Abstract
Background
Rapid Response Teams (RRTs) are groups of healthcare providers that are used by many hospitals to respond to acutely deteriorating patients admitted to the wards. We sought to identify outcomes of patients assessed by RRTs outside standard working hours.
Methods
We used a prospectively collected registry from two hospitals within a single tertiary care-level hospital system between May 1, 2012, and May 31, 2016. Patient information, outcomes, and RRT activation information were stored in the hospital data warehouse. Comparisons were made between RRT activation during daytime hours (0800–1659) and nighttime hours (1700–0759). The primary outcome was in-hospital mortality, analyzed using a multivariable logistic regression model.
Results
A total of 6023 RRT activations on discrete patients were analyzed, 3367 (55.9%) of which occurred during nighttime hours. Nighttime RRT activation was associated with increased odds of mortality, as compared with daytime RRT activation (adjusted OR 1.34, 95% CI 1.26–1.40, P = 0.02). The time periods associated with the highest odds of mortality were 0600–0700 (adjusted OR 1.30, 95% CI 1.09–1.61) and 2300–2400 (adjusted OR 1.34, 95% CI 1.01–1.56). Daytime RRT activation was associated with increased odds of intensive care unit admission (adjusted OR 1.40, 95% CI 1.31–1.50, P = 0.02). Time from onset of concerning symptoms to RRT activation was shorter among patients assessed during daytime hours (P < 0.001).
Conclusions
Acutely deteriorating ward patients assessed by an RRT at nighttime had a higher risk of in-hospital mortality. This work identifies important shortcomings in health service provision and quality of care outside daytime hours, highlighting an opportunity for quality improvement.
Electronic supplementary material
The online version of this article (10.1186/s13054-018-2005-1) contains supplementary material, which is available to authorized users.
Keywords: Rapid Response Team, Intensive care unit, Critical care, Resuscitation
Background
Patients admitted to the hospital wards are at risk of deterioration, and recognition of deterioration can be delayed [1, 2]. This puts these patients at increased risk of morbidity and mortality. Previous work has demonstrated that various adverse events, including cardiac arrest, unplanned intensive care unit (ICU) admission, and unexpected death, are usually preceded by objective signs of deterioration, often over the course of many hours [2]. In order to monitor such cases, many hospitals have instituted Rapid Response Teams (RRTs), which are a group of interprofessional critical care providers, often led by an intensivist, who immediately respond to ward patients experiencing clinical deterioration [3, 4]. RRTs do not routinely patrol wards, but are rather summoned by physicians or other health professionals who are caring for these patients, or in some instances, by patients’ families. Common causes for activation include respiratory distress, vital sign abnormalities (such as hypotension), or decreased level of consciousness; but healthcare providers are encouraged to activate RRT assistance if they are at all concerned about a patient’s condition [5].
The existing literature on the use of RRTs in clinical practice highlights many potential benefits. Most consistently, RRT use has been shown to reduce the incidence of in-hospital cardiac arrest, which has been attributed to early identification and treatment of at-risk patients [6–8]. Furthermore, a dose-response relationship between increasing RRT review rates and decreasing in-hospital cardiac arrest rates has been demonstrated [9]. Although the findings are less robust, RRTs have also been shown to improve overall hospital mortality, with particular benefit in reducing the incidence of unexpected in-hospital death [10]. RRTs are also thought to be useful for early involvement in end-of-life care and ensuring that patient treatment occurs within patient-specific limits of care [11].
At most centers, RRT availability exists at all times during the day. However, there may be differences in the amount and level of training of staff who are available at night [12]. Most hospital wards in Canada (including our centers) have reduced nursing-to-patient ratios at night, which has been shown to be associated with poorer performance in various quality-of-care indicators [13]. Additionally, attending physicians are less likely to be in the hospital during nighttime hours [14, 15]. There is often diurnal variability in RRT staffing. Whereas during the day the RRT may be staffed by a single dedicated attending physician, during the night RRTs may be more commonly composed of residents or emergency department (ED) physicians who are on shift at the time. There is also diurnal variation in patient-physician ratios, as well as in patient throughput (namely patient discharge) in the ICU, with a higher ratio and greater throughput found during the day [16], as is the case at our center. It is also well-accepted that staffing levels and expertise have an inverse relationship with patient outcomes [17]. We therefore attempted to determine whether nighttime RRT activation was associated with worse patient outcomes.
Methods
Ethics approval for this study was obtained from The Ottawa Health Science Network Research Ethics Board (protocol number 20170016-01H).
Study design, setting, and subjects
The study was performed at The Ottawa Hospital, a Canadian tertiary care hospital network that consists of two individual campuses, each with its own tertiary-level ICU. The Ottawa Hospital is a 1163-bed facility than handles over 160,000 emergency visits, 50,000 inpatients, and roughly 35,000 surgical cases annually. Each hospital has approximately 28–30 ICU beds. These are mixed medical and surgical ICUs. We analyzed prospectively collected data contained in the Ottawa Hospital Data Warehouse, which is a health administrative database widely used in previous research [18–20]. It contains information from several of the hospital’s information systems, including the patient registration system, clinical data repository, case-costing system, and patient discharge abstracts. Extensive assessments of data quality were performed during database development, and quality assurance initiatives to ensure completeness and accuracy of the data are conducted regularly [18].
