Abstract
Background
Cardiac device infection (CDI) is a serious complication of cardiovascular implantable electronic device (CIED) implantations. Many risk factors have been identified, but several are still uncertain. This study aimed to identify and evaluate the risk factors. Moreover, an infection control protocol (ICP) was carried out, and its efficacy in reducing CDIs was investigated.
Material/Methods
A total of 1259 patients who received permanent pacemaker (PPM) implantations were enrolled in this study in a 3-year period in a high-volume center and low-volume centers in the central area of Shaanxi Province, China. Follow-up data of all enrolled patients were collected. The risk factors for CDIs were identified and analyzed. The ICP was adopted in the low-volume centers. Data, including CDI rates, medical costs, and microbiology, were collected and compared.
Results
Male gender, diabetes, CKD, operation duration, PPM replacement, and low center volume were identified as the risk factors for CDIs. Furthermore, CDI rates in low-volume centers were significantly higher than in high-volume centers. The adoption of an ICP dramatically reduced CDI rates in low-volume centers without significant increases in medical costs.
Conclusions
ICPs were easily carried out, effective, and economical in controlling CDIs in low-volume centers, which was identified as a risk factor of CDIs.
MeSH Keywords: Bacterial Infections; Pacemaker, Artificial; Risk Factors
Background
Cardiovascular implantable electronic device (CIED) implantations are recognized as standard therapies for multiple cardiac arrhythmias including sick sinus syndrome, high-grade atrioventricular blockage, and obstructive hypertrophic cardiomyopathy [1]. However, the spreading clinical applications of CIEDs are accompanied by an increasing cardiac device infection (CDI) rate, which is one of the fastest growing complications of CIED implantations [2]. CDIs not only result in prolonged hospitalizations and increased financial burdens, but also cause poor outcomes and elevated mortality. According to the guidelines, patients with limited pocket infection are recommended to receive lead extraction [3].
A CDI is extremely challenging in both diagnosis and management due to its complicated association with the pocket, device, leads, vessels, and endocardium. It was reported that in CDI patients with endocarditis who did not receive a lead extraction procedure, the mortality rates were as high as 66% [4]. Thus, it is important to prevent infection during the perioperative period and operative procedures because infections at 1 year after an operation are strongly correlated with the operation and patient management. A better understanding of the risk factors would be very helpful in preventing CDIs.
Many risk factors have been identified and are well established according to previous investigations [5]. Patient factors, including age, gender, and underlying chronic diseases, were proposed as risk factors. In addition to patient-related factors, treatment-related factors should also be taken into consideration. Additionally, there are some disputes regarding several risk factors. In 2014, Syed et al. published their work regarding a protocol that effectively reduced CDIs [6]. This so-called infection control protocol (ICP) was easy to follow and the costs were low. In the present article, we report the results from a multi-center study for CDIs in the CIED population. Both patient- and treatment-related factors were analyzed. We identified that a low-volume center was one of the risk factors of CDIs, which was rarely reported in previous investigations. We also made a comparison of CDIs before and after adoption of an ICP in these low-volume centers. We believe that our data will be helpful in improving the understanding of and preventing CDIs.
Material and Methods
Subjects
The data of this present retrospective observational study were from a central hospital (high-volume center) and 5 branch hospitals (low-volume centers). The central hospital was Shaanxi Provincial People’s Hospital. The branch hospitals were Xi’an Dianli Central Hospital, Shaanxi Yangling Demonstration Zone Hospital, Shaanxi Friendship Hospital, Shaanxi Pucheng People’s Hospital, and the Second Affiliated Hospital of Xi’an Medical University. The retrospective audit of CDIs occurred during 1 October 2014 to 1 October 2017. We enrolled hospitalized patients who received an implantation of a permanent pacemaker (PPM). The medical records of all enrolled patients were reviewed.
Demographic and clinical data collection
The demographic data were collected from the medical records of the enrolled patients. Clinical parameters, including gender, age, chronic disease history, drug history, operation duration, temporal wire implantation, type of PPM, and medical costs, were collected and recorded.
