Abstract
Background:
In type 1 diabetes mellitus (T1DM), patients play an active role in their own care and need to have the knowledge to adapt decisions to their daily living conditions. Artificial intelligence applications can help people with type 1 diabetes in decision making and allow them to react at time scales shorter than the scheduled face-to-face visits. This work presents a decision support system (DSS), based on glucose prediction, to assist patients in a mobile environment.
Methods:
The system’s impact on therapeutic corrective actions has been evaluated in a randomized crossover pilot study focused on interprandial periods. Twelve people with type 1 diabetes treated with insulin pump participated in two phases: In the experimental phase (EP) patients used the DSS to modify initial corrective decisions in presence of hypoglycemia or hyperglycemia events. In the control phase (CP) patients were asked to follow decisions without knowing the glucose prediction. A telemedicine platform allowed participants to register monitoring data and decisions and allowed endocrinologists to supervise data at the hospital. The study period was defined as a postprediction (PP) time window.
Results:
After knowing the glucose prediction, participants modified the initial decision in 20% of the situations. No statistically significant differences were found in the PP Kovatchev’s risk index change (–1.23 ± 11.85 in EP vs –0.56 ± 6.06 in CP). Participants had a positive opinion about the DSS with an average score higher than 7 in a usability questionnaire.
Conclusion:
The DSS had a relevant impact in the participants’ decision making while dealing with T1DM and showed a high confidence of patients in the use of glucose prediction.
Keywords: decision support, diabetes, m-health, glucose prediction
People with type 1 diabetes mellitus (T1DM) have to play an active role in their own care and need to have the knowledge to make decisions adapted to their daily living conditions. They need to perform a constant learning process about the disease and about how daily conditions (insulin administration, meals schedule and composition, physical activity, illness) affect blood glucose (BG) levels. All these elements have an impact on the success of metabolic control (avoidance of hyper- and hypoglycemia events).1 In such scenario, artificial intelligence (AI) applications make it possible to support patients’ decisions in any scenario of their daily living and open the door to react at time scales shorter than scheduled face-to-face visits.2
In the past decade, the diabetes management paradigm has been transformed by the combination of continuous glucose monitoring (CGM) and insulin pump data.3,4 Real-time CGM systems provide new alternatives of care and allow registering changes in glucose levels 24 hours a day. Instead, the amount of information available to both patients and clinicians makes decision-making more complex. This scenario raises the need to exploit the information in such a way that the users can benefit from decision-making tools for both health care professionals5 and patients,6 from enhanced graphical data visualization7 or from the automatic generation of warnings and reminders.8,9
Advances in the area of mobile communication for health care (mHealth) have created applications to ease daily monitoring of most parameters that affect the disease.10 However, the experiences that provide AI-based decision support directly to patients are quite limited in number. An early example was the DIACRONO portable microcomputer, created in the late 1980s to aid patients in ambulatory decision making.11 Participants registered self-monitoring data (glucose, insulin, diet, physical activity, and other events) and received automated feedback about insulin therapy adjustments. DIACRONO implemented Skyler’s algorithms12 in a simplified rule-based system that was updated in real time with self-monitoring data to provide patients with real-time advice.
In the following years, the availability of commercial portable devices and of always-on communications made it possible to send the data through telemedicine platforms and to execute the automatic analysis algorithms13,14 in computer servers with significant HW/SW resources. Patients usually receive feedback through alarms and/or advice in the form of SMS or email messages.15-17 Other experiences that support patients’ decision are insulin bolus calculators18-20 and glucose predictors that calculate near-future BG values.21 The prediction of glucose from CGM data is useful at different scenarios like the artificial pancreas22,23 or the prevention of hypoglycemia events.24,25
This article presents a decision support system (DSS) for people with type 1 diabetes that is based on a glucose predictor and evaluates its impact on their daily decision making.
