.8%, P<0.001) with a longer median duration (22.8 vs 8.5 months, P=0.001). Other comparisons are shown in Table 1.
Table 1.
Comparisons of patient characteristics and other parameters between baseline assessment and at the end of Phase 1 for 94 perinatally HIV-infected Thai adolescents
| Characteristics | Baseline, n=94 | End of Phase 1, n=94 | P-value |
|---|---|---|---|
| Antiretroviral treatment | |||
| CD4 T cell count (cell/mm3), median (IQR) | 662(506–796) | 651(518–822) | 0.47 |
| Plasma viral load<50(copies/mL), n (%) | 84(89.4) | 77(81.9) | 0.15 |
| Duration of ART(months), median(IQR) | 91.1(62.7129.0) | 115.8(86.0–158.7) | <0.001 |
| Current ART regimen, n(%) | |||
| 2NRTI+NNRTI | 45(47.9) | 46(48.9) | 0.56 |
| 1–2NRTI+PI | 47(50.0) | 46(48.9) | 0.56 |
| NNRTI+PI | 2(2.1) | 2(2.1) | 1.00 |
| Exposure to TDF, n(%) | 13(13.8) | 40(42.6) | <0.001 |
| Duration TDF(months), median(IQR) | 8.5(7.6–10.9) | 22.8(17.2–27.7) | 0.01 |
| Anthropometric parameters | |||
| Body mass index(Kg/m2), median(IQR) | 17.5(15.8–19.6) | 18.7(17.0–20.6) | <0.001 |
| Thai WAZ, median(IQR) | −0.86(−1.74–0.05) | −0.67(−1.61–0.42) | 0.05 |
| Thai HAZ, median(IQR) | −1.01(−1.80to−0.25) | −0.74(−1.45to−0.06) | <0.001 |
| Pubertal Tanner Stage 3–5, n(%) | 63(67.0) | 88(97.8) | <0.001 |
| Bone health parameters, median(IQR) | |||
| BMD(g/cm2) | 0.84(0.71–0.98) | 0.95(0.82–1.04) | <0.001 |
| BMD Z-score | −1.06(−2.00to−0.07) | −1.08(−2.07to−0.38) | 0.08 |
| 25(OH)D level(ng/mL) | 24.7(19.1–31.4) | 26.7(23.1–32.8) | 0.01 |
| iPTH(pg/mL) | 43.7(33.7–58.5) | 49.3(37.1–68.6) | 0.01 |
BMD: lumbar bone mineral density; EFV: efavirenz; HAZ: height-for-age Z score; iPTH: intact parathyroid hormone; IQR: interquartile range; NNRTI: non-nucleoside reverse transcriptase inhibitor; NRTI: nucleoside reverse transcriptase inhibitor; PI: protease inhibitor; TDF: tenofovir disoproxil fumarate; WAZ: weight-for-age Z score; 25(OH)D: 25-hydroxyvitamin D.
With the provision of dietary and lifestyle education over the Phase 1 period, the median lumbar (L2–L4) BMD (IQR) increased from 0.84 g/cm2 (0.71–0.98 g/cm2) to 0.95 g/cm2 (0.82–1.04 g/cm2, P<0.001). However, the median lumbar BMD Z-score (−1.06 vs −1.08, P=0.08) as well as the proportion of participants with low BMD (25.5 vs 27.7%, P=0.67) remained unchanged. Among 40 participants who received a TDF-containing regimen, the median BMD Z-score was also unchanged (−1.37 vs −1.35, P=0.88). Median serum 25(OH)D concentrations (IQR) slightly increased (24.7 vs 26.7 ng/mL, P=0.01), but the proportion of participants with VDD was not reduced (25.5% vs 14.9%, P=0.07). Changes in other parameters are shown in Table 1.
