Abstract
[Purpose] The purpose of the present study was to assess the relationship between age of onset and risk factors including family history and life style in Korean population with type 2 diabetes mellitus (T2D). [Subjects and Methods] Subjects with T2D patients who received outpatient care for blood sugar control were randomly sampled at 13 general hospitals and 969 subjects were included. Cox proportional hazard models were used to confirm associations between age of onset and risk factors including family history and life style in Korean population with T2D. [Results] Parent history of T2D was significantly associated with age of onset. Compared to none of family members with T2D, those whose both father and mother had a history showed the highest the risk of early-onset (HR=2.36; 95% CI=1.45–3.85). Mother and father’s history of T2D (HR=1.73; 95% CI=1.46–2.05; HR=1.83; 95% CI=1.40–2.37) were associated with the risk of early-onset. Moreover, exercise (HR=1.23, CI=1.08–1.40) smoking status (HR=1.62, CI=1.32–1.99), and drinking (HR=1.32, CI=1.13–1.54) were associated with a higher risk for the early-onset. [Conclusion] Family history as well as life style including exercise, smoking, and drinking are the risk factors for early-onset factor in Korean population with T2D.
Key words: Family history, Type 2 diabetes mellitus, Age of onset
INTRODUCTION
The prevalence of diabetes, comprised mostly by those with type 2 diabetes mellitus (T2D), is a growing public health concern worldwide accounting for substantial morbidity and premature mortality1, 2). The number of people with diabetes has more than doubled over the past three decades globally3). Around 415 million adults (20–79 years old) of the world’s population had diabetes in 2015. Furthermore, the public health burden of T2D may be underestimated because one in two adults with diabetes remain undiagnosed for years. The estimated annual economic cost of diabetes-related care is USD 673 billion, approximately 12% of global health expenditures1).
T2D is a multifactorial disease associated with the interactions of modifiable and nonmodifiable risk factors, including family history, age, gender, being overweight or obese, lifestyle, and the others4). Given that the onset and development of T2D can be delayed or prevented, understanding a mixture of genetic and epigenetic predispositions, as well as the variety of behavioral and environmental risk factors involved, is an important step toward its prevention and control. Among the many risks for T2D, family history is a strong and independent risk factor to reflect environmental and behavioral attributes in addition to genetics5). According to Hariri and colleagues6), family history is a more precise indicator of the presence of T2D than obesity, one of the well-established risk factors. Moreover, a family history has consistently been related to increased risk of type 2 diabetes mellitus5,6,7,8,9,10). When the father, mother, or both are diagnosed with T2D, the risk for the diagnosis of the condition increases approximately by 2–4 times11). Previous studies have suggested that maternal diabetes is more likely to increase the likelihood in the next generation than paternal7, 11). In addition, Jeong et al.8) found that family history was significantly associated with the onset of T2D at a younger age, which may lead to longer duration of the disease and more complications.
Given such family history knowledge and the burden of T2D on public health, family history provides a readily available, inexpensive screening tool to identify individuals in high risk groups for evaluation, detection, prevention, and risk reducing interventions6, 12). Furthermore, prevention efforts could be extended to family members who might be at an increased risk or possibly influential in assisting to change surroundings’ lifestyle. However, there are few studies about the family history of T2D among Asian populations8). Most of the studies have mainly been concentrated among western populations6, 13,14,15), and in-depth investigations among Asian populations are limited to a few studies9, 16, 17). Therefore, it is needed to study among Korean populations in clarifying the association between age of onset and risk factors including family history and life style of T2D. The purpose of the present study was to assess the relationship between age of onset and risk factors including family history and life style in Korean population with T2D.
