Abstract
Introduction:
ISO 15197:2013 recommends testing procedures and acceptance criteria for the evaluation of influence quantities such as hematocrit on measurement results with systems for self-monitoring of blood glucose (SMBG). In this study, hematocrit influence was evaluated for a novel SMBG system (system A) and five other systems with different hematocrit ranges based on ISO 15197:2013.
Methods:
Test procedures were performed with one test strip lot for each system. Each system was tested within the hematocrit range indicated in the manufacturer’s labeling (system A: 10-65%, B: 15-65%, C: 20-60%, D: 35-60%, E: 30-60%, F: 30-55%). According to ISO 15197:2013, clause 6.4.2, venous blood samples were used for the evaluation of hematocrit influence. The evaluation was performed for three glucose concentration categories (30-50 mg/dL, 96-144 mg/dL, and 280-420 mg/dL). For each glucose concentration category, at least five different hematocrit levels were investigated.
Results:
The novel system A and systems B, E, and F complied with the tested lot with the defined criteria and showed ≤10 mg/dL and ≤10% difference between the test sample and the respective control sample with a hematocrit value of 42% ± 2% for BG concentrations <100 mg/dL and ≥100 mg/dL, respectively. Two systems showed >10% difference at glucose concentrations ≥100 mg/dL.
Conclusions:
Remarkable hematocrit influence within the labeled hematocrit range was obtained in two systems with the tested reagent system lot. Adequate SMBG systems should be carefully chosen by patients and their health care professionals, particularly for patients with increased and decreased hematocrit values.
Keywords: blood glucose monitoring systems, hematocrit influence, EN ISO 15197:2015, ISO 15197:2013, self-monitoring of blood glucose
Systems for self-monitoring of blood glucose (SMBG) enable people with diabetes to measure their blood glucose levels to adjust their therapy and to prevent complications, such as microvascular and neurologic long-term complications.1 SMBG measurements are performed on capillary whole blood. For this purpose, most SMBG systems use an electrochemical sensor that is located within a capillary chamber on the reagent system, for example, test strip, as a thin, dry layer comprising a mixture of enzymes and other chemical components that react specifically with glucose.2-4 The reaction on the test strip and thus the reliability of the measurement result can be affected by a number of variables, including hematocrit, interfering substances (eg, acetaminophen), humidity, temperature, and partial pressure of oxygen (pO2).5-11
The International Organization for Standardization (ISO) standard 15197:201312 recommends test procedures and analytical performance requirements for SMBG systems. ISO 15197:2013 was harmonized in the European Union as EN ISO 15197:201513 with no changes regarding its requirements for performance studies. In Europe, manufacturers usually apply the ISO 15197:2013 standard to obtain the CE (Conformité Européenne) mark for their SMBG system. ISO 15197:2013 also requires the evaluation of influence quantities such as hematocrit and interfering substances (eg, acetaminophen, ascorbic acid) that can affect the analytical performance of an SMBG system. According to ISO 15197:2013, hematocrit effects are acceptable if the mean difference between the test sample at different hematocrit levels and the respective control sample with a hematocrit value of 42% ± 2% is ≤10 mg/dL and ≤10% for blood glucose (BG) concentrations <100 mg/dL and ≥100 mg/dL, respectively. Hematocrit influence shall be described in the instructions for use if the system exceeds these criteria. Currently available SMBG systems show variations in the labeled hematocrit ranges. Different studies showed that the blood sample’s hematocrit value can affect SMBG measurement results.5,6,14
Therefore, in this study, the hematocrit influence was evaluated for a novel SMBG system and five other systems from different manufacturers with one test strip lot each based on ISO 15197:2013. For the systems, different hematocrit ranges were labeled by the manufacturers. Each system was tested within the hematocrit range indicated in the manufacturer’s labeling.
Materials and Methods
The study was conducted between May and June 2017 in compliance with the German Medical Devices Act at the Institut für Diabetes-Technologie Forschungs- und Entwicklungsgesellschaft mbH an der Universität Ulm (IDT), Germany. The study was approved by the responsible Ethics Committee and exempted from approval by the German Federal Institute for Drugs and Medical Devices. Informed consent forms were signed by all participants prior to the study procedures.
