Abstract
Objective
This prospective longitudinal study investigated sleep disturbance (SD) and internalizing problems after traumatic injury, including traumatic brain injury (TBI) or extracranial/bodily injury (EI) in children and adolescents, relative to typically developing (TD) children. We also examined longitudinal relations between SD and internalizing problems post-injury.
Method
Participants (N=87) ages 8–15 included youth with TBI, EI, and TD children. Injury groups were recruited from a Level 1 trauma center after sustaining vehicle-related injuries. Parent-reported SD and internalizing problems were assessed at preinjury/baseline, and 6- and 12-months post-injury. Linear mixed models evaluated the relation of group and time of assessment on outcomes.
Results
Controlling for age, the combined traumatic injury group experienced significantly higher post-injury levels of SD (p=.042) and internalizing problems (p=.024) than TD children; however, TBI and EI injury groups did not differ from each other. Injury severity was positively associated with SD in the EI group only, but in both groups SD was associated to additional post-injury sequelae, including fatigue and externalizing behavior problems. Internalizing problems predicted subsequent development of SD but not vice-versa. The relation between injury and SD 1 year later was consistent with mediation by internalizing problems at 6 months post-injury.
Conclusions
Children with both types of traumatic injury demonstrated higher SD and internalizing problems than healthy children. Internalizing problems occurring either prior to or following pediatric injury may be a risk factor for post-traumatic SD. Consequently, internalizing problems may be a promising target of intervention to improve both SD and related adjustment concerns.
Keywords: sleep, internalizing problems, injury, outcome, adjustment, TBI
Unintentional injuries, including both head and non-head injury, are the leading cause of death and disability for individuals aged 1–19 in the U.S (Centers for Disease Control and Prevention, 2012). Per year, 12,000 people aged 0–19 die from such injuries, while more than 9.2 million children are treated in emergency departments for nonfatal injuries (Borse et al., 2008). Traumatic brain injury (TBI) is a leading cause of injury; an average of 1.7 million persons every year have a TBI either alone or in conjunction with other injury (Faul, Xu, Wald, & Coronado, 2010). These estimates are based on emergency-related visits, and do not include the large number of patients who either do not seek care or receive only outpatient care (Arbogast et al., 2016). Thus, the true incidence rate is likely even higher. Pediatric TBI remains a stark public health concern, as survivors are often struck with debilitating and cumulative deficits in multiple domains after injury. Similarly, studies examining outcome following orthopedic injury have identified a surprisingly high rate of psychological health and adaptive behavior difficulties (Ding et al., 2006; Fay et al., 2009; Zatzick et al., 2008a; Zatzick et al., 2008b). Consequently, it is critically important to evaluate these long-term sequelae of both TBI and bodily injuries. A growing base of research suggests sleep disturbances (SD) (Beebe et al., 2007; Milroy, Dorris, & McMillian, 2008; Pillar et al., 2003; Shay et al., 2014; Sumpter, Dorris, Kelly, & McMillan, 2013, Tham et al., 2012) and internalizing problems (Anderson et al., 2012; Kirkwood et al., 2000; Max et al., 2011; Max et al., 2012a; Max, 2014) are prevalent and debilitating problems after pediatric TBI and to a lesser extent after bodily injury. Given the importance of sleep for cognitive and emotional functioning (Alfano & Gamble, 2009), investigation of sleep quality and disturbances is essential to improving clinical care of children and adolescents with physical injury.
Pediatric Sleep Disturbance and Associated Impairment
SD is unfortunately common in children, and typically manifests in several ways. SD in children encompasses four general problem areas: insufficient sleep, fragmented or disrupted sleep, excessive daytime sleepiness, and circadian rhythm disorders (Owens, 2009). Up to 20–25% of children exhibit sleep problems at some point during development (Mindell, Owens, & Carskadon, 1999), and lifetime presence of insomnia in 13–16 year olds may be greater than 10% (Owens, 2009). SD in children and adolescents are associated with widespread problems related to cognitive, behavioral, socioemotional, and physical development. In other words, children who have persistent sleep problems are also at risk for experiencing additional psychopathology, particularly internalizing problems (Alfano & Gamble, 2009; McMakin & Alfano, 2015). In addition to increased anxiety, other psychological difficulties associated with SD include significantly reduced sustained attention and psychomotor vigilance (Sadeh, Raviv, & Gruber, 2000), higher rates of behavioral problems including oppositionality (Beebe, Fallone, Godiwala, & Flanigan, 2008), decreased ability to deal with complex cognitive tasks (Sadeh, Gruber, & Raviv, 2002), and physical/cardiovascular health problems (Amin et al., 2002; Gozal, & Kheirandish-Gozal, 2008). Clearly, SD is associated with a number of adverse cognitive and psychological health outcomes.
In addition to concurrent psychological health concerns, sleep problems independently predict development of emotional and behavioral problems later in life (Reynolds & Alfano, 2016). However, whether SD predicts the later occurrence of problems, including internalizing problems, or whether psychological health problems predict SD is still a point of investigation. Several studies demonstrate children with psychological problems and disorders are at risk for later SD (Alfano, Beidel, Turner, & Lewin, 2006; Alfano, Ginsburg, & Kingery, 2007; Gregory, Eley, O’Connor, & Plomin, 2004; Ong, Wickramaratne, Min, & Weissman, 2006) while others suggest that SD predicts later developing problems (Gregory et al., 2005; Johnson, Chilcoat, & Breslau, 2000; Reynolds & Alfano, 2016). As a whole, the evidence suggests there is a bidirectional relation between sleep and mental health. In the area of pediatric injury, parent-reported SD has predicted psychological health problems (Theadom et al., 2016, Shay et al., 2014); however, no studies have investigated bidirectional relations among these outcomes. This information is essential for developing targeted interventions to address sequelae after pediatric injury.
Sleep Disturbance and Related Difficulties Following Traumatic Brain Injury
Despite the relative dearth of empirical investigations and variability in methods, several studies have shown higher rates of subjective (parent and child) and objective reported SD in children with TBI than in typically developing or injured comparison groups (Beebe et al., 2007; Gosselin et al., 2009, Milroy et al., 2008, Shay et al., 2014, Sumpter et al., 2013, Tham et al., 2012). Findings converge in identifying disturbances in the acute and chronic stage of recovery, as well as across the range of injury severity. However, accurate assessment of prevalence of pediatric SD after TBI is difficult, given the paucity of rigorous research in the area. Epidemiological studies targeting sleep quality in children with TBI have not been published. Clinically oriented studies have varied substantially in terms of methods used to characterize SD. The breadth of coverage of SD behaviors afforded by the questionnaires ranges from single items rating SD to validated, multidimensional questionnaires assessing multiple dimensions of sleep quality (Shay et al., 2014; Tham et al., 2012). Thus far, only a small number of investigations have included either a healthy or other injury comparison group in their study design, and many focus on SD in samples of children with only a specific level of TBI severity, such as mild TBI (Milroy et al., 2008; Sumpter et al., 2012). Other methodological differences include the timing of assessment of SD, the respondent used, and whether preinjury SD was evaluated (Gagner, Landry-Roy, Lainé, & Beauchamp, 2015).
Consensus has not been reached as to whether children with bodily injury, often utilized as comparison groups in TBI research, also endure significant levels of SD. The few available studies comparing TBI with bodily injury suggest that SD is higher in children with TBI. However, a recent study compared SD in children with TBI versus orthopedic injury, finding over 17% of children in each group had new-onset sleep problems 12 months after injury (Ewing-Cobbs et al., 2014). The type and severity of bodily injury sustained in injury comparison groups is not consistent among studies. For instance, Tham et al. (2012) recruited children with isolated arm fractures, whereas Beebe et al. (2007) required at least one night of hospitalization after bodily injury. The influence of injury and non-injury related factors impacting SD over the course of child development has yet to be comprehensively studied. Pain occurring after traumatic injury has been associated with SD in children with other injury types, such as severe burns and children with chronic pain, although mechanism and pathology of injury is more specific in such cases (Kravitz et al., 1993; Mayes et al., 2013; Roth-Isigkeit, Thyen, Stöven, Schwarzenberger, & Schmucker, 2005).
Injury severity is commonly linked to SD after pediatric TBI. In the most methodologically rigorous study to date, Shay et al. (2014) examined parent reported SD and found that young children with mild and moderate/severe TBI experienced SD in distinct ways. While those with complicated mild and moderate TBI demonstrated more total sleep problems than children with orthopedic injury at 6 months, children with severe TBI reported more bedtime resistance than children with orthopedic injuries. Children with severe TBI also had persistently shorter sleep duration than those with complicated mild TBI 18 months after injury. In school-aged children and adolescents, the pattern of findings is less clear; while some studies noted that mild TBI was associated with actigraphy-, parent-, and child-reported SD (Milroy et al., 2008; Tham, Fales, & Palermo, 2015), Sumpter et al., (2013) found significantly more SD after moderate-severe TBI than in healthy controls. Injury severity has also been related to changes in sleep quality from preinjury to post-injury. Tham et al. (2012) found increased parent-reported SD post-injury after both mild TBI and bodily injury. In contrast, Beebe et al. (2007) showed increased parent-reported SD after severe TBI but not in children with mild TBI, moderate TBI, or orthopedic injury. Because of the many factors that influence recovery after injury, the relation between TBI severity and sleep problems is not yet well understood.
A wide range of psychological health difficulties has been observed in patients with SD after TBI. In young children with TBI, parent-reported sleep problems were associated with an increased risk for externalizing and internalizing problems (Shay et al., 2014). Additionally, Tham et al. (2012) reported that parent-assessed SD in moderate and severe TBI predicted poorer behavioral adjustment and participation levels. In another report of objective and subjective SD after TBI, children with TBI had poorer quality of life and more behavioral problems than sibling controls, but this was not statistically related to SD (Sumpter et al., 2003). Adding to potential interweaving pathways, the association between pain, fatigue, and SD has been demonstrated as well, especially in adult populations (Ponsford, Schönberger, & Rajaratnam, 2015; Ponsford & Sinclair, 2014). A study of 8–18 year olds 6 weeks following TBI demonstrated that parent reported symptoms of depression and SD predicted fatigue (Crichton et al., 2016). Child-reported fatigue, however, did not predict SD, adding to the intricate picture of SD after pediatric TBI. More work is needed to shed light on interrelations of these complex injury sequelae and SD and their impact on health outcomes.
Rationale
The literature on SD following pediatric injury is sparse. Few studies have targeted longitudinal associations between SD, injury type, and severity of injury. SD in children has been associated with psychological health difficulties such as internalizing problems; however, whether the relation between SD and psychopathology is unidirectional or bidirectional warrants further investigation in injured populations.
The purpose of this study was to further characterize the long-term course of SD and internalizing problems in children hospitalized following TBI or extracranial injury (EI) relative to a healthy comparison group. We examined the impact of traumatic injury on behavioral outcomes broadly by comparing injured to typically developing (TD) children without injuries, and then examining the effect of TBI above the effects of bodily injury by comparing each injury type to each other. The relation between SD and internalizing behavior problems was evaluated across the first year after injury. To examine the impact of co-occurring physical and behavioral symptoms, the relation of pain, fatigue, and externalizing disorders to SD was also examined.