SUBJECTS AND MEHTODS
Subjects with T2D patients who received outpatient care for blood sugar control were randomly sampled at 13 general hospitals. The study period was from Mar 19, 2013 to May 29, 2013. Initially, 2,610 patients who were more than 20 years old and had blood sugar control for more than six months were selected. This study was approved by the Institutional Review Board (IRB) of Korea (Asan Medical Center IRB: 2013–0334, Bucheon St. Mary’s Hospital IRB: CIRB-00062_1-032, Cheil General Hospital IRB: CGH-IRB-2013–3, Inje University Ilsan Paik Hospital IRB: IB-2–1304-014, Inje University Seoul Paik Hospital IRB: IIT-2013–059, Kyung Hee University Hospital at Gangdong IRB: KHNMC IRB 2013–042, Samsung Medical Center IRB: SMC 2013–01-019–001, Seoul National University Bundang Hospital IRB: B-1303–194-108, Seoul National University Hospital IRB: H-1303–057-47, Seoul St. Mary’s Hospital IRB: CIRB-00061_2-020, Yeouido St. Mary’s Hospital IRB: CIRB-00062_1-034, Yonsei University Gangnam Severance Hospital IRB: IRB No. 3–2013-0007). All study subjects were provided with written informed consent for the survey. Data of 2,610 patients were collected by retrospective chart review. Missing data for age of onset for T2D (N=196), body mass index (BMI) (N=76), smoking status (N=281), drinking (N=327), and family history of T2D (N=751) were excluded. Ultimately, a total of 969 subjects were included in this study.
The age of onset for T2D was set as the dependent variable. The age of onset for T2D was defined as the day when a doctor diagnosis a patient as a T2D patient. Diagnostic criteria for T2D were followed Korean diagnostic criteria of Korean Diabetes Association. The specific diagnostic criteria were as follows: 1) fasting plasma glucose ≥126 mg/dl; 2) 2-hour plasma glucose ≥200 mg/dl during the oral glucose tolerance test; 3) Classic symptoms of hyperglycemia or hyperglycemic crisis, a random plasma glucose level ≥200 mg/dl; 4) HbA1c ≥6.5%18). The independent variables were BMI, gender, health behaviors (exercise, smoking status, and drinking), and family history of T2D. Family history of T2D was investigated in each of ‘father+mother’, ‘father’, ‘mother’, ‘only siblings’, and ‘none’. The ‘father+mother’, ‘father’, and ‘mother’ categories included siblings as well. BMI was categorized into groups as underweight (BMI ≤18.5 kg/m2), normal (BMI 18.5–22.9 kg/m2), overweight (BMI 23.0–24.9 kg/m2), and obese (BMI ≥25.0 kg/m2). Exercise and drinking was divided into binary as ‘no’ or ‘yes’. Exercise was collected based on the question ‘Do you do any kinds of physical activities?’. A criterion of drinking was recommended for an average of 3 cups/day, regardless of type. Smoking status was categorized as ‘never’, ‘past’, ‘current’.
ANOVA and t-test analyses were used to compare the general characteristics and age of onset for T2D. The crude association between family history and the age of onset for T2D was examined by Kaplan-Meier curves. Then, Cox proportional hazard models were used to identify the associations between family history of T2D and age of onset. The significance level was 0.05. All results from testing the proportional hazard assumption in the Cox proportional hazard model had p>0.05. All statistical analyses were performed with SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Table 1 shows the general characteristics of the study subjects. Among all 969 subjects, the average age of onset for T2D was 50.3 ± 11.2. For the cases where no one had T2D in the family, the average age of onset was 51.7 ± 11.6. The average age of onset for T2D was 52.9 ± 10.0 with those with siblings who had a history, 46.1 ± 10.1 with those whose mother had, 46.0 ± 9.1 with father, and 43.0 ± 10.1 with both the father and mother.
Table 1. General characteristics of study population.
| N (%) | Age of onsetfor T2D | Significance | ||
|---|---|---|---|---|
| Total | 969 (100.0) | 50.3 ± 11.2 | ||
| Family history of T2D | None | 542 (55.9) | 51.7 ± 11.6 | *** |
| Siblings | 158 (16.3) | 52.9 ± 10.0 | ||
| Mother | 186 (19.2) | 46.1 ± 10.1 | ||
| Father | 66 (6.8) | 46.0 ± 9.1 | ||
| Father+Mother | 17 (1.8) | 43.0 ± 10.1 | ||
| Gender | Women | 415 (42.8) | 52.1 ± 11.3 | *** |
| Men | 554 (57.2) | 49.0 ± 10.9 | ||
| BMI | Underweight | 11 (1.1) | 50.8 ± 12.2 | ** |
| Normal | 493 (50.9) | 51.1 ± 11.3 | ||
| Overweight | 214 (22.1) | 50.6 ± 10.2 | ||
| Obese | 251 (25.9) | 48.5 ± 11.7 | ||
| Exercise | No | 404 (41.7) | 51.0 ± 12.1 | ns |
| Yes | 565 (58.3) | 49.8 ± 10.5 | ||
| Smoking status | Never | 555 (57.3) | 51.6 ± 11.1 | *** |
| Past | 225 (23.2) | 51.4 ± 11.1 | ||
| Current | 189 (19.5) | 45.2 ± 10.1 | ||
| Drinking | No | 640 (66.1) | 51.9 ± 11.3 | *** |
| Yes | 329 (34.0) | 47.2 ± 10.3 |
T2D: type 2 diabetes mellitus.