Definitions
The definition of a CDI was in accordance with previous investigations. Both local infection of the generator pocket and device-associated infective endocarditis were defined as a CDI. Either detected valvular/lead vegetation or meeting Duke criteria were defined as infective endocarditis [7]. Three consecutive blood culture examinations were carried out in all suspected CDI patients. Swabs were used to collect samples from infectious pockets. Diabetes was defined as fasting serum glucose above 7.0 mmol/l or random serum glucose above 11.1 mmol/l, HbA1c above 6.5%, or a positive history of diabetes [8]. COPD was defined according to the European Respiratory Society (ERS) diagnostic criteria for COPD: coughing, sputum production and/or dyspnoea, an history of exposure to risk factors for COPD. The diagnosis was confirmed by a post-bronchodilator FEV1/FVC < 0.7 in spirometry as a sign that the airflow limitation is not fully reversible [9]. CKD was defined as GFR <60 mL per min per 1.73 m2 for 3 months or more, with or without kidney damage [10]. Heart failure was defined by use of the Framingham criteria for congestive heart failure [11]. A low-volume center was defined as a cardiac center performing fewer than 200 cases of CIED implantations per year.
ICP
The ICP carried out in this study was adopted from a previous study with several modifications. The detailed protocol for an ICP is outlined below. The ICP was carried out in all low-volume centers from 1 January 2016 to the end of this study.
Preoperational methicillin-resistant Staphylococcus aureus (MRSA) screening
A MRSA screening procedure was carried out in every patient after admission. Nasal, oral, axillary, and groin areas were sampled with swabs. If results of MRSA detection were positive, topical bacterial suppression, including a 0.5% hydrogen peroxide mouth wash, 4% chlorhexidine skin wash, and nasal mupirocin, were used prior to the operation for 5 days. An antibiotic prophylaxis strategy was carried out with reference to the consultations of the Infection Control Department. Teicoplanin/gentamicin.
Antibiotics
Routinely, cephazolin or clarithromycin were administered intravenously 30 min prior to the beginning of the operations. In patients identified as high risk for CDIs, such as those with replacement, diabetes, CKD, a recent infection, previous endocarditis, prosthetic valves, or congenital heart disease, we administered teicoplanin or gentamicin intravenously 30 min prior to the beginning of the operations. The dosages of antibiotics were adjusted according to the instructions in patients with renal dysfunctions.
Preoperative preparations
Serum glucose level control was required for diabetic patients to achieve a serum glucose level <11 mmol/l. Implantation operations were deferred or cancelled in patients with clinical signs of sepsis or raised infective markers such as fever, and leukocytosis or increased C reactive protein levels.
Hair removal and operational area preparation
The surgical areas were defined as the skin areas receiving the incision and vascular access points. Electric clippers were used to remove the hair over these areas. Disinfectant liquids and gels were used to prepare the areas. A solution of 2% chlorhexidine gluconate/70% isopropyl alcohol was used instead of betadine. Double-gloving was mandatory during disinfection and draping. The outer gloves were removed prior to skin incision and vascular punctures.
Surgical procedures
Disposable adhesive plastic incision drapes were used with all patients. Cutting diathermy (40 W) was used for cutting and hemostasis after incisions. Antibacterial-coated Vicryl sutures were used for subcutaneous closures. Wound dressings were changed every 2 days 3 times and wounds were disinfected at the same time.
Statistical analysis
Statistical software SPSS (version 16.0, SPSS Inc) was used to perform the statistical analyses. Continuous variables are presented as the mean ± standard deviation. Categorical variables are presented as proportions. The Mann-Whitney U test was used to analyze the differences in continuous variables, and the chi-squared test was used to analyze the differences in categorical variables. A logistic regression model was established to determine the correlation between CDIs and candidate factors. Statistical significance was set as p<0.05.
