Abstract
Background:
The objective of this study was to identify the minimum basal insulin infusion rates and bolus insulin doses that would result in clinically relevant changes in blood glucose levels in the most insulin sensitive subjects with type 1 diabetes.
Methods:
The UVA/PADOVA Type 1 Diabetes Simulator in silico population of children, adolescents, and adults was administered a basal insulin infusion rate to maintain blood glucose concentrations at 120 mg/dL (6.7 mmol/L). Two scenarios were modeled independently after 1 hour of simulated time: (1) basal insulin infusion rates in increments of 0.01 U/h were administered and (2) bolus doses in increments of 0.01 U were injected. Subjects were observed for 4 hours to determine insulin delivery required to change blood glucose by 12.5 mg/dL (0.7 mmol/L) and 25 mg/dL (1.4 mmol/L) in only 5% of the in silico population.
Results:
The basal insulin infusion rates required to change blood glucose by 12.5 mg/dL and 25 mg/dL in 5% of children, adolescents, and adults were 0.03, 0.11, and 0.10 U/h and 0.06, 0.21, and 0.19 U/h, respectively. The bolus insulin doses required to change blood glucose by the target amounts in the respective populations were 0.10, 0.28, and 0.30 U and 0.19, 0.55, and 0.60 U.
Conclusions:
In silico modeling suggests that only a very small percentage of individuals with type 1 diabetes, corresponding to children with high insulin sensitivity and low body weight, will exhibit a clinically relevant change in blood glucose with very low basal insulin rate changes or bolus doses.
Keywords: CSII, insulin pump, insulin sensitivity, pediatric
An important consideration for the use of continuous subcutaneous insulin infusion (CSII) systems is the ability to meet the treatment needs of the most insulin sensitive patients. In this context, it is critical to understand the impact that variations in insulin delivery can have on this population.1 While clinical trials will continue to be the definitive method for such evaluations, simulation technology has been increasingly used to perform such assessments.
The UVA/PADOVA Type 1 Diabetes Simulator is accepted by the US Food and Drug Administration (FDA) as a substitute for preclinical trials and has been used extensively to model insulin treatments, including closed-loop algorithms.2-5 This platform includes an in silico population of 300 subjects—100 children, 100 adolescents, and 100 adults—and provides advantages for modeling under controlled experimental conditions with the full variability seen in individuals with type 1 diabetes. The present investigation relies on UVA/PADOVA simulation to understand the impact of changes to insulin delivery to ultimately understand the minimal insulin doses that would result in a clinically relevant impact on blood glucose levels.
Materials and Methods
The objective of this study was to use the UVA/PADOVA Type 1 Diabetes Simulator version S20135 to identify the minimum basal infusion rates and bolus insulin doses that would result in clinically relevant changes in blood glucose concentrations of ±12.5 mg/dL (0.7 mmol/L) and ±25 mg/dL (1.4 mmol/dL) from a baseline level of 120 mg/dL (6.7 mmol/L) in only 5% (the most insulin sensitive subjects) of the in silico population of children, adolescents and adults. In these scenarios, 95% of the in silico cohorts would have a change in blood glucose of <25 mg/dL. Two in silico simulation scenarios were performed to quantify the effect of changes in basal and bolus insulin delivery on blood glucose concentrations at the individual and population level in the simulator populations.
Scenario 1—Changes to Basal Insulin Infusion Rate
Beginning at 08:00, subjects were maintained at a blood glucose concentration of 120 mg/dL (6.7 mmol/L) by administering an adequate amount of insulin, which was computed offline. After 1 hour of simulated time (09:00), basal insulin infusion rates in increments of 0.01 U/h up to 1.5 U/h were modeled independently to characterize blood glucose concentration changes over 4 hours.
Additional simulations over 10 hours, to approximate an overnight period, at a baseline blood glucose of 120 mg/dL were performed for all cohorts and also at a lower baseline blood glucose of 100 mg/dL for the adult cohort.