Changes in BMD and vitamin D status during Phase 2 (supplementation)
There were 26 (27.7%) adolescents found to have low BMD, among whom 14 (54%) had low BMD from the first measurement and 12 (46%) were identified with low BMD at the second measurement. Of these, five (19%) had low BMD with VDD and 21 (81%) had only low BMD. All were enrolled into the Phase 2 study period. However, two were lost to follow-up (both had low BMD with VDD), resulting in a total of 24 participants receiving a complete schedule of calcium and vitamin D supplementation during this study period. Of the 24 participants, 19 (79%) self-reported taking >80% of the supplementation. Characteristics of the 24 participants enrolled into the Phase 2 are summarised in Table 2. Antiretroviral treatment included eight non-nucleoside reverse transcriptase inhibitors (NNRTI)-based regimens: four TDF/lamivudine (3TC)/efavirenz (EFV); two zidovudine (AZT)/3TC/EFV; and two AZT/3TC/nevirapine (NVP). There were 16 protease inhibitor (PI)-based regimens: five patients were taking TDF/3TC/lopinavir/ritonavir (LPV/r); five taking AZT/3TC/LPV/r; three taking TDF/3TC/atazanavir/ritonavir (ATV/r); one taking abacavir (ABC)/3TC/LPV/r; one taking didanosine (ddI/)3TC/LPV/r; and one was taking 3TC/LPV/r).
Table 2.
Comparison of patient characteristics and other parameters between Phase 1 and Phase 2 for perinatally HIV-infected Thai adolescents
| Characteristics | Phase 1 study period, n=24 | Phase 2 study period, n=24 | P-value |
|---|---|---|---|
| Current CD4 T cell count (cell/mm3), median (IQR) | 693(510–852) | 641(463–897) | 0.65 |
| Plasma viral load<50(copies/mL), n(%) | 20(83.3) | 18(75.0) | 0.50 |
| Current ART regimens, n(%) | |||
| 2NRTI+NNRTI | 8(33.3) | 8(33.3) | 1.00 |
| 2NRTI+PI | 16(66.7) | 16(66.7) | 1.00 |
| Exposure to TDF, n(%) | 9(37.5) | 12(50.0) | 0.25 |
| Exposure to PI, n(%) | 18(75.0) | 18(75.0) | 1.00 |
| Anthropometric parameters | |||
| Body mass index(Kg/m2), median(IQR) | 17.2(15.6–18.3) | 17.0(15.5–18.8) | 0.54 |
| Thai WAZ, median(IQR) | −1.97(−2.25to−1.20) | −1.81(−2.48to−1.03) | 0.84 |
| Thai HAZ, median(IQR) | −1.36(−2.13to−1.09) | −1.41(−2.08to−0.86) | 0.54 |
| Bone health parameters, median(IQR) | |||
| BMD(g/cm2) | 0.76(0.70–0.86) | 0.82(0.78–0.92) | <0.001 |
| BMD Z-score | −2.59(−3.02to−2.35) | −1.70(−2.76to−1.10) | <0.001 |
| 25(OH)D level(ng/mL) | 31.2(23.6–37.3) | 28.7(24.2–35.8) | 0.57 |
| iPTH(pg/mL) | 58.2(35.7–84.0) | 42.8(33.3–51.7) | 0.06 |
| Calcium(mg/dL) | 9.3(9.0–9.5) | 9.5(9.2–9.9) | 0.04 |
BMD: lumbar bone mineral density; EFV: efavirenz; HAZ: height-for-age Z score; iPTH: intact parathyroid hormone; IQR: interquartile range; NRTI: nucleoside reverse transcriptase inhibitor; NNRTI: non-nucleoside reverse transcriptase inhibitor; PI: protease inhibitor; TDF: tenofovir disoproxil fumarate; WAZ: weight-for-age Z score; 25(OH)D: 25-hydroxyvitamin D.
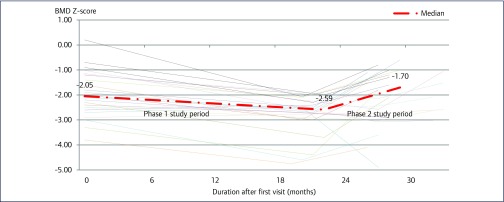
After a 6-month calcium and vitamin D supplementation period, the median lumbar (L2–L4) BMD (IQR) increased from 0.76 (0.70–0.86) to 0.82 (0.78–0.92) g/cm2 (P<0.001). Similarly, the median lumbar BMD Z-score (IQR) improved from −2.59 (−3.02 to −2.35) to −1.70 (−2.76 to −1.10) (P<0.001) (Figure 1). Additionally, the proportion of participants with low BMD reduced from 24 (100%) to 10 (42%) (P< 0.001), and median serum calcium level (IQR) increased from 9.3 (9.0–9.5) to 9.5 (9.2–9.9) mg/dL (P=0.04). However, median serum 25(OH)D levels (31.2 vs 28.7 ng/mL, P=0.57) remained unchanged. Changes in other parameters are shown in Table 2.