***p<0.001; **p<0.01; *p<0.05; ns: not significant.
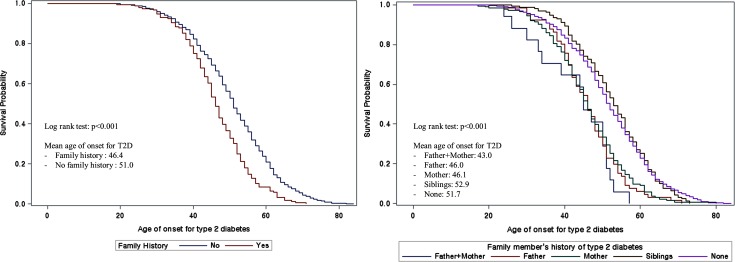
Figure 1 shows the survival probability for family history of T2D. Both the dichotomous categorization of family history of T2D and each family member’s history were associated with the age of onset. Both of the Kaplan-Meier curves’ log rank tests were p<0.001. There was a significant difference in survival times between dichotomous categorization of family history of T2D in left side of Fig. 1. In right side of Fig. 1, the Kaplan-Meier survival curves were categorized into two groups: (1) Siblings and None; (2) Father and Mother.
Fig. 1.
Kaplan-Meier curves for family history of type 2 diabetes mellitus and age of onset for type 2 diabetes mellitus.
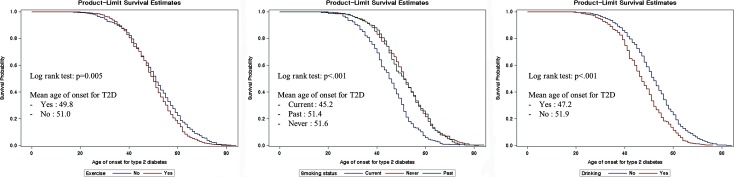
Figure. 2 shows results of Kaplan-Meier curves for exercise, smoking status, and drinking. All log rank tests were significant (p<0.05). Mean ages of onset for T2D are showed in Fig. 2. Mean ages of onset for T2D of those who did exercise or were drinkers were higher than those who were not. For smoking status, Kaplan-Meier survival curves were divided into never+past smoker group and current smoker group. Mean age of onset for T2D of current smoker was 45.2 years old, but it was 51.4 and 51.6 years old for past smoker and never smoked group.
Fig. 2.
Kaplan-Meier curves for life style and age of onset for type 2 diabetes mellitus.
The association between age of onset for T2D and risk factors including family history and life style is identified in Table 2. Family history of T2D was associated with a higher risk for the early-onset (hazard ratio (HR)=1.37; 95% confidence interval (CI)=1.20–1.56). Moreover, exercise (HR=1.23, CI=1.08–1.40), smoking status (HR=1.62, CI=1.32–1.99), and drinking (HR=1.32, CI=1.13–1.54) were also associated with a higher risk for the early-onset.
Table 2. Adjusted hazard ratios for the association between family history and age of onset for type 2 diabetes mellitus.
| HR | CI | ||
|---|---|---|---|
| Family history of T2D | No | 1.00 | |
| Yes | 1.37 | (1.20–1.56)*** | |
| Sex | Women | 1.00 | |
| Men | 1.11 | (0.93–1.33) | |
| BMI | Underweight | 1.00 | |
| Normal | 0.98 | (0.54–1.79) | |
| Overweight | 1.03 | (0.56–1.89) | |
| Obese | 1.20 | (0.65–2.20) | |
| Exercise | No | 1.00 | |
| Yes | 1.23 | (1.08–1.40)** | |
| Smoking status | Never | 1.00 | |
| Past | 0.87 | (0.72–1.05) | |
| Current | 1.62 | (1.32–1.99)*** | |
| Drinking | No | 1.00 | |
| Yes | 1.32 | (1.13–1.54)** |
HR: Hazard ratio; CI: Confidence interval; T2D: Type 2 diabetes mellitus.