Results
Demographic data of enrolled patients
There were 1300 patients enrolled in this study, and 41 patients withdrew during the follow-up. The baseline characteristics of enrolled patients are listed in Table 1. The total CDI rate was 1.906%. The male/female ratio was 1.004 and the average age was 59.00 y. All patients received PPM implantation procedures. Among these patients, 1103 patients (87.609%) received implantation of double-chamber PPMs, 96 patients (7.625%) received replacement procedures, and a total of 476 patients (37.808%) received implantations of temporal wires during the procedures. The operation durations of 121 patients (9.611%) were over 2 h. Operations of 592 patients (47.021%) were performed in high-volume centers. Regarding underling chronic diseases, 178 patients (14.138%) were diagnosed as having COPD; 110 patients (8.737%) were diagnosed as having diabetes; 105 patients (8.340%) were diagnosed as having heart failure; and 70 patients (5.560%) were diagnosed as having CKD. A total of 251 patients (19.936%) were taking anti-coagulation drugs during the perioperative period. The average medical cost was 32 656 in RMB.
Table 1.
Demographic data of enrolled patients.
| Variable | Descriptions |
|---|---|
| Infection | n, % |
| 24, 1.906% | |
| Gender | n, % |
| Male | 631, 50.119 |
| Female | 628, 49.880 |
| Age | Median, 25–75% percentile range |
| 59.000, 52.000–66.000 | |
| COPD | n, % |
| 178, 14.138% | |
| Diabetes | n, % |
| 110, 8.737% | |
| Heart failure | n, % |
| 105, 8.340% | |
| CKD | n,% |
| 70, 5.560% | |
| Anti-coagulation drugs | n, % |
| 251, 19.936% | |
| Operation duration >2 hours | n, % |
| 121, 9.611% | |
| Temporal lead implantation | n, % |
| 476, 37.808% | |
| Center volume >200/year | n, % |
| 592, 47.021% | |
| Replacement | n, % |
| 96, 7.625% | |
| Double chamber | n, % |
| 1103, 87.609% | |
| Costs (RMB) | Median, 25–75% percentile range |
| 32656, 30146–33333 |
Risk factors associated with CDI
Logistic regression analysis for CDI using candidate risk factors in enrolled patients is demonstrated in Table 2. Candidate factors, including gender, age, COPD, diabetes, heart failure, anti-coagulation drugs, operation duration, temporal lead implantation, center volume, replacement, and double-chamber PPM, were enrolled as variants and analyzed. The results indicated that male gender, diabetes, CKD, operation duration, PPM replacement, and low center volume were significantly associated with CDI in enrolled patients (p<0.05).
Table 2.
Logistic regression analysis of risk factors contributing to CDI.
| Odd ratios | 95% CI | p Value | |
|---|---|---|---|
| Age | 0.210 | 1.164–1.307 | <0.001* |
| Gender | 1.113 | 0.102–1.654 | 0.019* |
| Diabetes | 3.186 | 0.030–0.377 | <0.001* |
| Heart failure | 1.094 | 0.430–0.871 | 0.053 |
| CKD | 3.992 | 0.013–0.187 | <0.001* |
| Anticoagulation | 0.162 | 0.199–3.627 | 0.827 |
| Operation duration | 2.360 | 0.025–0.359 | 0.001* |
| COPD | 0.723 | 0.042–2.940 | 0.131 |
| Temporal wire | 0.510 | 0.281–9.862 | 0.574 |
| Replacement | 2.359 | 0.015–0.592 | 0.012* |
| Double chamber | 0.942 | 0.501–13.142 | 0.258 |
| Center volume | 0.996 | 0.607–12.645 | 0.036* |
P<0.05.
Comparison of CDI in high-volume centers and low-volume centers
In the above section, center volume was identified as one of the risk factors of CDI. We compared the total CDI rate in the high-volume center and low-volume centers. The CDI rate was 1.013% in the high-volume center and 3.976% in the low-volume centers and the difference was significant (p<0.01). The results are demonstrated in Figure 1.
Figure 1.
