Abstract
OBJECTIVES
This study sought to determine whether Holter-based parameters of heart rate variability (HRV) are independently associated with incident heart failure among older adults in the CHS (Cardiovascular Health Study) as evidenced by an improvement in the predictive power of the Health Aging and Body Composition Heart Failure (Health ABC) score.
BACKGROUND
Abnormal HRV, a marker of autonomic dysfunction, has been associated with multiple adverse cardiovascular outcomes but not the development of congestive heart failure (CHF).
METHODS
Asymptomatic CHS participants with interpretable 24-h baseline Holter recordings were included (n = 1,401). HRV measures and premature ventricular contraction (PVC) counts were compared between participants with (n = 260) and without (n = 1,141) incident CHF on follow-up. Significantly different parameters between groups were added to the components of the Health ABC score, a validated CHF prediction tool, using stepwise Cox regression.
RESULTS
The final model included components of the Health ABC score, In PVC counts (adjusted hazard ratio [aHR]: 1.12; 95% confidence interval [CI]: 1.07 to 1.19; p < 0.001) and the following HRV measures: abnormal heart rate turbulence onset (aHR: 1.52; 95% CI: 1.11 to 2.08; p = 0.009), short-term fractal scaling exponent (aHR: 0.27; 95% CI: 0.14 to 0.53; p < 0.001), in very low frequency power (aHR: 1.28; 95% CI: 1.02 to 1.60; p = 0.037), and coefficient of variance of N-N intervals (aHR: 0.94; 95% CI: 0.90 to 0.99; p = 0.009). The C-statistic for the final model was significantly improved over the Health ABC model alone (0.77 vs. 0.73; p = 0.0002).
CONCLUSIONS
Abnormal HRV parameters were significantly and independently associated with incident CHF in asymptomatic, older adults. When combined with increased PVCs, HRV improved the predictive power of the Health ABC score.
Keywords: heart failure, heart rate variability, risk prediction
The development of congestive heart failure (CHF) is associated with significant morbidity and mortality. It is estimated that the annual cost of care for CHF patients in the United States will increase from $31 billion in 2012 to $70 billion in 2030, largely due to aging of the American population (1). Currently, care for these patients begins after the development of symptoms that lead to the diagnosis of the disease. Improved tools to identify patients at risk for the development of CHF may result in targeted interventions for primary prevention. Several risk models have been created to predict the development of CHF using known risk factors such as diabetes mellitus, coronary artery disease (CAD), hypertension, and chronic kidney disease (2,3). Although predictive in the specific populations from which they were derived, most of these models have not been externally validated (4). However, the Health Aging and Body Composition Heart Failure (Health ABC) score, originally developed in the Health ABC study, was externally validated in the CHS (Cardiovascular Health Study), a longitudinal study of community dwelling, older adults (5). In addition, a subset of CHS participants volunteered to undergo 24-h Holter monitoring, making it possible to determine whether Holter-based parameters, including heart rate variability (HRV), improves the ability of the Health ABC model to identify participants who are at risk of developing CHF.
HRV is a set of parameters that capture the magnitude and organization of the intervals between consecutive normal heart beats. These parameters reflect cardiac autonomic function and are abnormal in patients with CAD, diabetes mellitus, and CHF, but are also affected by the normal aging process (6–9). Abnormal HRV has been shown to be an independent predictor of mortality after myocardial infarction (MI) and sudden cardiac death in patients with CHF (10–13). In the CHS, abnormal HRV has been shown to be associated with increased cardiovascular mortality in community-dwelling adults without signs or symptoms of heart failure (14).
Ventricular ectopy is a commonly recognized electrocardiogram (ECG) finding that can be an ominous sign in the post-MI phase, but may also be seen on routine ECGs in otherwise healthy individuals (15–17). Multiple studies have examined the prognostic significance of premature ventricular contractions (PVCs) in the general population, and a 2013 meta-analysis showed that frequent PVCs were associated with a markedly increased risk of both sudden death and cardiac death (18). In the CHS, PVCs from 24-h Holter recordings were significantly associated with the development of heart failure (19).
Given the relationship among HRV, ectopy, and cardiovascular mortality, we hypothesized that abnormal HRV on 24-h Holter recordings could improve the ability of the Health ABC model to identify participants who are at an increased risk for the development of CHF in the CHS.
METHODS
STUDY POPULATION.
The design, rationale, and recruitment methods for the CHS have been previously described (20,21). Briefly, noninstitutionalized individuals who were 65 years of age or older were recruited from Medicare eligibility lists in 4 communities. The original cohort included 5,201 participants, recruited from 1989 to 1990. An additional 687 African-American individuals were recruited from 1992 to 1993. Baseline physical examinations, laboratory tests, and questionnaires were obtained for all participants. A subset of participants from the CHS cohort that had baseline 24-h Holter monitoring were studied (n = 1,764) (14). The original cohort underwent Holter recording at year 2 of the CHS, whereas the African-American cohort, which had baseline assessments at year 5, had Holter recordings performed in year 7. Participants with unusable Holter data (i.e., paced rhythms, atrial fibrillation, wandering atrial pacemaker, or >20% ectopic beats) were excluded (n = 275). Additionally, those with heart failure at the time of Holter monitoring were excluded (n = 42), as well as those with unknown baseline heart failure status (n = 4). Participants with missing components of the Health ABC score were also excluded, as only complete case data were utilized in our analysis (n = 39). Three participants were excluded due to incomplete follow-up data. This yielded a final sample size of 1,401 participants.