Subjects
In this study, 11 subjects (≥ 18 years) with diabetes mellitus type 1, type 2, or without diabetes were included to generate samples within each of the 3 specified glucose concentration intervals. The subjects’ anamnesis and medication as well as inclusion and exclusion criteria for study participation (eg, pregnancy or lactation period, severe acute disease, and/or chronic disease) were reviewed and interfering substances given in the manufacturers’ labeling were checked by the study physician.
Blood Glucose Monitoring Systems
The Accu-Chek® Instant (Roche Diabetes Care GmbH, Mannheim, Germany) system (system A) was evaluated and compared with five other SMBG systems (systems B to F) (Table 1). All systems are CE-marked. All systems are available on the European market. Systems A, D, and E utilize a glucose dehydrogenase (GDH) enzyme reaction on test strips and systems B, C, and F utilize a glucose oxidase (GOD) enzyme reaction on test strips. The systems show variations in their labeled hematocrit range (Table 1) with the broadest range indicated for system A (10-65%) and the smallest range indicated for system D (35-60%) and system F (30-55%).
Table 1.
Characteristics of the Six SMBG Systems Evaluated.
| System | Enzyme (Reagent system) | Hematocrit (%) | Measurement conditions |
|
|---|---|---|---|---|
| Temperature (°C) | Humidity (%) | |||
| A | Glucose dehydrogenase | 10-65 | 4-45 | 10-90 |
| B | Glucose oxidase | 15-65 | 5-50 | 10-90 |
| Ca | Glucose oxidase | 20-60 15-65 |
10-40 | 10-90 |
| D | Glucose dehydrogenase | 35-60 | 10-40 | <85 |
| E | Glucose dehydrogenase | 30-60 | 10-40 | <85 |
| F | Glucose oxidase | 30-55 | 10-44 | 10-90 |
The hematocrit range specified in the meter’s manual was 20-60%; the hematocrit range specified in the package insert of the test strips was 15-65%.
The meter and test strips of system A were procured by the manufacturer. Test strips of system A were provided by the manufacturer, who also funded the study, because at the time of study performance the CE-marked system was not yet available in the European market. Meter and test strips of systems B, C, D, and F were purchased from a local pharmacy. Meters and test strips of system E were provided by the distributor free of charge. All meters displayed plasma equivalent glucose concentrations. The systems were stored, adjusted, and used in accordance to the manufacturers’ labeling. Control measurements were performed daily prior to the test procedure and for each test strip vial according to the manufacturer’s labeling to ensure proper function of each system.
Laboratory Measurement Method
Laboratory measurements were performed in duplicate with a hexokinase method (Cobas Integra® 400 plus; Roche Instrument Center, Rotkreuz, Switzerland). Glucose values were provided in mg/dL. For the Cobas Integra 400 plus, conformity to the traceability requirements of ISO 1751115 were confirmed by the manufacturer. Trueness and precision of the analyzer were verified during the test procedures by regular internal and external quality control measures as required by the German national standard (Rili-BÄK).16 In addition, daily quality control measurements were performed applying IDT-internal standard operating procedures using National Institute of Standards and Technology (NIST) standard reference materials (SRM) 965.
Test Procedures
The evaluation was performed by trained study personnel in a laboratory setting with controlled room temperature (21.5 to 24.2°C) and humidity (37.4% to 44.4%) based on the procedures described in detail in ISO 15197:2013, clause 6.4.3. According to ISO 15197:2013, clause 6.4.1, three test strip lots shall be used for the evaluation of influence quantities. In this study, hematocrit effects were evaluated for one test strip lot of each system.
Venous blood samples were used for the evaluation. Samples were collected from different subjects to generate samples in three glucose concentration categories based on ISO 15197:2013: 30-50 mg/dL, 96-144 mg/dL, and 280-420 mg/dL. Samples were assigned to the respective category according to the mean glucose result of the laboratory method. Samples could be adjusted to achieve target glucose concentrations. For this purpose, venous blood samples were collected in lithium heparin tubes and adjustment of samples was performed by either incubation to allow for glycolysis or by glucose supplementation (stock solution: 40% glucose in 0.9% NaCl). The samples’ starting temperature was checked to be 23°C ± 5°C and temperature was maintained within ±3°C of the starting temperature during the test procedures.