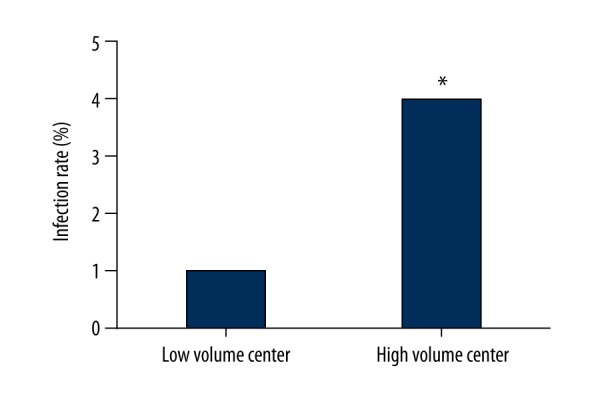
Comparison of CDI rate in low-volume centers and the high-volume center. Columns indicated the CDI rates in low-volume centers and high-volume center. [* difference is significant when compared with low-volume centers (P<0.05)]
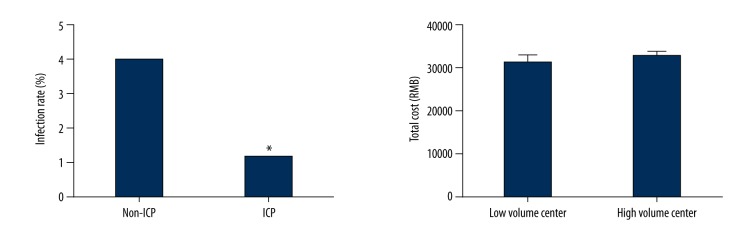
Comparison of CDI rates and medical costs before and after adoption of an ICP in low-volume centers
The results from comparisons of CDI rates and medical costs before and after adoption of an ICP in low-volume centers are shown in Figure 2. From 1 January 2016 to the end of this study, a total of 335 patients received an ICP in the low-volume centers. The CDI rates were significantly lower in patients who received an ICP (p<0.05). The average medical costs for patients who did not receive an ICP was 31263±2216 RMB vs. 32804±1459 RMB for patients who received an ICP and the difference was not significant between the medical costs. These results are shown in Figure 2.
Figure 2.
Comparisons of CDI rates and medical costs before and after adoption of ICP. Columns on the left indicate the CDI rates in patients did not receive ICP and patients who received ICP; Columns on the right indicate the medical costs in patients did not receive ICP and patients who received ICP. [* difference was significant when compared with non-ICP (P<0.05)].
Figure 3 shows a comparison of microbiology analysis results before and after an ICP in low-volume centers. Results of blood and wound cultures are also shown in. Infections of Staphylococcus aureus, Pseudomonas aeruginosa and coagulase-negative staphylococcus were identified in patients with CDIs. After adoption of an ICP, no infection of staphylococcus aureus was found.
Figure 3.
Microbiology before and after ICP. Pie charts indicate the compositions of detected pathogens from blood and wound cultures in patients who did not receive ICP and in patients who received ICP.
Discussion
The CDI rate has been rising over time along with increasing device implantation numbers. CDI is one of the primary causes of severe systemic infection and even septic shock, which is difficult to treat. Thus, prevention of CDIs is of critical importance. In the present study, 1259 patients who received PPM implantations were enrolled. The risk factors for CDIs were analyzed. Of note, male gender, PPM replacement, a long operation duration, low-volume center, and an underlying chronic disease, including diabetes and CKD, were identified as the contributors to CDI. Our data also indicated that the CDI rates in low-volume centers were indeed higher than the rate in the high-volume center. More importantly, the adoption of an ICP significantly lowered the CDI rate in the low-volume centers, and the medical costs were not dramatically increased.
The CDI rate in the present study was 1.906%, which is similar to previous reports. We enrolled several candidate risk factors contributing to CDIs in this investigation, including patient-related, procedure-related, and provider-related factors. Concerning patient-related factors, male gender and several underling chronic diseases were identified as CDI risk factors. Male gender was identified as a risk factor, which was consistent with previous studies. The reasons for this remain unclear. It may be that younger patients are more likely to have non-transvenous systems, which have a higher rate of infection [12]. Similar to previous studies [13], our analysis also suggested that patients with diabetes and CKD had a higher risk for CDI. Disorder of immune-defensive systems and long-term immunosuppressive therapies may be involved. Importantly, there were also several procedure-related risk factors. At first glance, the replacement should not be one of the risk factors because the replacement procedure is simple, and only a minor surgical revision is needed compared with the primary implantations. However, replacement was found to be a risk factor for CDI. The pockets that were fibrous and poorly vascularized limited immunological responses [14]. In addition, the pockets were actually colonized by bacteria, even without obvious clinical signs of infection [15]. Thus, when the pocket were reopened, the latent or perioperative inoculation of pathogens may have facilitated the formation of microbial biofilms, which can lead to pocket infections [5]. It is rational to speculate that a long operation duration was another risk factor for CDIs because it entails longer environmental exposure and more opportunity for infection.