COMPONENTS OF THE HEALTH ABC HEART FAILURE SCORE.
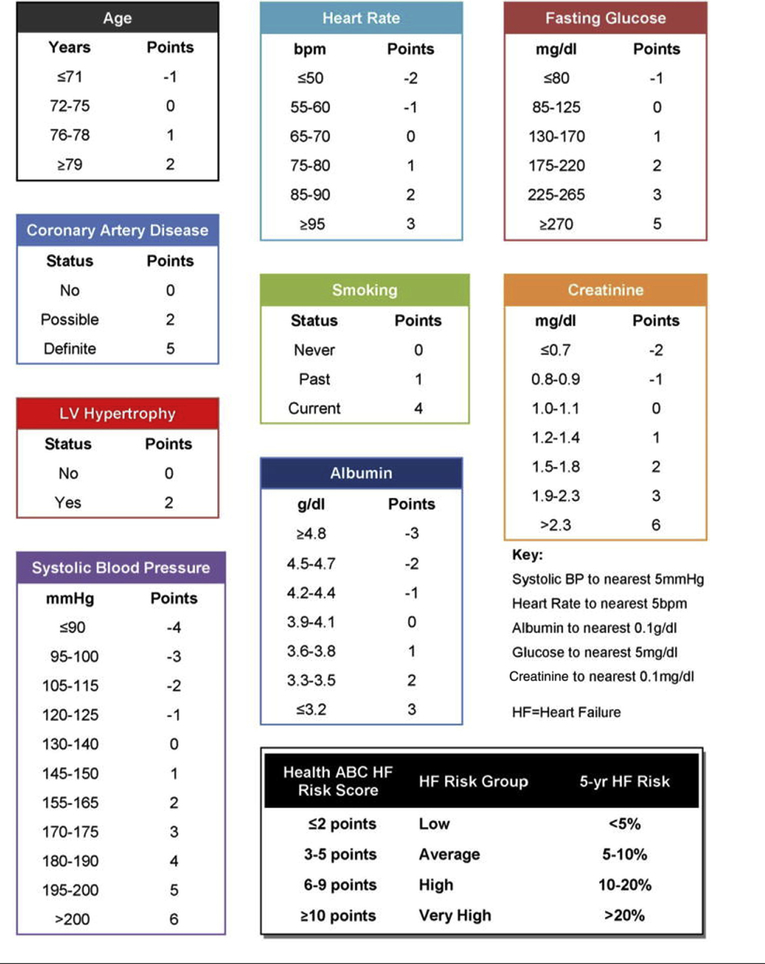
The Health ABC score components were recorded in all participants who underwent Holter monitoring using baseline physical examination findings, laboratory values, and testing, as shown in Figure 1. Of note, the 687 African-American participants who were recruited later had baseline laboratory values assessed 2 years before their 24-h Holter monitoring. The individual components of the Health ABC score, as defined in Table 1, were compared between participants who did and did not develop CHF by the end of follow-up.
FIGURE 1. The Health ABC Heart Failure Risk Score.
Individual components of the score with their corresponding point values as outline by the original Health ABC Heart Failure study. Reprinted with permission from Butler et al. (2). BP = blood pressure; bpm = beats/min; HF = heart failure; LV = left ventricular.
TABLE 1.
Comparison of Baseline Characteristics in Participants With Useable Holter Data
| No CHF (n = 1,141) | Incident CHF (n = 260) | p Value | |
|---|---|---|---|
| Sex | 0.002 | ||
| Male | 41.6 | 51.5 | |
| Female | 58.4 | 48.5 | |
| Age, yrs* | 71.3 ± 4.7 | 73.0 ± 5.3 | <0.001 |
| Race | 0.172 | ||
| White | 77.6 | 83.1 | |
| Black | 21.9 | 16.1 | |
| Other | 0.6 | 0.8 | |
| BMI, kg/m2† | 26.8 ± 4.3 | 27.8 ± 4.5 | 0.001 |
| Heart rate on resting ECG, beats/min* | 63.1 ± 9.7 | 66.9 ± 12.7 | <0.001 |
| Systolic blood pressure, mm Hg* | 134.4 ± 21.0 | 140.0 ± 20.5 | <0.001 |
| Fasting glucose, mg/dl | 108.6 ± 33.2 | 127.0 ± 61.8 | <0.001 |
| Coronary artery disease*‡ | <0.001 | ||
| No | 81.2 | 62.7 | |
| Probable | 8.2 | 14.2 | |
| Definite | 10.6 | 23.1 | |
| LVH* | 3.0 | 7.7 | 0.001 |
| Smoking* | 0.874 | ||
| Never | 45.6 | 45.8 | |
| Former | 44.2 | 43.1 | |
| Current | 10.2 | 11.1 | |
| Creatinine, mg/dl* | 1.03 ± 0.27 | 1.15 ± 0.39 | <0.001 |
| Albumin, g/dl* | 4.01 ± 0.29 | 4.01 ± 0.30 | 0.954 |
| Total Health ABC score | 0.75 ± 3.55 | 3.50 ± 3.81 | <0.001 |
| Alcoholic drinks per week | 2.25 ± 5.30 | 2.15 ± 6.40 | 0.776 |
| NT-proBNP, pg/ml§ | 144.1 ± 187.9 | 388.3 ± 912.1 | <0.001 |
Values are % or mean ± SD.