For each glucose concentration category, individual samples (combination of glucose concentration and hematocrit value) with at least five different hematocrit levels were generated including a midlevel sample with a hematocrit value of 42% ± 2% (Table 2). The highest and the lowest hematocrit levels represent the upper and lower limit of a system’s acceptable hematocrit range as indicated in the manufacturer’s labeling (Table 1). For the generation of individual samples with different hematocrit values, an aliquot of the sample was centrifuged, plasma and packed cells were separated and defined volumes of plasma, cells and whole blood were mixed.
Table 2.
Evaluated hematocrit levels.
| Hematocrit (%) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| System | 10 | 15 | 20 | 25 | 30 | 35 | 42 | 50 | 55 | 60 | 65 |
| A | x | x | − | − | − | − | x | – | x | x | x |
| B | – | x | x | – | – | – | x | – | x | x | x |
| Ca | – | x | x | x | – | – | x | – | x | x | x |
| D | – | – | – | – | – | x | x | x | x | x | – |
| E | – | – | – | – | x | x | x | x | x | x | – |
| F | – | – | – | – | x | x | x | x | x | – | – |
For each system, at least five different hematocrit levels were evaluated including a midlevel sample with a hematocrit value of 42%. Lowest and highest hematocrit levels represent the upper and lower limits of the acceptable range of a system as indicated in the manufacturer’s labeling. Generated hematocrit values were within ±2% for the midlevel sample and within ±3% for the other samples, except for the samples at the lower and the upper limits (within +3% and –3%, respectively).
The hematocrit range specified in the meter’s manual was 20-60%; the hematocrit range specified in the package insert of the test strips was 15-65%.
The hematocrit value of each individual sample was determined in duplicate to verify the correct generation of hematocrit values (within ±2% for the midlevel sample and within ±3% for the other samples, except for the samples at the lower and the upper limits [within +3% and –3%, respectively]) (Table 2). For this purpose, samples were collected in heparinized capillaries, the capillaries were centrifuged and the hematocrit values were determined using an alignment chart.
Each individual sample was measured within 8 hours of sample collection. Ten consecutive measurements with each system (one test strip lot) were performed on each individual sample (combination of glucose concentration and hematocrit value) using 10 different meters. Samples were applied to the test strip directly from a syringe. Before and after the measurements with the SMBG systems, aliquots for measurements with the laboratory method were removed from the sample. Aliquots were centrifuged and measurements were performed on separated plasma. The difference between the first (aliquot collected before the measurements with the test systems) and second (aliquot collected after the measurements with the test systems) laboratory measurement result was checked to be ≤4 mg/dL at BG concentrations <100 mg/dL and ≤4% at BG concentrations ≥100 mg/dL to verify sample stability.
In addition, the pO2 was determined before and after the test procedure in each individual sample using a blood gas analyzer (Opti™ Check; OPTI Medical Systems Incorporation, Roswell, GA) to ensure a pO2 that is comparable to the pO2 in native capillary blood samples.17 In addition, the difference between the pO2 determined before and after the measurement procedure was checked to be within ±5 mmHg.
Data Analysis
Data Exclusions
Data were excluded from analysis for the following reasons: difference between the pO2 determined before and after the measurement procedure exceeded ±5 mmHg; the hematocrit value was outside the acceptable range.
Analysis of Hematocrit Influence
Data analysis was performed in mg/dL; for systems displaying results in mmol/L, values were converted (1 mmol/L = 18.02 mg/dL).
For each system, hematocrit influence was evaluated for each of the three glucose concentrations separately. The assessment of hematocrit influence with regard to the calculation of hematocrit effects is not clearly described in ISO 15197:2013 (differences in content between section 6.4.3.2 [acceptance criteria] and section 6.4.3.5 [data analysis and presentation of results]). In this study, hematocrit influence was assessed based on the requirements in section 6.4.3.5. For each individual sample (combination of glucose concentration and hematocrit value), the bias between the mean glucose result measured with the test system (mean of 10 measurements) and the mean result of the laboratory method was calculated. To assess the hematocrit influence for each glucose concentration, the difference between the bias at higher and lower hematocrit levels and the bias at the midlevel (42±2%) was calculated (normalized bias).