Methods
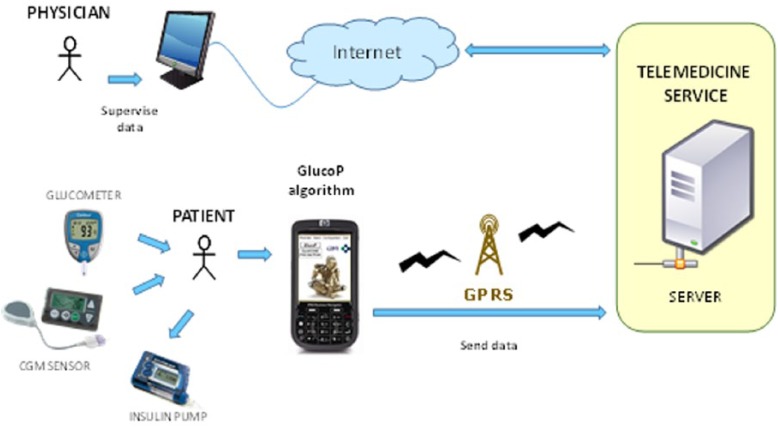
The DSS is designed to help patients in real time while performing therapeutic corrective actions such as (1) administration of insulin bolus to correct hyperglycemia or (2) intake of carbohydrates in case of hypoglycemia. The DSS, named GlucoP, is implemented in a portable device and is executed as a stand-alone application. The DSS is connected to a telemedicine platform to allow remote supervision by endocrinologists at the hospital. Figure 1 shows the architecture of the system. The final aim of the system is to improve metabolic control.
Figure 1.
System architecture, DSS, which provides real-time glucose prediction to patients and telemedicine platform to allow physicians’ remote supervision.
Glucose Predictor
The glucose predictor is based on an artificial neural network (ANN) trained with CGM profiles.24 The network architecture has three layers with a first layer of 10 neurons and a second layer of 5 neurons. Layers have a sigmoidal transfer function, with totally connected and feed forward neurons. The dataset for training and validation includes data from 9 patients who wore the CGM system intermittently for 72 hours/week over a 4-week period. The network inputs are the recent glucose measurements (up to 20 minutes before the current time) and the network output is the glucose prediction for a specific prediction horizon (PH). A Levenberg-Marquardt back-propagation algorithm was applied for the training of the ANN. In general terms, the predictor provided a good accuracy (root mean square error [RMSE] of 17.45 ± 5.44 mg/dl for a PH = 30 minutes and 9.74 ± 2.71 mg/dl for a PH = 15 minutes).
In this work we selected a PH = 30 minutes as we considered that it provides enough anticipation to make corrective decisions able to revert hypoglycemia or hyperglycemia risk situations.
Mobile DSS
The glucose predictor was integrated in a mobile application that was implemented in Java using the Mysaifu virtual machine and the Windows Mobile 6.0 Professional operating system. The graphical user interface (GUI) allows the patient to manually register the last 5 CGM (measurements are available every 5 minutes). The application runs the ANN algorithm to calculate the glucose prediction and presents it to the patient both numerically and graphically (Figure 2).
Figure 2.
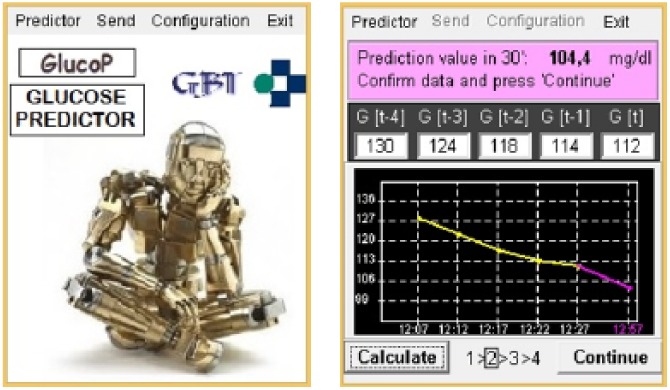
DSS graphical user interface.
Once the patient checks the glucose prediction, he or she can use this information to make a decision regarding either an insulin bolus administration (to correct hyperglycemia situations) or a carbohydrate intake (to correct hypoglycemia situations). The corrective action is registered by the patient in the mobile application (Figure 3). The patient’s decision can be retrospectively evaluated both by the patient or the physician in charge, so that the patient’s education can be reinforced in case it is necessary.