Component of the Health ABC Heart Failure Score.
3 participants who did not develop CHF had missing data.
Probable CAD defined as history of self-reported CAD without documented antianginal medication (beta-blocker, calcium-channel blocker, or nitrates). Definite CAD defined as history of self-reported CAD with documented antianginal medication use.
Data were missing for 70 participants.
BMI = body mass index; CAD = coronary artery disease; LVH = left ventricular hypertrophy; NT-proBNP = N-terminal pro-B-type natriuretic peptide.
OUTCOMES.
The primary outcome was incident CHF. The methods used to define incident CHF have been reported previously (22,23). To summarize, in the CHS, incident events (e.g., heart failure, MI, and stroke) were tabulated through regular surveys, clinic visits, and calls through which participants were questioned regarding any recent hospitalizations or new diagnoses. Additionally, participants were able to contact the local field centers to report any changes in their medical care. Finally, incident events could be explored through review of medical records including discharge summaries, hospital face sheets, or using International Classification of Diseases-Ninth Revision-Clinical Modification diagnostic codes through the Centers for Medicare and Medicaid Services data (24). Once identified, CHF diagnoses were formally adjudicated by the CHS Events Subcommittee through the use of signs, symptoms, clinical tests, and/or medical therapies (25).
AMBULATORY ECG MONITORING AND ASSESSMENT OF HRV.
Holter tapes were recorded on Del Mar Avionics recorders, which have a calibrated timing signal. Data were processed by research technicians at the Washington University School of Medicine Heart Rate Variability Laboratory (St. Louis, Missouri), using a GE Marquette MARS 8000 Holter Analyzer (GE-Marquette, Milwaukee, Wisconsin). All Holter analyses were reviewed in detail by an investigator (P.K.S.) primarily to ensure that only beats with uniformly detected onsets were labeled as normal. The longest and shortest true N–N intervals were identified for each tape, and intervals outside of these limits, including blocked atrial premature contractions, were excluded from all HRV calculations.
Time domain, frequency domain, and nonlinear HRV measures were determined. Heart rate turbulence (HRT), a relatively novel measure of heart rate responses to isolated PVCs, was also calculated (26). HRT is generally reported as the categorical variables turbulence onset (TO) and turbulence slope (TS) (27,28). On the basis of previous studies in the CHS, a TS ≥3.0 ms/RR was categorized as normal (29), whereas a TO ≤0% was considered normal. HRV and HRT were calculated from annotated beat-to-beat files exported to a Sun Enterprise 450 server (Sun Microsystems, Santa Clara, California) using validated research software.
STATISTICAL ANALYSIS.
To determine which HRV measurements were candidate predictors for the development of CHF, independent sample Student t tests were performed to compare continuous 24-h Holter-based HRV measures and ectopy counts between participants who did and did not develop CHF. Chi-square tests were used to compare categorical HRV-based variables of interest.
The initial survival model tested the Health ABC score components alone. HRV measures and ectopy counts that were significantly different on univariate analysis were then added to the Health ABC score components using a stepwise regression with forward selection and a p value <0.05 for inclusion.
The C-statistic, a measure of the accuracy of discrimination that can be thought of as an extension of the binary receiver-operating characteristic curve to multivariate survival analysis, was calculated for the Health ABC model and covariate-adjusted risk model. Greenwood-Nam-D’Agostino (GND) test was performed to assess goodness of fit of the adjusted regression model (30). The statistical significance of the difference between the 2 models was calculated using STATA version 12.1 (StataCorp, College Station, Texas). SAS version 9.4 (SAS Institute, Inc., Cary, North Carolina) was used to perform the GND test. SPSS version 23 (SPSS, Inc., Chicago, Illinois) software was used for all other analyses. A p value < 0.05 was considered to be statistically significant.
SUBGROUP ANALYSIS.
A subgroup analysis was performed on participants stratified by baseline N-terminal pro–B-type natriuretic peptide (NT-proBNP) to identify HRV measures significantly associated with heart failure in an otherwise low-risk cohort. The CHS has previously documented that participants with a baseline NT-proBNP of >190 pg/ml were at an elevated risk of developing CHF (31). To reduce the possibility of including asymptomatic participants with subclinical heart failure, as manifested by an NT-proBNP >190 pg/ml, we repeated the previously described analysis and limited it to participants with baseline NT-proBNP levels ≤190 pg/ml.
RESULTS
A total of 260 participants (19%) developed CHF during follow-up (median 10.5 years). Comparisons of the individual components of the Health ABC score between participants who did and did not develop CHF are shown in Table 1. Participants who developed CHF were more likely to be male, be older, and have a higher body mass index. They were also more likely to have higher baseline heart rate and systolic blood pressure, as well as higher baseline NT-proBNP, creatinine, and fasting glucose levels. Additionally, they were more likely to have left ventricular hypertrophy and CAD.