We included all patients who met all of the following eligibility criteria: (a) over the age of 18 years; (b) admitted to either campus of The Ottawa Hospital; and (c) received RRT activation between May 1, 2012, and May 31, 2016. Patients with incomplete demographic or outcome data were excluded. We also excluded cases of routine scheduled RRT follow-up. We did not have data related to cases of cardiac arrest (because they involve a different response team). Patients were categorized by time of initial RRT activation. Patients with multiple activations during their admission were categorized on the basis of the time of their initial activation. Activations were classified as occurring during either daytime hours (0800–1659) or nighttime hours (1700–0759). At The Ottawa Hospital, RRTs during daytime hours are composed of an attending critical care specialist, a registered nurse, and a respiratory therapist. Nighttime RRT activations are managed by a resident physician on the critical care service, also with support from a registered nurse and respiratory therapist. An on-call intensivist is available but is not in-house. The same model is used at both sites. During daytime hours, most wards are under the direct supervision of an attending faculty physician. During nighttime hours, most hospital wards are under the supervision of resident physicians, with an attending physician available on-call. The RRT responds only to inpatients, outpatients experiencing distress (e.g., in radiology and endoscopy suites), or patients and family members requiring immediate care in hospital clinics or waiting rooms. The RRT does not respond to patients being assessed in the ED (who have not yet been admitted). Specific criteria for RRT activation at our center have been published previously [21]. They include concerns surrounding airway patency, tachypnea, systolic blood pressure ≤ 90 mmHg or ≥ 200 mmHg, decreased level of consciousness, and hypoxia, but healthcare providers are encouraged to activate the RRT for any reason of concern, even in the absence of objective changes in vital signs or laboratory values.
Data collection
Patient information, including demographic data, comorbidities, previous ED visits, previous hospital admissions, and previous ICU admissions in the year prior to the index admission, was collected by registration clerical staff at the time of admission and stored in the data warehouse [18]. Information related to RRT activation is gathered by the RRT nursing staff at the time of patient assessment, and it is also stored in the data warehouse. This includes the most recent vital signs and laboratory values at the time of activation, reason for activation, admitting service, and whether the patient was admitted to the ICU. Data on latency time from onset of concerning symptoms or signs (e.g., hypotension, altered mental status) to RRT activation are also estimated by the RRT during assessment for quality improvement purposes. Outcome at hospital discharge (including death or disposition) and lengths of hospital and ICU stay are also stored in the data warehouse.
The primary outcome was in-hospital mortality, comparing patients assessed during daytime vs. nighttime hours. Secondary outcomes included ICU admission following RRT assessment and overall hospital length of stay.
Statistical analysis
All statistical analyses were performed with commercially available statistical software packages (R version 3.3.3 [R Foundation for Statistical Computing, Vienna, Austria] and IBM SPSS Statistics version 24.0 [IBM, Armonk, NY, USA]). Data are presented as mean values (with SD) or as medians (with IQR), where indicated. Descriptive statistics were employed for between-group comparisons between daytime and nighttime hours, using Student’s t test for continuous variables and the χ2 test for categorical variables. In evaluating the outcomes of in-hospital mortality and ICU admission after RRT activation, we used multivariable logistic regression modeling to adjust for potential confounders, including patient characteristics (age, sex, comorbidities, comorbidity index, previous ED visits in the past year, previous hospital admissions in the past year, and previous ICU admissions in the past year), number of RRT activations, latency to RRT activation, most recent laboratory investigations at the time of RRT activation, and vital signs at the time of RRT activation. We also performed a secondary analysis using time of day as the exposure variable (with 1200–1300 as a reference, as reported previously [22]) to evaluate changes in adjusted mortality over the course of the day. We analyzed disposition of survivors at hospital discharge using a similarly constructed multivariable logistic regression model but restricted to patients originally admitted from home, assuming that patients initially admitted from peripheral acute or long-term care centers were likely to return to those centers. Adjusted ORs with 95% CIs and adjusted P values are provided. A P value ≤ 0.05 was taken to represent statistical significance.
Results
Patient cohort
The RRT was activated for 6132 discrete patients during the study period. Of these, 109 patients were excluded because of incomplete data, leaving 6023 patients in the study. A total of 2656 patients (44.1%) had calls that occurred during daytime hours (0800–1659), and 3367 (55.9%) had calls that occurred during nighttime hours (1700–0759).
Characteristics of the included patients, along with between-group comparisons between daytime and nighttime RRT activation are depicted in Table 1. The mean age of patients with RRT activation during daytime hours was 67.3 years (SD 16.7), whereas the mean age of those with RRT activation during nighttime hours was 67.9 years (SD 16.7) (P = 0.18). There was no significant difference in admission source (home, acute care facility transfer, long-term care facility transfer) between groups. There were no significant differences between groups in the median number of previous ED visits, hospital admissions, or ICU admissions in the past year.