***p<0.001; **p<0.01; *p<0.05; ns: not significant.
In Fig. 3, it was analyzed the association between family members’ history of T2D and age of onset. Parent history of T2D was significantly associated with age of onset. After adjusting all independent variables, the cases of T2D family history in ‘father+mother’ showed the highest risk of early-onset compared to ‘none’ (of family members with T2D) (HR=2.36; 95% CI=1.45–3.85). Compared to none of family members with T2D, those whose mother had a history increased the risk of early-onset (HR=1.73; 95% CI=1.46–2.05) and those whose father had a history also increased the risk of early-onset (HR=1.83; 95% C I=1.40–2.37).
Fig. 3.
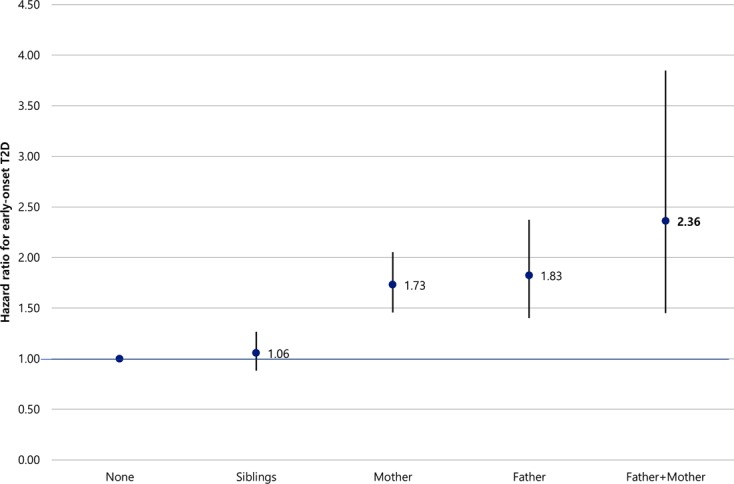
Hazard ratio between family member’s history of type 2 diabetes mellitus and age of onset for type 2 diabetes mellitus.
DISCUSSION
T2D is a multifactorial disease associated with the interactions of various risk factors, including family history, age, gender, being overweight or obese, lifestyle, and the others. Especially, T2D has a genetic predisposition as family history and it remains to be an independent risk indicator6, 7, 9, 19, 20). While family history is fixed and irreversible, the significance of family history lies within its value of being a premature indicator of diabetes and associated risks, which can contribute to the early detection and prevention of T2D. Therefore, this research attempts to establish effective management strategies of T2D high risk factors by comprehending the family history and related factors of patients with T2D in Korea.
In this study, the overall average age of onset for diabetes was 50.3. The average age without a family history of T2D were recorded at 51.7, whereas those with a family history were recorded at 47, which indicate that participants with a family history have a higher risk for the diagnosis of diabetes at a younger age than those without a family history (HR=1.37; 95% CI=1.20–1.56). This result was similar with previous studies, which showed that the age of onset for T2D is associated with a family member’s history of T2D6, 8, 9, 21,22,23). The average age of onset for T2D was 43 in the case where both parents had a history, in contrast to 46 in where only one parent had a history. When compared to the subjects whose parents exhibited no signs of family diabetes, the subjects who had both parents with a history reflected a faster onset age of 9 years, with a faster onset age of 6 years for those who only had one parent. This result was similar to the previous research which showed that the onset of diabetes for those with parents having a history of T2D is likely to be 8.5–9 years earlier than those who had parents without a history, and 3.5–4 years earlier than those with only one parent22, 24).
Many studies have suggested that direct family history is a high risk indicator of onset for diabetes19, 24). This means the fact for those with both parents having a history of diabetes. This disposition maintains the highest risk of onset for diabetes when compared to those without family history7, 22). In the results, it showed that compared to those without any family history, the risk of early-onset was 2.3 times higher for those with both parents having T2D, 1.8 times higher for those whose father only, and 1.7 times higher for those whose mother only. These results were similar with previous studies that those with both parents having history of T2D have the highest risk of onset for diabetes.