Hypotheses
We expected that greater severity of TBI and EI would be associated with greater SD and internalizing behavior problems at 6 and 12 months post-injury.
Children suffering traumatic injury were expected to show higher SD than TD children across time and children with TBI were expected to show higher SD than children with EI.
Similarly, internalizing behavior problems were expected to vary by group. Children with traumatic injury would have higher levels of internalizing behaviors than TD children at 6 and 12 months. In addition, internalizing behavior problems in children with TBI would be higher than in children with EI, and this pattern would be consistent across time.
An additional research question addressed possible bidirectional relations between SD and psychopathology. SD has been shown to predict the development of anxiety and depression; conversely, internalizing symptoms have been shown to predict SD. Therefore, relations among baseline and post-injury SD and internalizing symptoms were examined to determine whether SD influenced the development of internalizing symptoms, whether internalizing symptoms influenced SD, or whether the relations were bidirectional. In addition, we investigated whether the relation of internalizing problems and chronic SD differed in injured children relative to healthy children.
Methods
Participants
Children and adolescents ages 8–15 injured in vehicle accidents (n=60) were enrolled in a longitudinal prospective study. In addition, healthy children (n=27) in the same age range were recruited and enrolled using informational flyers that were posted throughout the community in public spaces (e.g., health and community centers, children’s sports events, YMCA, museums, etc.). All participants with TBI or EI were recruited from a Level 1 Pediatric Trauma Center at Children’s Memorial Hermann Hospital/University of Texas Health Science Center at Houston. Additional demographic and injury data are presented in Table 1.
Table 1.
Demographics and Clinical Characteristics by Group
| Group | ||||
|---|---|---|---|---|
| Traumatic brain
injury (n = 36) |
Extracranial
injury (n = 24) |
Typically
developing (n = 27) |
Statistic | |
| Age at baseline in years, M (SD) | 12.2 (2.4) | 11.7 (2.4) | 11.9 (2.7) | F(2,84)=.270, p=.766 |
| Males, n (%) | 63.9% | 70.8% | 37.0% | χ2(2, N=87) = 6.97, p<.031 |
| Hollingshead, M (SD) | 38.8 (14.8) | 34.6 (14.6) | 38.8 (12.9) | F(2,84)=.760, p=.470 |
| Race/Ethnicity | χ2(8, N=87) = 9.08, p=.336 | |||
| African American | 11.1% | 20.8% | 25.9% | |
| Asian | 2.8% | 0.0% | 3.7% | |
| Caucasian | 30.6% | 25.0% | 14.8% | |
| Hispanic/Latino | 50.0% | 54.2% | 40.7% | |
| Mixed ethnicity | 5.6% | 0.0% | 14.8% | |
| Injury characteristics | ||||
| M(SD) | ||||
| Months from injury to | 1.5 (0.7) | 1.7 (0.8) | ||
| baseline | t(58)=1.07, p=.291 | |||
| Length of hospital stay | 6.4(6.7) | 3.3(3.6) | t(58)=−2.06, p=.044 | |
| ISS score | 17.7(11.8) | 10.3(6.8) | t(58)=−2.48, p=.012 | |
| Modified ISS score | 7.31(9.95) | 10.13(6.79) | t(58)=1.21, p=.231 | |
| GCS score distribution | ||||
| 3–8 | 30.6% | |||
| 9–12 | 8.3% | |||
| 13–15 | 61.1% | |||
Note. TBI=traumatic brain injury, EI=extracranial injury, TD=typically developing, M = mean, SD = standard deviation, GCS = Glasgow Coma Scale, ISS = Injury Severity Score.
Children with EI were included in the study design to account for potential preinjury characteristics, such as propensity for risk-taking behaviors, for the potential traumatic stress of injury and hospitalization, and to allow comparison of the effects of TBI above and beyond that of bodily injury (Langeland & Olff, 2008; Mellman, David Bustamante, Fins, & Esposito, 2001). Inclusion criteria for all injured participants were: 1) age at injury between 8 and 15 years, 2) hospitalization for TBI, including uncomplicated mild TBI, or EI, 3) proficiency in English, 4) residing within a 125 mile catchment radius, 5) no history of major neuropsychiatric disorder such as intellectual deficiency or low functioning autism spectrum disorder to control for complications of the assessment of impact of injury on behavioral outcomes, and 6) no prior diagnosed TBI. The latter two criteria were assessed during screening using a brief parent interview as well as review of medical records for children in the injury groups. The healthy comparison group met criteria 3–6.
For the TBI group, a total of 48 eligible children were hospitalized, three withdrew participation after 6 weeks, one withdrew after 6 months, and eight were lost to follow up, resulting in a sample of 36 children with TBI. Of the 33 patients hospitalized for EI, one withdrew participation after 6 weeks, and four were lost to follow up, one was excluded due to pregnancy, and one was excluded due to the diagnosis of a major psychiatric disorder, resulting in a sample of 24 children with EI. Of 31 eligible healthy children enrolled in the typically developing (TD) group, two withdrew after 6 weeks and two withdrew after 6 months, resulting in 27 children in the TD group.
TBI severity was assessed using the Glasgow Coma Scale (GCS; Teasdale & Jennett, 1974). Accepted classification considers GCS scores of 13–15 rated as mild, 13–15 with evidence of parenchymal injury on acute computed tomography (CT) scan rated as complicated mild, 9–12 rated as moderate TBI, and 3–8 rated as severe TBI (Levin et al., 2008; Williams, Levin, & Eisenberg, 1990). Participants in the EI group had no history of head trauma or symptoms of concussion at hospitalization. The Injury Severity Score (ISS) was used to assess bodily injury severity, and was calculated by summing the squares of the 3 most severely injured body regions as rated by the Abbreviated Injury Scale (AIS; Buckley et al., 1994). Children with bodily injury suffered a wide range of extremity injury and/or internal injuries involving the abdomen or chest. In children with mild or moderate TBI, skeletal or body AIS scores were limited to 2 or less to minimize any confounding influence of severe EI on accurate assessment of GCS scores. Modified ISS (mISS), defined as ISS not including the head, was measured for children in the TBI group (Osler, Baker, & Long, 1997). A mISS of 0–9 was considered minimal or minor, whereas mISS>10 was considered moderate to severe.
Procedure
Informed written consent was obtained from the child’s guardian, in accordance with guidelines established by the hospital and university institutional review board. Children who were ages 11 and older signed the written adolescent ascent while those under 11 signed the written child assent.
Parents completed questionnaires in a quiet testing room at 6 week, 6 month, and 12 month time points. Data collected at 6 weeks reflected initial level of functioning for children in the TD group, and functioning just prior to the injury for children in EI and TBI groups. Children were administered a comprehensive neuropsychological battery as part of a larger study at the same time intervals.
Measures
Sleep disturbance
The Sleep Disturbance Scale for Children (SDSC) is a well-validated parent-report measure of childhood SD, demonstrating internal consistency (0.71) and test-retest reliability (.71) that has been normed with children aged 6 to 15 (Bruni et al., 1996; Spruyt, Cluydts, & Verleye, 2004). The 26-item measure uses a 5-point rating scale (0 = least severe and 5 = most severe) and contains 6 subscales previously categorized using factor analysis. The sleep disturbance subscales are: sleep initiation and maintenance (insomnia), sleep-related breathing, sleep arousal/nightmares, sleep-wake transition, excessive somnolence (daytime sleepiness or fatigue), and hyperhidrosis (bed sweating). The SDSC has been effectively used as a routine measure of sleep in children with neurological disorders as well (Cohen, Halevy, & Shuper, 2013). The current study utilized the total score, representing overall SD, which is stable across age in both healthy and SD populations (Bruni et al., 1996). Bruni and colleagues suggested a diagnostic cut-off raw score of 39, which is less than one standard deviation above the normative group mean (raw score of 43 = T score of 60). Internal consistency of the total score in the current study was high for baseline (α = 0.87), 6 month (α = 0.89), and 12 month time points (α = 0.89).
Internalizing and externalizing behavior problems
The Child Behavior Checklist (CBCL) is a widely accepted parent-reported measure consisting of 112 items targeting various domains of behavioral functioning. The measure has demonstrated high test-retest reliability and validity for 6–18 year olds (Achenbach & Rescorla, 2001). Intraclass correlations in the .90s have been demonstrated for inter-parent agreement, 1-week test-retest reliability, and inter-interviewer reliability. The current study used the CBCL scale T-score for internalizing behavior problems, and explored the externalizing behavior problems broadband scale T-score post-hoc.
Pain
Items related to physical pain on the CBCL were used in post-hoc analyses to inform relations with injury severity and SD. Items consisted of rating physical problems of aches or pains, headaches, or stomachaches without known medical cause using a three-point scale of “not true,” “somewhat or sometimes true,” or “very true.”
Fatigue
The Post Concussion Symptom Inventory (PCSI) is a 22-item self-report scale used to assess frequent symptoms occurring following concussion in children and adolescents. Post-hoc analyses used the PCSI’s factor analyzed fatigue scale, which has demonstrated moderate to strong internal consistency (0.79) and test-retest reliability (0.69–0.73) (Sady, Vaughan, & Gioia, 2014). Parent-reported fatigue was based on CBCL items, which asked whether children were “overtired without good reason,” or “underactive, slow-moving, or lacks energy.”
Pubertal development
The Pubertal Development Scale (Petersen, Crockett, Richards, & Boxer, 1988) is a validated self-report measure of pubertal status with favorable reliability (α =.77–.81). Children and their parent independently rated changes associated with puberty, including growth in height, body hair, and skin, and sex-specific changes. Each item was then coded on a 5-point scale similar to Tanner staging (Shirtcliff & Essex, 2008). Parent and child ratings were averaged to yield a score ranging from 1 (pre-pubertal) to 5 (post-pubertal).
Statistical Analyses
Hypothesis 1 investigated the association between injury severity with SD and internalizing behavior problems. The GCS was used to rate TBI severity, while the ISS represented EI severity. Spearman’s rho correlations were used due to the ordinal nature of the GCS. These analyses were repeated at 6 and 12-month time points for each injury group. Relations of age and pubertal status with SD and internalizing behavior problems were also examined using Spearman’s rho.
Post hoc analyses further examined whether SD and internalizing problems differed across TBI severity groups. One-way Analyses of Covariance (ANCOVA), controlling for age, compared children with uncomplicated mild TBI with those with complicated mild, moderate, or severe TBI. To investigate the influence of bodily injury within the TBI group, ANCOVA compared SD and internalizing behavior problems between those with TBI without significant bodily injury (mISS≤9) and those with significant bodily injury (mISS>10).
Hypothesis 2 investigated the relation between type of injury and change in SD across the follow-up using linear mixed models. Orthogonal planned group comparisons investigated the impact of 1) both injury groups versus the TD group, and 2) the TBI versus the EI group on level of SD reported at each time of assessment (baseline, 6 month, and 12 month) as well as the group by time interaction. Age was included as a covariate. Mixed models used unstructured covariance matrices to allow for unequal variances/covariances across time. The comparison between TBI and EI groups examined whether TBI influenced SD above and beyond the influence of bodily injury.
Post-hoc analyses examined explored whether pain and fatigue symptoms varied by group using one-way ANCOVA. Relations between pain, fatigue, and SD were explored for each injury group using Spearman’s rho.