Table 1.
Characteristics of patients with daytime and nighttime Rapid Response Team activation
| Daytime hours (0800–1659) (n = 2656) |
Nighttime hours (1700–0759) (n = 3367) |
P value | |
|---|---|---|---|
| Age, years, mean (SD) | 67.3 (16.7) | 67.9 (16.7) | 0.18 |
| Male sex, n (%) | 1377 (53.0) | 1816 (53.9) | 0.91 |
| Admission source, n (%) | 0.97 | ||
| Home | 1854 (69.8) | 2357 (70.0) | |
| Acute care facility transfer | 296 (11.1) | 379 (11.3) | |
| Long-term care facility transfer | 240 (9.0) | 306 (9.1) | |
| Unknown | 266 (10.0) | 325 (9.6) | |
| Comorbidities, n (%) | |||
| Congestive heart failure | 419 (15.8) | 553 (16.4) | 0.54 |
| Arrhythmia | 587 (22.1) | 780 (23.2) | 0.37 |
| Valvular disease | 80 (3.0) | 100 (3.0) | 0.90 |
| Peripheral vascular disease | 174 (6.6) | 235 (7.0) | 0.54 |
| Hypertension | 1007 (37.9) | 1081 (32.1) | < 0.001 |
| Chronic obstructive pulmonary disease | 421 (15.9) | 533 (15.8) | 0.93 |
| Diabetes mellitus | 1183 (44.5) | 1499 (44.5) | 0.89 |
| Renal failure | 262 (9.9) | 352 (10.5) | 0.48 |
| Liver disease | 172 (6.5) | 189 (5.6) | 0.15 |
| Metastatic cancer | 376 (14.2) | 523 (15.5) | 0.15 |
| Elixhauser comorbidity score, mean (SD) | 9.0 (8.3) | 9.1 (8.5) | 0.51 |
| Emergency department visits in past year, median (IQR) | 1 (0–2) | 1 (0–2) | 0.20 |
| Hospital admissions in past year, median (IQR) | 0 (0–1) | 0 (0–1) | 0.35 |
| ICU admissions in past year, median (IQR) | 0 (0–0) | 0 (0–0) | 0.39 |
ICU Intensive care unit
Boldface font indicates statistical significance
Characteristics of RRT activations
Between-group differences in characteristics of RRT activation calls are depicted in Table 2. There was a trend toward lower RRT use (number of RRT activations per 1000 hospital admissions, also termed the RRT “dose” [23]) during nighttime hours, though this difference was not statistically significant. Vital signs and laboratory values at the time of RRT activation are displayed. Reasons for RRT activation were significantly different between daytime and nighttime RRT calls (P < 0.001). Respiratory distress and tachycardia/arrhythmias were more common causes of activation at nighttime, whereas altered level of consciousness was more common during daytime hours. Calls arising from general nonspecific concerns about the patient were more common during the daytime. Nighttime RRT activation was associated with longer latency from symptom onset (P < 0.001). More RRT activations occurred within 1 h of symptom onset during daytime than during nighttime hours (81.0% vs. 73.2%, P < 0.001). There was no difference between groups in time to RRT response. A comparison of admitting services at the time of RRT activation is shown in Table 3. Some services (such as orthopedic surgery and hematology) more commonly requested RRT assistance during daytime hours, whereas others (such as neurosurgery and medical oncology) more often activated the RRT at nighttime.
Table 2.
Characteristics of initial Rapid Response Team call for patients with daytime and nighttime Rapid Response Team activation
| Daytime hours (0800–1659) (n = 2656) |
Nighttime hours (1700–0759) (n = 3367) |
P value | |
|---|---|---|---|
| Number of RRT activations during admission, median (IQR) | 1 (1–1) | 1 (1–1) | 0.24 |
| Patients with multiple RRT activations during admission, n (%) | 473 (17.8) | 629 (18.7) | 0.38 |
| RRT activations/1000 admissions | 21.5 | 19.3 | 0.12 |
| Most recent vital signs | |||
| Systolic blood pressure, mmHg, mean (SD) | 121 (29) | 124 (32) | 0.11 |
| Diastolic blood pressure, mmHg, mean (SD) | 70 (15) | 71 (16) | 0.05 |
| Heart rate, beats/minute, mean (SD) | 98 (30) | 102 (30) | < 0.001 |
| Temperature, °C, mean (SD) | 36.8 (0.7) | 36.8 (0.7) | 0.28 |
| Oxygen saturation, %, mean (SD) | 94 (5) | 94 (5) | 0.66 |
| Most recent laboratory values | |||
| White blood cell count, ×109/L, median (IQR) | 10.1 (7.1–14.4) | 10.7 (7.4–14.9) | < 0.01 |
| Hemoglobin, g/L, mean (SD) | 106.8 (22.9) | 108.1 (22.6) | 0.03 |
| Platelets, ×109/L, mean (SD) | 225.4 (126.6) | 229.6 (134.7) | 0.22 |
| Potassium, mmol/L, mean (SD) | 4.1 (0.7) | 4.1 (0.7) | 0.87 |