Figure 1.
Longitudinal plots of median BMD Z-score during the Phase 1 and Phase 2 study periods for 24 HIV-infected Thai adolescents with low BMD. Phase 1 study period (pre-supplementation) represented change in median BMD Z-score over a period of 12–24 months of diet and life style modification. Phase 2 study period (supplementation) represented change in median BMD Z-score after 6 months of calcium and vitamin D supplementation.
Comparison of BMD and vitamin D changes between Phase 1 (pre-supplementation) and Phase 2 (supplementation)
Focusing on 13 individuals with persistent low BMD and who had completed both study phases, the median intervals during pre-supplementation and supplementation periods were 20.9 and 6.0 months, respectively. Antiretroviral regimens among these 13 individuals include ten PI-based regimens: five AZT/3TC/LPV/r; two TDF/3TC/LPV/r; two TDF/3TC/ATV/r; and one ddI/3TC/LPV/r). There were three NNRTI-based regimens: two AZT/3TC/EFV; and one AZT/3TC/NVP.
Median changes in BMD Z-scores (−0.05 vs 0.65, P=0.03) and serum calcium levels (−0.5 vs 0.45 mg/dL, P=0.047) were more marked during the supplementation period. However, there was no significant change in median serum 25(OH)D and iPTH levels (P>0.05). Comparisons between other parameters are shown in Table 3.
Table 3.
Comparison of the changes in BMD, vitamin D status, and other parameters between Phase 1 and Phase 2 for HIV-infected Thai adolescents with persistent low BMD
| Parameters | Change during Phase 1, n=13 | Change during Phase 2, n=13 | P-value |
|---|---|---|---|
| Median (IQR) | Median (IQR) | ||
| Interval, month | 20.9(20.5–21.6) | 6.0(5.8–6.7) | – |
| Change of BMD(g/cm2) | 0.063(0.047–0.096) | 0.057(0.040–0.066) | 0.13 |
| Change of BMD Z-score | −0.50(−1.00–0.06) | 0.65(0.13–1.20) | 0.03 |
| Change of 25(OH)D level(ng/mL) | 5.51(2.02–11.95) | 0.10(−4.80–3.31) | 0.08 |
| Change of iPTH(pg/mL) | 11.57(−11.87–22.87) | −3.33(−42.45–0.36) | 0.31 |
| Change of calcium(mg/dL) | −0.5(−0.7to−0.1) | 0.45(−0.2–0.9) | 0.047 |
BMD: bone mineral density; iPTH: intact parathyroid hormone; 25(OH)D: 25-hydroxyvitamin D.
Discussion
This is a longitudinal study aimed at describing BMD changes among perinatally HIV-infected Thai adolescents over periods with and without calcium and vitamin D supplementation among individuals with low baseline BMD. BMD Z-scores were unchanged during a 2-year period of dietary and lifestyle education in the absence of supplementation. However, BMD Z-scores and serum calcium levels were improved after a 6-month daily supplementation with 1200 mg of elemental calcium and 400 IU of cholecalciferol.
In this study, we observed an increase in bone mass index but not in BMD Z-scores over almost 2 years in the Phase 1. The BMD physiological increase during adolescence underscores the need to use age- and ethnically matched references to calculate Z-scores to accurately interpret BMD results in this age group [2] as compared to adults who have relatively stable values over time [23–25]. In contrast to BMD Z-scores, a slight but significant improvement in vitamin D levels following the 2-year Phase 1 study period was observed. This could be the effect of education with increased sunlight exposure or simply because of improved health associated with a longer duration of successful ART. Unlike Western countries, calcium- or vitamin D-fortified foods are not available in Thailand.