Results
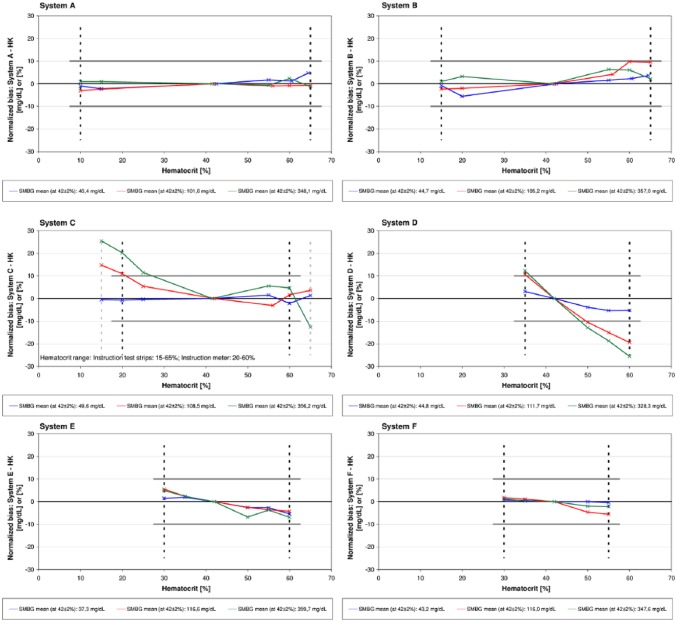
A complete presentation of results based on ISO 15197:2013 is provided in the supplements. The novel system A and the systems B, E and F showed with the tested reagent system lot ≤10% difference (normalized bias) between the bias at each hematocrit level and the bias at the midlevel at glucose concentrations ≥100 mg/dL and ≤10 mg/dL difference at glucose concentrations <100 mg/dL (Tables 3 and 4, Figure 1), thus fulfilling criteria of ISO 15197:2013 for the hematocrit ranges given in the manufacturers labeling. System C and D showed with the tested lot >10% difference (normalized bias) at glucose concentrations ≥100 mg/dL (Tables 3 and 4, Figure 1). System C exceeded the defined criteria at lower hematocrit levels (20%, 25%) when applying the hematocrit range specified in the manual of the meter (Table 1). System D exceeded with all investigated hematocrit levels the defined criteria at glucose concentrations ≥100 mg/dL (Tables 3 and 4, Figure 1).
Table 3.
Normalized Bias (mg/dL or %) for Each Glucose Concentration and Each Hematocrit Level.
| System A | |||||||
|---|---|---|---|---|---|---|---|
| Glucose concentration | 30-50 mg/dL | ||||||
| Hematocrit (%) | 10.0 | 15.0 | 42.5 | 55.0 | 60.5 | 64.5 | |
| Normalized bias (mg/dL) | –1.0 | –2.2 | 0.0 | 1.7 | 1.2 | 4.7 | |
| Glucose concentration | 96-144 mg/dL | ||||||
| Hematocrit (%) | 10.0 | 15.0 | 42.0 | 56.0 | 60.0 | 65.0 | |
| Normalized bias (%) | −3.1 | −2.3 | 0.0 | −1.0 | −0.9 | −0.7 | |
| Glucose concentration | 280-420 mg/dL | ||||||
| Hematocrit (%) | 10.0 | 15.0 | 41.5 | 55.0 | 60.0 | 65.0 | |
| Normalized bias (%) | 0.9 | 0.9 | 0.0 | −0.6 | 2.3 | −1.3 | |
| System B | |||||||
| Glucose concentration | 30-50 mg/dL | ||||||
| Hematocrit (%) | 15.0 | 20.0 | 42.5 | 55.0 | 60.5 | 64.5 | |
| Normalized bias (mg/dL) | −0.8 | −5.5 | 0.0 | 1.6 | 2.3 | 3.6 | |
| Glucose concentration | 96-144 mg/dL | ||||||
| Hematocrit (%) | 15.0 | 20.0 | 42.0 | 56.0 | 60.0 | 65.0 | |
| Normalized bias (%) | −2.3 | −2.0 | 0.0 | 4.2 | 9.7 | 9.5 | |
| Glucose concentration | 280-420 mg/dL | ||||||
| Hematocrit (%) | 15.0 | 20.0 | 41.5 | 55.0 | 60.0 | 65.0 | |
| Normalized bias (%) | 1.0 | 3.2 | 0.0 | 6.3 | 6.1 | 2.1 | |