| Creatinine, μmol/L, median (IQR) | 83 (60–142) | 84 (60–142) | 0.48 |
| Urea, mmol/L, median (IQR) | 7.4 (4.7–12.9) | 7.6 (4.9–12.9) | 0.18 |
| Lactate, mmol/L, median (IQR) | 2.2 (1.7–3.1) | 2.2 (1.7–3.1) | 0.92 |
| Albumin, g/L, mean (SD) | 27.1 (6.8) | 26.9 (6.8) | 0.20 |
| INR, median (IQR) | 1.2 (1.1–1.4) | 1.2 (1.1–1.4) | 0.62 |
| Reason for call, n (%) | < 0.001 | ||
| Respiratory distress | 604 (22.7) | 927 (27.5) | |
| Tachycardia/bradycardia/arrhythmia | 395 (14.9) | 643 (19.1) | |
| Altered level of consciousness | 485 (18.3) | 537 (15.9) | |
| Hypotension | 356 (13.4) | 388 (11.5) | |
| Hypertension | 40 (1.5) | 122 (3.6) | |
| Airway concern | 90 (3.4) | 124 (3.6) | |
| Seizure | 30 (1.1) | 30 (0.9) | |
| Worried about patient | 336 (12.7) | 304 (9.1) | |
| Other | 152 (5.7) | 152 (4.5) | |
| Unknown | 168 (6.3) | 140 (4.1) | |
| Latency from concerning symptom/sign onset to RRT activation | < 0.001 | ||
| < 1 h | 2151 (81.0) | 2402 (73.2) | |
| 1–4 h | 437 (16.4) | 667 (20.3) | |
| 5–8 h | 11 (0.4) | 109 (3.3) | |
| 9–12 h | 8 (0.3) | 26 (0.8) | |
| 13–18 h | 6 (0.2) | 22 (0.7) | |
| 19–24 h | 4 (0.2) | 18 (0.6) | |
| 25–36 h | 4 (0.2) | 9 (0.3) | |
| 37–48 h | 2 (0.1) | 2 (0.1) | |
| > 48 h | 33 (0.4) | 29 (0.9) | |
| RRT response time, minutes, median (IQR) | 5 (3–7) | 5 (3–7) | 0.74 |
Abbreviations: RRT Rapid Response Team, ICU Intensive care unit, INR International normalized ratio
Boldface font indicates statistical significance
Table 3.
Admitting service of patients with daytime and nighttime Rapid Response Team activation
| Daytime hours (0800–1659) (n = 2656) |
Nighttime hours (1700–0759) (n = 3367) |
P value | |
|---|---|---|---|
| Service, n (%) | < 0.001 | ||
| Surgical services | 0.06 | ||
| General surgery | 285 (10.7) | 382 (11.4) | |
| Orthopedic surgery | 240 (9.0) | 248 (7.4) | |
| Vascular surgery | 116 (4.4) | 136 (4.0) | |
| Neurosurgery | 85 (3.2) | 152 (4.5) | |
| Thoracic surgery | 81 (3.1) | 103 (3.1) | |
| Obstetrics and gynecology | 66 (2.5) | 73 (2.2) | |
| Urology | 64 (2.4) | 82 (2.4) | |
| Otolaryngology | 27 (1.0) | 42 (1.3) | |
| Plastic surgery | 4 (0.2) | 9 (0.3) | |
| Nonsurgical Services | < 0.001 | ||
| General internal medicine | 715 (26.9) | 934 (27.7) | |
| Hematology | 208 (7.8) | 197 (5.9) | |
| Intensive care | 144 (5.4) | 140 (4.2) | |
| Medical oncology | 112 (4.2) | 230 (6.8) | |
| Family medicine | 103 (3.9) | 130 (3.9) | |
| Nephrology | 92 (3.5) | 112 (3.3) | |
| Radiation oncology | 83 (3.1) | 79 (2.4) | |
| Neurology | 76 (2.9) | 96 (2.9) | |
| Respirology | 52 (2.0) | 91 (2.7) | |
| Psychiatry | 49 (1.8) | 53 (1.6) | |
| Cardiology | 31 (1.2) | 48 (1.4) | |
| Rehabilitation | 16 (0.6) | 19 (0.6) | |
| Gastroenterology | 7 (0.3) | 11 (0.3) | |
Boldface font indicates statistical significance
Nighttime RRT activation and patient mortality
Logistic regression using multivariable models with adjustment for patient variables demonstrated that nighttime RRT activation was associated with significantly higher odds of in-hospital mortality (adjusted OR 1.34, 95% CI 1.26–1.40) (Table 4). The logistic regression model, including all covariables, is depicted in Additional file 1. Daytime RRT activation was associated with significantly higher odds of ICU admission (adjusted OR 1.40, 95% CI 1.30–1.48). No differences in overall hospital length of stay (P = 0.92) or disposition of survivors (P = 0.47) were found between groups. The frequency of RRT calls, with associated risk of mortality across the day, is depicted graphically in Fig. 1. Of note, the frequency of RRT activation increases dramatically at approximately 0800, shortly after the beginning of daytime hours. Two statistically significant time periods were associated with increased adjusted odds of in-hospital mortality: 0600–0700 (adjusted OR 1.30, 95% CI 1.09–1.61) and 2300–2400 (adjusted OR 1.34, 95% CI 1.01–1.56). A sensitivity analysis excluding patients admitted to the ICU was performed (Additional file 2). There were no differences in mortality or hospital length of stay between groups.
