Abstract
Macrophage activation syndrome (MAS) refers to acute overwhelming inflammation caused by a ‘cytokine storm’. Although increasingly recognized as a life-threatening complication of various rheumatic diseases, clinically, MAS is strikingly similar to primary and secondary forms of haemophagocytic lymphohistiocytosis (HLH). Not surprisingly, many rheumatologists prefer the term secondary HLH rather than MAS to describe this condition, and efforts to change the nomenclature are in progress. The pathophysiology of MAS remains elusive, but observations in animal models, as well as data on the effects of new anticytokine therapies on rates and clinical presentations of MAS in patients with systemic juvenile idiopathic arthritis (sJIA), provide clues to the understanding of this perplexing clinical phenomenon. In this Review, we explore the latest available evidence and discuss potential diagnostic challenges in the era of increasing use of biologic therapies.
Macrophage activation syndrome (MAS) is a potentially fatal complication of rheumatic diseases caused by excessive activation and expansion of T lymphocytes and of macrophages that exhibit haemophagocytic activity1–7. These events lead to overproduction of cytokines and a hyperinflammatory state associated with cytopenias, liver dysfunction and coagulopathy, resembling disseminated intravascular coagulation. Another prominent feature of MAS is extremely high levels of serum ferritin, presumably originating from activated macrophages. MAS remains a major cause of mortality in paediatric rheumatology with reported death rates as high as 20–30%6,7. Although this complication has been associated with most rheumatic diseases, in paediatrics it is by far most common in systemic juvenile idiopathic arthritis (sJIA)2,6,7. The pathophysiology of sJIA seems to be driven by continuous activation of innate immune pathways leading to dysregulated production of proinflammatory cytokines. Therefore, many paediatric rheumatologists view sJIA as an autoinflammatory disorder rather than a classic auto-immune disease8–10. IL -1β11–13 and IL-6 (REFS 14–16) have been suggested as essential cytokines in the pathogenesis of this condition, although the source of the excess IL-6 and IL-1β activity has not yet been defined.
Due to increasing awareness of MAS, this condition is now recognized more frequently than before and the interest in this syndrome is growing. New observations in animal models and increasing clinical experience with MAS treatment with various biologics shed new light on the role of cytokines in its pathophysiology. Here, we describe the current terminology and the newly proposed classification criteria for MAS, as well as the animal models commonly used to study this condition. In addition, we explore new concepts of the underlying pathophysiology. Finally, we review the effects of existing biologic therapies on MAS and likely new therapeutic targets, and discuss the potential impact of these drugs on the performance of current diagnostic criteria.
Defining MAS and HLH
The inflammatory infiltrate in MAS consists mainly of activated T lymphocytes and histologically benign, well-differentiated macrophages (or histiocytes) that engulf normal haematopoietic cells17,18 (FIG. 1). Among T lymphocytes, CD8 T cells predominate markedly over CD4 T cells (FIG. 2). The abundance of highly activated haemophagocytic histiocytes suggests that MAS belongs to the group of histiocytic disorders known as haemophagocytic lymphohistiocytosis (HLH).
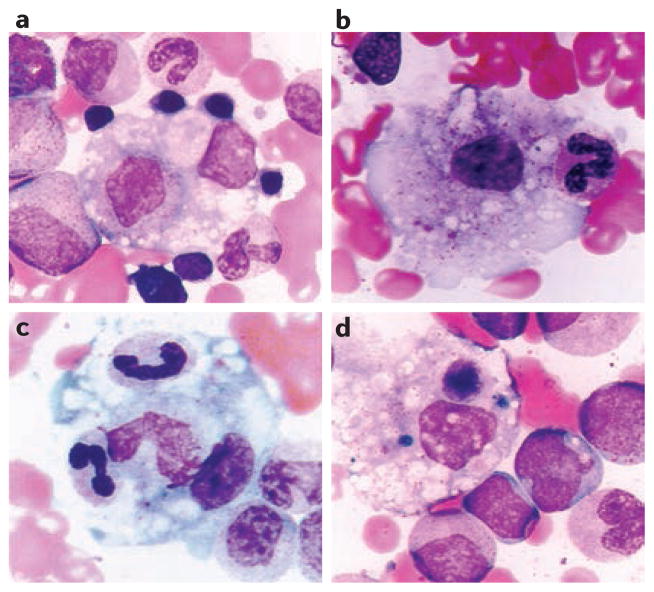
Figure 1. Activated macrophages in bone marrow inflammatory infiltrate in MAS.
a | Myelocyte within an activated macrophage. In addition, there are multiple adherent red blood cell and myeloid precursors. b | Activated macrophage engulfing a band neutrophil. c | Band neutrophil and metamyelocyte within an activated macrophage. Nuclei of neutrophil band appear condensed. d | Activated macrophage with haemosiderin deposits and a degenerating phagocytosed nucleated cell. H&E stain, original magnification ×1000. MAS, macrophage activation syndrome. Reproduced with permission from Prahalad, S. et al. Etanercept in the treatment of macrophage activation syndrome. J. Rheumatol. 28, 2120–2124 (2001). All rights reserved.
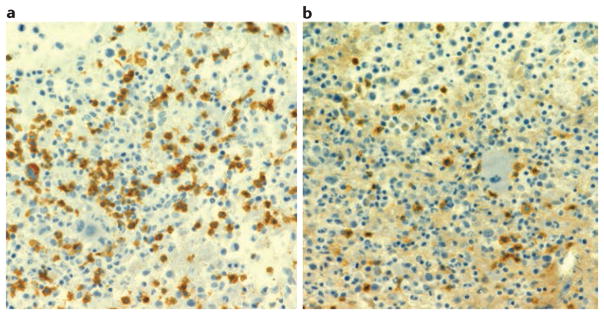
Figure 2. T cells in bone marrow inflammatory infiltrate in MAS.
Immunohistochemical assessment of the T lymphocyte infiltrate in a bone marrow biopsy from a patient with macrophage activation syndrome (MAS) presenting as a complication of systemic juvenile idiopathic arthritis (sJIA). a | Immunohistochemical staining with anti-CD8 antibodies shows numerous CD8+ T lymphocytes; b | CD4 staining shows only rare CD4+ T lymphocytes.
In the current classification of histiocytic disorders, HLH is further divided into primary (or familial) HLH and secondary HLH (also known as acquired or reactive HLH)19–21.
Primary HLH
Primary HLH is not a single disease, but rather a group of rare, autosomal recessive, immune disorders linked to various genetic defects, all affecting the perforin-mediated cytolytic pathway. Symptoms of primary HLH usually become evident during the first year of life, although cases with onset in adulthood are reported with increasing frequency. The clinical course of primary HLH can be rapidly progressive; such cases eventually require haematopoietic stem cell transplantation as definitive treatment20.