| System Ca | |||||||
| Glucose concentration | 30-50 mg/dL | ||||||
| Hematocrit (%) | 15.0 | 20.0 | 25.0 | 42.0 | 55.0 | 60.0 | 65.0 |
| Normalized bias (mg/dL) | −0.5 | −0.7 | −0.4 | 0.0 | 1.6 | −2.1 | 1.4 |
| Glucose concentration | 96-144 mg/dL | ||||||
| Hematocrit (%) | 15.0 | 20.0 | 25.0 | 42.0 | 56.0 | 60.0 | 65.0 |
| Normalized bias (%) | 14.8 | 11.0 | 5.4 | 0.0 | −3.0 | 1.7 | 3.6 |
| Glucose concentration | 280-420 mg/dL | ||||||
| Hematocrit (%) | 15.0 | 20.0 | 25.0 | 41.5 | 55.0 | 60.0 | 65.0 |
| Normalized bias (%) | 25.4 | 20.2 | 11.5 | 0.0 | 5.6 | 4.7 | −12.7 |
| System D | |||||||
| Glucose concentration | 30-50 mg/dL | ||||||
| Hematocrit (%) | 35.0 | 42.0 | 50.0 | 55.0 | 60.0 | ||
| Normalized bias (mg/dL) | 3.1 | 0.0 | –3.8 | −5.3 | −5.2 | ||
| Glucose concentration | 96-144 mg/dL | ||||||
| Hematocrit (%) | 35.0 | 42.0 | 50.0 | 55.0 | 60.0 | ||
| Normalized bias (%) | 10.6 | 0.0 | –10.5 | −15.0 | −19.4 | ||
| Glucose concentration | 280-420 mg/dL | ||||||
| Hematocrit (%) | 35.0 | 42.0 | 50.0 | 55.0 | 60.0 | ||
| Normalized bias (%) | 12.2 | 0.0 | –12.9 | −18.7 | −25.5 | ||
| System E | |||||||
| Glucose concentration | 30-50 mg/dL | ||||||
| Hematocrit (%) | 30.0 | 35.0 | 42.0 | 50.0 | 55.0 | 60.0 | |
| Normalized bias (mg/dL) | 1.5 | 2.0 | 0.0 | −2.6 | −2.7 | −5.4 | |
| Glucose concentration | 96-144 mg/dL | ||||||
| Hematocrit (%) | 30.0 | 35.0 | 42.0 | 50.0 | 55.0 | 60.0 | |
| Normalized bias (%) | 5.5 | 2.4 | 0.0 | −2.5 | −3.5 | −4.2 | |
| Glucose concentration | 280-420 mg/dL | ||||||
| Hematocrit (%) | 30.0 | 35.0 | 42.0 | 50.0 | 55.0 | 60.0 | |
| Normalized bias (%) | 4.9 | 2.4 | 0.0 | −6.8 | −3.7 | −6.9 | |
| System F | |||||||
| Glucose concentration | 30-50 mg/dL | ||||||
| Hematocrit (%) | 30.0 | 35.0 | 42.0 | 50.0 | 55.0 | ||
| Normalized bias (mg/dL) | 0.8 | 0.5 | 0.0 | 0.0 | −0.4 | ||
| Glucose concentration | 96-144 mg/dL | ||||||
| Hematocrit (%) | 30.0 | 35.0 | 42.0 | 50.0 | 55.0 | ||
| Normalized bias (%) | 1.8 | 1.1 | 0.0 | −4.6 | −5.5 | ||
| Glucose concentration | 280-420 mg/dL | ||||||
| Hematocrit (%) | 30.0 | 35.0 | 42.0 | 50.0 | 55.0 | ||
| Normalized bias (%) | 1.3 | 0.2 | 0.0 | −2.0 | −2.1 | ||
Normalized bias was calculated using a hexokinase laboratory measurement method.