Notably, in the present study, we found that center volume was associated with CDIs as a risk factor. In the high-volume center, the CDI rate was significantly lower than in the low-volume centers. A previous retrospective study indicated that the risks of implantable cardioverter defibrillator (ICD) complications, including infection, were decreased with higher physician volume [16]. In another recent large population-based cohort study, low center volume was also identified as one of the risk factors of CDI [17]. In these low-volume centers, physicians may be less experienced and take longer to perform complex operational procedures. An ICP that was shown to be effective in controlling CDI [6] was adopted in these low-volume centers. Several steps were carried out during peri-operational and operational periods. Patients were required to be screened for MRSA, which can lead to serious sepsis. Precautions, including topical and systemic decolonization, should be used prior to the implantations [18]. In skin preparation, iodine was replaced by a chlorhexidine-alcohol solution, which has been demonstrated to be superior in inhibiting bacterial colonization and is not inactivated by tissue fluid and blood [19]. More effective antibiotics were used prior to operations in high-risk patients. During surgical procedures, disposable adhesive plastic incision drapes, cutting diathermy, and antibacterial-coated Vicryl sutures were used. The underlining chronic diseases were carefully assessed and treated during the perioperational period.
Our data indicate that the adoption of an ICP dramatically decreased the CDI rate in the low-volume centers. The results from blood and wound cultures suggested a change in the composition of pathogens. In patients who received an ICP, no case of Staphylococcus aureus infection, which is often community-acquired, was found. The medical costs analysis suggested there were no significant extra medical costs in patients who received an ICP, which was also easily carried out. Thus, it is reasonable for us to speculate that adoption of an ICP in a low-volume center could offset the increasing risk of CDI brought by the low volume itself. It is a valuable experience, which is worthy of implementation in branch hospitals.
Limitation
There were several limitations to this study. Firstly, although this was a multi-center study, the sample size was relatively small. Secondly, the study was regional, which may have biased the results and conclusions. Thirdly, patients received ICD or CRT-D implantations were not enrolled because the sample size was very small in the branch hospitals. A national-wide multi-center trial would be valuable.
Conclusions
Male gender, diabetes, CKD, operation duration, PPM replacement, and low center volume were identified as the risk factors for CDIs.
Adoption of an ICP dramatically lowered CDI rates, which were higher in low-volume centers compared with high-volume centers.
ICPs were easy to carry out, effective, and economical in controlling CDIs and are worthy of implementation in low-volume centers.
Footnotes
Source of support: This study was supported by Shaanxi Provincial Social Developmental Technology R&D Program (No. 2015SF024)
References
- 1.Margey R. Cardiac implantable electronic device infections: The enemy that lurks beneath the skin. J Long Term Eff Med Implants. 2010;20(3):203–17. doi: 10.1615/jlongtermeffmedimplants.v20.i3.40. [DOI] [PubMed] [Google Scholar]
- 2.Durante Mangoni E, Carbonara S, Iacobello C, et al. [Management of infections from cardiac implantable electronic devices: Recommendations from a study panel]. Infez Med. 2011;19:207–23. [in Italian] [PubMed] [Google Scholar]
- 3.Harrison JL, Prendergast BD, Sandoe JA. Guidelines for the diagnosis, management and prevention of implantable cardiac electronic device infection. Heart (British Cardiac Society) 2015;101:250–52. doi: 10.1136/heartjnl-2014-306873. [DOI] [PubMed] [Google Scholar]
