Key Points
Fifteen percent of NCI high-risk, Ph-negative, B-ALL patients harbored a kinase-activating fusion, and often associated with IKZF1 deletion.
IKZF1 deletion represents an independent prognostic factor of poor outcomes, regardless of fusion-positivity.
Abstract
Recurrent chromosomal rearrangements carry prognostic significance in pediatric B-lineage acute lymphoblastic leukemia (B-ALL). Recent genome-wide analyses identified a high-risk B-ALL subtype characterized by a diverse spectrum of genetic alterations activating kinases and cytokine receptor genes. This subtype is associated with a poor prognosis when treated with conventional chemotherapy but has demonstrated sensitivity to the relevant tyrosine kinase inhibitors. We sought to determine the frequency of kinase-activating fusions among National Cancer Institute (NCI) high-risk, Ph-negative, B-ALL patients enrolled on Dana-Farber Cancer Institute ALL Consortium Protocol 05-001 and to describe their associated clinical characteristics and outcomes. Among the 105 patients screened, 16 (15%) harbored an ABL-class fusion (ETV6-ABL1: n = 1; FOXP1-ABL1: n = 1; SFPQ-ABL1: n = 1; ZC3HAV1-ABL2: n = 1) or a fusion activating the JAK-STAT pathway (P2RY8-CRLF2: n = 8; PAX5-JAK2: n = 4). Sixty-nine percent of patients with an identified fusion had a concomitant IKZF1 deletion (n = 11). In univariate analysis, fusion-positivity and IKZF1 deletion were each associated with inferior event-free survival; IKZF1 deletion retained statistical significance in multivariable analysis (hazard ratio, 2.64; P = .019). Our findings support therapy intensification for IKZF1-altered patients, irrespective of the presence of a kinase-activating fusion.
Visual Abstract
Introduction
Over the last several decades, cure rates for childhood acute lymphoblastic leukemia (ALL) have drastically improved, with overall survival now approaching 90%.1 However, relapse still occurs in approximately 15% of patients, and long-term cure rates postrelapse remain poor, making ALL the second leading cause of cancer-related death in children.2
Recent large-scale genome-wide studies have enabled the identification of actionable genomic alterations, providing the rationale to test targeted therapies in genomically defined patient subsets. In particular, BCR-ABL1-like ALL (or Ph-like ALL) constitutes a new high-risk B-lineage ALL (B-ALL) subgroup, comprising approximately 15% of children and adolescents with B-ALL.3 Lymphoblasts from these patients display a gene expression signature similar to that of Philadelphia chromosome–positive (Ph+) ALL but lack the canonical BCR-ABL1 gene fusion.4,5 Ph-like status is more common among National Cancer Institute (NCI) high-risk B-ALL patients and has been associated with inferior outcomes.3,6 A distinguishing feature of Ph-like ALL is a heterogeneous spectrum of genomic alterations activating kinases and cytokine receptor genes that are amenable to inhibition with the relevant tyrosine kinase inhibitors (TKIs).3 A high proportion of Ph-like ALL patients also exhibits IKZF1 deletion, which has also been reported to be an independent predictor of adverse outcome in pediatric B-ALL.7-9 The objectives of our study were to determine the frequency and prognostic significance of TKI-targetable kinase-activating fusions in NCI high-risk, Ph-negative B-ALL patients treated on the Dana-Farber Cancer Institute (DFCI) ALL Consortium protocol 05-001.
Methods
Patients and samples
Between 2005 and 2011, 219 newly diagnosed NCI high-risk, Ph-negative, B-ALL patients, aged 1 to 18 years old, were enrolled on DFCI ALL Consortium protocol 05-001 (NCT00400946).10 One hundred five of these patients had sufficient banked material to undergo kinase fusion testing by validated multiplex reverse transcriptase polymerase chain reaction (RT-PCR) assays, of whom 94 also had material available for assessment of IKZF1 gene deletion status. Patients, their parents/guardians, or both provided informed consent for trial participation, banking, and future research prior to enrollment.
Therapy
Treatment on DFCI 05-001 has been previously described.10 NCI high-risk, Ph-negative B-ALL patients with any of the following characteristics were treated in the very high risk group: KMT2A rearrangements, low hypodiploidy, and/or high end-induction minimal residual disease (MRD), defined as ≥10−3 (≥0.001) as assessed by real-time quantitative PCR analysis of patient-specific antigen receptor gene rearrangements. All other NCI high-risk, Ph-negative B-ALL patients were treated as high risk.