Table 4.
Outcomes in patients with daytime and nighttime Rapid Response Team activation
| Daytime hours (0800–1659) (n = 2656) |
Nighttime hours (1700–0759) (n = 3367) |
Adjusted OR (95% CI) | AdjustedP value | |
|---|---|---|---|---|
| In-hospital mortality, n (%) | 772 (29.6) | 1061 (32.0) | 1.34a (1.26–1.40) | 0.02 a |
| Admitted to ICU, n (%) | 760 (30.5) | 948 (27.8) | 1.40a (1.30–1.48) | 0.02 b |
| Hospital length of stay, days, median (IQR) | 14 (7–29) | 14 (7–28) | 0.92 | |
| Survivors discharged to home, n (%)b | 903 (48.7) | 1131 (48.0) | 1.01a (0.91–1.07) | 0.47 |
ICU Intensive care unit
aOR and P value were adjusted for age, sex, comorbidities, previous emergency department visits in the past year, previous hospital admissions in the past year, previous ICU admissions in the past year, total number of Rapid Response Team (RRT) calls, latency to RRT activation, laboratory values at the time of RRT activation, vital signs at the time of RRT activation, reason for RRT activation, and admitting service, using multivariate logistic regression
bAnalysis includes only patients originally admitted from home
Boldface font indicates statistical significance
Fig. 1.
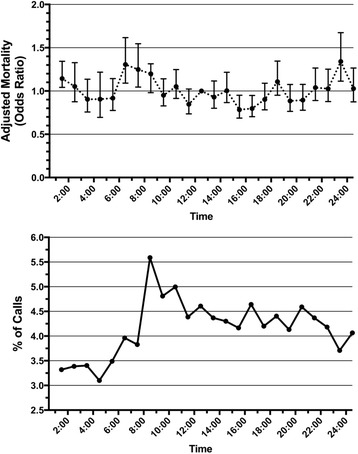
Percentage of total calls and adjusted in-hospital mortality by time of day of Rapid Response Team activation (using 1200 as a reference)
Discussion
We found that RRT activation during nighttime hours (1700–0759) was associated with an increased risk of in-hospital mortality compared with daytime (0800–1659) activation. The two time periods associated with highest risk of in-hospital mortality were 0600–0700 and 2300–2400. These times correspond to workforce shift changes at our institution, particularly with regard to ward nursing care. Patients who were seen by the RRT during nighttime were less commonly admitted to the ICU. These findings were evident even when adjusted for many possible confounding patient factors. Nighttime RRT activations had increased latency from symptom onset compared with daytime RRT activations.
Our results demonstrate variability in outcomes with RRT activation over the course of the day. Our findings are consistent with a recent large database study in the United States [22] and a smaller study in Australia [24], which also found increased mortality with overnight RRT activation and an increased adjusted risk of mortality for activations between 0700 and 0800. These studies also found that the lowest frequency of calls occurred in the early morning (immediately preceding the time associated with the highest risk of mortality) and that the highest frequency of calls occurred between 0800 and 0900. They suggested that this may be due to workforce shift changes, and the investigators hypothesized that there may be increased delay to RRT activation overnight, but they did not have this data available to them. Our study adds to this work not only by supporting these findings but also by adjusting for more confounding variables. We found that nighttime RRT activation was in fact associated with prolonged delays to activation. There are likely many reasons for this, including reduced patient/nursing ratios, coupled with fewer and less-experienced physicians (on both the ward as well as the RRT), on hospital wards at night. Similar work has demonstrated that RRT activation more commonly occurs after nurse shift change and vital sign checks [25, 26], which may explain the dramatic increase in frequency after 0800.
Understanding why these differences in outcomes occur is paramount in identifying strategies for quality improvement in recognition of deteriorating patients on hospital wards, as well as for improvement in RRT function. Diurnal differences in patient outcomes have been reported in various disease processes, including cardiac arrest, trauma, and myocardial infarction [27–29]. As mentioned, this may be related to known differences in the amount and experience of staffing (both physicians and nurses) overnight, particularly in critical care settings [14, 16, 17, 30, 31]. Although we found no differences in patient characteristics or most recent vital signs or laboratory work between daytime and nighttime RRT activation, patients admitted to the ICU during early morning hours tend to be older and sicker than those admitted later in the day [32]. We found that RRT activation during the daytime was more likely to result in ICU admission than activations occurring during nighttime. Because delayed ICU admission is a factor associated with worse outcomes in critically ill patients [33], moving these patients to a critical care setting may have partially accounted for the improved survival seen in activations during daytime hours. This may have been affected by bed availability because fewer ICU beds are available at night [34].