The hematocrit range specified in the meter’s manual was 20-60%; the hematocrit range specified in the package insert of the test strips was 15-65%.
Table 4.
Summarized Presentation of Results for Each of the Investigated Hematocrit Levels.
| Hematocrit (%) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| System | 10 | 15 | 20 | 25 | 30 | 35 | 42 | 50 | 55 | 60 | 65 |
| A | No | No | — | — | — | — | NA | — | No | No | No |
| B | — | No | No | — | — | — | NA | — | No | No | No |
| Ca | — | Yes | Yes | Yes | — | — | NA | — | No | No | Yes |
| D | — | — | — | — | — | Yes | NA | Yes | Yes | Yes | — |
| E | — | — | — | — | No | No | NA | No | No | No | — |
| F | — | — | — | — | No | No | NA | No | No | — | — |
Hematocrit influences are indicated with “yes” and show a calculated normalized bias >10% and >10 mg/dL for glucose concentrations ≥100 mg/dL and <100 mg/dL, respectively. No hematocrit influences are indicated with “no” and show a calculated normalized bias ≤10% and ≤10 mg/dL for glucose concentrations ≥100 mg/dL and <100 mg/dL, respectively. The normalized bias was calculated by determining the difference between the bias at higher and lower hematocrit levels and the bias at the midlevel (42 ± 2%).
The hematocrit range specified in the meter’s manual was 20-60%; the hematocrit range specified in the package insert of the test strips was 15-65%.
Figure 1.
The difference between the bias at each hematocrit level and the bias at the midlevel (42 ± 2%) was calculated (normalized bias) for the following concentration categories: 30-50 mg/dL, 96-144 mg/dL, 280-420 mg/dL. The bias was calculated using a hexokinase laboratory method. Lines connecting individual data points are provided for a simplified visualization and do not represent measured data. Each system was tested within the labeled hematocrit range (indicated as dashed line). Solid gray lines show ≤10% and ≤10 mg/dL limits at glucose concentrations ≥100 mg/dL and at glucose concentrations <100 mg/dL, respectively.
Discussion
In this study, evaluation of hematocrit influence was performed for a novel SMBG system and five systems with different hematocrit ranges with one test strip lot each under standardized controlled laboratory conditions based on ISO 15197:2013, clause 6.4.3.
The blood sample’s hematocrit value has long been recognized as a potential factor that can affect BG measurements.5,6,14 ISO 15197:2013 describes testing procedures and acceptance criteria for the premarket evaluation of hematocrit influence on SMBG measurement results; however, data obtained in a setting based on ISO 15197:2013 are rare.
In this study, all six systems were tested within the hematocrit range indicated in the respective manufacturer’s labeling. Labeled hematocrit ranges varied between the investigated systems with the broadest range indicated for system A (10-65%) and the smallest range indicated for system D (35-60%) and system F (30-55%). According to ISO 15197:2013, hematocrit influence shall be described in the instructions for use if it exceeds the specified limits (≤10 mg/dL and ≤10%). In this study, two systems (C and D) showed remarkable hematocrit influence at medium and higher glucose concentrations (96-144 mg/dL and 280-420 mg/dL). For system C, different hematocrit ranges were indicated in the manual of the meter (20-60%) and in the package inserts of the test strips (15-65%). This system showed overestimated glucose measurements at low hematocrit values (20%, 15%). When applying the upper hematocrit level as indicated in the package insert of the test strips (65%), underestimated glucose measurements were found at higher glucose concentrations (280-420 mg/dL). System D showed overestimated glucose measurements at lower hematocrit levels (35%) and underestimated glucose measurements at higher hematocrit levels (≥50%). To ensure that patients can rely on correct measurement results, manufacturers should provide high quality instructions for use that are correct and consistent in the system characteristics and information provided such as the indicated hematocrit range.