A previous randomised controlled trial (RCT) conducted by Arpadi et al. among HIV-infected adolescents with normal BMD did not find a BMD improvement after a 2-year calcium (1000 mg calcium carbonate daily) co-administered with cholecalciferol (1600 IU daily) supplementation [15]. In contrast, our study demonstrated a significant improvement of BMD and BMD Z-scores with a 6-month course of calcium (1200 mg elemental calcium daily) and cholecalciferol (400 IU daily) supplementation. These findings might be explained by the difference in age and pubertal status of participants. Our adolescents were older with a higher proportion in an advanced pubertal stage with a more extensive physiological bone mineral accumulation. More importantly, our study recruited only adolescents with low baseline BMD into the intervention Phase 2 study period, so this could result in a bias towards a positive effect from supplementation. Nevertheless, our results were similar to those from Gulati et al. that demonstrated an increase in total body and spinal BMD and BMD Z-scores among children with nephrotic syndrome, who were concomitantly receiving steroid treatment, after calcium (500 mg calcium carbonate daily) and cholecalciferol (400 IU daily) supplementation for about 1.5 years [26]. In further comparisons with previous RCTs conducted in HIV-infected adults with osteopenia or osteoporosis, a significant BMD improvement was not demonstrated after calcium and vitamin D supplementation [16,27]. This finding could be attributed to age, menopausal status and baseline BMD. In a study by McComsey et al., calcium (1000 mg calcium carbonate daily) and cholecaciferol (400 IU daily) supplements were given to participants with a median age of 48 years, half of them (54%) having already reached menopause [16]. Therefore, the lack of improved BMD might be due to a negative biological effect on bone metabolism, which enhances bone resorption in this age group. In addition, Mondy et al. gave the same regimen of calcium and vitamin D supplementation to patients with mean baseline lumbar BMD T-scores of −1.64 [27], suggesting that the baseline BMD might not have been low enough for the intervention to demonstrate a significant impact. Recently, Overton et al. have shown an attenuation of bone loss by taking calcium (1000 mg calcium carbonate daily) and cholecalcifeol (4000 IU daily) supplementation in individuals initiated with tenofovir, emtricitabine, and efavirenz [17]. This study was conducted in young HIV-positive adults with a median age of 33 years, an age of peak bone mass [28], with high-dose vitamin D supplementation. The above mentioned factors may contribute to the positive effect on bone health observed in the study. After reviewing these studies, it appears that supplementation might be useful in those with active bone accrual such as adolescents and young adults.
We did not see changes in vitamin D levels in the Phase 2 study period as there were only five participating adolescents with VDD. These participants received high-dose vitamin D treatment while those without VDD received regular supplement doses. The increased vitamin D levels in Phase 1 may have normalised vitamin D level in many subjects, included those enrolled into Phase 2. Previous RCTs have suggested that high-dose vitamin D supplementation, ranging from 1100–7000 IU/day, is more effective in improving serum vitamin D concentrations among HIV-infected children and adolescents [29,30]. Results from our study suggest that calcium and vitamin D supplementation are essential to improve bone health especially for those with suboptimal levels, and that inadequate calcium intake and VDD may be major contributors to the low BMD found among adolescents infected with HIV.
Our study is the first longitudinal study to evaluate BMD changes using a period with and without calcium and vitamin D supplementation among HIV-infected adolescent living in a resource-limited country. Nevertheless, it has its limitations. First, since this is not an RCT, confounding factors such as indication and selection bias could not be eliminated. However, we did perform a sub-analysis among 13 adolescents with persistent low BMD and results are similar to those from the 24 participants in the Phase 2 study period. Second, the BMD evaluation period of only 24 weeks is quite short, as a 12-month interval is more commonly used to quantify BMD changes. Thus, we cannot be completely sure whether the positive effect on BMD from supplementation will be sustained after supplementation cessation. Third, since most of our adolescents were in the peak period of physiological bone mineral accrual, the observed BMD improvement may be confounded. Fourth, we have analysed only the lumber spine and not total body BMD. However, it has been shown that the lumber spine BMD is associated with the risk for fractures and has less variation compared to total body BMD. Finally, the number of individuals enrolled into the Phase 2 supplementation period was small and heterogeneous. Therefore, a large RCT with appropriate dosing of supplements and duration of evaluation is warranted to confirm the benefits of calcium and vitamin D supplementation on bone health among perinatally HIV-infected adolescents.