Figure 3.
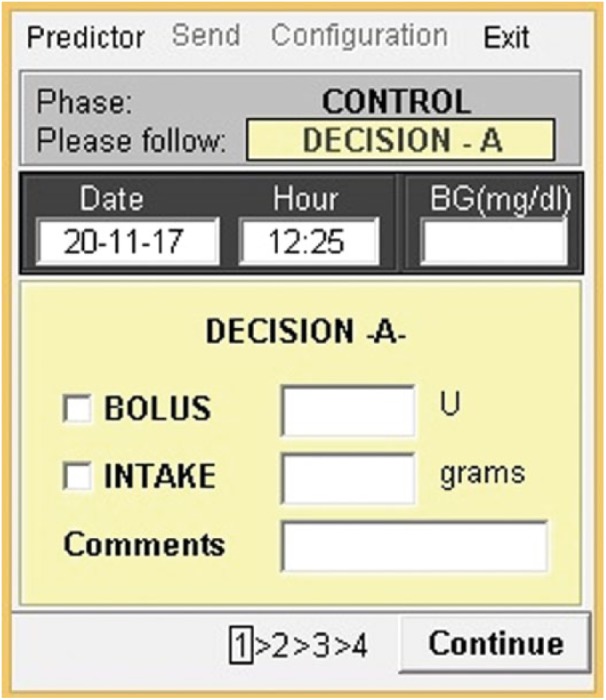
Registration of the patient’s corrective actions.
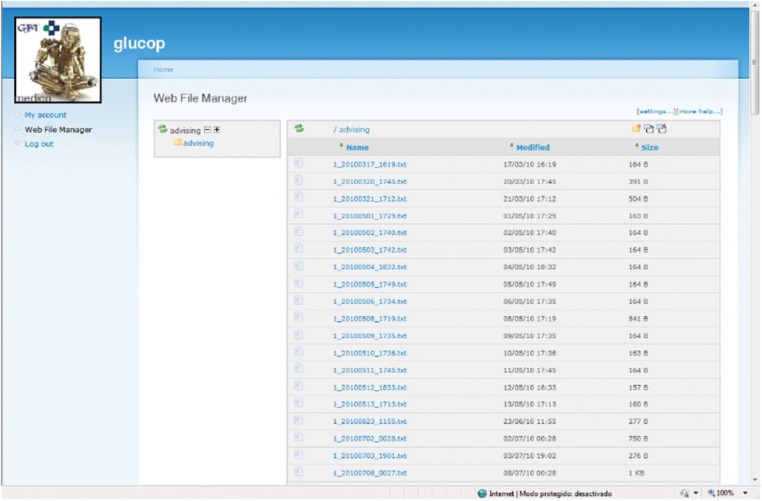
Telemedicine Service
The telemedicine platform receives and stores data associated to glucose predictions: CGM values, BG readings, and insulin/diet corrective actions. A web application allows remote supervision by endocrinologists at the hospital (Figure 4).
Figure 4.
Web interface for physicians: visualization of monitoring data registered.
Clinical Evaluation
The DSS was evaluated in a pilot study to analyze if it could provide helpful information to improve the patient’s decision making. In the pilot study participants had to correct interprandial situations (around 2 hours after lunch or at least 1-2 hours before dinner) of either hyperglycemia or hypoglycemia. The clinical evaluation was designed as a randomized crossover pilot study. We evaluated the impact of knowing the glucose prediction in the patient’s corrective decisions, its impact in glycemic control, and the DSS GUI usability.
Twelve people with type 1 diabetes (6 male, 6 female) with an average age of 41.97 ± 9.30 years (27.12-56.82) and 16.80 ± 6.63 years of T1DM duration participated in the study. Participants had followed a CSII treatment for more than 1 year. All of them completed the study.