In ~30% of patients with primary HLH, cytolytic dysfunction is due to loss-of- function mutations in the gene encoding perforin (PRF1), a protein utilized by cytolytic cells (such as cytotoxic CD8 T lymphocytes or natural killer [NK] cells) to induce apoptosis of target cells via granzyme B22. When released in the interface between the cytolytic and the target cell (immune synapse), perforin self-polymerizes, creating pores in the plasma membrane that enable granzymes to enter the target cell and trigger apoptosis. The genes implicated in three other types of primary HLH (MUNC13-4, STX11 and STXBP2) encode proteins involved in the transport of granules containing perforin and granzyme to the immune synapse23–25. The cytolytic cells in patients with these mutations produce sufficient amounts of perforin, but an impaired ability to release perforin into the immune synapse leads to profoundly decreased cytolytic activity. Although mutations in PRF1, MUNC13-4, STX11 and STXBP2 explain disease in the majority of patients with primary HLH, ~40% of familial cases are still awaiting molecular definition26. Depressed cytolytic function due to abnormal movements of intracellular granules also contributes to the development of HLH in Griscelli syndrome type 2, caused by mutations in RAB27A, and Chediak-Higashi syndrome, caused by mutations in LYST27–28. Both these syndromes are also considered primary HLH. In normal physiological conditions, cytolytic cells induce apoptosis of cells infected with viruses or cells undergoing malignant transformation. Even moderate defects in the cytolytic pathway might prolong the survival of target cells, ultimately leading to overproduction of proinflammatory cytokines29. Cytolytic cells can also be directly involved in the termination of immune responses by inducing apoptosis of overly activated immune cells30–32. These observations led to the hypothesis that, in HLH, failure to induce apoptosis of target cells by cytolytic cells might delay the contraction stage of the immune response, leading to persistent expansion of activated T lymphocytes and macrophages and escalated production of pro-inflammatory cytokines, thus creating a ‘cytokine storm’. X-linked lymphoproliferative syndromes type 1 and 2, caused by mutations in SH2D1A and XIAP, respectively, are two other hereditary immunodeficiencies associated with HLH. Genetic defects in these syndromes interrupt activation-induced apoptosis of immune cells, leading to prolonged survival of lymphocytes and thus increased production of cytokines33,34. The onset of HLH symptoms in these patients is usually triggered by Epstein–Barr virus (EBV) infection causing rapid expansion of activated lymphocytes.
Secondary HLH
Secondary HLH can occur at any age. In general, patients tend to have less severe clinical presentations than in primary HLH, but mortality in this group is still considered high20, and the emergence of the first clinical signs and symptoms can usually be linked to an infection (most commonly EBV or cytomegalovirus [CMV]) or malignancy19–21. Furthermore, the cytolytic pathway abnormalities that occur in this condition are generally considered to be acquired35. Over the past 3 years, however, the development of secondary HLH in some of these patients has been linked to compound heterozygous or heterozygous hypomorphic mutations that confer a partial dominant negative effect on cytolytic function36. These findings make it increasingly difficult to distinguish between primary and secondary HLH.
Two 2014 reports described patients with periodic fevers and MAS-like features linked to a gain-of-function mutation in NLRC4, which was in turn associated with overproduction of IL-1β and IL-18, as well as increased pyroptosis37,38. Pyroptosis is morphologically and mechanistically distinct from other forms of cell death. This process is mediated by caspase 1 and is characterized by rapid plasma-membrane rupture and release of proinflammatory intracellular contents. In patients with gain-of-function mutations in NLRC4, MAS-like clinical presentation seemed to be induced by a macrophage-intrinsic defect in the absence of primary cytotoxic abnormalities. Although haemophagocytosis has been observed in these patients38, the extent of patho-physiologic and clinical overlap between this clinical entity and primary HLH is yet to be characterized.
MAS as a form of HLH
The extent of clinical similarity between MAS and HLH is striking (TABLE 1); not surprisingly, many paediatric rheumatologists prefer to classify MAS as one of the categories among the secondary HLH forms, or ‘rheumatic HLH’. Efforts to update the terminology used to describe MAS and HLH are underway (see BOX 1).
Table 1.
Clinical and laboratory features of sJIA, MAS and HLH
| Feature | sIJA | MAS | HLH |
|---|---|---|---|
| Clinical features | |||
| Fever pattern | Quotidian | Unremitting | Unremitting |
| Rash | Evanescent, maculopapular | Papular, petechial or purpuric | Papular, petechial or purpuric |
| Hepatomegaly | + | +++ | +++ |
| Lymphadenopathy | + | +++ | ++ |
| Arthritis | + | – | – |
| Serositis | + | – | – |
| Encephalopathy | – | ++ | +++ |
| Laboratory features | |||
| WCC, neutrophil count | ↑↑ | ↓ | ↓↓ |
| Haemoglobin | Normal or ↓ | ↓ | ↓↓ |
| Platelets | ↑↑ | ↓ | ↓↓ |
| ESR | ↑↑ | Normal or sudden ↓ | Usually low |
| Bilirubin | Normal | Normal or ↑ | Normal or ↑ |
| ALT/AST | Normal or ↑ | ↑↑ | ↑↑ |
| PT | Normal | ↑ | ↑↑ |
| PTT | Normal | ↑ | ↑↑ |
| D-dimer | ↑ | ↑↑ | ↑↑ |
| Fibrinogen | ↑ | ↓ | ↓↓ |
| Ferritin | Normal or ↑ | ↑↑ | ↑↑↑ |
| sCD25 | Normal or ↑ | ↑↑ | ↑↑↑ |
| CD163 | Normal or ↑ | ↑↑ | ↑↑↑ |
| HPS in BM | +/− | ++ | +++ |
ALT, alanine aminotransferase; AST, asparate aminotransferase; BM, bone marrow; ESR, erythrocyte sedimentation rate; HLH haemophagocytic lymphohistiocytosis; HPS, haemophagocytosis; MAS, macrophage activation syndrome; PT, prothrombin time, PTT, partial thromboplastin time; sCD25, soluble CD25; sJIA, systemic juvenile idiopathic arthritis; WCC, white cell count; ↑, mildly elevated; ↑↑, moderately elevated; ↑↑↑, highly elevated; ↓, mildly decreased; ↓↓, moderately decreased; ↓↓↓, highly decreased; –, not observed; +/–, occasionally observed; + commonly observed; ++, observed very frequently; +++, observed almost always.
Box 1. Development of the terminology for MAS and HLH.
From a historical perspective, the terms haemophagocytic lymphohistiocytosis (HLH) and macrophage activation syndrome (MAS) were introduced at approximately the same time. The classification of histiocytic disorders was initially proposed by the Histiocyte Society in 1987 (REF. 84). The first comprehensive clinical descriptions of the syndrome now known as MAS were first published in the early 1980s1,2. In 1985, Hadchouel et al.2 linked this clinical phenomenon to extensive proliferation of macrophagic histiocytes with pronounced haemophagocytic activity, and the term MAS was eventually introduced by the same group a few years later3. Suggestions to replace the term MAS with secondary HLH first appeared in the literature in 2002 (REFS 85–86). A survey of paediatric rheumatologists and haematologists presented at the 2015 Annual Meeting ofthe International Histiocyte Society revealed that both groups were in favour of using a single term to describe these patients. Interestingly, many haematologists felt that, as a large proportion (up to 40%) of patients with HLH did not display overt haemophagocytosis, the word haemophagocytic within the term HLH might not be appropriate, whereas the term lymphohistiocytosis should certainly be preserved. Efforts to update the contemporary classification of the histiocytic disorders and align terminology used by haematologists and rheumatologists are currently in progress and, in collaboration with the WHO, the revised classification and nomenclature on histiocytic disorders is due to be included in the upcoming 11th version of the International Classification of Diseases.