In conclusion, this longitudinal study demonstrated that BMD Z-scores were unchanged during the 2-year period without calcium and vitamin D supplementation. The 6-month calcium and low-dose vitamin D supplementation improved BMD among HIV-infected Thai adolescents with low BMD. We conclude that there is a potential benefit in using calcium and vitamin D supplements among perinatally HIV-infected adolescents with impaired bone health.
Acknowledgements
Disclaimer
The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of any of the institutions to which authors are affiliated.
Conflict of interests
The authors declare no conflicts of interests.
Funding
TREAT Asia funding
This study was supported by a grant to amfAR, the Foundation for AIDS Research, as part of the International Epidemiologic Databases to Evaluate AIDS (IeDEA; U01AI069907), by the US National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (NIAID), Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and National Cancer Institute (NCI), with additional support from AIDS Life Association.
References
- 1. Hazra R, Siberry GK, Mofenson LM. Growing up with HIV: children, adolescents, and young adults with perinatally acquired HIV infection. Ann Rev Med 2010; 61: 169– 185. [DOI] [PubMed] [Google Scholar]
- 2. McKay HA, Bailey DA, Mirwald RL et al. Peak bone mineral accrual and age at menarche in adolescent girls: a 6-year longitudinal study. J Pediatr 1998; 133: 682– 687. [DOI] [PubMed] [Google Scholar]
- 3. Puthanakit T, Saksawad R, Bunupuradah T et al. Prevalence and risk factors of low bone mineral density among perinatally HIV-infected Thai adolescents receiving antiretroviral therapy. J Acquir Immune Defic Syndr 2012; 61: 477– 483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schtscherbyna A, Pinheiro MF, Mendonca LM et al. Factors associated with low bone mineral density in a Brazilian cohort of vertically HIV-infected adolescents. Int J Infect Dis 2012; 16: e872– 878. [DOI] [PubMed] [Google Scholar]
- 5. Bunders MJ, Frinking O, Scherpbier HJ et al. Bone mineral density increases in HIV-infected children treated with long-term combination antiretroviral therapy. Clin Infect Dis 2013; 56: 583– 586. [DOI] [PubMed] [Google Scholar]
- 6. DiMeglio LA, Wang J, Siberry GK et al. Bone mineral density in children and adolescents with perinatal HIV infection. AIDS 2013; 27: 211– 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Puthanakit T, Siberry GK. Bone health in children and adolescents with perinatal HIV infection. J Int AIDS Soc 2013; 16: 18575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chokephaibulkit K, Saksawad R, Bunupuradah T et al. Prevalence of vitamin D deficiency among perinatally HIV-infected Thai adolescents receiving antiretroviral therapy. Pediatr Infect Dis J 2013; 32: 1237– 1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Meyzer C, Frange P, Chappuy H et al. Vitamin D deficiency and insufficiency in HIV-infected children and young adults. Pediatr Infect Dis J 2013; 32: 1240– 1244. [DOI] [PubMed] [Google Scholar]
- 10. Bischoff-Ferrari HA, Dietrich T, Orav EJ, Dawson-Hughes B. Positive association between 25-hydroxy vitamin D levels and bone mineral density: a population-based study of younger and older adults. Am J Med 2004; 116: 634– 639. [DOI] [PubMed] [Google Scholar]
- 11. Heaney RP, Dowell MS, Hale CA, Bendich A. Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Am Coll Nutr 2003; 22: 142– 146. [DOI] [PubMed] [Google Scholar]
- 12. Vieth R, Ladak Y, Walfish PG. Age-related changes in the 25-hydroxyvitamin D versus parathyroid hormone relationship suggest a different reason why older adults require more vitamin D. J Clin Endocrinol Metab 2003; 88: 185– 191. [DOI] [PubMed] [Google Scholar]
- 13. O'Brien KO, Razavi M, Henderson RA et al. Bone mineral content in girls perinatally infected with HIV. Am J Clin Nutr 2001; 73: 821– 826. [DOI] [PubMed] [Google Scholar]
- 14. Sherwood JE, Mesner OC, Weintrob AC et al. Vitamin D deficiency and its association with low bone mineral density, HIV-related factors, hospitalization, and death in a predominantly black HIV-infected cohort. Clin Infect Dis 2012; 55: 1727– 1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Arpadi SM, McMahon DJ, Abrams EJ et al. Effect of supplementation with cholecalciferol and calcium on 2-y bone mass accrual in HIV-infected children and adolescents: a randomized clinical trial. Am J Clin Nutr 2012; 95: 678– 685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McComsey GA, Kendall MA, Tebas P et al. Alendronate with calcium and vitamin D supplementation is safe and effective for the treatment of decreased bone mineral density in HIV. AIDS 2007; 21: 2473– 2482. [DOI] [PubMed] [Google Scholar]
- 17. Overton ET, Chan ES, Brown TT et al. 2014. High-dose vitamin D and calcium attenuates bone loss with ART initiation: results from ACTG A5280. Conference on Retroviruses and Opportunistic Infections. Boston, MA, USA. Abstract 133.