Scenario 2—Changes to Bolus Insulin Delivery
Subjects were maintained at a blood glucose concentration of 120 mg/dL (6.7 mmol/L) as in Scenario 1. After 1 hour at 09:00, bolus doses in increments of 0.01 U up to 0.5 U were simulated independently and effects on blood glucose concentrations were observed over 4 hours.
Results
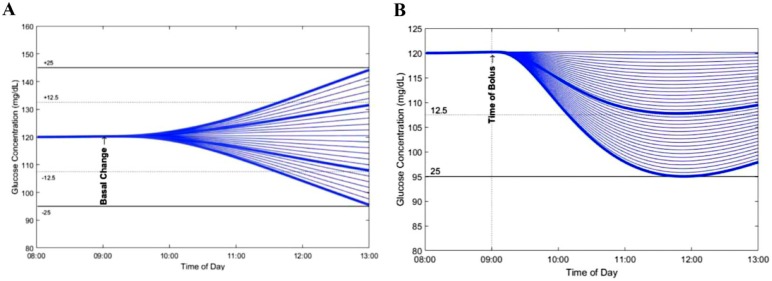
A simulation diagram illustrating the blood glucose response was generated for each subject and basal insulin infusion rate (Scenario 1) and bolus dose combination (Scenario 2). A representative diagram of an adult subject is presented in Figure 1.
Figure 1.
Effect of changes in basal insulin infusion rate and bolus insulin doses on blood glucose in a representative in silico adult subject. (A) Scenario 1: Effect of changes in basal insulin infusion rate in increments of 0.01 U/h. Changes in blood glucose in the amount of 12.5 (.….) and 25 mg/dL (—) are identified by the blue lines. (B) Scenario 2: Effect of bolus insulin doses ranging from 0.01 U to 0.5 U delivered in increments of 0.1 U. Bold blue lines represent a decrease in blood glucose of 12.5 md/dL and 25 mg/dL, respectively.
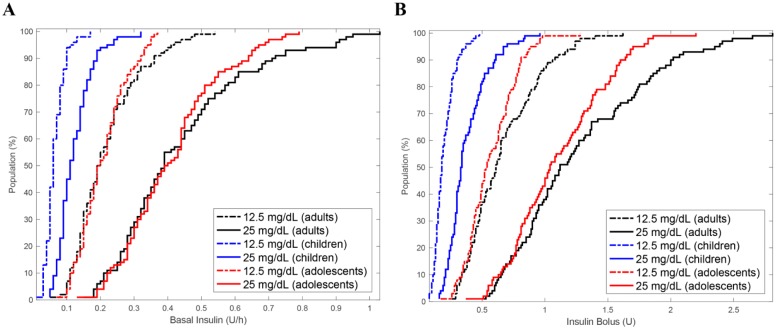
The cumulative distribution of basal and bolus insulin delivery required to produce changes of 12.5 mg/dL (0.7 mmol/L) and 25 mg/dL (1.4 mmol/L) in blood glucose concentrations in the complete in silico population over 4 hours is presented in Figure 2.
Figure 2.
Cumulative distribution of insulin doses required to change blood glucose by 12.5 mg/dL (0.7 mmol/L) and 25 mg/dL (1.4 mmol/L) in the in silico population. (A) Scenario 1: Minimum basal insulin infusion rates. (B) Scenario 2: minimum bolus doses required to change blood glucose by 12.5 mg/dL (0.7 mmol/L) and 25 mg/dL (1.4 mmol/L).
The basal insulin infusion rates and bolus doses required to change blood glucose levels by the target amounts in different proportions of the in silico population are also presented in Table 1. The basal insulin infusion rates required to change blood glucose by the target amounts in 5% of children, adolescents and adults were 0.03, 0.11, and 0.10 U/h and 0.06, 0.21, and 0.19 U/h, respectively, over 4 hours. Similar but slightly lower infusion rates were observed for the 10 hour simulation at the baseline blood glucose level of 120 mg/dL. At the lower baseline blood glucose of 100 mg/dL a nonlinear response to basal infusion rates was observed. A basal rate of 0.03 U/h resulted in a 12.5 mg/dL (0.7 mmol/L) change in blood glucose in 5% of the adult cohort whereas a 4-fold higher rate of 0.12 U/h resulted in change blood glucose by 25 mg/dL (1.4 mmol/L).