Kinase fusion and IKZF1 status detection
Diagnostic marrow samples were subject to multiplex PCR for identifying kinase fusions in Ph-like ALL, as has been previously described.6 Each purified PCR product was sequenced individually using the reverse primer present in the amplification master mix and analyzed by using the NCBI database to identify the forward partner of the fusion. A singleplex PCR reaction was set up to confirm the identity of the positive RT-PCR result using the appropriate forward and reverse primers. IKZF1 deletion status was assessed by multiplex ligation-dependent probe amplification (MLPA) (SALSA MLPA P202 IKZF1; MRC-Holland), as has been previously described.7
Statistical analysis
Categorical and continuous patient characteristics were compared by fusion status with the Fisher’s exact test and the Wilcoxon rank sum test, respectively. Event-free survival (EFS), overall survival (OS), and disease-free survival (DFS) were estimated with the Kaplan-Meier method and compared using a log-rank test. DFS was defined for those patients entering a complete remission. Univariate and multivariable Cox proportional hazards models of EFS and DFS were also constructed by considering fusion-positivity, IKZF1 deletion, and diagnostic white blood cell count (WBC). DFS also considered end-induction MRD.
Results
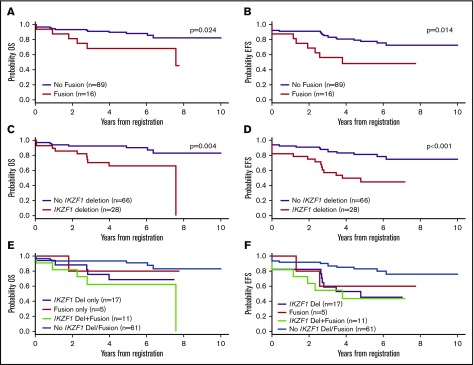
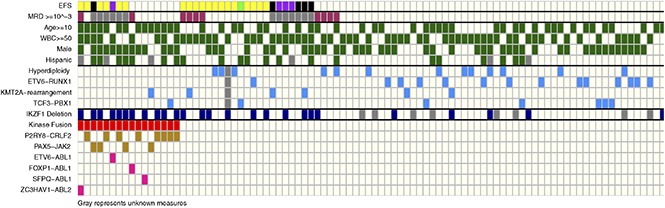
Of the NCI high-risk, Ph-negative, B-ALL patients enrolled on DFCI 05-001, a higher proportion of patients screened for fusions were age <10 years (47% vs 20%; P < .001) and had WBC ≥50 × 109/L at diagnosis (68% vs 25%; P < .001) in comparison with those who were not screened. Among the 105 patients screened, 16 (15%) were found to harbor either an ABL-class fusion (ETV6-ABL1: n = 1; FOXP1-ABL1: n = 1; SFPQ-ABL1: n = 1; ZC3HAV1-ABL2: n = 1) or a fusion activating the JAK-STAT pathway (P2RY8-CRLF2: n = 8; PAX5-JAK2: n = 4). Of the 16 patients with an identified kinase fusion (Fusion+), 11 (69%) had a concomitant IKZF1 deletion. Features associated with fusion-positivity were age ≥10 years (P = .003), male sex (P = .031), Hispanic ethnicity (P = .014), and IKZF1 deletion (P < .001) (Table 1). None of the Fusion+ patients had favorable cytogenetics such as hyperdiploidy or ETV6-RUNX1. Fifty percent of Fusion+ patients experienced an event (induction death: n = 1; induction failure: n = 1; relapse: n = 6) in comparison with 24% of patients without a kinase fusion. Fusion+ patients had significantly inferior OS and EFS than did those without kinase fusions (Figure 1A-B). These findings are comparable to previous reports in terms of the frequency, genomic distribution, clinical characteristics, and outcomes of Ph-like ALL patients within this risk group.3
Table 1.