CGM data were collected with a Guardian® Real-Time CGM sensor (Medtronic-Minimed, Northridge, CA) whose sampling period is 5 minutes or a Paradigm® Veo™ (Medtronic-Minimed, Northridge, CA) for participants who had the CSII system. The Animas® 2020™ (Animas corporation, West Chester, PA) and the Accu-Check® Spirit™ (Roche Diagnostics, Indianapolis, IN) were also used to retrieve administered insulin bolus. All the participants followed a training session where the DSS tool and the experiment protocol were explained. Participants wore the CGM monitor during both the experimental (EP) and control (CP) phases. Physicians could follow the study and check data using the telemedicine service. Participants were randomly assigned to CP or EP the first week of the study. After a one-week washout period, they were assigned to the other phase.
The week before beginning the trial, each patient carried a CGM sensor (72 hours). The registered profiles were used to validate the ANN prediction model trained in a generic way (section 2.A). If the precision (measured in terms of RMSE) was lower than 20% of the ANN model precision (for PH = 30),24 the ANN was retrained, including the registered profiles of the particular patient. In most cases (9/12) the generic network was used.
Experimental Phase (1 Week)
Every day the participants had to use the DSS system at an interprandial moment (Figure 5). In this period, the patient had to measure a capillary BG and decide whether a corrective action (insulin administration or carbohydrates intake) was required if indicated by an altered BG level (A decision). After that, the patient had to review the CGM measurements provided by the monitor and check the glucose prediction with the DSS. Due to the unavailability of an automatic connection with the CGM monitor, the participants manually entered the CGM measurements in the DSS application. Once the glucose prediction was known by the patient, he or she had to decide either to maintain the initial decision (A decision), or to perform a different one (B decision). Participants had to register both A and B decisions in the application, so that it could be evaluated whether the patient changed his or her initial decision after knowing the glucose prediction and the effect of either A or B decision in metabolic control.
Figure 5.
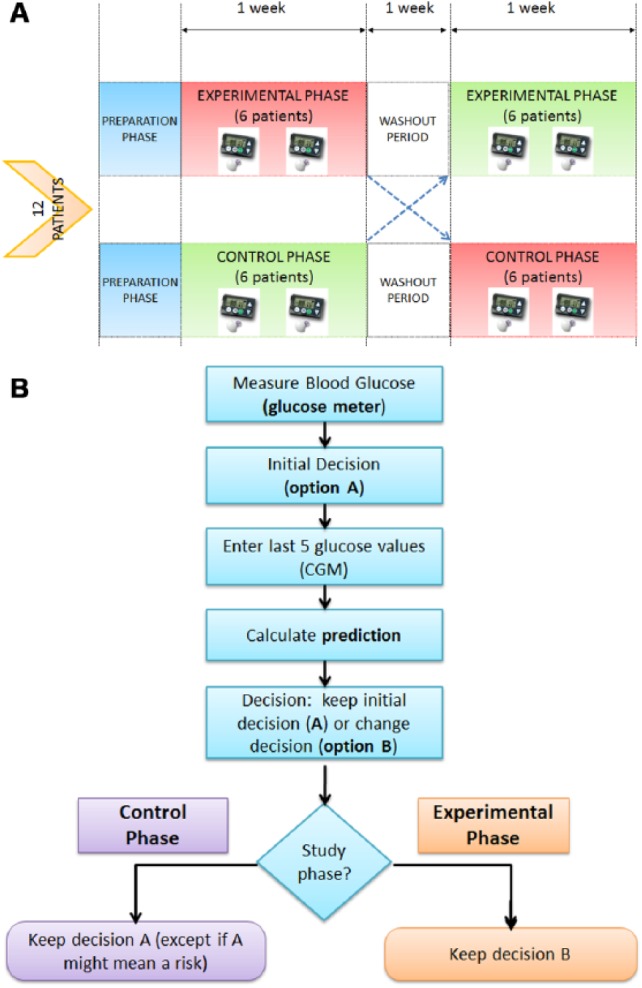
(a) Study design. (b) User actions according to the study phase.
Control Phase (1 Week)
The protocol is similar to the one followed in the EP (see Figure 5), but the patient was requested to always execute the initial decision (A). However, for safety reasons, the patient had the option to modify the initial decision and to perform B decision if he or she considered that decision A could mean a health risk.
Methodology of Evaluation
The following parameters were calculated:
- Percentage of times when the patient modifies his or her initial decision.