Importantly, we found that nighttime RRT activation was associated with prolonged latency from the onset of concerning symptoms and signs. Delays in RRT activation have been shown to be associated with worse outcomes [35, 36]. These increased delays may occur because of impaired recognition and/or monitoring of clinical deterioration. Diurnal variation in shift times and durations also influence staff performance, which has been shown to decline during the night [37]. In tertiary care centers, most nighttime services are under the supervision of medical residents, who not only have less experience than their attending physician counterparts but also often work many consecutive hours, with concomitant sleep deprivation. These factors together result in poorer cognitive performance [38, 39] and may contribute to reduced recognition of clinical deterioration among admitted patients. Additionally, these residents may be busy performing other clinical duties, owing to a reduced number of personnel available during nighttime hours [16]. In keeping with this, we found a trend toward lower “RRT dose” during nighttime hours, though the difference was not statistically significant. Increasing RRT dose has been associated with a reduction in adverse events among hospitalized patients [23], and lower dosing has previously been demonstrated during nighttime hours [40].
This study has several strengths, including a large sample size as well as data related to various patient and RRT activation variables. The main outcomes were assessed using logistic regression models to control for the influence of important patient factors. However, there are several limitations that hinder the generalizability of our results. First, the observational nature of our dataset allows detection of associations but does not reveal the causal pathways that lead to increased mortality risk after nighttime RRT activations. Second, although the data were gathered from two different hospitals, the hospitals are located within the same city and use the same RRT model, which may limit the generalizability of the results. However, as mentioned, our findings are similar to those of recent studies in the United States [22] and Australia [24]. Although we attempted to control for many confounding patient and RRT factors, including laboratory values and vital signs, there are some factors that we were unable to include in the model, such as the degree of acute physiological derangement. We were also unable to compute the “Score to Door Time,” which is an indicator of quality of healthcare delivery, for patients requiring ICU admission [41]. Finally, although we were able to gather data related to the latency of RRT response, we did not have data related to time until treatment, which may have been delayed during nighttime RRT activations.
Conclusions
We found that nighttime RRT activation was associated with increased mortality, decreased ICU admission, and increased latency to activation in hospitalized patients with acute deterioration. These findings underscore important shortcomings in both the recognition of these patients by ward staff and the function of RRTs, and they serve as an important avenue for future quality improvement.
Additional files
Multivariate logistic regression analysis of factors associated with in-hospital mortality (n = 6023). Multivariate logistic regression variables, with associated ORs and 95% CIs. (DOCX 136 kb)
Time of rapid response team activation and patient outcomes among patients not admitted to the intensive care unit. Subgroup analysis of outcomes among patients not admitted to the intensive care unit. (DOCX 77 kb)
Acknowledgements
The authors thank Deanna Rothwell and Dr. Alan J. Forster (The Ottawa Hospital Data Warehouse) for assistance.
Funding
None to report.
Availability of data and materials
The datasets generated and analyzed are not publicly available, owing to patient privacy considerations, but they are available from the corresponding author on reasonable request.
Abbreviations
- ED
Emergency department
- ICU
Intensive care unit
- INR
International normalized ratio
- RRT
Rapid Response Team
Authors’ contributions
SMF, PMR, and KK designed the study. PMR, KM, JS, PT, and KK gathered the data. SMF, SMB, DCS, DKH, and KK analyzed the data. SMF, PMR, SMB, DCS, KM, JS, PT, DKH, and KK wrote the manuscript and agree to be responsible for its contents. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Ethics approval for this study was obtained from The Ottawa Health Science Network Research Ethics Board.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s13054-018-2005-1) contains supplementary material, which is available to authorized users.
Contributor Information
Shannon M. Fernando, Email: sfern014@uottawa.ca
Peter M. Reardon, Email: peter.reardon1@gmail.com
Sean M. Bagshaw, Email: bagshaw@ualberta.ca
Damon C. Scales, Email: damon.scales@sunnybrook.ca
Kyle Murphy, Email: kymurphy@toh.ca.
Jennifer Shen, Email: jenniferxshen@gmail.com.
Peter Tanuseputro, Email: ptanuseputro@ohri.ca.
Daren K. Heyland, Email: dkh2@queensu.ca
Kwadwo Kyeremanteng, Email: kkyeremanteng@toh.ca.