The similarities between HLH and MAS are not limited to clinical features. Similarly to patients with HLH, patients with MAS in association with a rheumatic disease also have profoundly decreased cytolytic function, although this impairment tends to improve with better control of the activity of the underlying rheumatic disease39. These observations suggest that background inflammation is at least partially responsible for this functional abnormality in MAS. Indeed, IL-6, a major contributor to the pathogenesis of sJIA, has been shown to induce defective expression of perforin and decreased NK cell cytotoxic activity40. The development of cytolytic dysfunction in sJIA and MAS might also be influenced by a genetic component: a study using whole-exome sequencing demonstrated the presence of hypomorphic mutations in primary HLH-associated genes in approximately one-third of patients with sJIA and MAS41. Targeted sequencing of primary HLH genes from patients with sJIA-associated MAS led to similar results in another study42. These studies showed a markedly increased frequency of rare protein-altering variants of genes involved in the intracellular transport of perforin-containing granules to the cell surface, as well as in the perforin gene itself. Interestingly, PRF1 variants were observed mainly in populations in Europe43, but not in North America41–44.
Animal models of HLH and MAS
Perforin-deficient mice develop many of the clinical features of HLH after infection with lymphocytic choriomeningitic virus, and are considered the model organism for primary HLH45. Levels of several cytokines were reported to be elevated in this model — a pattern reminiscent of the cytokine storm seen in HLH. Remarkably, all clinical and laboratory features of this condition as well as associated mortality can be almost completely prevented by elimination of CD8 T lymphocytes or neutralization of IFN-γ 45. IFN-γ is known to activate macrophages; therfore, IFN-γ might be critical for triggering the expansion of macrophages in perforin-deficient mice. Similar findings have been observed in Ras-related protein Rab-27A knockout mice46, another model of primary HLH. These findings implicate IFN-γ-producing CD8 T lymphocytes as the main driving force of HLH, and suggest that this pathway is a potential therapeutic target.
HLH-like features can also be induced in healthy mice by repeated administration of CpG, a Toll-like receptor 9 (TLR9) ligand47. This model is thought to closely reflect the pathology of secondary HLH that occurs in the context of infections, as continuous stimulation of TLR9 mimics infection. Although serum ferritin levels are only mildly elevated in these animals, and despite the need for additional blockade of IL-10 to induce overt haemo-phagocytosis, many clinical features seen in this model are reminiscent of HLH (such as cytopenias and liver dysfunction)47. The role of IFN-γ in this model has been assessed by several groups. Behrens et al.47 demonstrated that in these animals, IFN-γ was produced mainly by den-dritic cells (DCs) and NK cells rather than CD8 T lymphocytes. Interestingly, in a later study by the same group, IFN -knockout mice subjected to repeated administration of CpG developed immunopathologies and haemo-phagocytosis comparable to wild-type mice48. However, IFN-γ-knockout mice did not become anaemic and had greater numbers of splenic erythroid precursors, suggesting that IFN-γ contributes to the development of anaemia but might not be required for other MAS features48. In a more recent study using the same model, De Min et al.49 neutralized IFN-γ by repeated administration of anti-IFN-γ antibodies. The investigators confirmed neutralization of IFN-γ activity by measuring circulating levels of IFN-γ-induced chemokines such as CXCL9. In this study, clinical and laboratory features dependent on IFN-γ were not limited to anaemia, but included weight loss, splenomegaly, hyperferritinaemia, cytopenia and liver inflammation49. Despite some discrepancies, all studies utilizing the CpG model of MAS described herein clearly link chronic TLR stimulation with the development of an HLH-like phenotype. These findings might be relevant for the pathogenesis of MAS, as gene expression signatures reflecting continuous activation of TLR–IL-1 receptor (IL-1R)-induced signalling pathways have also been reported in sJIA50.
Other observations with potential relevance to MAS have been made in mice genetically modified to overproduce IL-6 (REF. 51). The rationale for the development of this model was based on data implicating IL-6 as the main cytokine in the pathogenesis of sJIA14–16 Findings in mice that overproduce IL-6 might reflect the pathology of MAS that occurs in the setting of autoinflammation or autoimmunity more accurately than other animal models of haemophagocytic syndromes. In these mice, macrophages chronically exposed to IL-6 have an exaggerated response to TLR stimulation, which is used as a surrogate for acute infection51. Mice overexpressing IL-6 have reduced survival when compared with wild-type mice, and develop MAS-like features, including cytopenia and increased serum levels of ferritin. These observations suggest that IL-6-driven background inflammation, as seen in sJIA, can lead to exaggerated responses of macrophages to inflammatory stimuli induced by infection and thus contribute to MAS development. Background inflammatory activity also seems to have a role in the emergence of MAS-like phenotypes in patients with a gain-of-function mutation in the NLRC4 gene, leading to overproduction of IL-1β and IL-18 (REFS 37,38).
Proposed MAS pathophysiology model
Serum IFN-γ levels in patients with MAS are markedly high compared with patients with active sJIA without MAS52,53. The emergence of clinical features of MAS in sJIA patients often corresponds to an increase in levels of IFN-γ-induced chemokines53 and neopterin, a catabolic product of guanosine triphosphate released by IFN-γ activated macrophages54. Furthermore, extremely high serum levels of soluble IL-2 receptor subunit-α (sIL2Rα), presumably shed by cytotoxic CD8 T lymphocytes, are a consistent finding in patients with MAS that could be a useful diagnostic marker55. Histopathological evaluation of inflammatory infiltrates in MAS lesions also reveals abundant CD8 T lymphocytes that produce proinflammatory cytokines, including IFN-γ 17. Combined, these observations suggest that, in keeping with findings from animal models, extensive activation and expansion of cytotoxic CD8 T lymphocytes producing IFN-γ and other macrophage-activating cytokines are also likely to be central to the pathogenesis of MAS. Subsequently, prolonged stimulation of monocytes and macrophages with these cytokines results in excessive activation and expansion of these cells. These final pathways leading to the development of overt MAS seem similar to those in primary HLH. The upstream events, however, might be more complex and have several components (FIG. 3). Similar to primary HLH, depressed cytolytic activity also seems to be an important factor in MAS. The genetic defects contributing to the development of this cytolytic dysfunction are usually low-penetrance, mainly heterozygous variants and, therefore, might only trigger MAS in combination with the background inflammatory activity of sJIA. In other words, persistent activation of TLR signalling pathways as observed in sJIA and chronic IL-6 exposure might further suppress cytolytic function and exaggerate responses of macrophages to inflammatory stimuli. Intercurrent infection, a common trigger of MAS, associated with an additional surge in macrophage activation by cytokines such as IFN-γ , further amplifies the inflammatory response, leading to escalating production of cytokines and ultimately creating a cytokine storm.