A comparison of Holter-based variables between participants who did and did not develop CHF is shown in Online Table 1. Detailed definitions for each HRV parameter can be seen in the table legends. CHS participants who developed CHF had decreased values for measures reflecting total 24-h HRV (ln total power) and circadian HRV (ln ultra low frequency power). Significant differences were also seen in ln very low frequency (VLF) power, which captures variance in heart rate patterns at a scale of every 20 s to every 5 min. Also, participants who developed CHF had decreased HRV when evaluated in 5-minsegmentsand then averaged over 24 h (CV%, normalized low frequency power, normalized high frequency power). Further, in participants who developed CHF, nonlinear HRV measures that capture the organization of the heart rate time series (DFA1, DFA2, and SD12) were significantly more abnormal, reflecting greater disorganization in heart rate control. Both abnormal HRT slope and onset were more prevalent in those who developed CHF. Also, both ventricular and atrial ectopy counts were significantly higher in the CHF group. Notably, 24-h mean heart rate was not different between participants with and without incident CHF (74 ± 10 beats/min vs. 74 ± 9 beats/min; p = 0.73).
The unadjusted risk model for incident CHF, created from the individual Health ABC score components, can be seen in Table 2. Neither albumin levels nor smoking status were significant in the unadjusted risk model; however, all other components of the Health ABC score were statistically significant in the model. Participants who developed CHF had significantly higher Health ABC scores (0.75 ± 3.55 vs. 3.50 ± 3.81; p < 0.001). The C-statistic for the unadjusted model alone was 0.73. The final adjusted Cox regression model is also shown in Table 2. The final model included the following Health ABC score components: creatinine (p < 0.001), albumin (p = 0.0787), heart rate on resting ECG (p < 0.001), systolic blood pressure (p =0.001), fasting glucose (p <0.001), age (p <0.001), left ventricular hypertrophy (p =0.01), smoking status (p = 0.41), and CAD (p < 0.001). The final model also included the following 24-h Holter-based measures: increased In PVC counts (p < 0.001), decreased CV% (p=0.009),decreased DFA1(p<0.001),abnormal HRT onset (p =0.009), and increased In very low frequency power (p = 0.037).
TABLE 2.
Components of the Health ABC Heart Failure Before and After Adjustment for Holter-Based Risk Predictors
| Unadjusted Model | Adjusted Model | |||||
|---|---|---|---|---|---|---|
| aHR | 95% CI | p Value | aHR | 95% CI | p Value | |
| Creatinine | 3.08 | 2.20–4.31 | <0.001 | 3.06 | 2.14–4.38 | <0.001 |
| Albumin | 0.71 | 0.46–1.09 | 0.115 | 0.68 | 0.44–1.04 | 0.078 |
| Heart rate on resting ECG | 1.03 | 1.02–1.04 | <0.001 | 1.03 | 1.02–1.04 | <0.001 |
| Systolic blood pressure | 1.01 | 1.004–1.015 | 0.001 | 1.01 | 1.004–1.016 | 0.001 |
| Fasting glucose | 1.006 | 1.004–1.008 | <0.001 | 1.005 | 1.003–1.008 | <0.001 |
| Age | 1.07 | 1.05–1.10 | <0.001 | 1.06 | 1.03–1.09 | <0.001 |
| LVH | 0.017 | 0.010 | ||||
| No | 1.00 | 1.00 | ||||
| Yes | 1.79 | 1.11–2.90 | 1.87 | 1.16–3.02 | ||
| Smoking | 0.220 | 0.41 | ||||
| Never | 1.00 | 1.00 | ||||
| Former | 1.11 | 0.85–1.46 | 1.02 | 0.78–1.34 | ||
| Current | 1.45 | 0.95–2.22 | 1.34 | 0.87–2.06 | ||
| CAD | <0.001 | <0.001 | ||||
| No | 1.00 | 1.00 | ||||
| Probable | 1.72 | 1.19–2.48 | 1.65 | 1.13–2.41 | ||
| Definite | 2.47 | 1.82–3.25 | 2.56 | 1.89–3.48 | ||
| DFA1 | 0.27 | 0.14–0.53 | <0.001 | |||
| ln (PVC + 1) | 1.12 | 1.07–1.19 | <0.001 | |||
| ln (VLF24) | 1.28 | 1.02–1.60 | 0.037 | |||
| CV (%) | 0.94 | 0.90–0.99 | 0.009 | |||
| Turbulence onset | 0.009 | |||||
| Normal (≤0%) | 1.00 | |||||
| Abnormal (>0%) | 1.52 | 1.11–2.08 | ||||
Turbulence onset indicates a signal average of the magnitude of tachycardia, if any, as the percentage of change in N-N intervalofthe sinus rhythm 2 beats afterthe prematureventricularcontraction (PVC) compared with the 2 beats before. Although calculation of HRT requires at least 5 qualifying PVCs, when HRT is categorized, participants with fewer than 5 PVCs can be categorized as having normal heart rate variability and are therefore included in the analysis. Very low frequency power indicates variance in heart rate variability at underlying frequencies of every 25 s to every 5 min calculated for every 15-min segment and averaged.
CV (%) = average coefficient of variance of N-N intervals for 5-min segments for 24 h; DFA1 = short-term fractal scaling exponent calculated over 3 to 11 beats and averaged over 1,000 beats for 24 h; ECG = electrocardiogram; Health ABC = Health Aging and Body Composition Heart Failure; ln (PVC + 1) = natural log transformation ofthe number of prematureventricular contractions + 1; ln (VLF24) = natural log transformation of very low frequency power which indicates variance in heart rate variability at underlying frequencies of every 25 s to every 5 min, calculated for every 15-min segment and averaged; other abbreviations as in Table 1.