Hypothesis 3 used the same approach to investigate group comparisons across time for internalizing problems. Cohen’s d was calculated as an estimate of effect size when p < .1. Post hoc analyses were conducted to assess relations between externalizing behavior problems and SD using Spearman’s rho.
Given the potential bidirectional relations that have been demonstrated between SD and psychopathology, the current study sought to characterize directional relations between SD and internalizing behavior problems over time. To address the research question, a cross-lagged panel path analysis was performed to characterize relations between SD and internalizing symptoms at baseline, 6 month, and 12 month time points. Within each time point for the cross-lagged panel, SD and internalizing behavior problems were allowed to correlate with each other. Paths were included from baseline to 6 month SD, from 6 month to 12 month SD, from baseline to 6 month internalizing, and from 6 month to 12 month internalizing. The cross-lag paths went from baseline SD to 6 month internalizing, from 6 month SD to 12 month internalizing, from baseline internalizing to 6 month SD, and from 6 month internalizing to 12 month SD. Significant cross-lag paths would indicate the possibility of causality over time. Due to the sample size, we were not able to include group in the cross-lagged model. However, directional pathways and time points identified in the model informed a multicategorical mediation model examining whether internalizing problems at 6 months mediated the relation between group and chronic sleep disturbance.
The multicategorical mediation model reflects a causal sequence in which a multicategorical independent variable (e.g., group), relates to a dependent variable (e.g., SD), indirectly through a mediator variable (e.g., internalizing behavior problems). Following the recommendations of Preacher & Hayes (2008), a bootstrapping procedure was run using 10,000 samples with replacement to assess the indirect effect. Based on these samples, approximations of the distribution of the indirect effect are obtained and a point estimate and confidence interval are calculated. The point estimate of an indirect effect was considered significant when the confidence interval did not straddle zero.
Results
Data analyses were run using IBM SPSS Version 22 (2013), and SAS 9.4 (SAS Institute, Cary NC). Data screening strategies and goodness-of-fit checks were conducted before analyses. Outliers were examined for inclusion or exclusion from the current study. Screening of outliers identified two extreme values greater than three standard deviations above the mean only for the baseline SD; consequently, these values were trimmed and replaced with consecutive values just above the cutoff. Examination of the distribution of the data revealed that skewness and kurtosis were found to be within acceptable limits for all variables.
Demographic variables of interest were examined using independent samples t-test, analysis of variance, chi-square and linear regression analyses (Table 1). Developmental influences, as measured by age and pubertal status at initial evaluation, did not vary significantly by group. Socioeconomic status as measured by the Hollingshead-Redlich Index (Hollingshead, 1975), and race/ethnicity also did not differ significantly by group. As expected, sex varied significantly by group, in that injury groups included more males than the TD group as expected based on the epidemiology of pediatric injury (Faul et al., 2010). None of the above demographic variables correlated significantly with baseline SD or internalizing scores; all p’s >.05. Regarding injury variables, children with TBI had longer length of hospital stay than children with EI. As expected, length of stay was also correlated with severity of injury in both injury groups, however it was not significantly associated with outcomes. mISS scores did not vary across injury groups, suggesting similar severity of extracranial injury. Expectedly, the ISS score was significantly higher in the TBI group compared to the EI group. Medications including pain/anti-inflammatory, psychotropic, and allergy medications, as well as stimulants and other prescribed medications, did not vary by group at either 6 months or 12 months. Mean SDSC scores for the TBI and EI group at baseline were above a diagnostic cutoff found by Bruni and colleagues (1996), whereas the number of children above this cutoff at baseline did not vary by group χ2(2, N=87) = 2.56, p=0.28 (Table 2).
Table 2.
Longitudinal Sleep Disturbances and Internalizing Outcomes by Group
| Measure | Typically developing | Extracranial injury | Traumatic brain injury |
|---|---|---|---|
| Sleep Disturbance Scale for Children | |||
| Baseline M(SD) | 37.7(6.6) | 39.3(9.3) | 41.9(10.4) |
| 6M M(SD) | 37.7(8.4) | 43.3(15.8) | 43.0(14.1) |
| 12M M(SD) | 35.4(6.3) | 41.1(16.5) | 39.7(10.6) |
| CBCL Internalizing Scale | |||
| Baseline M(SD) | 50.9(9.2) | 53.0(12.9) | 52.7(12.6) |
| 6M M(SD) | 47.3(9.6) | 54.8(12.9) | 54.6(13.8) |
| 12M M(SD) | 45.5(7.5) | 54.1(13.3) | 52.7(13.0) |
Note. Baseline, 6, and 12 month outcomes are provided. 6M = 6 month, 12M = 12 month, M = Mean, SD = Standard deviation, CBCL = Child Behavior Checklist.
Injury Severity and Behavioral Outcomes
Hypothesis 1 investigated the association of injury severity with SD and internalizing problems. Age and pubertal stage were not correlated with SD or internalizing behavior problems. In children with TBI, severity of injury as indicated by GCS was not associated with either SD or with internalizing behavior problems at 6 or 12 months. Likewise, severity of injury as indicated by ISS was not associated with SD at 6 or 12 months, nor with internalizing behavior problems at 6 or 12 months post-injury. In children with EI, the ISS score was significantly positively associated with SD at 6 months, r(22) = .45, p = .03, but not at 12 months, r(22) = .27, p = .20. However, the ISS score in children with EI was significantly positively related to internalizing behavior problems at 6 months, r(22) = .47, p = .02, and 12 months post-injury, r(22) = .59, p = .01. Results remained the same when using the mISS score.
Post-hoc analyses were conducted to examine the impact of the severity of TBI and co-occurring bodily injury in the TBI group on SD and internalizing problems. Controlling for age, an ANCOVA comparing children with uncomplicated mild TBI to those with complicated mild-moderate-severe TBI revealed that SD did not significantly differ at baseline or follow-up intervals. Similarly, comparison of children with TBI but without bodily injury and those with TBI and bodily injury did not demonstrate significantly differing levels of SD or internalizing behavior problems at baseline, 6 months, or 12 months after injury.
Group Differences in Sleep Disturbance and Related Physical Symptoms
Regarding hypothesis 2, in order to determine SD levels across time in those with traumatic injury and those in the TD group, a linear mixed model analysis was conducted with age as a covariate. The group by time interaction was not significant and was trimmed from the models. Above and beyond the influence of age, children with bodily injury or head injury experienced significantly higher levels of SD than typically developing children, demonstrating a moderate effect size (Table 3). Although the combined TBI and EI groups did not differ from the TD group at baseline, injured children trended towards having higher SD scores than the TD group at 6 (d = −0.39) and 12 months (d = −0.41), with moderate effect sizes. With respect to the TBI versus EI group contrast, SD did not significantly differ between TBI and EI groups overall or at any of the assessments.
Table 3.
Statistics for Linear Mixed Models for Planned Group Comparisons of Sleep Disturbances and Internalizing Problems by Time
| Sleep disturbances | Internalizing behavior problems | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| df | Statistic | p | d | df | Statistic | p | d | |
| Typically developing vs. injured groups | ||||||||
| Group | 83 | t(−2.04) | 0.044 | −0.45 | 83 | t(−2.30) | 0.024 | −0.50 |
| Baseline | 83 | t(−1.41) | 0.162 | – | 83 | t(−0.73) | 0.464 | – |
| 6 Months | 83 | t(−1.78) | 0.078 | −0.39 | 83 | t(−2.59) | 0.011 | −0.57 |
| 12 Months | 83 | t(−1.87) | 0.065 | −0.41 | 83 | t(−2.73) | 0.008 | −0.60 |
| Traumatic brain injury vs. extracranial injury group | ||||||||
| Group | 83 | t(−0.18) | 0.856 | – | 83 | t(0.04) | 0.970 | – |
| Baseline | 83 | t(1.14) | 0.257 | – | 83 | t(0.01) | 0.991 | – |
| 6 Months | 83 | t(0.02) | 0.981 | – | 83 | t(−0.05) | 0.958 | – |
| 12 Months | 83 | t(0.42) | 0.675 | – | 83 | t(0.14) | 0.885 | – |
| Main effect | ||||||||
| Time | 83 | F(2.68) | 0.075 | – | 83 | F(2.28) | 0.109 | – |
Note. Planned contrasts using linear mixed models demonstrated children with traumatic brain injury or extracranial injury had greater sleep disturbances and internalizing problems than those in the typically developing group. d refers to effect size via Cohen’s d.
To assess whether other post-traumatic symptoms commonly associated with injury were related to SD, pain and fatigue symptoms at were examined post-hoc. However, only fatigue at 12 months varied by group F(83)=3.33, p = .04, with higher fatigue in both injury groups. Parent-reported pain in the TBI group was significantly associated with SD at 6 months but not at 12 months (Table 4). Parent-reported fatigue was positively associated with levels of SD at 6 months and at 12 months in both injury groups. Additionally, child-reported fatigue in both injury groups was positively associated with SD at 12 months. Parent-reported fatigue at 12 months was positively correlated with ISS in the bodily injury group, and child-reported fatigue at 12 months was positively related to GCS in the TBI group. Child-reported fatigue did not significantly vary when comparing children with uncomplicated mild TBI to children with complicated mild-moderate-severe TBI at 6 weeks F(33)=0.00, p = .97, 6 months F(33)=1.31, p = .26, or 12 months F(33)=0.04, p = .85.
Table 4.
Post-hoc correlations among sleep disturbance, fatigue, pain, and injury severity
| Parent Report | Child Report | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Fatigue | Pain | Fatigue | ||||
|
| ||||||
| TBI | EI | TBI | EI | TBI | EI | |
|
Sleep Disturbance |
||||||
| 6M | 0.57** | 0.48* | 0.39* | 0.35 | 0.24 | 0.33 |
| 12M | 0.60** | 0.58** | 0.15 | 0.26 | 0.41* | 0.46* |
|
Injury Severity |
||||||
| GCS | ||||||
| 6M | 0.14 | – | 0.11 | – | 0.11 | – |
| 12M | −0.05 | – | –0.22 | – | 0.36* | – |
| ISS | ||||||
| 6M | 0.02 | 0.34 | −0.31 | 0.44* | −0.02 | 0.08 |
| 12M | 0.09 | 0.42* | −0.14 | 0.39 | −0.08 | 0.14 |
Note.
= p<.05,
= p<.01, TBI = traumatic brain injury, EI = extracranial injury, GCS = Glasgow Coma Scale, ISS = Injury Severity Score.
Group Differences in Internalizing Behavior Problems
Regarding hypothesis 3, internalizing behavior problems were expected to be elevated after injury in the TBI relative to the EI group. Planned group comparisons were investigated via a linear mixed model. The group by time interaction was nonsignificant and was trimmed. This hypothesis was partially supported; accounting for the influence of age, internalizing problems were higher in the combined TBI and EI group when compared to the TD group, with a moderate effect size observed (Table 3). Although the TBI and EI groups did not differ from the TD group at baseline, injured children had significantly higher internalizing scores than the TD group at 6 and 12 months with moderate effect sizes. For the TBI versus EI group comparison, internalizing behavior problems did not differ significantly between TBI and EI groups overall or at any time point.