Table 1.
Basal and Bolus Insulin Delivery Required for Clinically Meaningful Changes in Blood Glucose Concentrations in the In Silico Population.
| Children |
Adolescents |
Adults |
||||
|---|---|---|---|---|---|---|
| Insulin delivery | 12.5 mg/dL (0.7 mmol/L) | 25 mg/dL (1.4 mmol/L) | 12.5 mg/dL (0.7 mmol/L) | 25 mg/dL (1.4 mmol/L) | 12.5 mg/dL (0.7 mmol/L) | 25 mg/dL (1.4 mmol/L) |
| Basal (U/h) | ||||||
| 5% | 0.03 | 0.06 | 0.11 | 0.21 | 0.10 | 0.19 |
| 25% | 0.05 | 0.09 | 0.15 | 0.30 | 0.14 | 0.29 |
| 50% | 0.06 | 0.11 | 0.19 | 0.39 | 0.19 | 0.39 |
| 75% | 0.08 | 0.15 | 0.25 | 0.49 | 0.27 | 0.52 |
| 100% | 0.17 | 0.32 | 0.37 | 0.79 | 0.54 | 1.03 |
| Bolus (U) | ||||||
| 5% | 0.10 | 0.19 | 0.28 | 0.55 | 0.30 | 0.60 |
| 25% | 0.14 | 0.27 | 0.42 | 0.80 | 0.44 | 0.88 |
| 50% | 0.18 | 0.33 | 0.53 | 1.04 | 0.61 | 1.12 |
| 75% | 0.25 | 0.46 | 0.70 | 1.37 | 0.85 | 1.66 |
| 100% | 0.48 | 0.96 | 1.28 | 2.20 | 1.62 | 2.81 |
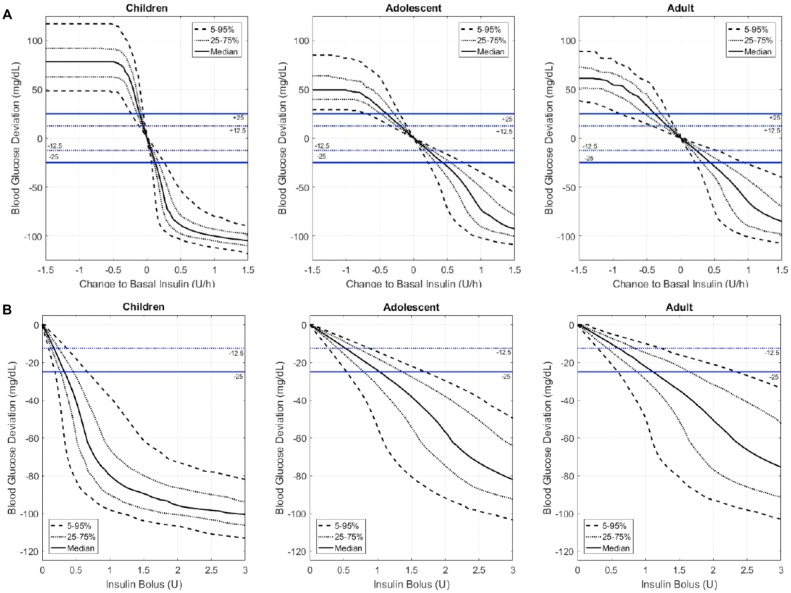
The bolus insulin doses required to change blood glucose by the target amounts in 5% in the respective populations were 0.10, 0.28, and 0.30 U and 0.19, 0.55, and 0.60 U, respectively. Changes in blood glucose levels above and below the study targets across a continuum of basal rates and bolus doses are depicted in Figures 3A and 3B.
Figure 3.
Changes in blood glucose concentrations across a continuum of basal insulin doses in the in silico population. Blood glucose response to changes in basal insulin rate (A) or bolus dose (B) amount is indicated for the median ( ) and the least and most insulin sensitive 5% to 95% (- - -) and 25% to 75% (….) segments of children, adolescents, and adults. The blue dotted line indicates a change of 12.5 mg/dL and the solid blue line indicates a change of 25 mg/dL.