Variations in the hematocrit influence on BG measurements depending on the glucose concentration and depending on the hematocrit levels have been observed in previous studies.5,6,14,18 The blood sample’s hematocrit value can affect the glucose reaction on test strips. Increasing or decreasing hematocrit values increase or decrease blood viscosity which decelerates or accelerates the diffusion of blood components into the reaction chamber of the test strip.4,18,19 Today, many SMBG systems include compensating technologies to correct the measurement for hematocrit interferences.19,20
Regarding the clinical impact of different hematocrit levels, hematocrit reference ranges are 35%-47% and 40%-52% in adult women and men, respectively, and 42%-82% in newborns.21 Hematocrit values between approximately 10% and 73% were observed in hospitalized and nonhospitalized patients.22 To prevent a potential hematocrit influence on measurement results, patients and health care professionals should choose an SMBG system with adequate hematocrit range. As hematocrit depends on a variety of factors, for example, health conditions including anemia or pregnancy, patients and health care professionals should be aware of the specific patient’s needs.
When interpreting results of this study it must be considered that the study design has several limitations which can contribute to the outcome of a given system. ISO 15197:2013, clause 6.4.1, requires the evaluation of three different test strip lots for each system. The results shall be presented separately for every test strip lot. In this study, only one test strip lot was used for each system. It cannot be ruled out that additional test strip lots per system may have shown differing results between test strip lots. Specific information about differences in hematocrit influence between test strip lots is scarce, but the limited information suggests clinically irrelevant differences.6 However, some of the results obtained in this study showed remarkably biased results in dependence of hematocrit. Because system A was not yet commercially available, it was procured by the manufacturer, and test strips were randomly chosen. Materials for system E were provided by the distributor. Both cases deviate from how patients with diabetes typically obtain their meters and test strips. According to ISO 15197:2013, clause 6.4.2, venous blood is the preferred sample for the evaluation of influence quantities. However, three systems investigated in this study (system B, C, and F) are only indicated for use with capillary blood samples. In addition, blood samples from only a small number of different subjects were included.
Conclusion
In this study, remarkable hematocrit influence was observed in two systems with the evaluated reagent system lot, although the systems were tested within the hematocrit range indicated in the respective manufacturer’s labeling. Thus, hematocrit variations are an important SMBG error source that is particularly relevant in patients with increased or decreased hematocrit values, for example, critically ill patients. To choose an adequate system, patients should be aware that there are variations in the labeled hematocrit range. In addition, manufacturers should ensure that the hematocrit range indicated in their systems’ labeling is correct.
Supplementary Material
Acknowledgments
We would like to thank the study personnel and other employees of the IDT who conducted the study and helped prepare the manuscript.
Footnotes
Abbreviations: BG, blood glucose; CE, Conformité Européenne; GDH, glucose dehydrogenase; GOD, glucose oxidase; ISO, International Organization for Standardization; NIST, National Institute of Standards and Technology; pO2, partial pressure of oxygen; SMBG, self-monitoring of blood glucose; SRM, standard reference material.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AH and SW are employees of Roche Diabetes Care GmbH.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by a grant from Roche Diabetes Care GmbH, Mannheim, Germany.