- Parameters of glycemic control associated to the postprediction (PP) time window, which has been defined as the interval from the glucose prediction execution up to the next meal intake (and preprandial BG measurement), limited to a maximum duration of 3 hours. In the PP period, the following parameters are considered: (1) average daily glucose; (2) initial CGM value when the PP period starts; (3) difference between subcutaneous glucose values at the initial and final points of the PP period (ΔG); (4) Kovatchev’s risk index26 change (ΔRI) calculated for 1 hour before the start and 1 hour before the end of the PP.
- Usability Questionnaire Quis 7.27 This questionnaire allows registering the user’s opinion about 84 items classified into 9 categories: previous experience with the system, previous experience with ICT technologies, general impression, screen factors, terminology and system feedback, learning factors, system capabilities, technical manuals, and multimedia. Each area measures the user’s overall satisfaction with that facet of the GUI, as well as the factors that make up that facet, on a 9-point scale. The questionnaire is designed to be configured according to the needs of each interface analysis, by including only the sections that are of interest to the user.
Results
All the participants finished the study. No patient had hypoglycemia during the PP period. Five participants have been discarded in the provided results due to errors in the manual registration of CGM measurements. The results consider 64 situations when the patients evaluated their glycemic control and registered decisions related to corrective actions before and after activating the glucose prediction provided by the DSS.
Average daily subcutaneous glucose was 152.49 ± 60.94 mg/dl in EP versus 143.68 ± 49.24 mg/dl in CP. The subcutaneous glucose at the start of the PP period was 148.00 ± 61.36 mg/dl in EP versus 141.44 ± 49.00 mg/dl in CP.
After knowing the glucose prediction, the participants decided to modify the initial decision (A) in 20% of the situations (13/64), 46% of the modified decisions (6/13) were done in the EP while 54% (7/13) in the CP (see Table 1). Even though the participants were instructed to follow decision A in the CP, in all the cases but one (in the CP), participants decided to execute the registered postprediction decision B (Table 1).
Table 1.
Situations When the Participants Decided to Modify the Initial Decision After Knowing the Glucose Prediction
| Phase | BG (t) | CGM (t) | PANN (t+30) | Initial decision (A) |
Postpredicction decision (B) |
Executed decision | Changes in metabolic state (CGM) |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bolus (ui) | Intake (gr) | Bolus (ui) | Intake(gr) | ΔGPP | GiniPP | GfinPP | |||||
| CP | 89 | 106 | 97 | 0 | 40 | 0 | 60 | B | 142 | Normal | Hyper |
| CP | 145 | 172 | 177 | 0 | 10 | 1 | 0 | B | −62 | Normal | Normal |
| CP | 72 | 93 | 92.8 | 0 | 0 | 0 | 10 | B | −11 | Normal | Normal |
| CP | 227 | 205 | 202.7 | 2 | 0 | 4 | 0 | A | −75 | Hyper | Normal |
| CP | 246 | 230 | 229.4 | 2.65 | 0 | 2.35 | 0 | B | −56 | Hyper | Normal |
| CP | 189 | 182 | 189.8 | 0 | 0 | 0.95 | 0 | B | −52 | Hyper | Normal |
| CP | 277 | 220 | 211 | 1.5 | 0 | 1.8 | 0 | B | −52 | Hyper | Normal |
| EP | 222 | 186 | 190.5 | 2 | 0 | 3 | 0 | B | −20 | Hyper | Normal |
| EP | 152 | 191 | 190.5 | 0 | 0 | 1 | 0 | B | 53 | Hyper | Hyper |
| EP | 188 | 232 | 248.6 | 2 | 0 | 3 | 0 | B | 26 | Hyper | Hyper |
| EP | 176 | 180 | 192.6 | 0 | 0 | 1 | 0 | B | −62 | Hyper | Normal |
| EP | 288 | 282 | 276.7 | 1 | 0 | 2.5 | 0 | B | −60 | Hyper | Hyper |
| EP | 190 | 144 | 137.8 | 1.25 | 0 | 1.75 | 0 | B | −60 | Normal | Normal |
BG, glucose meter value at time t; PANN, predicted glucose at t + 30; ΔG: increment of glucose in the PP time window. Metabolic state: hypoglycemia G ≤ 70; normal 70 < G < 180; hyperglycemia G ≥ 180.