Parent-reported externalizing problems were examined post-hoc. Expectedly, externalizing and internalizing behavior problems were highly intercorrelated following TBI and EI; coefficients ranged from .81 to .87 at 6 months and .67 to .89 at 1 year (all ps < .001). SD was significantly positively associated with externalizing problems in the TBI group (baseline: r(34) = .38, p = .02; 6 months: r(34) = .71, p = .00 ; 12 months: r(34) = .52, p = .00) and in the EI group (baseline: r(22) = .71, p = .00; 6 months: r(22) = .82, p = .00 ; 12 months: r(22) = .76, p = .00), but not in the TD group (baseline: r(22) = .18, p = .36; 6 months: r(22) = .29, p = .14 ; 12 months: r(22) = −.07, p = .72.
Longitudinal Relations Between Sleep Disturbance and Internalizing Problems
This study used a cross-lagged panel design to further investigate potential bidirectional relations between SD and internalizing problems over time, and to help specify mediational pathways of interest. The full sample original cross-lag panel model did not fit the data well (χ2(4) = 50.932; p < 0.001; CFI = 0.745; TLI = 0.107; SRMR = 0.112). Examination of the residuals and modification indices indicated the need for a path between baseline internalizing behaviors to sleep disturbance at 12 months. When this path was added, the model fit the data reasonably well (χ2(3) = 3.360; p = 0.3394; RMSEA = 0.037, p = 0.434; CFI = 0.999; TLI = 0.994; SRMR = 0.024). Figure 1 provides the path coefficients for the longitudinal relations of sleep and internalizing problems. The results showed that the covariances between the measures at the same time point were significantly greater than zero, as expected and that autoregressive paths (paths relating a variable to itself over time) were as well. Paths from earlier internalizing behavior to later SD were positive and significant, suggesting that higher internalizing scores predicted greater SD at subsequent time intervals. In contrast, the cross-lag paths from SD to later internalizing problems were not significant (p = 0.364, 0.752, respectively), indicating that SD did not predict later levels of internalizing problems. Initial baseline internalizing scores predicted SD at 6 months, p = 0.02, and internalizing problems at 6 months predicted SD at 12 months, p < 0.001. Although the path from baseline internalizing to SD at 12 months was inverse (p < 0.001), the path from the immediately preceding assessment indicates the most important time-linked relation of internalizing problems and subsequent SD.
Figure 1.
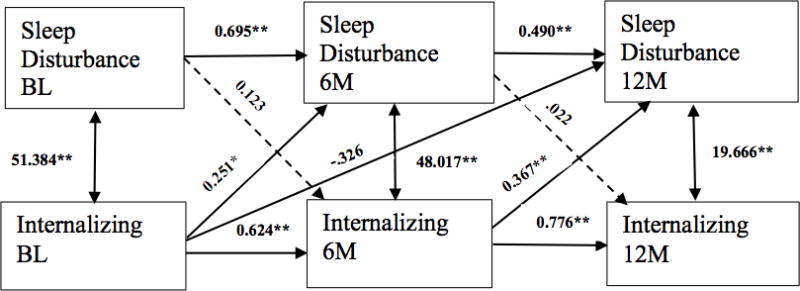
Longitudinal cross-lagged panel of sleep disturbance and internalizing problems. Parameter estimates are labeled for each pathway. The model indicates internalizing behavior problems lead to sleep disturbance, but not that sleep disturbance leads to internalizing. * =p<.01; ** =p<.001; BL = Baseline/initial evaluation; 6M = 6 months; 12M =12 months.
A multicategorical mediation model was run to investigate whether internalizing problems at 6 months mediated the relation between group and chronic SD, using the TD group as the reference group (Hayes & Preacher, 2014). The model demonstrated that the relation between each injury group and SD at 12 months was consistent with mediation by the level of internalizing behavior problems at 6 months. Therefore, this significant pathway illustrates time precedence such that an increase in internalizing at 6 months for either the TBI or EI group was associated with an increase in chronic SD (Figure 2). However, as this model does not include any potential confounding variables, it is possible that other models could be tenable.
Figure 2.
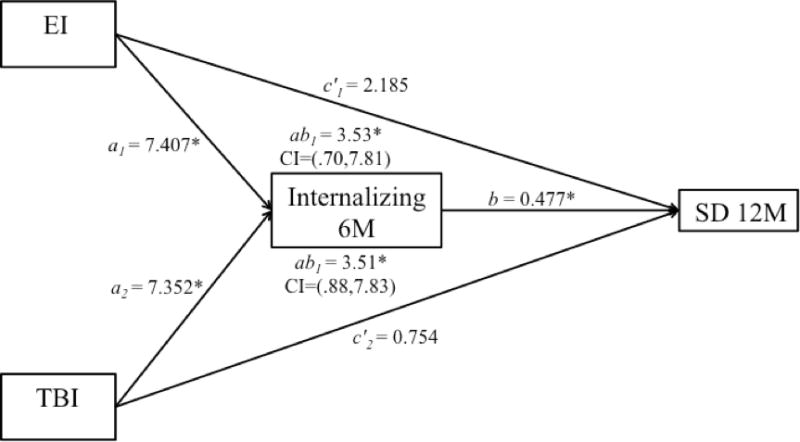
The effect of group on 12 month sleep disturbances was mediated through the effect of injured groups internalizing behavior problems at 6 months. EI = extracranial injury, TBI = traumatic brain injury, SD = sleep disturbance, 6M = 6 month, 12 = 12 month, CI = confidence interval, path ab1 = indirect effect of EI on internalizing, path ab2 = indirect effect of TBI on internalizing. * p<.05.
Discussion
The aims of the current study were to compare SD among children and adolescents with TBI, bodily injury, and typically developing children, and to better understand the relation between such SD and internalizing behavior problems during the year following injury. To fill a notable gap in the research literature, we included all three groups of children in order to be able to differentiate problems related to post head injury versus post bodily injury functioning. Neither parent-reported SD nor internalizing problems differed by group at baseline, suggesting similar pre-injury levels of functioning. However, all children with traumatic injury developed higher levels of SD and internalizing problems than the healthy comparison group at both 6 and 12 month follow-ups, as indicated by moderate effect sizes. Contrary to expectations, TBI and EI groups did not differ on either outcome at any time point. Severity of EI, but not TBI, predicted post-injury SD and internalizing problems. The similarity in post-injury SD and internalizing problems in children with TBI and EI raise the question of whether some symptoms commonly attributed to TBI may be associated with effects of traumatic injury more generally.
We also examined the directionality of the relation between parent-reported SD and internalizing problems and the impact of injury on this relation. Using a cross-lagged model, bidirectional pathways between SD and internalizing problems were examined across time. Consistent with a host of previous research, internalizing problems predicted SD at all subsequent time points. However, the opposite was not true (i.e., SD did not predict later internalizing problems), suggesting a unidirectional pathway in children with injuries. This pathway was similar in both injury groups. Mediation analysis demonstrated that for both the TBI and EI groups, increased internalizing problems at 6 months post-injury mediated the relation of group with chronic SD one year post-injury. These findings expand on the current literature by further illustrating the dynamic and persistent psychological and behavioral problems children experience after both TBI and bodily injury, with substantial symptom burden persisting across the first year after injury.
Injury Severity and Behavioral Outcomes
Contrary to prior research, we did not find a significant relation between severity of TBI and parent-reported SD. In the current study, EI injury severity, as indexed by the ISS, was positively associated with SD at 6-months but not at 12 months post-injury. Alternative to our hypothesis, neither GCS nor mISS scores were related to SD in participants with TBI. Several recent studies reported that SD was positively associated with level of TBI severity as indexed by the GCS score or classification based on clinical findings (Beebe et al., 2007; Osorio et. al., 2013; Shay et al., 2014; Tham et al., 2012). Current literature on relations between SD and pediatric TBI severity is limited, such that studies have shown SD relates to mild TBI but not severe TBI, and vice versa.
In children with EI, injury severity was also positively associated with internalizing behavior problems at 6 and 12 months following injury. To the authors’ knowledge, the current study is the first to demonstrate associations between greater bodily injury severity in children with EI and higher SD and internalizing behavior problems. This relation was not significant in children with TBI irrespective of TBI severity using continuous GCS scores or categorical severity groups. It should be noted however that some children in the TBI group also suffered bodily injury, limiting our ability to determine precise pathophysiological relationships. It is possible that bodily injury in addition to TBI compounded negative outcomes in these children. However, levels of SD and internalizing problems did not vary between children with TBI and minor or no bodily injury, and those with TBI and moderate to severe bodily injury. Still, this result was somewhat confounded due to study design: those with mild TBI were only included in the study if AIS scores were 2 or lower, but those with higher severity of TBI and higher AIS scores were included. Thus, bodily injury in the current study did not appear to have an impact on SD above and beyond TBI. It may be that negative impacts of traumatic injury play a role in the progression of behavioral outcomes whether suffering TBI, bodily injury, or both. Future work is needed to better delineate etiological differences using larger samples or excluding children with TBI who have also suffered bodily injury.
Although some studies have found a dose-response relation between TBI severity and psychological outcomes, others have been unable to find a significant relation between severity and internalizing problems (Max et al., 2015; Peterson et al., 2013). Inconsistency of prior findings on injury severity and behavioral outcomes may be due to several factors, including varying methods of assessing TBI severity, SD, and internalizing problems, sampling variation across studies, differing assessment time intervals after injury, and non-injury related factors that may moderate the effect of injury severity. Research on both pediatric TBI and bodily injury severity is necessary to improve understanding of these relations.
Group Differences in Sleep Disturbance and Internalizing Problems
The relation between SD, TBI, and body injury is complex. Children with bodily injury and/or TBI demonstrated significantly higher levels of parent-reported SD compared to typically developing children. This supplements existing research illustrating the range of functional difficulties children experience after both TBI and bodily injury, such that both injury types can result in chronic impairment (Ding et al., 2006; Ewing-Cobbs, et al., 2014; Pillar, et al., 2003; Stancin et al., 2001; Sumpter, et al., 2013). There is a lack of published studies assessing SD specifically in traumatic injury populations, however one showed parent-reported SD in 16.7% of a sample of children one to five years post-TBI. Still, this rate was not significantly different from parent-reported SD in the bodily injury group (Necajauskaite, et al., 2005). Although Milroy et al. (2008) found greater parent-reported SD between children with TBI than with bodily injury, over half of those with bodily injury still experienced SD levels above a commonly used cutoff score on their sleep measure. Thus, in cases where significant TBI group differences emerge, often a substantial proportion of those in bodily injury groups still report clinically relevant SD.
Although SD levels did not significantly differ by group at baseline, mean baseline scores for the TBI and EI group were above an established clinical cutoff (Bruni et al., 1996). However, the number of children above this diagnostic cutoff at baseline did not vary by group and more recent studies have used a higher cutoff of T = 70, corresponding to a raw score of 50 (Cohen, Halevy, & Shuper, 2013; Romeo et al., 2013). Still, elevated levels of SD in these groups highlight important considerations. First, this finding is in line with a wealth of research emphasizing the importance of early sleep for emotional and physical health. Second, along with research finding increased rates of SD among children with high injury rates and more injury-prone behaviors (Owens, Fernando, & Guinn, 2005), the possibility that children in both injury groups had high levels of SD prior to injury is remarkable.