Patient characteristics by fusion status
| Kinase-activating fusion | P | |||||
|---|---|---|---|---|---|---|
| No | Yes | |||||
| N | N | % | N | % | ||
| Number screened | 105 | 89 | 85 | 16 | 15 | |
| Age at diagnosis | .003 | |||||
| <10 y | 49 | 47 | 96 | 2 | 4 | |
| ≥10 y | 56 | 42 | 75 | 14 | 25 | |
| Median (range) | 10.6 (1.3-18.0) | 7.4 (1.3-17.1) | 13.5 (1.8-18.0) | .016 | ||
| Leukocyte count at diagnosis, ×109/L | .38 | |||||
| <50 | 34 | 27 | 79 | 7 | 21 | |
| ≥50 | 71 | 62 | 87 | 9 | 13 | |
| Median (range) | 70.0 (1.3-726.3) | 75.2 (1.3-726.3) | 51.8 (5.5-363.8) | .43 | ||
| Sex | .031 | |||||
| Female | 46 | 43 | 93 | 3 | 7 | |
| Male | 59 | 46 | 87 | 13 | 22 | |
| Ethnicity* | .014 | |||||
| Hispanic or Latino | 24 | 17 | 71 | 7 | 29 | |
| Non-Hispanic | 74 | 68 | 92 | 6 | 8 | |
| Ethnicity not known | 7 | 4 | 57 | 3 | 43 | |
| IKZF1 deleted*† | <.001 | |||||
| No | 66 | 61 | 92 | 5 | 8 | |
| Yes | 28 | 17 | 61 | 11 | 39 | |
| Cytogenetics | ||||||
| Hyperdiploidy (51-65 chromosomes) |
14 | 14 | 100 | 0 | 0 | .12 |
| ETV6-RUNX1 | 16 | 16 | 100 | 0 | 0 | .12 |
| KMT2A rearrangement | 8 | 7 | 88 | 1 | 13 | 1.00 |
| TCF3-PBX1 | 8 | 7 | 88 | 1 | 13 | 1.00 |
| Achieve CR | 96 | 82 | 85 | 14 | 15 | .62 |
| MRD* | .08 | |||||
| High | 10 | 8 | 80 | 2 | 20 | |
| Low | 72 | 64 | 89 | 8 | 11 | |
| Indeterminate | 14 | 10 | 71 | 4 | 28 | |
| Final risk | .45 | |||||
| High | 81 | 70 | 86 | 14 | 17 | |
| Very high | 15 | 12 | 80 | 3 | 20 | |
CR, complete remission.
The P value excludes unknowns.
Ninety-four of the 105 were also screened for IKZF1.
Figure 1.
Overall and event-free survival of NCI high-risk, Ph-negative, B-ALL patients by fusion and IKZF1 status. (A-B) The 5-year OS and EFS for patients with Fusion+ patients were 68% (95% confidence interval [CI], 39% to 85%) and 48% (95% CI, 22% to 70%), respectively, in comparison with 88% (95% CI, 79% to 93%) and 78% (95% CI, 67% to 85%) for fusion-negative patients. (C-D) The 5-year OS and EFS for patients with IKZF1 deletion were 66% (95% CI, 45% to 81%) and 45% (95% CI, 25% to 62%), respectively, in comparison with 90% (95% CI, 79% to 95%) and 81% (95% CI, 69% to 89%) for patients without IKZF1 deletion. (E-F) The 5-year OS and EFS were 62% (95% CI, 28% to 84%) and 44% (95% CI, 15% to 70%), respectively, for patients with both fusion-positivity and IKZF1 deletion in comparison with 91% (95% CI, 79% to 96%) (P = .005) and 83% (95% CI, 71% to 90%) (P = .006) for those with neither. Del, deletion.
Twenty-eight of 94 patients (30%) assessed were found to harbor IKZF1 deletions. Clinical features associated with the presence of the IKZF1 deletion were male sex (P = .045) and Hispanic ethnicity (P = .011). As with fusion-positivity, the presence of IKZF1 deletion was associated with inferior outcome (Figure 1C-D), as has been reported by others.7-9 In univariate analysis, fusion-positivity (hazard ratio [HR], 2.66, P = .019) and IKZF1 deletion (HR, 3.21, P = .002) were each significantly associated with inferior EFS (Table 2). In multivariable analysis, only IKZF1 deletion retained statistical significance (HR, 2.64, P = .016). Univariately, fusion-positivity (HR, 3.29, P = .015), IKZF1 deletion (HR, 3.27, P = .008), and high end-induction MRD (HR, 3.30, P = .027) were also significantly associated with inferior DFS (Table 3). None of the above variables retained significance in the multivariable analysis for DFS, although there was a trend toward inferiority for IKZF1 deletion (HR, 2.70, P = .087). Additionally, in analyses comparing OS and EFS in patients with both IKZF1 deletion and fusion-positivity, with one or neither of these alterations, patients with concomitant Fusion+/IKZF1 deletion (P = .005, P = .006) or IKZF1 deletion alone (P = .050, P = .003) had significantly worse outcomes than did those with neither alteration (Figure 1E-F).