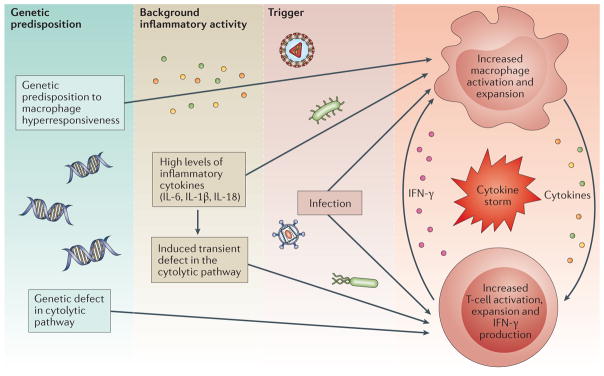
Figure 3. Multi-layer model of pathogenic events leading to the development of MAS in the context of rheumatic diseases.
Genetic factors and the inflammatory milieu created by the underlying rheumatic disease act synergistically to reach the threshold for macrophage activation syndrome (MAS) in the presence of an infectious trigger.
Indeed, in both MAS and HLH, remarkably high levels of circulating cytokines, including IFN-γ , IL-2, macrophage colony-stimulating factor (M-CSF), IL-1, IL-6, IL-18 and TNF, as well as cytokine inhibitors such as soluble TNF receptors and IL-1R antagonist (IL-1Ra), have been reported56–58. The relative importance of these cytokines, however, is not clear. High levels of a particular cytokine that strongly correlate with disease activity do not necessarily constitute causality. By contrast, the effects of biologic therapies that block specific cytokines on the risk of developing MAS and its clinical presentation can provide valuable clues for understanding the pathophysiology of this clinical phenomenon.
Effects of biologic therapy on MAS
As mentioned earlier, IL-1β11–13 and IL-6 (REFS 14–16) have been implicated as important cytokines in sJIA pathophysiology. Encouraging findings from small studies involving treatment of sJIA with drugs targeting these cytokines12,15 led to large phase III trials with the IL-6-receptor inhibitor tocilizumab and the IL-1β inhibitor canakinumab13,16. In these trials, 60–70% of patients achieved ACR70 (70% improvement according to ACR criteria). Furthermore, more than one-third of patients achieved clinical remission. Adequate control of the underlying disease was expected to protect against MAS, as sJIA disease activity was thought to contribute to the development of this complication. Surprisingly, complete protection was not observed in either trial, even in patients whose underlying sJIA was well-controlled13,16. In the following sections we review the effect of IL-1 and IL-6-inhibiting biologics on the rates and clinical features of MAS in patients with sJIA.
IL-1-inhibiting agents
Anakinra
Anakinra is a recombinant, non-glycosylated form of human IL-1Ra. Anakinra blocks the biologic activity of both IL-1α and IL-1β by competitively inhibiting their binding to IL-1R. Despite a lack of phase III trials, anakinra is now widely used for the treatment of sJIA and, occasionally, MAS. Marked improvement in response to anakinra in sJIA-associated MAS after inadequate response to corticosteroids and ciclosporin has been described in many case reports59–60. By contrast, in two reports of the experience with anakinra in sJIA in several paediatric rheumatology centres, occurrence of MAS was described in children treated with doses of 1–2 mg/kg daily61–62. In some of these patients, however, features of MAS improved after the dose of anakinra was increased. Overall, the exact effect of anakinra on rates of MAS in sJIA patients is unknown. The consensus is that anakinra, particularly at higher doses, might be effective at least in some patients with sJIA-associated MAS. However, MAS occurs even when disease activity of the underlying sJIA is controlled by regular treatment with anakinra.
Canakinumab
Canakinumab is a high-affinity, fully human monoclonal anti-IL-1β antibody designed to exclusively bind and neutralize human IL-1β13. It does not bind IL-1α or IL-1Ra. In the early stages of two phase III trials of canakinumab in sJIA, MAS was reported as an adverse event, prompting the formation of an independent adjudication committee. Members of the committee had full access to the entire sJIA clinical database for both trials and reviewed all events suspected to be MAS based on specific adjudication criteria (see Supplementary information S1 (table)). Of 323 patients with sJIA enrolled in the trials, including the open-label extension phase (total 669 patient-years of exposure)13, 17 (5.3%) experienced events classified as probable MAS (complete criteria are provided elsewhere63) whilst treated with canakinumab (2.8 events per 100 patient-years)63. Two patients had two separate episodes of MAS. The existing literature suggests that 7–17% of all patients with sJIA develop full-blown MAS7; the proportion of patients developing MAS in the canakinumab trials seems to be just below this range. Furthermore, the incidence of MAS observed in the trials was similar to the incidence of MAS in sJIA patients reported from a paediatric rheumatology centre in Cincinnati, USA (4–6 MAS events per 100 patient-years; A.A.G., personal observation). These observations suggest that IL-1 inhibition with canakinumab does not have a major effect on the risk of developing MAS. Surprisingly, even when underlying sJIA was controlled with this treatment in the two phase III trials, occurrence of MAS was not prevented. In almost all MAS events in these studies, infection was a trigger and clinical and laboratory features of MAS did not seem to be substantially modified by the treatment.
In addition to the 19 probable MAS events in 17 patients, 10 events in nine patients treated with canakinumab were classified as possible MAS63. These patients developed laboratory features consistent with MAS and fever, but no other typical clinical features of MAS. All these events were identified through a search of the clinical and laboratory database for the two phase III trials using predefined laboratory criteria (ferritin ≥500 μ/l; elevated transaminase(s); or leukopenia and/or thrombocytopenia). Surprisingly, none of these events were reported as MAS by the treating physicians, but had been interpreted as flares of sJIA triggered by intercurrent infection. Management of these events was limited to a moderate increase in the dose of corticosteroids63. The timely increase in corticosteroid dose is likely to have prevented the progression to overt life-threatening MAS in these patients, as laboratory abnormalities were consistent with MAS, perhaps reflecting the early stages of this syndrome (or ‘subclinical MAS’). In fact, five of the 10 events satisfied the new classification criteria for MAS in sJIA listed in BOX 2. One important conclusion from these observations is that even mild worsening of sJIA features in a patient treated with canakinumab, especially if triggered by infection, should prompt additional laboratory investigations to rule out subclinical MAS that might require modification of treatment and close monitoring.
Box 2. Classification criteria for MAS in sJIA.
A febrile patient with known or suspected systemic juvenile idiophatic arthritis (sJIA) is classified as having macrophage activation syndrome (MAS) if the following criteria are met:
Serum ferritin >684 ng/ml
Plus any two of the following:
Platelet count ≤181 × 109/l
Aspartate aminotransferase >48 U/l
Triglycerides >156 mg/dl
Fibrinogen ≤360 mg/dl
Rilonacept
Rilonacept is a recombinant protein in which the extracellular domains of the IL-1R type I and IL-1R accessory protein are fused with each other as well as with the Fc portion of human IgG1. The extracellular domains of the IL-1R components have strong affinity for both IL-1α and IL-1β and, therefore, intravenous administration of this fusion protein leads to neutralization of both cytokines. Another unique feature of rilonacept is that it can also potentially bind to IL-1Ra64. In a phase III trial of rilonacept in sJIA, patients were randomly allocated in a 1:1 ratio to receive either 4 weeks of placebo followed by 20 weeks of rilonacept or to receive 24 weeks of rilonacept, resulting in a double-blind placebo-controlled phase (weeks 0–4) and a phase of active treatment for all participants (weeks 4–24). Patients who benefited from rilonacept treatment were eligible for enrolment in the long-term open-label extension phase (24 weeks to 21 months)64. In this trial, corticosteroids were increased or started in case of emergence of any clinical feature suggestive of MAS. Overall, rilonacept demonstrated efficacy in controlling active sJIA. One episode of definite MAS was reported by the investigator. In this patient, MAS was triggered by EBV infection during the open-label extension phase when underlying sJIA was controlled.