In 6 out of 7 situations when participants in the CP decided to change their initial decision, the patients’ actions improved their metabolic state and they achieved a normal glucose level at the end of the study window. In 4 out of 6 situations participants in the EP changed their initial decision and the patients’ actions improved their metabolic state (ΔGPP). In 3 out of 6 situations they achieved a normal glucose level at the end of the study window.
There were no statistically significant differences in the average subcutaneous glucose during the PP period (142.35 ± 59.28 mg/dl in EP vs 142.02 ± 46.03 mg/dl in CP). The average ΔG in both EP and CP was –8.88 ± 53.13 mg/dl, with –13.84 ± 54.13 mg/dl in EP versus –3.64 ± 52.29 mg/dl in CP. No statistically significant differences were found in the Kovatchev’s risk index change ΔRI (–1.23 ± 11.85 in EP vs –0.56 ± 6.06 in CP).
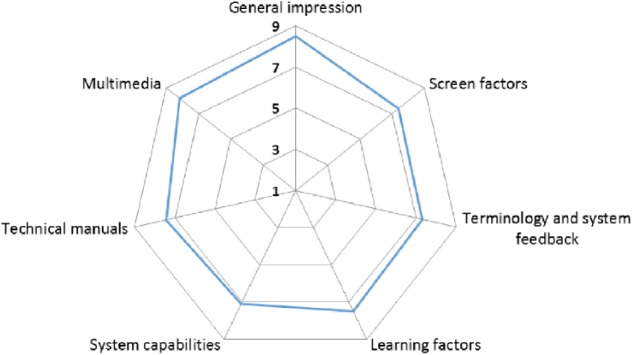
The usability questionnaire was filled by 7 participants. Figure 6 shows the results grouped into categories. Patients had a positive opinion about the tool with average score higher than 7 in all the categories. The “general impression” category had the higher score (8.48): participants declared to have a pleasant experience while using the DSS. They positively assessed aspects such as the GUI design and the format and screen sequences. Regarding the terminology used in the application (messages, warnings, and general information provided in the GUI), the participants considered that they achieved the expected purpose. Answers by patients related to learning factors show that the application is easy to learn and use. The category with a lower score was the system’s capacity (7.10), but it also obtained a high score. The assessment of technical manuals regarding utility and reading comprehension was positive. The quality of graphical design (images and colors) was also positive.
Figure 6.
Usability questionnaire results.
Discussion
One limitation of this study is that the participants had to manually register CGM measurements in the DSS application. This caused several transcription errors, and we had to eliminate data from 5 patients in the final results. The errors could have been avoided with an automatic CGM reading and data entering to the glucose prediction algorithm integrated in the DSS. For this reason it is considered that the DSS utility is conditioned to the availability of the automatic CGM reading.
The participants decided to modify their initial decision in a relevant number of situations after knowing the glucose prediction (20%). This means that the DSS provides useful data to improve patients’ decision making while managing T1DM. The patients had a positive subjective assessment of the tool.
The changes in glycemic control between the EP and the CP are not statistically significant, which might be explained by the small number of patients who participated in the clinical study and also by the fact that, in several cases, participants decided to execute the postprediction decision B in the CP phase. In 10 out of 13 cases the patients achieved better control (Table 1). In view of these results, it can be said that knowing the glucose prediction has a positive effect on patients’ corrective actions and is able to improve their glycemic control. Six changes in the initial decision were carried out in the CP. These changes contributed to improve the average of glycemic control during the control phase and justify that no significant differences appear between the two groups.
We consider that the DSS based on a glucose prediction algorithm is an interesting tool to create a proactive system. The use of automatic CGM readings for 24 hours without patient intervention would allow implementing an automatic alarm system triggered when the glucose prediction anticipates glucose levels out of the defined normal range.
We expect that a prediction model that includes exogenous variables such as meal information and administered insulin boluses will improve the model precision. However, the use of additional parameters, such as meals, would increase the patients’ workload significantly and could generate typing errors, causing undesirable side effects.