- 4.Da Costa A, Kirkorian G, Cucherat M, et al. Antibiotic prophylaxis for permanent pacemaker implantation: A meta-analysis. Circulation. 1998;97:1796–801. doi: 10.1161/01.cir.97.18.1796. [DOI] [PubMed] [Google Scholar]
- 5.Johansen JB, Jorgensen OD, Moller M, et al. Infection after pacemaker implantation: infection rates and risk factors associated with infection in a population-based cohort study of 46299 consecutive patients. Eur Heart J. 2011;32:991–98. doi: 10.1093/eurheartj/ehq497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahsan SY, Saberwal B, Lambiase PD, et al. A simple infection-control protocol to reduce serious cardiac device infections. Europace. 2014;16:1482–89. doi: 10.1093/europace/euu126. [DOI] [PubMed] [Google Scholar]
- 7.Topan A, Carstina D, Slavcovici A, et al. Assesment of the Duke criteria for the diagnosis of infective endocarditis after twenty-years. An analysis of 241 cases. Clujul Med. 2015;88:321–26. doi: 10.15386/cjmed-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alonso GT, Pyle L, Frohnert B. Change in hemoglobin A1c one year following the 2014 American Diabetes Association guideline update. Diabetes Res Clin Pract. 2017;129:169–72. doi: 10.1016/j.diabres.2017.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wedzicha JA, Calverley PMA, Albert RK, et al. Prevention of COPD exacerbations: A European Respiratory Society/American Thoracic Society guideline. Eur Respir J. 2017;50(3) doi: 10.1183/13993003.02265-2016. pii: 1602265. [DOI] [PubMed] [Google Scholar]
- 10.Inker LA, Astor BC, Fox CH, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014;63:713–35. doi: 10.1053/j.ajkd.2014.01.416. [DOI] [PubMed] [Google Scholar]
- 11.Jimeno Sainz A, Gil V, Merino J, et al. [Validity of Framingham criteria as a clinical test for systolic heart failure]. Rev Clin Esp. 2006;206:495–98. doi: 10.1016/s0014-2565(06)72875-2. [in Spanish] [DOI] [PubMed] [Google Scholar]
- 12.Lai KK, Fontecchio SA. Infections associated with implantable cardioverter defibrillators placed transvenously and via thoracotomies: epidemiology, infection control, and management. Clin Infect Dis. 1998;27:265–69. doi: 10.1086/514673. [DOI] [PubMed] [Google Scholar]
- 13.Polyzos KA, Konstantelias AA, Falagas ME. Risk factors for cardiac implantable electronic device infection: A systematic review and meta-analysis. Europace. 2015;17:767–77. doi: 10.1093/europace/euv053. [DOI] [PubMed] [Google Scholar]
- 14.Klopfleisch R, Jung F. The pathology of the foreign body reaction against biomaterials. J Biomed Mater Res A. 2017;105:927–40. doi: 10.1002/jbm.a.35958. [DOI] [PubMed] [Google Scholar]
- 15.Subbiahdoss G, Kuijer R, Grijpma DW, et al. Microbial biofilm growth vs. tissue integration: “The race for the surface” experimentally studied. Acta Biomater. 2009;5:1399–404. doi: 10.1016/j.actbio.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 16.Al-Khatib SM, Lucas FL, Jollis JG, et al. The relation between patients’ outcomes and the volume of cardioverter-defibrillator implantation procedures performed by physicians treating Medicare beneficiaries. J Am Coll Cardiol. 2005;46:1536–40. doi: 10.1016/j.jacc.2005.04.063. [DOI] [PubMed] [Google Scholar]
- 17.Lin YS, Hung SP, Chen PR, et al. Risk factors influencing complications of cardiac implantable electronic device implantation: Infection, pneumothorax and heart perforation: A nationwide population-based cohort study. Medicine. 2014;93:e213. doi: 10.1097/MD.0000000000000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khalighi K, Aung TT, Elmi F. The role of prophylaxis topical antibiotics in cardiac device implantation. Pacing Clin Electrophysiol. 2014;37:304–11. doi: 10.1111/pace.12280. [DOI] [PubMed] [Google Scholar]
- 19.Brown TR, Ehrlich CE, Stehman FB, et al. A clinical evaluation of chlorhexidine gluconate spray as compared with iodophor scrub for preoperative skin preparation. Surg Gynecol Obstet. 1984;158:363–66. [PubMed] [Google Scholar]