Does the specific agent matter?
Data generated in the trials described above raise the question of whether the various IL-1-inhibiting agents might differ in terms of their effect on MAS. As discussed earlier, several case reports suggest that anakinra might be effective in at least some patients with sJIA-associated MAS, while canakinumab administered at doses of 4 mg/kg monthly does not have a major effect on the risk of developing MAS or on its clinical features. The reason for the observed differences is unclear, as both biologics neutralize IL-1β activity. Anakinra neutralizes both IL-1α and IL-1β activity whereas canakinumab is specific only for IL-1β, so it would seem important to assess the potential role of IL-1α in the pathogenesis of MAS. However, the fact that MAS has been seen in sJIA patients treated with rilonacept, which also neutralizes IL-1α, makes this possibility less likely. The effect of dosing could be another important factor. In most case reports describing successful use of anakinra in MAS, daily doses of up to 10 mg/kg were administered, whereas MAS is known to occur in patients treated with doses of 1–2 mg/kg per day, although exact rates have yet to be assessed. In the clinical trials of canakinumab in sJIA, the monthly dose did not exceed 4 mg/kg. It is possible that this dose may not be sufficient to neutralize excessive IL-1β activity in MAS. The effect of higher doses of canakinumab on MAS has not been studied.
IL-6- inhibiting agents: tocilizumab
Tocilizumab is a humanized monoclonal antibody that inhibits IL-6-mediated signalling by binding to the soluble and membrane-bound IL-6 receptors (sIL-6R and mIL-6R, respectively). An independent adjudication committee examined all cases reported as MAS by investigators, as well as all cases of increased transaminases, in a phase III clinical trial of tocilizumab in sJIA performed in Europe, Australia and the USA (112 patients, 403 patient-years of exposure), in a separate sJIA phase III trial (149 patients, 326 patient-years), and in a post-marketing surveillance program in Japan (366 patients, 524 patient-years)16,65,66. In total, 22 events were adjudicated as definite (n = 11) or potential (n = 11) MAS in 21 (3.3%) of the 627 patients with sJIA included in the entire database (see Supplementary information S1 (table) for criteria). On the basis of exposure to tocilizumab, the calculated rates of definite and potential MAS in the three cohorts were 1.24, 1.84 and 2.10 events per 100 patient-years, respectively. Combining all definite and potential MAS cases from the three cohorts yielded a rate of 1.8 events per 100 patient-years. In two of the five cases reported in the phase III trial performed in the USA, Europe and Australia, the development of MAS might have been secondary to withdrawal of tocilizumab therapy, to administration of a partial dose, or to both.
Treatment with tocilizumab has also been suggested to modify some MAS features. In the events that occurred during tocilizumab treatment in the phase III trial in the USA, Europe and Australia, clinical features of MAS were milder than expected. Moreover, these episodes also seemed to present less commonly with hepatomegaly. In almost three-quarters of all cases, C-reactive protein (CRP) levels remained within the normal range16,66. Given the role of IL-6 in inducing the acute-phase response of the liver, this observation is not unexpected. Ferritin levels were also lower than expected in at least some cases. Similar observations have been documented by other groups67,68. In addition, a trend towards decreased levels of serum fibrinogen, lowered platelet counts and more-extensive elevation of hepatic enzymes than in typical episodes of MAS was observed in all three cohorts.
In conclusion, data from the tocilizumab clinical trials and post-marketing surveillance suggest that IL-6 inhibition does not provide full protection against MAS, despite reports that clinical presentation of this syndrome might be modified in tocilizumab-treated patients. Moreover, the excellent response of sJIA features to tocilizumab with simultaneous development of MAS features in some patients also suggests that the role of IL-6 in MAS development might be limited68.
Pathophysiological implications
Taken together, the observed rates of MAS in phase III clinical trials of tocilizumab and canakinumab show that, in sJIA, therapeutic strategies aimed at the inhibition of either IL-1 or IL-6 do not provide full protection against MAS, even if the underlying sJIA is well controlled. One conclusion is that neither IL-1 nor IL-6 is the only driver contributing to development of MAS. Although direct evidence from animal models and indirect evidence from humans suggest that IL-6 might be a contributing factor in the development of MAS, data from clinical trials on IL-1 or IL-6 inhibitors in sJIA suggest that IL-1 or IL-6, or both, might be dispensable.
These findings also suggest that the risk of developing MAS has additional components, such as genetic factors or other inflammatory cytokines, that are not completely abrogated by controlling the underlying sJIA activity with agents that inhibit IL-6 or IL-1. Considering the close clinical resemblance between MAS and secondary HLH, the potential role of hypomorphic genetic variants in genes associated with primary HLH should be considered. In the presence of these variants in HLH-associated genes, an encounter with certain microorganisms could trigger MAS in patients with sJIA even if the underlying disease has responded well to agents inhibiting IL-1 or IL-6. Consistent with this idea, the development of MAS in patients with well-controlled sJIA from the trials described above was almost always triggered by infection63–66. Furthermore, targeted sequencing of HLH-associated genes41–44, and whole-exome sequencing in patients with sJIA-associated MAS41, revealed an enrichment for rare protein-altering variants in genes capable of affecting the cytolytic pathway that is dependent on granules. These findings suggest that the genetic contribution of this clinical phenomenon needs to be explored further. One should also consider the possibility that cytokines other than IL-1 or IL-6 have a central role in MAS pathophysiology.
A role for IL-18?
Over the past 5 years, interest in the role of IL-18 in the pathogenesis of sJIA in general and in MAS in particular has increased. Strikingly high serum levels of IL-18 have been observed in patients with sJIA69–71, in sharp contrast to only moderately elevated levels of IL-18 seen in other rheumatic diseases such as rheumatoid arthritis (RA) or systemic lupus erythematosus (SLE)72,73. Patients with high levels of IL-18 are more likely to have systemic manifestations than arthritis as the predominant feature of sJIA, and also seem to be more likely to develop MAS69. The emergence of MAS features in these patients is associated with a further increase in IL-18 levels69, which possibly reflect the extent of macrophage activation given that macrophages seem to be the main source of IL-18 in patients with MAS70. Consistent with this hypothesis, levels of IL-18 correlate with ferritin — another marker of macrophage activation — in adult-onset Still disease71. Finally, comparable IL-18 levels have also been observed in primary and secondary HLH74, as well as in patients with a MAS-like syndrome linked to NLRC4 mutations37. Therefore, the use of IL-18 levels as a potential marker for those at risk of developing MAS in adult-onset Still disease, and perhaps also in sJIA, has been suggested69–71.