Proactivity and prediction accuracy could be increased by integrating other physiological parameters (ie, heart rate, sleep quality, physical activity, etc) that have a close relationship with metabolic control and start to be available in commercial wearable devices that register them in a seamless way.
Conclusion
The DSS had a relevant impact on patients’ decision making while dealing with type 1 diabetes. Patients’ confidence in the glucose prediction was high, and they had a positive subjective assessment of the tool. No clear benefit in glucose control was found, but no hypoglycemic events were observed.
Acknowledgments
We would like to thank the volunteers who participated in the study for their collaboration and support in this research.
Footnotes
Abbreviations: AI, artificial intelligence; ANN, artificial neural network; BG, blood glucose; CGM, continuous glucose monitoring; CP, control phase; DSS, decision support system; EP, experimental phase; GUI, graphical user interface; HW, hardware; PH, prediction horizon; PP, postprediction; RMSE, root mean square error; SW, software; T1DM, type 1 diabetes mellitus.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by Spanish grant FITCloop (PI14/00109, PI14/00010), co-funded by FEDER, and a mobility grant from CIBER.
ORCID iDs: Gema García-Sáez  https://orcid.org/0000-0002-8396-8185
https://orcid.org/0000-0002-8396-8185
M. Elena Hernando  https://orcid.org/0000-0001-6182-313X
https://orcid.org/0000-0001-6182-313X
References
- 1. American Diabetes Association. Standards of medical care in diabetes 2018. Diabetes Care. 2018;41(suppl 1):s38-s50. [DOI] [PubMed] [Google Scholar]
- 2. Rigla M, García-Sáez G, Pons B, Hernando ME. Artificial intelligence methodologies and their application to diabetes [published online ahead of print May 1, 2017]. J Diabetes Sci Technol. doi: 10.1177/1932296817710475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hanaire H. Continuous glucose monitoring and external insulin pump: towards a subcutaneous closed loop. Diabetes Metab. 2006;32:534-538. [DOI] [PubMed] [Google Scholar]
- 4. Bergenstal RM, Tamborlane WV, Ahmann A, et al. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010; 363:311-320. [DOI] [PubMed] [Google Scholar]
- 5. Montani S, Bellazzi R, Portinale L, d’Annunzio G, Fiocchi S, Stefanelli M. Diabetic patients management exploiting case-based reasoning techniques. Comput Methods Programs Biomed. 2000;62:205-218. [DOI] [PubMed] [Google Scholar]
- 6. Zisser H, Palerm CC, Bevier WC, Doyle FJ, III, Jovanovic L. Clinical update on optimal prandial insulin dosing using a refined run-to-run control algorithm. J Diabetes Sci Technol. 2009;3:487-491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Popow C, Horn W, Rami B, Schober E. VIE-DIAB: a support program for telemedical glycaemic control. In: 9th Conference on Artificial Intelligence Proceedings Dordrecht, Netherlands: Springer; 2003:350-354. [Google Scholar]
- 8. Hanauer DA, Wentzell K, Laffel N, Laffel LM. Computerized Automated Reminder Diabetes System (CARDS): e-mail and SMS cell phone text messaging reminders to support diabetes management. Diabetes Technol Ther. 2009;11:99-106 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Farmer AJ, Gibson OJ, Dudley C, et al. A randomized controlled trial of the effect of real-time telemedicine support on glycemic control in young adults with type 1 diabetes (ISRCTN 46889446). Diabetes Care. 2005;28(11):2697-2702. [DOI] [PubMed] [Google Scholar]
- 10. Arnhold M, Quade M, Kirch W. Mobile applications for diabetics: a systematic review and expert-based usability evaluation considering the special requirements of diabetes patients age 50 years or older. J Med Internet Res. 2014;16(4):e104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gómez EJ, del Pozo F, Serrano R. DIACRONO: a new portable microcomputer system for diabetes management. In: Proceedings, 9th IEEE Annual Conference of the Engineering in Medicine and Biology Society. New York, NY: IEEE Press; 1987:1231-1232. [Google Scholar]