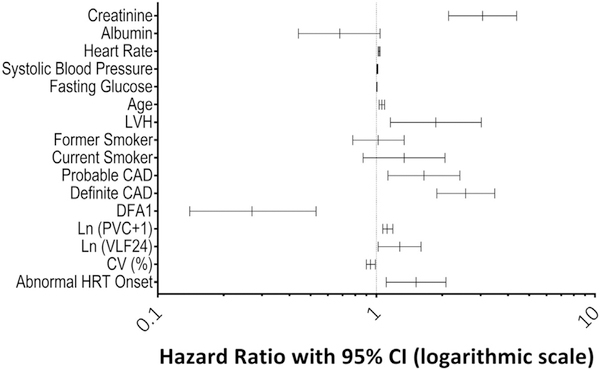
A forest plot showing the hazard ratios of the variables in the adjusted model and their corresponding 95% confidence intervals is presented in Figure 2. The final adjusted model had a C-statistic of 0.77 and an insignificant GND test, indicating good model fit (p = 0.727). The improvement in the adjusted model’s C-statistic over that of the Health ABC model was statistically significant (p = 0.0002).
FIGURE 2. Forest Plot of Adjusted Model Hazard Ratios and 95% Confidence Intervals.
Components of the final model, as defined in Tables 1 and 2, are shown in graphical form with corresponding hazard ratios. Very low-frequency power indicates variance in heart rate variability at underlying frequencies of every 25 s to every 5 min calculated for every 15-min segment and averaged. CAD = coronary artery disease; CI = confidence interval; CV (%) = average coefficient of variance of N–N intervals for 5-min segments for 24 h; DFA1 = short-term fractal scaling exponent calculated over 3 to 11 beats and averaged over 1,000 beats for 24 h; HRT = heart rate turbulence; ln (PVC + 1) = natural log transformation of the number of premature ventricular contractions + 1; ln (VLF24) = natural log transformation of very low frequency power which indicates variance in heart rate variability at underlying frequencies of every 25 s to every 5 min, calculated for every 15-min segment and averaged. LVH = left ventricular hypertrophy; PVC = premature ventricular contraction.
SUBGROUP ANALYSIS.
A comparison of Holter-based parameters in the subset of participants with a baseline NT-proBNP ≤190 pg/ml (n = 1,000), who did (n = 142, 14.2%) and did not (n = 858, 85.8%) develop CHF can be seen in Online Table 2. Participants who developed CHF in this subcohort had significantly higher Health ABC scores (0.31 ± 3.37 vs. 2.86 ± 3.68; p < 0.001). The final Cox regression model for this cohort included all of the components of the Health ABC score as well as decreased DFA1 (p < 0.001). Notably, albumin and smoking status were not significant in the adjusted model, similar to the model derived from the entire cohort. In contrast tothe original adjusted model, CV% (p = 0.41), heart rate TO (p = 0.14) and ln very low frequency power (p = 0.73) were no longer significant in the adjusted model. PVC count was not significant in the univariate analysis (p = 0.066), but was tested in the final model and did not enter the model (p = 0.12). Unadjusted and adjusted models for the subgroup analysis are presented in Table 3.
TABLE 3.
Unadjusted and Adjusted Health ABC Heart Failure Model in Participants With NT-proBNP ≤190 pg/ml
| Unadjusted Model | Adjusted Model | |||||
|---|---|---|---|---|---|---|
| aHR | 95% CI | p Value | aHR | 95% CI | p Value | |
| Creatinine | 1.99 | 1.06–3.71 | 0.032 | 2.10 | 1.13–3.91 | 0.019 |
| Albumin | 1.05 | 0.58–1.90 | 0.864 | 1.17 | 0.65–2.12 | 0.605 |
| Heart rate on resting ECG | 1.03 | 1.01–1.04 | <0.001 | 1.02 | 1.01–1.04 | 0.001 |
| Systolic blood pressure | 1.01 | 1.006–1.022 | 0.001 | 1.01 | 1.005–1.022 | 0.001 |
| Fasting glucose | 1.007 | 1.005–1.01 | <0.001 | 1.006 | 1.004–1.009 | <0.001 |
| Age | 1.07 | 1.03–1.11 | 0.001 | 1.05 | 1.01–1.09 | 0.008 |
| LVH | 0.06 | 0.035 | ||||
| No | 1.00 | 1.00 | ||||
| Yes | 2.15 | 0.97–4.79 | 2.35 | 1.06–5.20 | ||
| Smoking | 0.540 | 0.471 | ||||
| Never | 1.00 | 1.00 | ||||
| Former | 1.23 | 0.85–1.76 | 1.21 | 0.85–1.74 | ||
| Current | 1.06 | 0.56–2.03 | 0.90 | 0.47–1.73 | ||
| CAD | <0.001 | <0.001 | ||||
| No | 1.00 | 1.00 | ||||
| Probable | 1.14 | 0.64–2.04 | 1.08 | 0.60–1.94 | ||
| Definite | 2.86 | 1.89–4.33 | 2.70 | 1.78–4.08 | ||
| DFA1 | 0.14 | 0.06–0.36 | <0.001 | |||
DISCUSSION
In this retrospective analysis, we found that increased ventricular ectopy counts and abnormal HRV measures from 24-h Holter recordings, including decreased DFA1, decreased CV%, increased ln VLF, and abnormal HRT onset, added to the predictive power of the Health ABC score to identify asymptomatic older adults who are at increased risk of developing CHF. The model C-statistic increased from 0.73 to 0.77 when HRV was included. Furthermore, in a low-risk subcohort, as defined by NT-proBNP ≤190 pg/ml, the Health ABC components significantly associated with CHF were unchanged, and decreased DFA1 remained independently associated with incident CHF. To our knowledge, this is the first time that a relationship between abnormal 24-h Holter-based HRV measures at baseline and incident CHF over an extended follow-up period has been described in community-dwelling older adults. Although the Health ABC score incorporates many traditional CHF risk factors (e.g., age, fasting glucose, and coronary artery disease) that can result in abnormal HRV, we found that HRV parameters were associated with incident CHF independent of the Health ABC score components. This suggests that otherwise asymptomatic older adults who have cardiac autonomic dysfunction, as manifested by abnormal HRV, may be predisposed to the development of CHF.