References
- 1.Kause J, Smith G, Prytherch D, et al. A comparison of antecedents to cardiac arrests, deaths and emergency intensive care admissions in Australia and New Zealand, and the United Kingdom—the ACADEMIA study. Resuscitation. 2004;62:275–282. doi: 10.1016/j.resuscitation.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 2.Sax FL, Charlson ME. Medical patients at high risk for catastrophic deterioration. Crit Care Med. 1987;15:510–515. doi: 10.1097/00003246-198705000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Jones DA, DeVita MA, Bellomo R. Rapid-response teams. N Engl J Med. 2011;365:139–146. doi: 10.1056/NEJMra0910926. [DOI] [PubMed] [Google Scholar]
- 4.Jones D, Lippert A, DeVita M, et al. What’s new with rapid response systems? Intensive Care Med. 2015;41:315–317. doi: 10.1007/s00134-014-3567-2. [DOI] [PubMed] [Google Scholar]
- 5.Amaral AC, McDonald A, Coburn NG, et al. Expanding the scope of Critical Care Rapid Response Teams: a feasible approach to identify adverse events: a prospective observational cohort. BMJ Qual Saf. 2015;24:764–768. doi: 10.1136/bmjqs-2014-003833. [DOI] [PubMed] [Google Scholar]
- 6.Chan PS, Jain R, Nallmothu BK, et al. Rapid Response Teams: a systematic review and meta-analysis. Arch Intern Med. 2010;170:18–26. doi: 10.1001/archinternmed.2009.424. [DOI] [PubMed] [Google Scholar]
- 7.Winters BD, Weaver SJ, Pfoh ER, et al. Rapid-response systems as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158:417–425. doi: 10.7326/0003-4819-158-5-201303051-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maharaj R, Raffaele I, Wendon J. Rapid response systems: a systematic review and meta-analysis. Crit Care. 2015;19:254. doi: 10.1186/s13054-015-0973-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones D, Bellomo R, Bates S, et al. Long term effect of a medical emergency team on cardiac arrests in a teaching hospital. Crit Care. 2005;9:R808–R815. doi: 10.1186/cc3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Jong A, Jung B, Daurat A, et al. Effect of rapid response systems on hospital mortality: a systematic review and meta-analysis. Intensive Care Med. 2016;42:615–617. doi: 10.1007/s00134-016-4263-1. [DOI] [PubMed] [Google Scholar]
- 11.Jones D, Moran J, Winters B, et al. The rapid response system and end-of-life care. Curr Opin Crit Care. 2013;19:616–623. doi: 10.1097/MCC.0b013e3283636be2. [DOI] [PubMed] [Google Scholar]
- 12.Sundararajan K, Flabouris A, Thompson C. Diurnal variation in the performance of rapid response systems: the role of critical care services—a review article. J Intensive Care. 2016;4:15. doi: 10.1186/s40560-016-0136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stegenga J, Bell E, Matlow A. The role of nurse understaffing in nosocomial viral gastrointestinal infections on a general pediatrics ward. Infect Control Hosp Epidemiol. 2002;23:133–136. doi: 10.1086/502022. [DOI] [PubMed] [Google Scholar]
- 14.Wallace DJ, Angus DC, Barnato AE, et al. Nighttime intensivist staffing and mortality among critically ill patients. N Engl J Med. 2012;366:2093–2101. doi: 10.1056/NEJMsa1201918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kerlin MP, Small DS, Cooney E, et al. A randomized trial of nighttime physician staffing in an intensive care unit. N Engl J Med. 2013;368:2201–2209. doi: 10.1056/NEJMoa1302854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neuraz A, Guerin C, Payet C, et al. Patient mortality is associated with staff resources and workload in the ICU: a multicenter observational study. Crit Care Med. 2015;43:1587–1594. doi: 10.1097/CCM.0000000000001015. [DOI] [PubMed] [Google Scholar]
- 17.Needleman J, Buerhaus P, Pankratz VS, et al. Nurse staffing and inpatient hospital mortality. N Engl J Med. 2011;364:1037–1045. doi: 10.1056/NEJMsa1001025. [DOI] [PubMed] [Google Scholar]
- 18.Ronksley PE, McKay JA, Kobewka DM, et al. Patterns of health care use in a high-cost inpatient population in Ottawa, Ontario: a retrospective observational study. CMAJ Open. 2015;3:E111–E118. doi: 10.9778/cmajo.20140049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobewka DM, van Walraven C, Turnbull J, et al. Quality gaps identified through mortality review. BMJ Qual Saf. 2017;26:141–149. doi: 10.1136/bmjqs-2015-004735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McIsaac DI, Abdulla K, Yang H, et al. Association of delay of urgent or emergency surgery with mortality and use of health care resources: a propensity score-matched observational cohort study. CMAJ. 2017;189:E905–e912. doi: 10.1503/cmaj.160576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baxter AD, Cardinal P, Hooper J, et al. Medical emergency teams at The Ottawa Hospital: the first two years. Can J Anaesth. 2008;55:223–231. doi: 10.1007/BF03021506. [DOI] [PubMed] [Google Scholar]