Table 2.
Univariate and multivariable Cox proportional hazards models for event-free survival
| Univariate (n = 94) | Multivariable (n = 94) | |||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Fusion (yes vs no) | 2.66 (1.17-6.02) | .019 | 1.45 (0.58-3.61) | .43 |
| WBC (≥50 vs <50 × 109/L) | 1.64 (0.70-3.85) | .25 | 1.48 (0.63-3.50) | .25 |
| IKZF1 deletion (yes vs no) | 3.21 (1.55-6.68) | .002 | 2.73 (1.20-6.21) | .016 |
Table 3.
Univariate and multivariable Cox proportional hazards models for disease-free survival
| Univariate (n = 72) | Multivariable (n = 72) | |||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Fusion (yes vs no) | 3.29 (1.26-8.59) | .015 | 0.46 (0.09-2.23) | .34 |
| WBC (≥50 vs <50 × 109/L) | 0.99 (0.39-2.48) | .98 | 1.26 (0.40-3.96) | .69 |
| IKZF1 deletion (yes vs no) | 3.27 (1.36-7.88) | .008 | 2.70 (0.90-8.14) | .087 |
| MRD (high vs low) | 3.30 (1.15-9.51) | .027 | 2.19 (0.72-6.66) | .17 |
Discussion
Our study identified that 30% of NCI high-risk, Ph-negative B-ALL patients harbored an IKZF1 gene deletion and 15% harbored a kinase-activating fusion, frequently in association with an IKZF1 deletion. We demonstrated that the presence of a kinase-activating fusion was associated with an inferior outcome; however, IKZF1 deletion status represents an independent predictor of adverse outcome in NCI high-risk B-ALL patients, regardless of fusion status. Although screening for fusion status may identify high-risk patients who may respond to targeted therapies, our data would suggest that screening for IKZF1 deletions may enhance current risk classification strategies, identifying additional, kinase-negative patients who may benefit from intensified or novel therapies.
The inferior outcomes among fusion-positive patients in the DFCI cohort validate the need to find alternative therapeutic approaches for this patient population. These kinase fusions have been shown to be sensitive to the relevant TKIs in vitro and in patient-derived xenograft models.3,11 A number of clinical trial groups, including our own, are now conducting prospective studies to identify patients with actionable kinase-activating fusions at diagnosis and determine whether combining the relevant TKI with a high-risk chemotherapy backbone will improve their outcomes.
Our study has several limitations. First, the frequency of fusion-positive patients in our cohort is underestimated because our RT-PCR assays are not suitable for detecting IGH-CRLF2 and EPOR rearrangements. Alternative approaches, such as the Archer anchored multiplex PCR and transcriptome sequencing, may uncover more fusions than would our focused RT-PCR assay. Additional limitations of our study include the restriction to only NCI high-risk patients and retrospective testing of banked patient samples on the basis of specimen availability. These limitations are addressed in our current trial, DFCI ALL Consortium protocol 16-001, which utilizes a next-generation sequencing assay to identify IKZF1 deletions and an unbiased RNA-sequencing approach to prospectively identify targetable kinase fusions in all newly diagnosed B-ALL patients, regardless of NCI risk group, to enhance risk classification and stratify therapy.
Acknowledgments
M.L.L. is the Deborah and Arthur Ablin Endowed Professor of Pediatric Molecular Oncology and the University of California, San Francisco, Benioff Chair of Children’s Health. This work was supported by the Innovation Award from Alex’s Lemonade Stand Foundation (T.H.T. and M.L.L.).