Parents of children with TBI or bodily injury consistently reported more internalizing problems during the first year after injury than parents of TD children (Table 3). The presence of higher levels of internalizing problems after injury, but not at baseline, points to the influence of experiencing a traumatic injury playing a key role in subsequent internalizing behavior problems. This finding is in line with past work demonstrating greater internalizing problems following TBI when compared to baseline functioning (Anderson et al., 2012; Max et al, 2011; Max et al., 2015; Peterson et al., 2013). Such findings support previous literature demonstrating internalizing behavior problems in children and adolescents after bodily injury (Zatzick et al, 2006; Zatzick et al., 2008a, Zatzick et al., 2008b).
Contrary to expectations, children with TBI and those with EI in the current study did not differ in their level of post-injury internalizing problems. This contrasts with prior literature reporting that children with TBI suffer from higher levels of internalizing behavior problems than children with bodily injury. For instance, children with mild and moderate/severe TBI had a higher incidence of new onset mood/anxiety disorders 6 months following injury than orthopedic comparison children (Luis & Mittenberg, 2002). Higher parent-reported internalizing problems were found in 6–12 year old children with moderate and severe TBI than in those with bodily injury at 6 and 12 months (Kirkwood et al., 2000). However, our sample also included children with mild TBI. It is possible the range of severity accounted for some variation in the level of behavioral problems reported after different anatomical injuries. Also, bodily injury groups often consist of children with heterogeneous injuries, as they did in the current study, which could account for some variability. Overall, following traumatic injury of varying types, literature suggests children experience higher levels of internalizing behavior problems in chronic stages when compared to problems in typically developing children, but there is a lack of robust consensus when comparing children with TBI to children with bodily injury.
In addition to internalizing problems, we identified significant relations of externalizing problems with SD. Internalizing and externalizing scores were highly intercorrelated in both injury groups but not in the healthy group. Pediatric injury may have a more generalized impact on internalizing and externalizing dimensions of behavior than previously recognized. However, the multicollinearity of our measures indicates very substantial shared variance and limits the separation of the independent effects of internalizing and externalizing problems. Future work using specific measures for each construct may be able to minimize the multicollinearity, further delineate underlying constructs, and improve interpretability of both constructs.
Additional injury-related variables such as pain and fatigue are also associated with SD (Crichton et al., 2016; Gagner et al., 2015; Ponsford et al., 1999; Tham et al., 2012). In adults with TBI, SD and fatigue are among the most commonly reported difficulties, particularly following moderate and severe TBI (Fogelberg, Hoffman, Dikmen, Temkin, & Bell, 2012; Ponsford & Sinclair, 2014; Ruff & Blake, 2016). However, less has been published in the pediatric literature. Considering past work demonstrating relations between SD and other behavioral outcomes such as pain and fatigue, we examined if such factors also played a role in outcomes. Post-hoc analyses showed parent-reported pain and fatigue were associated with SD in the chronic stages post-injury. Though SD did not relate to TBI severity, more mild TBI was associated with higher levels of child-reported fatigue. Conversely, parent-reported fatigue was significantly associated with higher bodily injury severity. Still, child- and parent-reported fatigue did not vary by severity group at any time point when comparing uncomplicated mild TBI to more severe TBI.
A limitation of these post-hoc analyses, however, is the multicollinearity of pain and fatigue-related items. The CBCL pain and fatigue items used are included on the measure’s internalizing scale, and both child and parent-reported fatigue items describe symptoms typically associated with SD, such as seeming “overtired,” rather than including items addressing other forms of fatigue, such as cognitive (sustaining mental effort) or physical fatigue (e.g., moving slow) (Aaronson et al., 1999). Future studies would benefit from using separate and validated pain and fatigue measures in a larger sample to assess their relations with SD and internalizing behavior problems after injury, especially considering evidence of relations between fatigue, internalizing, and SD following head injury (Ponsford et al., 2012; Ponsford, Schönberger, & Rajaratnam, 2015).
Longitudinal Relations Between Sleep Disturbance and Internalizing Behavior Problems
Several prior studies examining relations between childhood internalizing disorders including anxiety and depression and SD in children without injuries have yielded inconsistent findings. In a cross-sectional study, Johnson et al (2000) reported that parent-assessed sleep quality at 6 years did not predict anxiety or depressive symptoms at age 11. In contrast, some longitudinal studies demonstrated a reciprocal relation between anxiety and SD over extended periods of time. For instance, objective and subjectively measured SD at age 8 was predictive of anxiety and depression over the course of a 5-year follow up, while psychological adjustment also predicted sleep changes to a lesser extent (Kelly & El-Sheikh, 2014). In addition, in a sample of 1,420 children assessed 4 to 7 times from age 9 to 16, parent and child-reported SD predicted higher levels of anxiety, depression, and oppositional behavior problems, while such behavioral symptoms also predicted increases in SD over time (Shanahan, Copeland, Angold, Bondy, & Costello, 2014). Overall, although studies of uninjured children show SD and internalizing problems influence each other; there is stronger evidence that SD predicts later internalizing problems than vice versa (Willis & Gregory, 2015).
In the current sample examining children with diverse injuries, a bidirectional relation was not shown over the course of 1-year follow up. Results demonstrated higher levels of earlier internalizing behavior consistently led to greater SD at later time points. For instance, Figure 1 shows that higher internalizing problems at baseline were related to greater SD at 6 and 12 months. Additionally, higher internalizing scores at 6 months were associated with higher concurrent and chronic SD. This is in line with extensive research demonstrating children with higher anxiety and depressive symptoms are at risk for SD later on in life (Alfano et al., 2006; Alfano et al., 2007; Gregory et al., 2004; Ong et al., 2006), and current results expand on this literature by showing this relation with a sample of injured and non-injured children. Investigating predictive pathways in the other direction, SD at baseline and 6 months was not significantly related to later levels of internalizing problems, supporting a more unidirectional relation. In contrast, Shay et al. (2014) found those with SD were at an increased risk for internalizing and externalizing behavior problems after injury, however this was in a sample of 3–6 year old children. Aforementioned studies have shown SD to be associated with later psychological and behavioral problems as well, yet these studies also associated such psychological problems with early childhood SD or examined problems over the course of several years (Gregory & O’Connor, 2002; Johnson, Chilcoat, & Breslau, 2000). It is possible the varying presentation and course of SD and behavioral problems across developmental stages may play a role in conflicting findings.
Internalizing behavior problems in the current sample consistently predicted later SD. Specifically, in comparison to typically developing children, the effect of either type of traumatic injury on chronic SD at 12 months was fully mediated by internalizing problems at 6 months (Figure 2). This follows a recent study showing depressive symptoms predicted concurrent self-reported sleep quality in adolescents during the year after mild TBI and in healthy controls (Tham, Fales, & Palermo, 2015). Tham, Fales, and Palermo (2015) relied on one assessment of functioning after injury, thus the relation over time was not examined. Adding to this finding, the current study provides evidence that internalizing behavior problems occur across injury type, persist over time, and contribute to later SD. This investigation contributes to the developmental psychopathology literature on the association between SD and behavioral problems by showing that such behavioral problems predict SD in the chronic stages after injury, and by demonstrating a unidirectional but not bidirectional relation after childhood injury. Following injury, the path between SD and internalizing problems may operate uniquely, with internalizing risk increased more directly through trauma exposure and secondarily impacting SD.
Considering the high incidence of pediatric traumatic injury and the strong relation between physical and psychological health problems and SD (Alfano & Gamble, 2009, Borse, et al., 2008), current findings present clear grounds for early and follow up clinical management of traumatic injury populations. Acute and follow up screening of sleep disturbances and internalizing behavior problems can assist in providing referral to timely treatment interventions. Cognitive-behavioral therapy and pharmacotherapy has been shown to be effective in TBI populations with psychosocial problems, and growing research has demonstrated improved outcomes when specifically targeting SD and internalizing problems after TBI (Beebe, 2011; Fann, Hart, & Schomer, 2009; Orff, Ayalon, & Drummond, 2009; Ouellet & Morin, 2004; Ouellet & Morin, 2007).
Limitations and Future Directions
The current study is limited by several factors, including sample size, restricted range of outcomes, and reliance on primarily parent-reported outcomes. Statistical power in the current study was limited by sample size, which reduced the ability to detect possible differences between TBI and EI groups at different points during the first year of recovery after injury. Future studies with larger samples may confirm whether longitudinal pathways between internalizing and SD are similar in children and adolescents with TBI and bodily injury in relation to typically developing groups.
Measures used are not without limitations. Measurement of TBI severity would benefit from the use of other clinical markers in addition to GCS scores, such as neuroimaging findings. A primary challenge to research on SD includes the discrepancies often demonstrated by child-report, parent-report, and objective measures. As such, gold standard sleep assessment is typically considered to include both objective and subjective measures. Therefore, inclusion of polysomnography and/or actigraphy measures; along with parent and child report of SD in future studies would provide a more comprehensive evaluation of sleep patterns (Willis & Gregory, 2015). The current study identified that pain and fatigue were significantly associated with behavioral outcomes, particularly in the injury groups, using parent and child report. However, future research on pediatric traumatic injury can benefit from measures that more comprehensively assess constructs of pain, physical fatigue, and cognitive fatigue to identify more precise correlates of injury-related physical and psychological health concerns and suggest targeted interventions.
Consideration of development in the current study used age and pubertal stage and did not find relations with outcomes; however prior work has demonstrated that both variables play a role in the course SD across “normal” development (El-Sheikh & Buckhalt, 2015; McMakin & Alfano, 2015). Additionally, a recent longitudinal study found that pubertal sleep changes may antedate physical pubertal changes (Sadeh, Dahl, Shahar, and Rosenblat-Stein (2009). Thus, including other assessments of pubertal development, such as hormone levels may provide more comprehensive examination of developmental relations following injury. Given that the age of current sample spans a wide range, developmental influences in findings cannot be ruled out. Future work can examine this further by targeting specific pubertal stages or using samples with a more limited age range.
The focus of this study was to examine longitudinal relations between SD and internalizing problems. In addition to internalizing behavior problems, sleep characteristics have also been associated with risk-taking and other externalizing behavior problems (Calhoun et al., 2016; Kelly & El-Sheikh, 2014; Shay et al., 2014). While the relation between internalizing behavior problems and SD in children appears to be stronger than the relation between SD and externalizing behavior problems, externalizing problems have been associated with SD in uninjured populations (Alfano & Gamble, 2009; Gregory et al., 2004) and following TBI and bodily injury (Shay et al., 2014) and should be the focus of future studies.
In addition, SD in children may be associated with an increased risk of injury (Koulouglioti, Cole, and Kitzman, 2008; Owens, Fernando, and McGuinn, 2005). Consequently, many children with traumatic injury may already have prior SD, placing them at a higher risk of injury. This further highlights the importance of including bodily injury and typically developing comparison groups in future study designs, as well as inclusion of baseline levels of functioning in models. Future investigations should examine the longitudinal relation between SD, pediatric injury, risk-taking behavior, and externalizing behavior problems, and should explore additional psychological health variables such as posttraumatic stress symptoms (Kovachy et al., 2013) that may influence the relation of SD with injury type and severity.