- 22.Churpek MM, Edelson DP, Lee JY, et al. Association between survival and time of day for rapid response team calls in a national registry. Crit Care Med. 2017;45:1677–1682. doi: 10.1097/CCM.0000000000002620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones D, Bellomo R, DeVita MA. Effectiveness of the Medical Emergency Team: the importance of dose. Crit Care. 2009;13:313. doi: 10.1186/cc7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molloy J, Pratt N, Tiruvoipati R, et al. Relationship between diurnal patterns in Rapid Response Call activation and patient outcome. Aust Crit Care. 2018;31(1):42–46. doi: 10.1016/j.aucc.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 25.Medical Emergency Team End-of-Life Care investigators. The timing of Rapid-Response Team activations: a multicentre international study. Crit Care Resusc. 2013;15:15–20. [PubMed]
- 26.Jones D, Bates S, Warrillow S, et al. Circadian pattern of activation of the medical emergency team in a teaching hospital. Crit Care. 2005;9:R303–R306. doi: 10.1186/cc3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peberdy MA, Ornato JP, Larkin GL, et al. Survival from in-hospital cardiac arrest during nights and weekends. JAMA. 2008;299:785–792. doi: 10.1001/jama.299.7.785. [DOI] [PubMed] [Google Scholar]
- 28.Dybdal B, Svane C, Hesselfeldt R, et al. Is there a diurnal difference in mortality of severely injured trauma patients? Emerg Med J. 2015;32:287–290. doi: 10.1136/emermed-2013-202754. [DOI] [PubMed] [Google Scholar]
- 29.Sorita A, Ahmed A, Starr SR, et al. Off-hour presentation and outcomes in patients with acute myocardial infarction: systematic review and meta-analysis. BMJ. 2014;348:f7393. doi: 10.1136/bmj.f7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pronovost PJ, Angus DC, Dorman T, et al. Physician staffing patterns and clinical outcomes in critically ill patients: a systematic review. JAMA. 2002;288:2151–2162. doi: 10.1001/jama.288.17.2151. [DOI] [PubMed] [Google Scholar]
- 31.Parshuram CS, Kirpalani H, Mehta S, et al. In-house, overnight physician staffing: a cross-sectional survey of Canadian adult and pediatric intensive care units. Crit Care Med. 2006;34:1674–1678. doi: 10.1097/01.CCM.0000218808.13189.E7. [DOI] [PubMed] [Google Scholar]
- 32.Bisbal M, Pauly V, Gainnier M, et al. Does admission during morning rounds increase the mortality of patients in the medical ICU? Chest. 2012;142:1179–1184. doi: 10.1378/chest.11-2680. [DOI] [PubMed] [Google Scholar]
- 33.Bing-Hua YU. Delayed admission to intensive care unit for critically surgical patients is associated with increased mortality. Am J Surg. 2014;208:268–274. doi: 10.1016/j.amjsurg.2013.08.044. [DOI] [PubMed] [Google Scholar]
- 34.Stelfox HT, Hemmelgarn BR, Bagshaw SM, et al. Intensive care unit bed availability and outcomes for hospitalized patients with sudden clinical deterioration. Arch Intern Med. 2012;172:467–474. doi: 10.1001/archinternmed.2011.2315. [DOI] [PubMed] [Google Scholar]
- 35.Barwise A, Thongprayoon C, Gajic O, et al. Delayed rapid response team activation is associated with increased hospital mortality, morbidity, and length of stay in a tertiary care institution. Crit Care Med. 2016;44:54–63. doi: 10.1097/CCM.0000000000001346. [DOI] [PubMed] [Google Scholar]
- 36.Chen J. Saving patients’ lives through activating a rapid response system: willing is not enough, we must do. Crit Care Med. 2016;44:239–240. doi: 10.1097/CCM.0000000000001437. [DOI] [PubMed] [Google Scholar]
- 37.Amirian I, Andersen LT, Rosenberg J, et al. Working night shifts affects surgeons’ biological rhythm. Am J Surg. 2015;210:389–395. doi: 10.1016/j.amjsurg.2014.09.035. [DOI] [PubMed] [Google Scholar]
- 38.Sharpe R, Koval V, Ronco JJ, et al. The impact of prolonged continuous wakefulness on resident clinical performance in the intensive care unit: a patient simulator study. Crit Care Med. 2010;38:766–770. doi: 10.1097/CCM.0b013e3181cd122a. [DOI] [PubMed] [Google Scholar]
- 39.Olson EJ, Drage LA, Auger RR. Sleep deprivation, physician performance, and patient safety. Chest. 2009;136:1389–1396. doi: 10.1378/chest.08-1952. [DOI] [PubMed] [Google Scholar]
- 40.Jones D, Bellomo R, Bates S, et al. Patient monitoring and the timing of cardiac arrests and medical emergency team calls in a teaching hospital. Intensive Care Med. 2006;32:1352–1356. doi: 10.1007/s00134-006-0263-x. [DOI] [PubMed] [Google Scholar]
- 41.Oglesby KJ, Durham L, Welch J, et al. ‘Score to Door Time’, a benchmarking tool for rapid response systems: a pilot multi-centre service evaluation. Crit Care. 2011;15:R180. doi: 10.1186/cc10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multivariate logistic regression analysis of factors associated with in-hospital mortality (n = 6023). Multivariate logistic regression variables, with associated ORs and 95% CIs. (DOCX 136 kb)
Time of rapid response team activation and patient outcomes among patients not admitted to the intensive care unit. Subgroup analysis of outcomes among patients not admitted to the intensive care unit. (DOCX 77 kb)
Data Availability Statement
The datasets generated and analyzed are not publicly available, owing to patient privacy considerations, but they are available from the corresponding author on reasonable request.