HRV quantifies the variation of normal sinus intervals and reflects cardiac autonomic function (27). This variation is attributed to the different regulatory effects of the parasympathetic and sympathetic branches of the autonomic nervous systems on the firing of the sinoatrial node (27,28). Abnormal HRV has been linked to multiple adverse cardiac outcomes including mortality after MI and has been described in patients with established CHF (6,11).
Early studies of HRV for risk stratification focused on time and frequency domain measures of HRV. Since then, novel measures of HRV, such as DFA1 and HRT, have been explored (32,33). DFA1, first described by Peng et al. (32), measures the randomness of the N-N intervals (i.e., the intervals between normal heart beats) in a time series. A lower value of DFA1 is believed to reflect a more disorganized, less predictable sinus rhythm (32). Makikallio et al. (33) first elucidated the relationship between cardiomyopathy and DFA1 when they discovered that a lower DFA1 was associated with increased mortality in patients with reduced ejection fraction after MI. Furthermore, decreased DFA1 has been shown to be associated with increased heart failure hospitalizations after MI (34). In the CHS, decreased DFA1 has previously been shown to be a strong risk factor for cardiovascular death (14). Despite this known link between DFA1 and adverse cardiovascular outcomes, this is the first study, to our knowledge, to demonstrate a relationship between DFA1 and incident CHF in asymptomatic older adults. Furthermore, this relationship was also seen in the subgroup of participants with NT-proBNP ≤190 pg/ml, indicating that DFA1 may identify otherwise low-risk older adults with an elevated risk of developing CHF.
HRT characterizes the autonomic response of the cardiac system to the loss of cardiac output associated with a PVC. Thus, it is a surrogate for the ability of the cardiovascular system to appropriately respond to a perturbation in cardiac output. TO, defined as the ability of the heart rate to immediately accelerate in response to a drop in cardiac output from a PVC, reflects the function of the parasympathetic nervous system. Abnormal TO, as evidenced by no acceleration or a deceleration in heart rate after a PVC, reflects a disturbance in vagally mediated cardiac regulation and, in our study, was associated with incident CHF.
CV%, the average variance of N-N intervals in 5-min segments over 24 h when corrected for the average heart rate, was found to correlate with incident CHF in the final model. Lower CV%, which suggests cardiac autonomic dysfunction, appears to help identify individuals who are at an elevated risk of developing CHF.
A recent study showed that a greater frequency of PVCs on Holter monitoring was an independent predictor of the development of CHF in the CHS (19). Our study confirmed that an increased number of PVCs was associated with an increased risk of CHF, independent of the Health ABC score components, even though participants with >20% ectopic beats were excluded from the HRV analysis.
The relationship of increased ln VLF power with CHF in the adjusted model is surprising in the light of the results of the Student t test, which showed that participants who developed CHF had significantly lower mean values for this parameter. Although any explanation would be speculative, it is likely that in this context, VLF reflects 2 different aspects of HRV. In most studies, decreased VLF power, which is believed to be vagally modulated in light of the fact that it is abolished by atropine (35), is associated with worse survival (36,37). However, VLF also captures the oscillations in heart rate associated with periodic respiration and sleep-disordered breathing (38). Thus, potentially, after adjustment for other parameters that capture reductions in normal HRV (e.g., CV % and abnormal TO), it is possible the relationship of higher ln VLF power with CHF captures the presence of undetected periodic oscillations in heart rate.
Although abnormal HRT onset, decreased CV%, higher VLF, and increased number of PVCs were associated with incident CHF in the adjusted model derived from the entire cohort, these variables were not associated with CHF in the subgroup of participants with an NT-proBNP ≤190 pg/ml. One possible explanation for this discrepancy may be that these variables are detecting subclinical heart failure, as manifested by elevated NT-proBNP levels. Alternatively, given the lower number of events in the subgroup analysis, the analysis may have lacked the power to detect small differences.
STUDY LIMITATIONS.
First, HRV parameters can only be measured in people who are in normal sinus rhythm, which would exclude those with atrial fibrillation, wandering atrial pacemaker, excessive ectopy, or an underlying paced rhythm, potentially excluding individuals who are at the greatest risk of developing CHF. Second, because Health ABC score components were obtained 2 years before the Holter recordings in the African-American cohort, these participants may have had a different Health ABC score at the time of their 24-h Holter measurements. Additionally, we did not correct for multiple comparisons, thereby increasing the risk of type I error. Last, full clinical application of these findings would require that 24-h Holter recordings be analyzed to research standards.