The Dana-Farber Cancer Institute (DFCI) ALL Consortium is multi-institutional group conducting clinical trials in children and adolescents with newly diagnosed ALL in the United States and Canada. DFCI serves as study sponsor and coordinating center. The following collaborating centers participated on the 05-001 protocol: Centre Hospitalier Université de Québec (Québec City, QC, Canada), Children's Hospital at Montefiore (Bronx, NY), Columbia University, New York–Presbyterian Morgan Stanley Children's Hospital (New York, NY), Hasbro Children's Hospital (Providence, RI), Sainte Justine Hospital (Montreal, QC, Canada), Inova Children's Hospital (Falls Church, VA), McMaster University (Hamilton, ON, Canada), San Jorge Children's Hospital (San Juan, Puerto Rico), Tulane Medical Center (New Orleans, LA), and University of Rochester Medical Center, Golisano Children's Hospital (Rochester, NY).
Authorship
Contribution: T.H.T., M.H.H., M.L.L., and L.B.S. designed the study; T.H.T. and J.V.N. performed the kinase fusion screen; M.H.H. performed the IKZF1 deletion screen; B.L.A., U.H.A., L.A.C., P.D.C., K.M.K., C.L., J.-M.L., B.M., M.A.S., J.J.G.W., L.B.S., and S.E.S. enrolled patients and collected data; E.S. and S.C.R. assisted with the kinase fusion screen; T.M.B., K.E.S., and D.S.N. did the statistical analysis; T.H.T., T.M.B., K.E.S., M.H.H., M.L.L., and L.B.S. analyzed the data and prepared the manuscript; all authors revised and approved the manuscript.
Conflict-of-interest disclosure: M.L.L. receives research funding from Incyte Corporation. The remaining authors declare no competing financial interests.
Correspondence: Thai Hoa Tran, Division of Pediatric Hematology-Oncology, CHU Sainte-Justine, 3175 Cote Sainte-Catherine, Local A-435, Montréal, QC H3T 1C5, Canada; e-mail: thai-hoa.tran@recherche-ste-justine.qc.ca.
References
- 1.Hunger SP, Lu X, Devidas M, et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children’s oncology group. J Clin Oncol. 2012;30(14):1663-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vrooman LM, Silverman LB. Treatment of childhood acute lymphoblastic leukemia: prognostic factors and clinical advances. Curr Hematol Malig Rep. 2016;11(5):385-394. [DOI] [PubMed] [Google Scholar]
- 3.Roberts KG, Li Y, Payne-Turner D, et al. Targetable kinase-activating lesions in Ph-like acute lymphoblastic leukemia. N Engl J Med. 2014;371(11):1005-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tran TH, Loh ML. Ph-like acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program. 2016;2016:561-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tasian SK, Loh ML, Hunger SP. Philadelphia chromosome-like acute lymphoblastic leukemia. Blood. 2017;130(19):2064-2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reshmi SC, Harvey RC, Roberts KG, et al. Targetable kinase gene fusions in high-risk B-ALL: a study from the Children’s Oncology Group. Blood. 2017;129(25):3352-3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clappier E, Grardel N, Bakkus M, et al. ; European Organisation for Research and Treatment of Cancer, Children’s Leukemia Group (EORTC-CLG). IKZF1 deletion is an independent prognostic marker in childhood B-cell precursor acute lymphoblastic leukemia, and distinguishes patients benefiting from pulses during maintenance therapy: results of the EORTC Children’s Leukemia Group study 58951. Leukemia. 2015;29(11):2154-2161. [DOI] [PubMed] [Google Scholar]
- 8.Dörge P, Meissner B, Zimmermann M, et al. IKZF1 deletion is an independent predictor of outcome in pediatric acute lymphoblastic leukemia treated according to the ALL-BFM 2000 protocol. Haematologica. 2013;98(3):428-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Veer A, Waanders E, Pieters R, et al. Independent prognostic value of BCR-ABL1-like signature and IKZF1 deletion, but not high CRLF2 expression, in children with B-cell precursor ALL. Blood. 2013;122(15):2622-2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Place AE, Stevenson KE, Vrooman LM, et al. Intravenous pegylated asparaginase versus intramuscular native Escherichia coli L-asparaginase in newly diagnosed childhood acute lymphoblastic leukaemia (DFCI 05-001): a randomised, open-label phase 3 trial. Lancet Oncol. 2015;16(16):1677-1690. [DOI] [PubMed] [Google Scholar]
- 11.Maude SL, Tasian SK, Vincent T, et al. Targeting JAK1/2 and mTOR in murine xenograft models of Ph-like acute lymphoblastic leukemia. Blood. 2012;120(17):3510-3518. [DOI] [PMC free article] [PubMed] [Google Scholar]