IL-18 was originally described as an IFN-γ-inducing factor75. The IL-18 receptor system is similar to the one utilized by IL-1 and, not surprisingly, IL-18 shares downstream effector pathways with critical immunoregulatory molecules such as TLRs and IL-1. The activity of IL-18 is counter-regulated by a high-affinity, naturally occurring IL-18 binding protein (IL-18BP). In humans, increased disease severity has been associated with an imbalance of IL-18 to IL-18BP, leading to high levels of unbound IL-18 (REFS 71,74,75). It has been assumed that the IL-18–IL-18BP imbalance might contribute to T-lymphocyte and macrophage activation in HLH74. However, in many patients with sJIA, plasma IL-18 levels remain above normal even in clinical remission69. In the absence of IL-12 (a cytokine that is not increased in sJIA), IL-18 can divert immune responsesfrom the proinflammatory type 1 helper T (TH1) cells to the anti-inflammatory TH2 cells; therefore, IL-18 might be part of an immunoregulatory negative-feedback loop. On the other hand, Chiossone and colleagues76 examined the role of IL-18 in perforin-deficient mice infected with murine CMV. Uncontrolled viral replication in these mice is associated with many features of HLH and MAS including pancytopenia, hepatic dysfunction, haemophagocytosis and death76. Administration of synthetic IL-18BP ameliorated liver damage in these mice; however, production of proinflammatory cytokines was still detected, and no change in overall survival was observed. These findings suggest that further work using different animal models is needed to better characterize the role of IL-18.
What about blocking IFN-γ?
As discussed earlier, IFN-γ has a pivotal role in several models of HLH; when this cytokine is neutralized, survival improved substantially in animal models45,46. Levels of IFN-γ are elevated in children with HLH, as are levels of IFN-γ-induced chemokines such as CXCL10 and CXCL9 (REF. 77). These observations suggest IFN-γ could be targeted therapeutically in HLH; a clinical trial evaluating this approach is underway78.
The role of IFN-γ in sJIA-associated MAS has not yet been fully determined. Interestingly, IFN-γ does not seem to be involved in the pathogenesis of sJIA itself. Levels of serum IFN-γ have been reported to be within the normal range in patients with sJIA, independently of disease activity79. Three independent gene expression studies have failed to find a prominent IFN-γ-induced signature in the peripheral blood monocytes of children with active sJIA but no clinical features of MAS11,50,80. The absence of IFN-γ activity is not limited to peripheral blood cells, but could also be observed in inflamed tissues. Thus, expression of IFN-γ-induced chemokines (CXCL9 and CXCL10) in synovial tissue from patients with sJIA is hardly detectable, in contrast to very high levels of these chemokines in tissue from patients with oligoarticular or polyarticular JIA79. The absence of the IFN-γ signature in sJIA does not seem to be caused by abnormal responsiveness to IFN-γ . In fact, monocytes from patients with sJIA incubated with exogenous IFN-γ often have exaggerated responses to this cytokine79.
In contrast to sJIA, evidence suggests that IFN-γ is essential for the pathogenesis of MAS. Episodes of MAS in sJIA commonly occur when elicited by viral infections, which are known to activate IFN-γ-induced pathways. One histopathological study of inflammatory infiltrates in tissues affected by MAS showed numerous IFN-γ-producing T cells in close proximity to activated haemophagocytic histiocytes17. Furthermore, children with MAS exhibit increased levels of neopterin, a product normally released by macrophages stimulated with interferons52. Another 2015 study, focused on longitudinal cytokine changes in serum of patients with sJIA, showed that IFN-γ itself and IFN-γ-induced chemokines increased markedly with the emergence of clinical features of MAS, and returned to normal ranges after resolution of this complication53. Furthermore, IFN-γ and IFN-γ-induced chemokines (CXCL9 in particular) strongly correlated with many laboratory features of MAS. Neutralization of IFN-γ in the MAS model using IL-6 transgenic mice led to a great improvement in survival and a considerable decrease in ferritin levels53. Collectively, these observations raise the question of whether IFN-γ could also be an appropriate therapeutic target in MAS.
Implications for diagnosis of MAS
Increasing experience of MAS in patients receiving various biologic anticytokine therapies provides valuable insights into the pathophysiology of this syndrome, but also underscores new diagnostic challenges in this population. The diagnosis of MAS is difficult, but owing to increased awareness of this complication, it is recognized more and more frequently. A fall in platelet count and erythrocyte sedimentation rate, in combination with persistently high CRP and increasing levels of serum D-dimers, are early signs of impending MAS in a febrile patient with an active rheumatologic condition. Other features indicative of MAS are hyperferritinaemia, cytopenias involving other cell lines (white blood cells and red blood cells), liver dysfunction, coagulopathy, decreasing serum fibrinogen and increasing triglycerides. Diagnosis is usually confirmed by presence of haemophagocytic macrophages (or histiocytes) in bone marrow, but this feature might not be apparent in the early stages of MAS.
In 2014, a set of classification criteria for MAS complicating sJIA was developed through a combination of expert consensus and analysis of patient data (see BOX 2). In cross-validation analyses, the criteria revealed a sensitivity of 0.72–0.76 and a specificity 0.97–0.99 (REFS 81–83). Prospective validation is still required to further scrutinize the performance of the new criteria. Another point to consider is that these criteria were developed using clinical data generated before the introduction of IL-1 and IL-6 inhibitors for the treatment of sJIA. How these criteria will perform in patients who develop MAS whilst treated with biologic therapy is unclear, as IL-6 inhibition tends to decrease ferritin levels and some patients treated with tocilizumab develop neutropenia, liver enzyme elevation and thrombocytopenia.
Conclusions
A cytokine storm is a consistent feature in patients with primary and secondary HLH, including MAS, as well as in animal models of this clinical phenomenon. Therefore, targeting specific cytokines might be an attractive therapeutic approach in such patients. The fact that biologic agents neutralizing IL-6 and IL-1 are highly effective treatments for sJIA — a rheumatic disease strongly associated with MAS — raised hopes that the same strategies would be successful to prevent MAS. However, phase III clinical trials of canakinumab and tocilizumab in sJIA clearly demonstrated that these treatments do not provide protection against MAS, and suggest that IL-1 and IL-6 might not have a central role in the pathogenesis of this syndrome. Current translational research has been focused on IL-18 and IFN-γ , with IFN-γ emerging as a new attractive therapeutic target. The conduct of these trials also underscored potential limitations of the new classification criteria for MAS in patients treated with biologics. As such, additional validation of these criteria will be necessary.
Supplementary Material
Key points.
A ‘cytokine storm’ is the final pathophysiological pathway in macrophage activation syndrome (MAS), and blocking various cytokines could be an attractive therapeutic strategy
Standard doses of anti-IL-1 and anti-IL-6 biologic therapies do not have a major effect on MAS rates even if the underlying disease responds well to the treatment
Several case reports suggest that anakinra might be effective at least in some patients with systemic juvenile idiophatic arthritis (sJIA)-associated MAS, particularly when used in high doses
Findings from several studies support IFN-γ blockade as a novel therapy for haemophagocytic lymphohistiocytosis (HLH); increasing evidence suggests the same approach could be beneficial in MAS presenting as a complication of rheumatic diseases
The exact mechanism of predisposition to MAS in sIJA is yet to be defined, but might be independent of underlying sJIA activity and similar to infection-associated secondary HLH
Whole-exome/genome sequencing approaches exploring hypomorphic mutations that affect the cytolytic pathway to support this theory might reveal promising therapeutic alternatives
Acknowledgments
A.A.G. is supported by NIH grants NIAMS R01-AR059049 and NIH P01-AR048929.