Meanwhile, with regards to family history and the onset risk of T2D previous studies has shown that the mother’s history of diabetes contributed to a higher risk of diabetes than those whose father only7, 9, 22, 24). In contrast, the present results showed that the contribution of the father’s diabetes history constituted not significant difference in the early-onset. More studies are needed to investigate the influential environmental factors that families share, as previous reports demonstrated that the child whose mother had a history of T2D was at a higher risk of onset for diabetes due to the mother’s dietary and habitual influences including diet, drinking, smoking, and level of obesity7).
Unlike the non-malleable family history of diabetes, the lifestyle habit and obesity are kown as modifiable risk indicators of diabetes. However, these indicators are difficult to change simultaneously. Those with a family history of diabetes are more likely to recognize the risk of diabetes onset and thus engage in a healthy lifestyle habit to prevent diabetes25). Because lifestyle habit and obesity cannot be improved shortly, there are high probabilities that weight and daily habits might have not altered even after the diabetes diagnosis.
In the present study, the relationships between the age of onset and the lifestyle habits of patients with a family history of T2D were assessed. The risk of onset for diabetes was 1.23 higher for those who exercise than those who do not exercise. However, this shows a contrary result to the previous result which stated that the lack of physical activity and exercise is a risk indicator of diabetes onset19). One potential explanation for this could be that since this result was shared with participants after the diabetes diagnosis, more exercise could have been done by the participants due to the anxiety associated with the result and the belief that exercise is an effective factor that is easily modifiable compared to other daily habits.
Moreover, in this result, the risk of early-onset for diabetes in drinkers was 1.32 times higher than non-drinkers and in smokers 1.62 times higher than non-smokers. This is the reason that drinking and smoking are severe risk indicators for the onset of diabetes19, 23), as well as daily habits, which are difficult to modify even after the diabetes diagnosis.
Although obesity is a comparatively less risky factor than having a family history in the onset of T2D, many studies have confirmed that weight is an independent risk factor for the onset of diabetes19, 20, 22, 23). However, our research showed that BMI had no association with the early-onset of diabetes. Although there is some research that show that BMI has no association with the early-onset of diabetes21), there is a high possibility of weight change due to the management of diabetes and blood sugar after the diagnosis of diabetes, which may lead to differences in BMI during diagnosis.
Because of variability of lifestyle habit and weight, this study has some limitations that we examined the patients’ weight and lifestyle habits at the time that the examination was conducted, rather than examining them immediately post-diagnosis. In the present study, the relationship between age of onset and risk factors including family history and life style in Korean population with T2D were assessed. Through our research, family history as well as life style including exercise, smoking, and drinking are the risk factors for early-onset factor in Korean population with T2D. Population with a family history of T2D need to actively modify their life style including exercise, smoking, and drinking in order to prevent or delay the onset of T2D.
Funding
This study was supported by a grant (K.H.S., 2012) from the Korean Diabetes Association and Korean Association of Diabetes Nurse Educators.
REFERENCES
- 1.International Diabetes Federation: IDF diabetes atlas: seventh edition. Brussels: International Diabetes Federation, 2015. [Google Scholar]
- 2.World Health Organization: Global report on diabetes. France: World Health Organization, 2016. [Google Scholar]
- 3.Danaei G, Finucane MM, Lu Y, et al. : Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Glucose): National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet, 2011, 378: 31–40. [DOI] [PubMed] [Google Scholar]
- 4.Chen L, Magliano DJ, Zimmet PZ: The worldwide epidemiology of type 2 diabetes mellitus—present and future perspectives. Nat Rev Endocrinol, 2011, 8: 228–236. [DOI] [PubMed] [Google Scholar]
- 5.Meigs JB, Shrader P, Sullivan LM, et al. : Genotype score in addition to common risk factors for prediction of type 2 diabetes. N Engl J Med, 2008, 359: 2208–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hariri S, Yoon PW, Qureshi N, et al. : Family history of type 2 diabetes: a population-based screening tool for prevention? Genet Med, 2006, 8: 102–108. [DOI] [PubMed] [Google Scholar]