CONCLUSIONS
Among participants in the CHS who underwent baseline 24-h Holter monitoring and had recordings eligible for HRV analysis, abnormal HRV parameters were significantly and independently associated with incident CHF. These 24-h HRV measures, when combined with increased PVCs, improved the predictive power of the Health ABC score. Furthermore, even among low-risk older adults with NT-proBNP ≤190 pg/ml, HRV added to the clinical risk model. Identification of asymptomatic older adults with abnormal HRV could potentially help direct targeted strategies for the primary prevention of heart failure.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE:
This is the first study to show an association between HRV measurements and the development of CHF in a community-dwelling, older population. Asymptomatic older adults who are identified as having a high risk of developing heart failure, as determined by abnormal HRV, could be targeted for preventive strategies.
TRANSLATIONAL OUTLOOK:
Future prospective studies are needed to validate this retrospective analysis, as well as to investigate this relationship in a younger population.
ACKNOWLEDGMENTS
The authors express their gratitude to the CHS participants. A full list of participating CHS investigators and institutions is available at: http://www.chs-nhlbi.org.
This work was supported in part by National Heart, Lung, and Blood Institute (NHLBI) contracts HHSN268201200036C, HHSN268200800007C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, and N01HC85086, and NHLBI grants U01HL080295 and U01HL130114, with additional contribution from the National Institute of Neurological Disorders and Stroke. Additional support was provided through R01AG023629 from the National Institute on Aging. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NHLBI, the National Institutes of Health. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- CAD
coronary artery disease
- CHF
congestive heart failure
- ECG
electrocardiogram
- GND
Greenwood-Nam-D’Agostino
- HRT
heart rate turbulence
- HRV
heart rate variability
- MI
myocardial infarction
- NT-proBNP
N-terminal pro–B-type natriuretic peptide
- PVC
premature ventricular contraction
- TO
turbulence onset
- TS
turbulence slope
Footnotes
APPENDIX For supplemental tables, please see the online version of this article.
REFERENCES
- 1.Heidenreich PA, Albert NM, Allen LA, et al. , for the American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; and Stroke Council. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail 2013;6:606–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butler J, Kalogeropoulos A, Georgiopoulou V, et al. , for the Health ABC Study. Incident heart failure prediction in the elderly: the health ABC heart failure score. Circ Heart Fail 2008;1:125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kannel WB, D’Agostino RB, Silbershatz H, Belanger AJ, Wilson PW, Levy D. Profile for estimating risk of heart failure. Arch Intern Med 1999; 159:1197–204. [DOI] [PubMed] [Google Scholar]
- 4.Echouffo-Tcheugui JB, Greene SJ, Papadimitriou L, et al. Population risk prediction models for incident heart failure: a systematic review. Circ Heart Fail 2015;8:438–47. [DOI] [PubMed] [Google Scholar]
- 5.Kalogeropoulos A, Psaty BM, Vasan RS, et al. , for the Cardiovascular Health Study. Validation of the health ABC heart failure model for incident heart failure risk prediction: the Cardiovascular Health Study. Circ Heart Fail 2010;3:495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casolo G, Balli E, Taddei T, Amuhasi J, Gori C. Decreased spontaneous heart rate variability in congestive heart failure. Am J Cardiol 1989;64: 1162–7. [DOI] [PubMed] [Google Scholar]
- 7.Casolo GC, Stroder P, Signorini C, et al. Heart rate variability during the acute phase of myocardial infarction. Circulation 1992;85: 2073–9. [DOI] [PubMed] [Google Scholar]
- 8.Umetani K, Singer DH, McCraty R, Atkinson M. Twenty-four hour time domain heart rate variability and heart rate: relations to age and gender over nine decades. J Am Coll Cardiol 1998;31: 593–601. [DOI] [PubMed] [Google Scholar]
- 9.Vinik AI, Freeman R, Erbas T. Diabetic autonomic neuropathy. Semin Neurol 2003;23:365–72. [DOI] [PubMed] [Google Scholar]
- 10.Jiang W, Hathaway WR, McNulty S, et al. Ability of heart rate variability to predict prognosis in patients with advanced congestive heart failure. Am J Cardiol 1997;80:808–11. [DOI] [PubMed] [Google Scholar]
- 11.Kleiger RE, Miller JP, Bigger JT Jr., Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol 1987;59:256–62. [DOI] [PubMed] [Google Scholar]
- 12.Pinnacchio G, Lanza GA, Stazi A, et al. Determinants of heart rate turbulence in individuals without apparent heart disease and in patients with stable coronary artery disease. Europace 2015;17:1855–61. [DOI] [PubMed] [Google Scholar]