Footnotes
Author contributions
A.A.G. contributed to researching data, discussions of content and writing of the article. A.C.H. contributed to discussions of content, review and editing of the manuscript before submission. F.D.B. contributed to discussions of content, review and editing of the manuscript before submission.
Competing interests statement
A.A.G declares that he has served as a consultant and speaker for Novartis and Roche and worked in collaboration with NovImmune. F.D.B declares that he has received unrestricted research grants from Pfizer, AbbVie, Novartis, NovImmune, Roche, and SOBI, and travel support from Roche. A.C.H. declares no competing interests.
References
- 1.Silverman ED, Miller JJ, Bernstein B, Shafai T. Consumption coagulopathy associated with systemic juvenile rheumatoid arthritis. J Pediatr. 1983;103:872–876. doi: 10.1016/s0022-3476(83)80704-5. [DOI] [PubMed] [Google Scholar]
- 2.Hadchouel M, Prieur AM, Griscelli C. Acute hemorrhagic, hepatic, and neurologic manifestations in juvenile rheumatoid arthritis: possible relationship to drugs or infection. J Pediatr. 1985;106:561–566. doi: 10.1016/s0022-3476(85)80072-x. [DOI] [PubMed] [Google Scholar]
- 3.Mouy R, et al. Efficacy of cyclosporine A in the treatment of macrophage activation syndrome in juvenile arthritis: report of five cases. J Pediatr. 1996;129:750–754. doi: 10.1016/s0022-3476(96)70160-9. [DOI] [PubMed] [Google Scholar]
- 4.Grom AA, Passo M. Macrophage activation syndrome in systemic juvenile rheumatoid arthritis. J Pediatr. 1996;129:630–632. doi: 10.1016/s0022-3476(96)70140-3. [DOI] [PubMed] [Google Scholar]
- 5.Ravelli A, De Benedetti F, Viola S, Martini A. Macrophage activation syndrome in systemic juvenile rheumatoid arthritis successfully treated with cyclosporine. J Pediatr. 1996;128:275–278. doi: 10.1016/s0022-3476(96)70408-0. [DOI] [PubMed] [Google Scholar]
- 6.Stephan JL, et al. Reactive haemophagocytic syndrome in children with inflammatory disorders. A retrospective study of 24 patients. Rheumatology (Oxford) 2001;40:1285–1292. doi: 10.1093/rheumatology/40.11.1285. [DOI] [PubMed] [Google Scholar]
- 7.Sawhney S, Woo P, Murray KJ. Macrophage activation syndrome: a potentially fatal complication of rheumatic disorders. Arch Dis Child. 2001;85:421–426. doi: 10.1136/adc.85.5.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mellins ED, Macubas C, Grom AA. Pathogenesis of systemic juvenile idiopathic arthritis: some answers, more questions. Nat Rev Rheumatol. 2011;7:416–426. doi: 10.1038/nrrheum.2011.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rossi-Semerano L, Kone-Paut I. Is Still’s disease an autoinflammatory syndrome? Int J Inflamm. 2012;2012:480373. doi: 10.1155/2012/480373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rigante D, Cantarini L. The systemic onset variant of juvenile idiopathic arthritis needs to be recorded as an autoinnflammatory syndrome: comment on the review by Nigrovic. Arthritis Rheumatol. 2014;66:2645. doi: 10.1002/art.38698. [DOI] [PubMed] [Google Scholar]
- 11.Pascual V, et al. Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. J Exp Med. 2005;201:1479–1486. doi: 10.1084/jem.20050473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quartier P, et al. A multicentre, randomised, double-blind, placebo-controlled trial with the interleukin-1 receptor antagonist anakinra in patients with systemic-onset juvenile idiopathic arthritis. Ann Rheum Dis. 2011;70:747–754. doi: 10.1136/ard.2010.134254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruperto N, et al. Two randomized trials of canakinumabin systemic juvenile idiopathic arthritis. N Engl J Med. 2012;367:2396–2406. doi: 10.1056/NEJMoa1205099. [DOI] [PubMed] [Google Scholar]
- 14.De Benedetti F, Martini A. Is systemic juvenile rheumatoid arthritis an IL-6 mediated disease? J Rheumatol. 1998;25:203–207. [PubMed] [Google Scholar]
- 15.Yokota S, et al. Efficacy and safety of tocilizumab in patients with systemic-onset juvenile idiopathic arthritis: a randomised, double-blind, placebo-controlled, withdrawal phase III trial. Lancet. 2008;371:998–1006. doi: 10.1016/S0140-6736(08)60454-7. [DOI] [PubMed] [Google Scholar]
- 16.De Benedetti F, et al. Randomized trial of tocilizumab in systemic juvenile idiopathic arthritis. N Engl J Med. 2012;367:2385–2395. doi: 10.1056/NEJMoa1112802. [DOI] [PubMed] [Google Scholar]
- 17.Billiau AD, et al. Macrophage activation syndrome: characteristic findings on liver biopsy illustrating the key role of activated, IFN-γ-producing lymphocytes and IL-6- and TNF-α-producing macrophages. Blood. 2005;105:1648–1651. doi: 10.1182/blood-2004-08-2997. [DOI] [PubMed] [Google Scholar]
- 18.Prahalad S, Bove KE, Dickens D, Lovell DJ, Grom AA. Successful use of etanercept in the treatment of macrophage activation syndrome. J Rheumatol. 2001;28:2120–2124. [PubMed] [Google Scholar]
- 19.Favara BE, et al. Contemporary classification of histiocytic disorders. Med Pediatr Oncol. 1997;199:157–166. doi: 10.1002/(sici)1096-911x(199709)29:3<157::aid-mpo1>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 20.Jordan MB, et al. How I treat hemophagocytic lymphohistiocytosis. Blood. 2011;118:4041–4052. doi: 10.1182/blood-2011-03-278127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henter JI, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:124–131. doi: 10.1002/pbc.21039. [DOI] [PubMed] [Google Scholar]
- 22.Stepp SE, et al. Perforin gene defects in familial hemophagocytic lymphohistiocytosis. Science. 1999;286:1957–1959. doi: 10.1126/science.286.5446.1957. [DOI] [PubMed] [Google Scholar]
- 23.Feldmann J, et al. Munc13-4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3) Cell. 2003;115:461–473. doi: 10.1016/s0092-8674(03)00855-9. [DOI] [PubMed] [Google Scholar]
- 24.zur Stadt U, et al. Linkage of familial hemophagocytic lymphohistiocytosis (FHL) type-4 to chromosome 6q24 and identification of mutations in syntaxin 11. Hum Mol Genet. 2005;14:827–834. doi: 10.1093/hmg/ddi076. [DOI] [PubMed] [Google Scholar]
- 25.zur Stadt U, et al. Familial hemophagocytic lymphohistiocytosis type 5 (FHL-5) is caused by mutations in Munc18-2 and impaired binding to Syntaxin 11. Am J Hum Genet. 2009;85:482–492. doi: 10.1016/j.ajhg.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chandrakasan S, Filipovich AH. Hemophagocytic lymphohistiocytosis: advances in pathophysiology, diagnosis, and treatment. J Pediatr. 2013;163:1253–1259. doi: 10.1016/j.jpeds.2013.06.053. [DOI] [PubMed] [Google Scholar]