References
- 1. Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986. [DOI] [PubMed] [Google Scholar]
- 2. Hönes J, Müller P, Surridge N. The technology behind glucose meters: test strips. Diabetes Technol Ther. 2008;10(suppl 1):s10-s26. [Google Scholar]
- 3. Vashist SK, Zheng D, Al-Rubeaan K, Luong JH, Sheu FS. Technology behind commercial devices for blood glucose monitoring in diabetes management: a review. Anal Chim Acta. 2011;703:124-136. [DOI] [PubMed] [Google Scholar]
- 4. Yoo EH, Lee SY. Glucose biosensors: an overview of use in clinical practice. Sensors (Basel). 2010;10:4558-4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kilpatrick ES, Rumley AG, Myint H, Dominiczak MH, Small M. The effect of variations in haematocrit, mean cell volume and red blood cell count on reagent strip tests for glucose. Ann Clin Biochem. 1993;30:485-487. [DOI] [PubMed] [Google Scholar]
- 6. Tang Z, Lee JH, Louie RF, Kost GJ. Effects of different hematocrit levels on glucose measurements with handheld meters for point-of-care testing. Arch Pathol Lab Med. 2000;124:1135-1140. [DOI] [PubMed] [Google Scholar]
- 7. Baumstark A, Schmid C, Pleus S, Haug C, Freckmann G. Influence of partial pressure of oxygen in blood samples on measurement performance in glucose-oxidase-based systems for self-monitoring of blood glucose. J Diabetes Sci Technol. 2013;7:1513-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ginsberg BH. Factors affecting blood glucose monitoring: sources of errors in measurement. J Diabetes Sci Technol. 2009;3:903-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bamberg R, Schulman K, MacKenzie M, Moore J, Olchesky S. Effect of adverse storage conditions on performance of glucometer test strips. Clin Lab Sci. 2005;18:203-209. [PubMed] [Google Scholar]
- 10. Tang Z, Louie RF, Lee JH, Lee DM, Miller EE, Kost GJ. Oxygen effects on glucose meter measurements with glucose dehydrogenase- and oxidase-based test strips for point-of-care testing. Crit Care Med. 2001;29:1062-1070. [DOI] [PubMed] [Google Scholar]
- 11. Tang Z, Louie RF, Payes M, Chang KC, Kost GJ. Oxygen effects on glucose measurements with a reference analyzer and three handheld meters. Diabetes Technol Ther. 2000;2:349-362. [DOI] [PubMed] [Google Scholar]
- 12. International Organization for Standardization. In Vitro Diagnostic Test Systems—Requirements for Blood-Glucose Monitoring Systems for Self-Testing in Managing Diabetes Mellitus. 2nd ed. International Organization for Standardization; 2013. [Google Scholar]
- 13. International Organization for Standardization. In Vitro Diagnostic Test Systems—Requirements for Blood-Glucose Monitoring Systems for Self-Testing in Managing Diabetes Mellitus. International Organization for Standardization; 2015. [Google Scholar]
- 14. Arens S, Moons V, Meuleman P, Struyf F, Zaman Z. Evaluation of Glucocard Memory 2 and Accutrend sensor blood glucose meters. Clin Chem Lab Med. 1998;36:47-52. [DOI] [PubMed] [Google Scholar]
- 15. International Organization for Standardization. In Vitro Diagnostic Medical Devices—Measurement of Quantities in Biological Samples—Metrological Traceability of Values Assigned to Calibrators and Control Materials. International Organization for Standardization; 2003. [Google Scholar]
- 16. Bundesärztekammer. Richtlinie der Bundesärztekammer zur Qualitätssicherung laboratoriumsmedizinischer Untersuchungen [Guideline of the German Medical Association on the Quality Assurance of Laboratory Medical Examinations]. Deutsches Ärzteblatt. 2014;111:1583-1618. [Google Scholar]
- 17. Freckmann G, Schmid C, Baumstark A, Pleus S, Link M, Haug C. Partial pressure of oxygen in capillary blood samples from the fingertip. J Diabetes Sci Technol. 2013;7:1648-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vanavanan S, Santanirand P, Chaichanajarernkul U, et al. Performance of a new interference-resistant glucose meter. Clin Biochem. 2010;43:186-192. [DOI] [PubMed] [Google Scholar]
- 19. Teodorczyk M, Cardosi M, Setford S. Hematocrit compensation in electrochemical blood glucose monitoring systems. J Diabetes Sci Technol. 2012;6:648-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Demircik F, Ramljak S, Hermanns I, Pfutzner A, Pfutzner A. Evaluation of hematocrit interference with MyStar extra and seven competitive devices. J Diabetes Sci Technol. 2015;9(2):262-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lothar T. Labor und Diagnose: Indikation und Bewertung von Laborbefunden für die medizinische Diagnostik [Laboratory and Diagnosis: Indication and Evaluation of Laboratory Findings for Medical Diagnostics]. 8th ed. Frankfurt, Germany: TH-Books-Verlags-Gesellschaft; 2012. [Google Scholar]
- 22. Lyon ME, Lyon AW. Patient acuity exacerbates discrepancy between whole blood and plasma methods through error in molality to molarity conversion: “Mind the gap!” Clin Biochem. 2011;44:412-417. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.