- 12. Skyler JS, Skyler DL, Seigler DE, O’Sullivan MJ. Algorithms for adjustment of insulin dosage by patients who monitor blood glucose. Diabetes Care. 1981;4:311-318. [DOI] [PubMed] [Google Scholar]
- 13. Hernandon ME, García-Sáez G, Gómez EJ, del Pozo F. Intelligent alarms integrated in a multi-agent architecture for diabetes management. Trans Inst Meas Control. 2004;26:185-200. [Google Scholar]
- 14. Caballero-Ruiz E, García-Sáez G, Rigla M, Villaplana M, Pons B, Hernando ME. A web-based clinical decision support system for gestational diabetes: automatic diet prescription and detection of insulin needs. Int J Med Inform. 2017;102:35-49. [DOI] [PubMed] [Google Scholar]
- 15. Bellazzi R, Arcelloni M, Bensa G, et al. Design, methods, and evaluation directions of a multi-access service for the management of diabetes mellitus patients. Diabetes Technol Ther. 2003;5:621-629. [DOI] [PubMed] [Google Scholar]
- 16. Gómez EJ, Hernando ME, Vering T, et al. The INCA system: a further step towards a telemedical artificial pancreas. Inf Technol Biomed IEEE Trans. 2008;12:470-479. [DOI] [PubMed] [Google Scholar]
- 17. Peleg M, Shahar Y, Quaglini S, et al. Assessment of a personalized and distributed patient guidance system. Int J Med Inform. 2017;101:108-130. [DOI] [PubMed] [Google Scholar]
- 18. Pesl P, Herrero P, Reddy M, et al. An advanced bolus calculator for type 1 diabetes: system architecture and usability results. IEEE J Biomed Heal Informatics. 2016;20:11-17. [DOI] [PubMed] [Google Scholar]
- 19. Barnard K, Parkin C, Young A, Ashraf M. Use of an automated bolus calculator reduces fear of hypoglycemia and improves confidence in dosage accuracy in patients with type 1 diabetes mellitus treated with multiple daily insulin injections. J Diabetes Sci Technol. 2012;6:144-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brown D, Aldea A, Harrison R, Martin C, Bayley I. Temporal case-based reasoning for type 1 diabetes mellitus bolus insulin decision support [published online ahead of print October 3, 2017]. Artif Intell Med. 2017. doi: 10.1016/j.artmed.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 21. Albisser AM. A graphical user interface for diabetes management that integrates glucose prediction and decision support. Diabetes Technol Ther. 2005;7:264-273. [DOI] [PubMed] [Google Scholar]
- 22. Sparacino G, Zanderigo F, Corazza S, Maran A, Facchinetti A, Cobelli C. Glucose concentration can be predicted ahead in time from continuous glucose monitoring sensor time-series. Biomed Eng IEEE Trans. 2007;54:931-937. [DOI] [PubMed] [Google Scholar]
- 23. Oviedo S, Vehí J, Calm R, Armengol J. A review of personalized blood glucose prediction strategies for T1DM patients. Int J Numer Method Biomed Eng. 2017;33:e2833. [DOI] [PubMed] [Google Scholar]
- 24. Perez-Gandia C, Facchinetti A, Sparacino G, et al. Artificial neural network algorithm for online glucose prediction from continuous glucose monitoring. Diabetes Technol Ther. 2010;12:81-88. [DOI] [PubMed] [Google Scholar]
- 25. Buckingham B, Chase HP, Dassau E, et al. Prevention of nocturnal hypoglycemia. Diabetes Care. 2010;33:1013-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kovatchev BP, Clarke WL, Breton M, Brayman K, McCall A. Quantifying temporal glucose variability in diabetes via continuous glucose monitoring: mathematical methods and clinical application. Diabetes Technol Ther. 2005;7:849-862. [DOI] [PubMed] [Google Scholar]
- 27. Chin JP, Diehl VA, Norman KL. Development of an instrument measuring user satisfaction of the human-computer interface. In: CHI ’88 Proceedings of the SIGCHI Conference on Human Factors in Computing Systems . New York, NY: ACM; 2009:213-218. [Google Scholar]