Conclusion
Taken together, the current findings expand on the emerging number of studies on sleep after TBI, by demonstrating the multifaceted relation of TBI and bodily injury with SD, internalizing problems, and other psychological and physical health symptoms in a prospective, longitudinal study. Specifically, across a wide range of severity, children sustaining traumatic injury suffered from elevated levels of SD and internalizing problems, and this pattern persisted at chronic stages after injury. Pain, fatigue, and externalizing behaviors were also related to SD. This course of behavioral problems in injured children is compounded by our findings showing early internalizing problems consistently predict later SD in both injury groups. Similar to other studies examining psychological health outcomes, injury to brain and/or body was associated with significant sequelae and the relation between injury severity and psychosocial symptoms was weak (Ewing-Cobbs et al., 2017). Given the vast number of children who suffer from traumatic injury annually, results underscore the substantial chronic public health burden related to management of the psychological health sequelae of pediatric traumatic injury.
The current results, along with recent studies incorporating preinjury assessment of SD, also highlight the importance of including baseline functioning levels in study design to better define post-injury changes (Beebe et al., 2007; Shay et al, 2014). Importantly, children with TBI and those with bodily injury did not demonstrate significant differences in outcomes. When compared to typically developing children, earlier internalizing problems in both children with bodily injury and those with TBI predicted higher chronic SD. This supplements the idea that sustaining an injury was more predictive of later developing problems than whether the injury involved brain or body. Clearly, both bodily injury and typically developing comparison groups should be incorporated in future pediatric TBI study designs when examining SD and other post-traumatic physical and psychological health outcomes.
Public Significance Statement.
Sleep disturbance and internalizing problems are common and impairing sequelae of injury to the brain or body during childhood and predict adverse psychosocial outcomes later in life. Given the high incidence of injury during the childhood years, and associated long-term risk of adjustment difficulties, health providers should screen and manage sleep and related psychological health concerns across the spectrum of injury type and severity.
Acknowledgments
This work was funded by the National Institutes of Health R01 NS046308 awarded to Linda Ewing-Cobbs, Ph.D. The content is solely the responsibility of the authors and does not necessarily represent the official views of the granting institute.
Footnotes
Disclosures
The authors report no financial or other conflict of interest.
Contributor Information
Jesse T. Fischer, Department of Psychology, University of Houston
H. Julia Hannay, TIMES, University of Houston.
Candice A. Alfano, Department of Psychology, University of Houston.
Paul R. Swank, School of Public Health, University of Texas Health Science Center at Houston.
Linda Ewing-Cobbs, Department of Pediatrics and Children’s Learning Institute, University of Texas Health Science Center at Houston.
References
- Aaronson LS, Teel CS, Cassmeyer V, Neuberger GB, Pallikkathayil L, Pierce J, Wingate A. Defining and measuring fatigue. Image - the Journal of Nursing Scholarship. 1999;31(1):45–50. doi: 10.1111/j.1547-5069.1999.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Family; 2001. [Google Scholar]
- Alfano CA, Beidel DC, Turner SM, Lewin DS. Preliminary evidence for sleep complaints among children referred for anxiety. Sleep Medicine. 2006;7(6):467–473. doi: 10.1016/j.sleep.2006.05.002. http://doi.org/10.1016/j.sleep.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Alfano CA, Gamble AL. The role of sleep in childhood psychiatric disorders. Child & Youth Care Forum. 2009;38(6):327–340. doi: 10.1007/s10566-009-9081-y. http://doi.org/10.1007/s10566-009-9081-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfano CA, Ginsburg GS, Kingery JN. Sleep-related problems among children and adolescents with anxiety disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46(2):224–232. doi: 10.1097/01.chi.0000242233.06011.8e. http://doi.org/10.1097/01.chi.0000242233.06011.8e. [DOI] [PubMed] [Google Scholar]
- Amin RS, Kimball TR, Bean JA, Jeffries JL, Willging JP, Cotton RT, Daniels SR. Left ventricular hypertrophy and abnormal ventricular geometry in children and adolescents with obstructive sleep apnea. American Journal of Respiratory and Critical Care Medicine. 2002;165(10):1395–1399. doi: 10.1164/rccm.2105118. http://doi.org/10.1164/rccm.2105118. [DOI] [PubMed] [Google Scholar]
- Anderson V, Le Brocque R, Iselin G, Eren S, Dob R, Davern TJ, Kenardy J. Adaptive ability, behavior and quality of life pre and posttraumatic brain injury in childhood. Disability and Rehabilitation. 2012;34(19):1639–1647. doi: 10.3109/09638288.2012.656789. http://doi.org/10.3109/09638288.2012.656789. [DOI] [PubMed] [Google Scholar]
- Arbogast KB, Curry AE, Pfeiffer MR, Zonfrillo MR, Haarbauer-Krupa J, Breiding MJ, Master CL. Point of health care entry for youth with concussion within a large pediatric care network. JAMA Pediatrics. 2016;170(7):e160294. doi: 10.1001/jamapediatrics.2016.0294. https://doi.org/10.1001/jamapediatrics.2016.0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe DW. Cognitive, behavioral, and functional consequences of inadequate sleep in children and adolescents. Pediatric Clinics of North America. 2011;58(3):649–665. doi: 10.1016/j.pcl.2011.03.002. https://doi.org/10.1016/j.pcl.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe DW, Fallone G, Godiwala N, Flanigan M, Martin D, Schaffner L, Amin R. Feasibility and behavioral effects of an at-home multi-night sleep restriction protocol for adolescents. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2008;49(9):915–923. doi: 10.1111/j.1469-7610.2008.01885.x. http://doi.org/10.1111/j.1469-7610.2008.01885.x. [DOI] [PubMed] [Google Scholar]
- Beebe DW, Krivitzky L, Wells CT, Wade SL, Taylor HG, Yeates KO. Brief report: Parental report of sleep behaviors following moderate or severe pediatric traumatic brain injury. Journal of Pediatric Psychology. 2007;32(7):845–850. doi: 10.1093/jpepsy/jsm003. http://doi.org/10.1093/jpepsy/jsm003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borse NN, Gilchrist J, Dellinger A, Rudd RA, Ballesteros MF, Sleet DA. CDC Childhood Injury Report: Patterns of Unintentional Injuries among 0-19 Year Olds in the United States, 2000-2006. Atlanta (GA): Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2008. [Google Scholar]
- Bruni O, Ottaviano S, Guidetti V, Romoli M, Innocenzi M, Cortesi F, Giannotti F. The Sleep Disturbance Scale for Children (SDSC). Construction and validation of an instrument to evaluate sleep disturbances in childhood and adolescence. Journal of Sleep Research. 1996;5(4):251–261. doi: 10.1111/j.1365-2869.1996.00251.x. [DOI] [PubMed] [Google Scholar]
- Buckley SL, Gotschall C, Robertson W, Sturm P, Tosi L, Thomas M, Eichelberger M. The relationships of skeletal injuries with trauma score, injury severity score, length of hospital stay, hospital charges, and mortality in children admitted to a regional pediatric trauma center. Journal of Pediatric Orthopedics. 1994;14(4):449–453. doi: 10.1097/01241398-199407000-00005. [DOI] [PubMed] [Google Scholar]
- Calhoun SL, Fernandez-Mendoza J, Vgontzas AN, Mayes SD, Liao D, Bixler EO. Behavioral profiles associated with objective sleep duration in young children with insomnia symptoms. Journal of Abnormal Child Psychology. 2016 doi: 10.1007/s10802-016-0166-4. https://doi.org/10.1007/s10802-016-0166-4. [DOI] [PMC free article] [PubMed]
- Centers for Disease Control and Prevention (CDC) Vital signs: Unintentional injury deaths among persons aged 0–19 years - United States, 2000–2009. MMWR Morbidity and Mortality Weekly Report. 2012;61:270–276. [PubMed] [Google Scholar]
- Cohen R, Halevy A, Shuper A. Children’s sleep disturbance scale in differentiating neurological disorders. Pediatric Neurology. 2013;49(6):465–468. doi: 10.1016/j.pediatrneurol.2013.06.010. http://doi.org/10.1016/j.pediatrneurol.2013.06.010. [DOI] [PubMed] [Google Scholar]
- Crichton AJ, Babl F, Oakley E, Greenham M, Hearps S, Delzoppo C, Anderson VA. Prediction of multidimensional fatigue after childhood brain injury. The Journal of Head Trauma Rehabilitation. 2016 doi: 10.1097/HTR.0000000000000248. https://doi.org/10.1097/HTR.0000000000000248. [DOI] [PubMed]
- Ding R, McCarthy ML, Houseknecht E, Ziegfeld S, Knight VM, Korehbandi P, CHAT Study Group The health-related quality of life of children with an extremity fracture: a one-year follow-up study. Journal of Pediatric Orthopedics. 2006;26(2):157–163. doi: 10.1097/01.bpo.0000218521.98244.7e. https://doi.org/10.1097/01.bpo.0000218521.98244.7e. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Buckhalt JA. Ii. Moving sleep and child development research forward: priorities and recommendations from the SRCD-sponsored forum on sleep and child development. Monographs of the Society for Research in Child Development. 2015;80(1):15–32. doi: 10.1111/mono.12142. https://doi.org/10.1111/mono.12142. [DOI] [PubMed] [Google Scholar]
- Ewing-Cobbs L, Bloom DR, Prasad MR, Waugh JK, Cox CS, Swank PR. Assessing recovery and disability after physical trauma: the Pediatric Injury Functional Outcome Scale. Journal of Pediatric Psychology. 2014;39(6):653–665. doi: 10.1093/jpepsy/jsu018. http://doi.org/10.1093/jpepsy/jsu018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing-Cobbs L, Prasad MR, Cox CS, Granger DA, Duque G, Swank PR. Altered stress system reactivity after pediatric injury: Relation with post-traumatic stress symptoms. Psychoneuroendocrinology. 2017;84:66–75. doi: 10.1016/j.psyneuen.2017.06.003. https://doi.org/10.1016/j.psyneuen.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fann JR, Hart T, Schomer KG. Treatment for depression after traumatic brain injury: a systematic review. Journal of Neurotrauma. 2009;26(12):2383–2402. doi: 10.1089/neu.2009.1091. https://doi.org/10.1089/neu.2009.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul M, Xu L, Wald MM, Coronado VG. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006. Atlanta (GA): Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010. [Google Scholar]