- 18. Nakavachara P, Pooliam J, Weerakulwattana L et al. A normal reference of bone mineral density (BMD) measured by dual energy X-ray absorptiometry in healthy thai children and adolescents aged 5–18 years: a new reference for Southeast Asian Populations. PLoS One 2014; 9: e97218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fan B, Lu Y, Genant H et al. Does standardized BMD still remove differences between Hologic and GE-Lunar state-of-the-art DXA systems? Osteoporos Int 2010; 21: 1227– 1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gordon CM, Bachrach LK, Carpenter TO et al. Dual energy X-ray absorptiometry interpretation and reporting in children and adolescents: the 2007 ISCD Pediatric Official Positions. J Clin Densitom 2008; 11: 43– 58. [DOI] [PubMed] [Google Scholar]
- 21. Holick MF, Binkley NC, Bischoff-Ferrari HA et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011; 96: 1911– 1930. [DOI] [PubMed] [Google Scholar]
- 22. Expected values and S.I. unit conversion pocket book Available at: www.esoterix.com/endocrinology-services/endocrinology-tools ( accessed October 2017).
- 23. Bolland MJ, Grey AB, Horne AM et al. Bone mineral density remains stable in HAART-treated HIV-infected men over 2 years. Clin Endocrinol 2007; 67: 270– 275. [DOI] [PubMed] [Google Scholar]
- 24. Mondy K, Yarasheski K, Powderly WG et al. Longitudinal evolution of bone mineral density and bone markers in human immunodeficiency virus-infected individuals. Clin Infect Dis 2003; 36: 482– 490. [DOI] [PubMed] [Google Scholar]
- 25. Nolan D, Upton R, McKinnon E et al. Stable or increasing bone mineral density in HIV-infected patients treated with nelfinavir or indinavir. AIDS 2001; 15: 1275– 1280. [DOI] [PubMed] [Google Scholar]
- 26. Gulati S, Sharma RK, Gulati K et al. Longitudinal follow-up of bone mineral density in children with nephrotic syndrome and the role of calcium and vitamin D supplements. Nephrol Dial Transplant 2005; 20: 1598– 1603. [DOI] [PubMed] [Google Scholar]
- 27. Mondy K, Powderly WG, Claxton SA et al. Alendronate, vitamin D, and calcium for the treatment of osteopenia/osteoporosis associated with HIV infection. J Acquir Immune Defic Syndr 2005; 38: 426– 431. [DOI] [PubMed] [Google Scholar]
- 28. Berger C, Goltzman D, Langsetmo L et al. Peak bone mass from longitudinal data: implications for the prevalence, pathophysiology, and diagnosis of osteoporosis. J Bone Miner Res 2010; 25: 1948– 1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Giacomet V, Vigano A, Manfredini V et al. Cholecalciferol supplementation in HIV-infected youth with vitamin D insufficiency: effects on vitamin D status and T-cell phenotype: a randomized controlled trial. HIV Clin Trials 2013; 14: 51– 60. [DOI] [PubMed] [Google Scholar]
- 30. Havens PL, Mulligan K, Hazra R et al. Serum 25-hydroxyvitamin D response to vitamin D3 supplementation 50,000 IU monthly in youth with HIV-1 infection. J Clin Endocrinol Metab 2012; 97: 4004– 4013. [DOI] [PMC free article] [PubMed] [Google Scholar]