- 13.Wu L, Jiang Z, Li C, Shu M. Prediction of heart rate variability on cardiac sudden death in heart failure patients: a systematic review. Int J Cardiol 2014;174:857–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stein PK, Barzilay JI, Chaves PH, et al. Novel measures of heart rate variability predict cardiovascular mortality in older adults independent of traditional cardiovascular risk factors: the Cardiovascular Health Study (CHS). J Cardiovasc Electrophysiol 2008;19:1169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheriyath P, He F, Peters I, et al. Relation of atrial and/or ventricular premature complexes on a two-minute rhythm strip to the risk of sudden cardiac death (the Atherosclerosis Risk in Communities [ARIC] study). Am J Cardiol 2011;107: 151–5. [DOI] [PubMed] [Google Scholar]
- 16.Kostis JB, Byington R, Friedman LM, Goldstein S, Furberg C. Prognostic significance of ventricular ectopic activity in survivors of acute myocardial infarction. J Am Coll Cardiol 1987;10: 231–42. [DOI] [PubMed] [Google Scholar]
- 17.Simpson RJ Jr., Cascio WE, Schreiner PJ, Crow RS, Rautaharju PM, Heiss G. Prevalence of premature ventricular contractions in a population of African American and white men and women: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J 2002;143: 535–40. [DOI] [PubMed] [Google Scholar]
- 18.Ataklte F, Erqou S, Laukkanen J, Kaptoge S. Meta-analysis of ventricular premature complexes and their relation to cardiac mortality in general populations. Am J Cardiol 2013;112: 1263–70. [DOI] [PubMed] [Google Scholar]
- 19.Dukes JW, Dewland TA, Vittinghoff E, et al. Ventricular ectopy as a predictor of heart failure and death. J Am Coll Cardiol 2015;66:101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tell GS, Fried LP, Hermanson B, Manolio TA, Newman AB, Borhani NO. Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann Epidemiol 1993;3: 358–66. [DOI] [PubMed] [Google Scholar]
- 21.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1991;1:263–76. [DOI] [PubMed] [Google Scholar]
- 22.Ives DG, Fitzpatrick AL, Bild DE, et al. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol 1995;5:278–85. [DOI] [PubMed] [Google Scholar]
- 23.Psaty BM, Kuller LH, Bild D, et al. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol 1995; 5:270–7. [DOI] [PubMed] [Google Scholar]
- 24.Medicode. ICD-9-CM: International Classification of Diseases, 9th Revision, Clinical Modification. Salt Lake City, Utah: Medicode; 1996. [Google Scholar]
- 25.Schellenbaum GD, Rea TD, Heckbert SR, et al. Survival associated with two sets of diagnostic criteria for congestive heart failure. Am J Epidemiol 2004;160:628–35. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt G, Malik M, Barthel P, et al. Heartrate turbulence after ventricular premature beats as a predictor of mortality after acute myocardial infarction. Lancet 1999;353:1390–6. [DOI] [PubMed] [Google Scholar]
- 27.Stein PK, Bosner MS, Kleiger RE, Conger BM. Heart rate variability: a measure of cardiac autonomic tone. Am Heart J 1994;127:1376–81. [DOI] [PubMed] [Google Scholar]
- 28.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation 1996;93:1043–65. [PubMed] [Google Scholar]
- 29.Stein PK, Sanghavi D, Sotoodehnia N, Siscovick DS, Gottdiener J. Association of Holterbased measures including T-wave alternans with risk of sudden cardiac death in the communitydwelling elderly: the Cardiovascular Health Study. J Electrocardiol 2010;43:251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Demler OV, Paynter NP, Cook NR. Tests of calibration and goodness-of-fit in the survival setting. Stat Med 2015;34:1659–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.deFilippi CR, Christenson RH, Gottdiener JS, Kop WJ, Seliger SL. Dynamic cardiovascular risk assessment in elderly people: the role of repeated N-terminal pro–B-type natriuretic peptide testing. J Am Coll Cardiol 2010;55:441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peng CK, Havlin S, Stanley HE, Goldberger AL. Quantification of scaling exponents and crossover phenomena in nonstationary heartbeat time series. Chaos 1995;5:82–7. [DOI] [PubMed] [Google Scholar]
- 33.Makikallio TH, Hoiber S, Kober L, et al. , for the TRACE Investigators. Fractal analysis of heart rate dynamics as a predictor of mortality in patients with depressed left ventricular function after acute myocardial infarction. TRAndolapril Cardiac Evaluation. Am J Cardiol 1999;83:836–9. [DOI] [PubMed] [Google Scholar]
- 34.Perkiomaki JS, Hamekoski S, Junttila MJ, Jokinen V, Tapanainen J, Huikuri HV. Predictors of long-term risk for heart failure hospitalization after acute myocardial infarction. Ann Noninvasive Electrocardiol 2010;15:250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor JA, Carr DL, Myers CW, Eckberg DL. Mechanisms underlying very-low-frequency RR-interval oscillations in humans. Circulation 1998;98:547–55. [DOI] [PubMed] [Google Scholar]
- 36.Bigger JT Jr., Fleiss JL, Steinman RC, Rolnitzky LM, Kleiger RE, Rottman. Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation 1992;85:164–71. [DOI] [PubMed] [Google Scholar]
- 37.Bigger JT Jr., Fleiss JL, Rolnitzky LM, Steinman RC. Frequency domain measures of heart period variability to assess risk late after myocardial infarction. J Am Coll Cardiol 1993;21:729–36. [DOI] [PubMed] [Google Scholar]
- 38.Mortara A, Sleight P, Pinna GD, et al. Abnormal awake respiratory patterns are common in chronic heart failure and may prevent evaluation of autonomic tone by measures of heart rate variability. Circulation 1997;96:246–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.