- Fay TB, Yeates KO, Wade SL, Drotar D, Stancin T, Taylor HG. Predicting longitudinal patterns of functional deficits in children with traumatic brain injury. Neuropsychology. 2009;23(3):271–282. doi: 10.1037/a0014936. https://doi.org/10.1037/a0014936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogelberg DJ, Hoffman JM, Dikmen S, Temkin NR, Bell KR. Association of sleep and co-occurring psychological conditions at 1 year after traumatic brain injury. Archives of Physical Medicine and Rehabilitation. 2012;93(8):1313–1318. doi: 10.1016/j.apmr.2012.04.031. https://doi.org/10.1016/j.apmr.2012.04.031. [DOI] [PubMed] [Google Scholar]
- Gagner C, Landry-Roy C, Lainé F, Beauchamp MH. Sleep-wake disturbances and fatigue after pediatric traumatic brain injury: A systematic review of the literature. Journal of Neurotrauma. 2015;32(20):1539–1552. doi: 10.1089/neu.2014.3753. https://doi.org/10.1089/neu.2014.3753. [DOI] [PubMed] [Google Scholar]
- Gosselin N, Lassonde M, Petit D, Leclerc S, Mongrain V, Collie A, Montplaisir J. Sleep following sport-related concussions. Sleep Medicine. 2009;10(1):35–46. doi: 10.1016/j.sleep.2007.11.023. http://doi.org/10.1016/j.sleep.2007.11.023. [DOI] [PubMed] [Google Scholar]
- Gozal D, Kheirandish-Gozal L. The multiple challenges of obstructive sleep apnea in children: morbidity and treatment. Current Opinion in Pediatrics. 2008;20(6):654–658. doi: 10.1097/MOP.0b013e328316ec2d. http://doi.org/10.1097/MOP.0b013e328316ec2d. [DOI] [PubMed] [Google Scholar]
- Gregory AM, Caspi A, Eley TC, Moffitt TE, Oconnor TG, Poulton R. Prospective longitudinal associations between persistent sleep problems in childhood and anxiety and depression disorders in adulthood. Journal of Abnormal Child Psychology. 2005;33(2):157–163. doi: 10.1007/s10802-005-1824-0. [DOI] [PubMed] [Google Scholar]
- Gregory AM, Eley TC, O’Connor TG, Plomin R. Etiologies of associations between childhood sleep and behavioral problems in a large twin sample. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43(6):744–751. doi: 10.1097/01.chi/0000122798.47863.a5. http://doi.org/10.1097/01.chi/0000122798.47863.a5. [DOI] [PubMed] [Google Scholar]
- Gregory AM, O’Connor TG. Sleep problems in childhood: A longitudinal study of developmental change and association with behavioral problems. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41(8):964–971. doi: 10.1097/00004583-200208000-00015. http://doi.org/10.1097/00004583-200208000-00015. [DOI] [PubMed] [Google Scholar]
- Hayes AF, Preacher KJ. Statistical mediation analysis with a multicategorical independent variable. The British Journal of Mathematical and Statistical Psychology. 2014;67(3):451–470. doi: 10.1111/bmsp.12028. http://doi.org/10.1111/bmsp.12028. [DOI] [PubMed] [Google Scholar]
- Hollingshead A. Four factor index of social status. Yale University; 1975. Unpublished manuscript. [Google Scholar]
- IBM Corp. Released 2013 IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp; [Google Scholar]
- Johnson EO, Chilcoat HD, Breslau N. Trouble sleeping and anxiety/depression in childhood. Psychiatry Research. 2000;94(2):93–102. doi: 10.1016/s0165-1781(00)00145-1. [DOI] [PubMed] [Google Scholar]
- Kelly RJ, El-Sheikh M. Reciprocal relations between children’s sleep and their adjustment over time. Developmental Psychology. 2014;50(4):1137–1147. doi: 10.1037/a0034501. https://doi.org/10.1037/a0034501. [DOI] [PubMed] [Google Scholar]
- Kirkwood M, Janusz J, Yeates KO, Taylor HG, Wade SL, Stancin T, Drotar D. Prevalence and correlates of depressive symptoms following traumatic brain injuries in children. Child Neuropsychology: A Journal on Normal and Abnormal Development in Childhood and Adolescence. 2000;6(3):195–208. doi: 10.1076/chin.6.3.195.3157. https://doi.org/10.1076/chin.6.3.195.3157. [DOI] [PubMed] [Google Scholar]
- Koulouglioti C, Cole R, Kitzman H. Inadequate sleep and unintentional injuries in young children. Public Health Nursing (Boston, Mass) 2008;25(2):106–114. doi: 10.1111/j.1525-1446.2008.00687.x. https://doi.org/10.1111/j.1525-1446.2008.00687.x. [DOI] [PubMed] [Google Scholar]
- Kovachy B, O’Hara R, Hawkins N, Gershon A, Primeau MM, Madej J, Carrion V. Sleep disturbance in pediatric PTSD: Current findings and future directions. Journal of Clinical Sleep Medicine: JCSM: Official Publication of the American Academy of Sleep Medicine. 2013;9(5):501–510. doi: 10.5664/jcsm.2678. https://doi.org/10.5664/jcsm.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz M, McCoy BJ, Tompkins DM, Daly W, Mulligan J, McCauley RL, Herndon DN. Sleep disorders in children after burn injury. The Journal of Burn Care & Rehabilitation. 1993;14(1):83–90. doi: 10.1097/00004630-199301000-00018. [DOI] [PubMed] [Google Scholar]
- Langeland W, Olff M. Psychobiology of posttraumatic stress disorder in pediatric injury patients: a review of the literature. Neuroscience and Biobehavioral Reviews. 2008;32(1):161–174. doi: 10.1016/j.neubiorev.2007.07.002. http://doi.org/10.1016/j.neubiorev.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Lesko MM, Jenks T, O’Brien SJ, Childs C, Bouamra O, Woodford M, Lecky F. Comparing model performance for survival prediction using total Glasgow Coma Scale and its components in traumatic brain injury. Journal of Neurotrauma. 2013;30(1):17–22. doi: 10.1089/neu.2012.2438. http://doi.org/10.1089/neu.2012.2438. [DOI] [PubMed] [Google Scholar]
- Levin HS, Hanten G, Roberson G, Li X, Ewing-Cobbs L, Dennis M, Swank P. Prediction of cognitive sequelae based on abnormal computed tomography findings in children following mild traumatic brain injury. Journal of Neurosurgery Pediatrics. 2008;1(6):461–470. doi: 10.3171/PED/2008/1/6/461. http://doi.org/10.3171/PED/2008/1/6/461. [DOI] [PubMed] [Google Scholar]
- Mayer T, Walker ML, Clark P. Further experience with the modified abbreviated injury severity scale. The Journal of Trauma. 1984;24(1):31–34. doi: 10.1097/00005373-198401000-00004. [DOI] [PubMed] [Google Scholar]
- Max JE. Neuropsychiatry of pediatric traumatic brain injury. The Psychiatric Clinics of North America. 2014;37(1):125–140. doi: 10.1016/j.psc.2013.11.003. http://doi.org/10.1016/j.psc.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Max JE, Keatley E, Wilde EA, Bigler ED, Levin HS, Schachar RJ, Yang TT. Anxiety disorders in children and adolescents in the first six months after traumatic brain injury. The Journal of Neuropsychiatry and Clinical Neurosciences. 2011;23(1):29–39. doi: 10.1176/jnp.23.1.jnp29. http://doi.org/10.1176/appi.neuropsych.23.1.29. [DOI] [PubMed] [Google Scholar]
- Max JE, Keatley E, Wilde EA, Bigler ED, Schachar RJ, Saunders AE, Levin HS. Depression in children and adolescents in the first 6 months after traumatic brain injury. International Journal of Developmental Neuroscience: The Official Journal of the International Society for Developmental Neuroscience. 2012b;30(3):239–245. doi: 10.1016/j.ijdevneu.2011.12.005. https://doi.org/10.1016/j.ijdevneu.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Max JE, Lopez A, Wilde EA, Bigler ED, Schachar RJ, Saunders A, Levin HS. Anxiety disorders in children and adolescents in the second six months after traumatic brain injury. Journal of Pediatric Rehabilitation Medicine. 2015;8(4):345–355. doi: 10.3233/PRM-150352. http://doi.org/10.3233/PRM-150352. [DOI] [PubMed] [Google Scholar]
- Max JE, Wilde EA, Bigler ED, MacLeod M, Vasquez AC, Schmidt AT, Levin HS. Psychiatric disorders after pediatric traumatic brain injury: a prospective, longitudinal, controlled study. The Journal of Neuropsychiatry and Clinical Neurosciences. 2012a;24(4):427–436. doi: 10.1176/appi.neuropsych.12060149. http://doi.org/10.1176/appi.neuropsych.12060149. [DOI] [PubMed] [Google Scholar]
- Mayes T, Gottschlich MM, Khoury J, McCall J, Simakajornboon N, Kagan RJ. A pilot review of the long-term impact of burn injury on sleep architecture in children. Journal of Burn Care & Research. 2013;34(1):E15–E21. doi: 10.1097/BCR.0b013e318272178e. http://doi.org/10.1097/BCR.0b013e318272178e. [DOI] [PubMed] [Google Scholar]
- McMakin DL, Alfano CA. Sleep and anxiety in late childhood and early adolescence. Current Opinion in Psychiatry. 2015;28(6):483–489. doi: 10.1097/YCO.0000000000000204. http://doi.org/10.1097/YCO.0000000000000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman TA, David D, Bustamante V, Fins AI, Esposito K. Predictors of post-traumatic stress disorder following severe injury. Depression and Anxiety. 2001;14(4):226–231. doi: 10.1002/da.1071. [DOI] [PubMed] [Google Scholar]
- Milroy G, Dorris L, McMillan TM. Sleep disturbances following mild traumatic brain injury in childhood. Journal of Pediatric Psychology. 2008;33(3):242–247. doi: 10.1093/jpepsy/jsm099. http://doi.org/10.1093/jpepsy/jsm099. [DOI] [PubMed] [Google Scholar]
- Mindell JA, Owens JA, Carskadon MA. Developmental features of sleep. Child and Adolescent Psychiatric Clinics of North America. 1999;8(4):695–725. [PubMed] [Google Scholar]
- Necajauskaite O, Endziniene M, Jureniene K. The prevalence, course and clinical features of post-concussion syndrome in children. Medicina (Kaunas, Lithuania) 2005;41(6):457–464. [PubMed] [Google Scholar]
- Ong SH, Wickramaratne P, Tang M, Weissman MM. Early childhood sleep and eating problems as predictors of adolescent and adult mood and anxiety disorders. Journal of Affective Disorders. 2006;96(1–2):1–8. doi: 10.1016/j.jad.2006.05.025. http://doi.org/10.1016/j.jad.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Orff HJ, Ayalon L, Drummond SPA. Traumatic brain injury and sleep disturbance: a review of current research. The Journal of Head Trauma Rehabilitation. 2009;24(3):155–165. doi: 10.1097/HTR.0b013e3181a0b281. https://doi.org/10.1097/HTR.0b013e3181a0b281. [DOI] [PubMed] [Google Scholar]
- Osler T, Baker SP, Long W. A modification of the injury severity score that both improves accuracy and simplifies scoring. The Journal of Trauma. 1997;43(6):922–925. 926. doi: 10.1097/00005373-199712000-00009. [DOI] [PubMed] [Google Scholar]
- Osorio MB, Kurowski BG, Beebe D, Taylor HG, Brown TM, Kirkwood MW, Wade SL. Association of daytime somnolence with executive functioning in the first 6 months after adolescent traumatic brain injury. PM & R: The Journal of Injury, Function, and Rehabilitation. 2013;5(7):554–562. doi: 10.1016/j.pmrj.2012.11.006. http://doi.org/10.1016/j.pmrj.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellet MC, Morin CM. Cognitive behavioral therapy for insomnia associated with traumatic brain injury: a single-case study. Archives of Physical Medicine and Rehabilitation. 2004;85(8):1298–1302. doi: 10.1016/j.apmr.2003.11.036. https://doi.org/10.1016/j.apmr.2003.11.036. [DOI] [PubMed] [Google Scholar]