- 7.Abbasi A, Corpeleijn E, van der Schouw YT, et al. : Maternal and paternal transmission of type 2 diabetes: influence of diet, lifestyle and adiposity. J Intern Med, 2011, 270: 388–396. [DOI] [PubMed] [Google Scholar]
- 8.Jeong SU, Kang DG, Lee DH, et al. : Clinical characteristics of type 2 diabetes patients according to family history of diabetes. Korean Diabetes J, 2010, 34: 222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakurai M, Nakamura K, Miura K, et al. : Family history of diabetes, lifestyle factors, and the 7-year incident risk of type 2 diabetes mellitus in middle-aged Japanese men and women. J Diabetes Investig, 2013, 4: 261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van’t Riet E, Dekker JM, Sun Q, et al. : Role of adiposity and lifestyle in the relationship between family history of diabetes and 20-year incidence of type 2 diabetes in U.S. women. Diabetes Care, 2010, 33: 763–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papazafiropoulou AK, Papanas N, Melidonis A, et al. : Family history of type 2 diabetes: does having a diabetic parent increase the risk? Curr Diabetes Rev, 2017, 13: 19–25. [DOI] [PubMed] [Google Scholar]
- 12.Harrison TA, Hindorff LA, Kim H, et al. : Family history of diabetes as a potential public health tool. Am J Prev Med, 2003, 24: 152–159. [DOI] [PubMed] [Google Scholar]
- 13.Mukherjee S, Schnur DB, Reddy R: Family history of type 2 diabetes in schizophrenic patients. Lancet, 1989, 1: 495. [DOI] [PubMed] [Google Scholar]
- 14.Freemark M, Bursey D: The effects of metformin on body mass index and glucose tolerance in obese adolescents with fasting hyperinsulinemia and a family history of type 2 diabetes. Pediatrics, 2001, 107: E55. [DOI] [PubMed] [Google Scholar]
- 15.Civitarese AE, Jenkinson CP, Richardson D, et al. : Adiponectin receptors gene expression and insulin sensitivity in non-diabetic Mexican Americans with or without a family history of Type 2 diabetes. Diabetologia, 2004, 47: 816–820. [DOI] [PubMed] [Google Scholar]
- 16.Ramachandran A, Snehalatha C, Mary S, et al. : Indian Diabetes Prevention Programme (IDPP): The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia, 2006, 49: 289–297. [DOI] [PubMed] [Google Scholar]
- 17.Tan JT, Tan LS, Chia KS, et al. : A family history of type 2 diabetes is associated with glucose intolerance and obesity-related traits with evidence of excess maternal transmission for obesity-related traits in a South East Asian population. Diabetes Res Clin Pract, 2008, 82: 268–275. [DOI] [PubMed] [Google Scholar]
- 18.Ko SH, Kim SR, Kim DJ, et al. : Committee of Clinical Practice Guidelines, Korean Diabetes Association: 2011 clinical practice guidelines for type 2 diabetes in Korea. Diabetes Metab J, 2011, 35: 431–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghassibe-Sabbagh M, Deeb M, Salloum AK, et al. : Multivariate epidemiologic analysis of type 2 diabetes mellitus risks in the Lebanese population. Diabetol Metab Syndr, 2014, 6: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ning F, Pang Z, Laatikainen T, et al. : Qingdao 2006 Diabetes Survey and FINRISK 2002 Study: Joint effect of family history of diabetes with obesity on prevalence of type 2 diabetes mellitus among Chinese and Finnish men and women. Can J Diabetes, 2013, 37: 65–71. [DOI] [PubMed] [Google Scholar]
- 21.Kim KS, Oh HJ, Kim JW, et al. : The clinical characteristics of the newly diagnosed early onset (<40 years old) diabetes in outpatients’ clinic. Korean Diabetes J, 2010, 34: 119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Velasco Mondragon HE, Charlton RW, Peart T, et al. : Diabetes risk assessment in Mexicans and Mexican Americans: effects of parental history of diabetes are modified by adiposity level. Diabetes Care, 2010, 33: 2260–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim DH, Ahn YO, Park SW, et al. : Incidence and risk factors for diabetes mellitus in Korean middle-age men: Seoul cohort DM follow-up study. Korean J Prev Med, 1999, 32: 526–537. [Google Scholar]
- 24.Annis AM, Caulder MS, Cook ML, et al. : Family history, diabetes, and other demographic and risk factors among participants of the National Health and Nutrition Examination Survey 1999–2002. Prev Chronic Dis, 2005, 2: A19. [PMC free article] [PubMed] [Google Scholar]
- 25.Baptiste-Roberts K, Gary TL, Beckles GL, et al. : Family history of diabetes, awareness of risk factors, and health behaviors among African Americans. Am J Public Health, 2007, 97: 907–912. [DOI] [PMC free article] [PubMed] [Google Scholar]