Discussion
The results of in silico modeling using the UVA/PADOVA Type 1 Diabetes Simulator suggest that only a very small percentage of individuals, corresponding to children with high insulin sensitivity and low body weight, will exhibit a clinically relevant change in blood glucose concentration with very low basal insulin rate changes or bolus doses.
The simulations performed in this study identified the basal insulin and bolus delivery doses required to obtain target changes in blood glucose of 12.5 mg/dL and 25 mg/dL across the entire in silico population of children, adolescents, and adults. The results indicate that relatively large insulin dose changes are required to result in blood glucose changes that are clinically relevant to a majority of the population.
The additional simulation at a low target blood glucose level over an extended time period demonstrated the scenario reported by Dalla Man and colleagues in which insulin action paradoxically increases when glucose decreases under a given threshold.5 This loss of counterregulation resulted in a nonlinear insulin-blood glucose response in 5% of the most insulin sensitive adult cohort: a very low basal infusion rate resulted in a change of 12.5 mg/dL in blood glucose, whereas a 4-fold higher basal infusion rate of 0.12 U/hr was required to impact blood glucose at a more clinically meaningful level of 25 mg/dL. This phenomenon has been described during hyperinsulemic clamps in type 1 diabetes.5
Although additional investigation is required to fully understand the impact of these variations during meals, exercise and other daily activities, the simulations show that a delivery capability of 0.05 U/h available in commercial insulin pumps adequately maintains a patient’s basal level across a representative population of subjects with type 1 diabetes.
The strengths of the present study include targeted modeling using the robust UVA/PADOVA Type 1 Diabetes Simulator which controls experimental conditions and minimizes confounders inherent in human subjects research. It also allows for a systematic exploration of different dosing disturbances, which is impractical in a clinical study. Limitations of in silico modeling also apply as controlled testing criteria may not fully account for physiologic factors such as intraindividual variability in insulin absorption and insulin action that can vary up to 25% at any given time.1 Thus, the results of these experiments may not translate to patient treatment outcomes. However, the results are instructive and address a clinically relevant topic for treatment of patients with type 1 diabetes.
Conclusion
While further study is needed, the results of in silico modeling experiments using the UVA/PADOVA Type 1 Diabetes Simulator suggest that low insulin doses impact blood glucose levels in a clinically relevant manner in only a very small percentage of children with type 1 diabetes.
Supplementary Material
Acknowledgments
Editorial support was provided by Christopher G. Parkin, MS of CGParkin Communications Inc, Boulder City, NV, which was supported by Insulet Corporation.
Footnotes
Abbreviations: CSII, continuous subcutaneous insulin infusion; FDA, US Food and Drug Administration; UVA, University of Virginia.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: ECN has duality of interest. JEL is an employee of Insulet Corporation, the study sponsor. HCZ was a full-time employee of Insulet Corporation during the development of this project.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Insulet Corporation provided funding for the study.
Supplemental Material: Supplementary material for this article is available online.
References
- 1. Heinemann L. Variability of insulin absorption and insulin action. Diabetes Technol Ther. 2002;4(5):673-682. [DOI] [PubMed] [Google Scholar]
- 2. Dalla Man C, Rizza RA, Cobelli C. Meal simulation model of the glucose-insulin system. IEEE Trans Biomed Eng. 2007;54(10):1740-1749. [DOI] [PubMed] [Google Scholar]
- 3. Dalla Man C, Raimondo DM, Rizza RA, Cobelli C. GIM, simulation software of meal glucose-insulin model. J Diabetes Sci Technol. 2007;1(3):323-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kovatchev BP, Breton M, Dalla Man CD, Cobelli C. In-silico preclinical trials: a proof of concept in closed-loop control of type 1 diabetes. J Diabetes Sci Technol. 2009;3(1):44-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Man C, Micheletto F, Lv D, Breton MD, Kovatchev BP, Cobelli C. The UVA/PADOVA Type 1 Diabetes Simulator: new features. J Diabetes Sci Technol. 2014;8(1):26-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.