- Ouellet MC, Morin CM. Efficacy of cognitive-behavioral therapy for insomnia associated with traumatic brain injury: a single-case experimental design. Archives of Physical Medicine and Rehabilitation. 2007;88(12):1581–1592. doi: 10.1016/j.apmr.2007.09.006. https://doi.org/10.1016/j.apmr.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Owens JA. Sleep and Sleep Disorders in Children. In: Carey WB, Crocker AC, Elias ER, Feldman HM, Coleman WL II, editors. Developmental-Behavioral Pediatrics 4th Edition: Expert Consult - Online and Print. Canada: Elsevier Health Sciences; 2009. pp. 619–627. [Google Scholar]
- Owens JA, Babcock D, Blumer J, Chervin R, Ferber R, Goetting M, Sheldon S. The use of pharmacotherapy in the treatment of pediatric insomnia in primary care: rational approaches. A consensus meeting summary. Journal of Clinical Sleep Medicine. 2005;1(1):49–59. [PubMed] [Google Scholar]
- Owens JA, Fernando S, Mc Guinn M. Sleep disturbance and injury risk in young children. Behavioral Sleep Medicine. 2005;3(1):18–31. doi: 10.1207/s15402010bsm0301_4. https://doi.org/10.1207/s15402010bsm0301_4. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17(2):117–133. doi: 10.1007/BF01537962. https://doi.org/10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Peterson RL, Kirkwood MW, Taylor HG, Stancin T, Brown TM, Wade SL. Adolescents’ internalizing problems following traumatic brain injury are related to parents’ psychiatric symptoms. The Journal of Head Trauma Rehabilitation. 2013;28(5):E1–12. doi: 10.1097/HTR.0b013e318263f5ba. http://doi.org/10.1097/HTR.0b013e318263f5ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillar G, Averbooch E, Katz N, Peled N, Kaufman Y, Shahar E. Prevalence and risk of sleep disturbances in adolescents after minor head injury. Pediatric Neurology. 2003;29(2):131–135. doi: 10.1016/s0887-8994(03)00149-8. [DOI] [PubMed] [Google Scholar]
- Ponsford J, Schönberger M, Rajaratnam SMW. A model of fatigue following traumatic brain injury. The Journal of Head Trauma Rehabilitation. 2015;30(4):277–282. doi: 10.1097/HTR.0000000000000049. https://doi.org/10.1097/HTR.0000000000000049. [DOI] [PubMed] [Google Scholar]
- Ponsford JL, Sinclair KL. Sleep and fatigue following traumatic brain injury. The Psychiatric Clinics of North America. 2014;37(1):77–89. doi: 10.1016/j.psc.2013.10.001. https://doi.org/10.1016/j.psc.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Ponsford J, Willmott C, Rothwell A, Cameron P, Ayton G, Nelms R, Ng KT. Cognitive and behavioral outcome following mild traumatic head injury in children. The Journal of Head Trauma Rehabilitation. 1999;14(4):360–372. doi: 10.1097/00001199-199908000-00005. [DOI] [PubMed] [Google Scholar]
- Ponsford JL, Ziino C, Parcell DL, Shekleton JA, Roper M, Redman JR, Rajaratnam SMW. Fatigue and sleep disturbance following traumatic brain injury - their nature, causes, and potential treatments. The Journal of Head Trauma Rehabilitation. 2012;27(3):224–233. doi: 10.1097/HTR.0b013e31824ee1a8. https://doi.org/10.1097/HTR.0b013e31824ee1a8. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods. 2008;40(3):879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Reynolds KC, Alfano CA. Childhood bedtime problems predict adolescent internalizing symptoms through emotional reactivity. Journal of Pediatric Psychology. 2016;41(9):971–982. doi: 10.1093/jpepsy/jsw014. https://doi.org/10.1093/jpepsy/jsw014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo DM, Bruni O, Brogna C, Ferri R, Galluccio C, De Clemente V, Mercuri E. Application of the sleep disturbance scale for children (SDSC) in preschool age. European Journal of Paediatric Neurology: EJPN: Official Journal of the European Paediatric Neurology Society. 2013;17(4):374–382. doi: 10.1016/j.ejpn.2012.12.009. https://doi.org/10.1016/j.ejpn.2012.12.009. [DOI] [PubMed] [Google Scholar]
- Roth-Isigkeit A, Thyen U, Stöven H, Schwarzenberger J, Schmucker P. Pain among children and adolescents: restrictions in daily living and triggering factors. Pediatrics. 2005;115(2):e152–162. doi: 10.1542/peds.2004-0682. http://doi.org/10.1542/peds.2004-0682. [DOI] [PubMed] [Google Scholar]
- Ruff RL, Blake K. Pathophysiological links between traumatic brain injury and post-traumatic headaches. F1000Research. 2016;5 doi: 10.12688/f1000research.9017.1. https://doi.org/10.12688/f1000research.9017.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh A, Dahl RE, Shahar G, Rosenblat-Stein S. Sleep and the transition to adolescence: a longitudinal study. Sleep. 2009;32(12):1602–1609. doi: 10.1093/sleep/32.12.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh A, Gruber R, Raviv A. Sleep, neurobehavioral functioning, and behavior problems in school-age children. Child Development. 2002;73(2):405–417. doi: 10.1111/1467-8624.00414. [DOI] [PubMed] [Google Scholar]
- Sadeh A, Raviv A, Gruber R. Sleep patterns and sleep disruptions in school-age children. Developmental Psychology. 2000;36(3):291–301. doi: 10.1037//0012-1649.36.3.291. [DOI] [PubMed] [Google Scholar]
- Sady MD, Vaughan CG, Gioia GA. Psychometric characteristics of the Postconcussion Symptom Inventory in children and adolescents. Archives of Clinical Neuropsychology. 2014;29(4):348–363. doi: 10.1093/arclin/acu014. https://doi.org/10.1093/arclin/acu014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute Inc. Base SAS® 9.4 Procedures Guide. Cary, NC: SAS Institute Inc.; 2013. [Google Scholar]
- Shanahan L, Copeland WE, Angold A, Bondy CL, Costello EJ. Sleep problems predict and are predicted by generalized anxiety/depression and oppositional defiant disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2014;53(5):550–558. doi: 10.1016/j.jaac.2013.12.029. https://doi.org/10.1016/j.jaac.2013.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shay N, Yeates KO, Walz NC, Stancin T, Taylor HG, Beebe DW, Wade SL. Sleep problems and their relationship to cognitive and behavioral outcomes in young children with traumatic brain injury. Journal of Neurotrauma. 2014;31(14):1305–1312. doi: 10.1089/neu.2013.3275. http://doi.org/10.1089/neu.2013.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Essex MJ. Concurrent and longitudinal associations of basal and diurnal cortisol with mental health symptoms in early adolescence. Developmental Psychobiology. 2008;50(7):690–703. doi: 10.1002/dev.20336. https://doi.org/10.1002/dev.20336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruyt K, Cluydts R, Verleye GB. Pediatric sleep disorders: Exploratory modulation of their relationships. Sleep. 2004;27(3):495–501. doi: 10.1093/sleep/27.3.495. [DOI] [PubMed] [Google Scholar]
- Stancin T, Kaugars AS, Thompson GH, Taylor HG, Yeates KO, Wade SL, Drotar D. Child and family functioning 6 and 12 months after a serious pediatric fracture. The Journal of Trauma. 2001;51(1):69–76. doi: 10.1097/00005373-200107000-00011. [DOI] [PubMed] [Google Scholar]
- Stores G, Stores R. Sleep disorders in children with traumatic brain injury: A case of serious neglect. Developmental Medicine and Child Neurology. 2013;55(9):797–805. doi: 10.1111/dmcn.12163. http://doi.org/10.1111/dmcn.12163. [DOI] [PubMed] [Google Scholar]
- Sumpter RE, Dorris L, Kelly T, McMillan TM. Pediatric sleep difficulties after moderate-severe traumatic brain injury. Journal of the International Neuropsychological Society: JINS. 2013;19(7):829–834. doi: 10.1017/S1355617713000465. http://doi.org/10.1017/S1355617713000465. [DOI] [PubMed] [Google Scholar]
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- Tham SW, Palermo TM, Vavilala MS, Wang J, Jaffe KM, Koepsell TD, Rivara FP. The longitudinal course, risk factors, and impact of sleep disturbances in children with traumatic brain injury. Journal of Neurotrauma. 2012;29(1):154–161. doi: 10.1089/neu.2011.2126. http://doi.org/10.1089/neu.2011.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tham SW, Fales J, Palermo TM. Subjective and objective assessment of sleep in adolescents with mild traumatic brain injury. Journal of Neurotrauma. 2015;32(11):847–852. doi: 10.1089/neu.2014.3559. https://doi.org/10.1089/neu.2014.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theadom A, Starkey N, Jones K, Cropley M, Parmar P, Barker-Collo S, Feigin VL. Sleep difficulties and their impact on recovery following mild traumatic brain injury in children. Brain Injury. 2016;30(10):1243–1248. doi: 10.1080/02699052.2016.1183171. https://doi.org/10.1080/02699052.2016.1183171. [DOI] [PubMed] [Google Scholar]
- Weinberg WA, Dietz SG, Penick EC, McAlister WH. Intelligence, reading achievement, physical size and social class. A study of St. Louis Caucasian boys aged 8-0 to 9-6 years, attending regular schools. The Journal of Pediatrics. 1974;85(4):482–489. doi: 10.1016/s0022-3476(74)80449-x. [DOI] [PubMed] [Google Scholar]
- Williams DH, Levin HS, Eisenberg HM. Mild head injury classification. Neurosurgery. 1990;27(3):422–428. doi: 10.1097/00006123-199009000-00014. [DOI] [PubMed] [Google Scholar]
- Willis TA, Gregory AM. Anxiety disorders and sleep in children and adolescents. Sleep Medicine Clinics. 2015;10(2):125–131. doi: 10.1016/j.jsmc.2015.02.002. https://doi.org/10.1016/j.jsmc.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Zatzick DF, Grossman DC, Russo J, Pynoos R, Berliner L, Jurkovich G, Rivara FP. Predicting posttraumatic stress symptoms longitudinally in a representative sample of hospitalized injured adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45(10):1188–1195. doi: 10.1097/01.chi.0000231975.21096.45. http://doi.org/10.1097/01.chi.0000231975.21096.45. [DOI] [PubMed] [Google Scholar]
- Zatzick DF, Jurkovich GJ, Fan MY, Grossman D, Russo J, Katon W, Rivara FP. Association between posttraumatic stress and depressive symptoms and functional outcomes in adolescents followed up longitudinally after injury hospitalization. Archives of Pediatrics & Adolescent Medicine. 2008;162(7):642–648. doi: 10.1001/archpedi.162.7.642. http://doi.org/10.1001/archpedi.162.7.642. [DOI] [PubMed] [Google Scholar]
- Zatzick D, Jurkovich GJ, Rivara FP, Wang J, Fan MY, Joesch J, Mackenzie E. A national US study of posttraumatic stress disorder, depression, and work and functional outcomes after hospitalization for traumatic injury. Annals of Surgery. 2008;248(3):429–437. doi: 10.1097/SLA.0b013e318185a6b8. http://doi.org/10.1097/SLA.0b013e318185a6b8. [DOI] [PubMed] [Google Scholar]