- 27.Menasche G, et al. Mutations in RAB27A cause Griscelli syndrome associated with haemophagocytic syndrome. Nat Genet. 2000;25:173–176. doi: 10.1038/76024. [DOI] [PubMed] [Google Scholar]
- 28.Barbosa MD, et al. Identification of the homologous beige and Chediak–Higashi syndrome genes. Nature. 1996;382:262–265. doi: 10.1038/382262a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jenkins MR, et al. Failed CTL/NK cell killing and cytokine hypersecretion are directly linked through prolonged synapse time. J Exp Med. 2015;212:307–317. doi: 10.1084/jem.20140964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Menasche G, Feldmann J, Fischer A, de Saint Basile G. Primary hemophagocytic syndromes point to a direct link between lymphocyte cytotoxicity and homeostasis. Immunol Rev. 2005;203:165–179. doi: 10.1111/j.0105-2896.2005.00224.x. [DOI] [PubMed] [Google Scholar]
- 31.Kagi D, Odermatt B, Mak TW. Homeostatic regulation of CD8+ T cells by perforin. Eur J Immunol. 1999;29:3262–3272. doi: 10.1002/(SICI)1521-4141(199910)29:10<3262::AID-IMMU3262>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 32.Lykens JE, Terrell CE, Zoller EE, Risma K, Jordan MB. Perforin is a critical physiologic regulator of T-cell activation. Blood. 2011;118:618–626. doi: 10.1182/blood-2010-12-324533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coffey AJ, et al. Host response to EBV infection in X-linked lymphoproliferative disease results from mutations in an SH2-domain encoding gene. Nat Genet. 1998;20:129–135. doi: 10.1038/2424. [DOI] [PubMed] [Google Scholar]
- 34.Marsh RA, et al. XIAP deficiency: a unique primary immunodeficiency best classified as X-linked familial hemophagocytic lymphohistiocytosis and not as X-linked lymphoproliferative disease. Blood. 2010;116:1079–1082. doi: 10.1182/blood-2010-01-256099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kogawa K, et al. Perforin expression in cytotoxic lymphocytes from patients with hemophagocytic lymphohistiocytosis and their family members. Blood. 2002;99:61–66. doi: 10.1182/blood.v99.1.61. [DOI] [PubMed] [Google Scholar]
- 36.Zhang KJ, et al. Hypomorphic mutations in PRF1, MUNC13-4, and STXBP2 are associated with adult-onset familial HLH. Blood. 2011;118:5794–5798. doi: 10.1182/blood-2011-07-370148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Canna SW, et al. An activating NLRC4 inflammasome mutation causes autoinflammation with recurrent macrophage activation syndrome. Nat Genet. 2014;46:1140–1146. doi: 10.1038/ng.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romberg N, et al. Mutation of NLRC4 causes a syndrome of enterocolitis and autoinflammation. Nat Genet. 2014;46:1135–1139. doi: 10.1038/ng.3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grom AA, et al. Natural killer cell dysfunction in patients with systemic-onset juvenile rheumatoid arthritis and macrophage activation syndrome. J Pediatr. 2003;142:292–296. doi: 10.1067/mpd.2003.110. [DOI] [PubMed] [Google Scholar]
- 40.Cifaldi L, et al. Inhibition of natural killer cell cytotoxicity by interleukin-6: implications for the pathogenesis of macrophage activation syndrome. Arthritis Rheum. 2003;67:3037–3046. doi: 10.1002/art.39295. [DOI] [PubMed] [Google Scholar]
- 41.Kaufman KM, et al. Whole exome sequencing reveals overlap between macrophage activation syndrome in systemic juvenile idiopathic arthritis and familial hemophagocytic lymphohistiocytosis. Arthritis Rheum. 2014;66:3486–3495. doi: 10.1002/art.38793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bracaglia C, et al. Mutations of familial hemophagocytic lymphohistiocytosis related genes and abnormalities of cytotoxicity function tests in patients with macrophage activation syndrome (MAS) occurring in systemic juvenile idiopathic arthritis. Pediatr Rheumatol Online J. 2014;12(Suppl 1):53. [Google Scholar]
- 43.Vastert SJ, et al. Mutations in the perforin gene can be linked to macrophage activation syndrome in patients with systemic onset juvenile idiopathic arthritis. Rheumatology (Oxford) 2010;49:441–449. doi: 10.1093/rheumatology/kep418. [DOI] [PubMed] [Google Scholar]
- 44.Zhang M, et al. Genetic defects in cytolysis in macrophage activation syndrome. Curr Rheumatol Rep. 2014;16:439–447. doi: 10.1007/s11926-014-0439-2. [DOI] [PubMed] [Google Scholar]
- 45.Jordan MB, Hildeman D, Kappler J, Marrack P. An animal model of hemophagocytic lymphohistiocytosis (HLH): CD8+ T cells and interferon gamma are essential for the disorder. Blood. 2004;104:735–743. doi: 10.1182/blood-2003-10-3413. [DOI] [PubMed] [Google Scholar]
- 46.Pachlopnik Schmid J, et al. Neutralization of IFNγ defeats haemophagocytosis in LCMV-infected perforin- and Rab27a-deficient mice. EMBO Mol Med. 2009;1:112–124. doi: 10.1002/emmm.200900009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Behrens EM, et al. Repeated TLR9 stimulation results in macrophage activation syndrome-like disease in mice. J Clin Invest. 2011;121:2264–2277. doi: 10.1172/JCI43157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Canna SW, et al. Interferon-γ mediates anemia but is dispensable for fulminant toll-like receptor 9-induced macrophage activation syndrome and hemophagocytosis in mice. Arthritis Rheum. 2013;65:1764–1775. doi: 10.1002/art.37958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Min C, et al. Interferon gamma (IFNg) is the driving mediator of secondary hemophagocytic lymphohistiocytosis (sHLH) in TLR9-mediated pathogenesis in mice and is correlated to disease parameters in children [abstract 3076] Arthritis Rheumatol. 2015;67(Suppl 10) [Google Scholar]
- 50.Fall N, et al. Gene expression profiling of peripheral blood from patients with untreated new-onset systemic juvenile idiopathic arthritis reveals molecular heterogeneity that may predict macrophage activation syndrome. Arthritis Rheum. 2007;56:3793–3804. doi: 10.1002/art.22981. [DOI] [PubMed] [Google Scholar]
- 51.Strippoli R, et al. Amplification of the response to toll-like receptor ligands by prolonged exposure to interleukin-6 in mice: implication for the pathogenesis of macrophage activation syndrome. Arthritis Rheum. 2012;64:1680–1688. doi: 10.1002/art.33496. [DOI] [PubMed] [Google Scholar]
- 52.Put K, et al. Cytokines in systemic juvenile idiopathic arthritis and haemophagocytic lymphohistiocytosis: tipping the balance between interleukin-18 and interferon-γ. Rheumatology (Oxford) 2015;54:1507–1517. doi: 10.1093/rheumatology/keu524. [DOI] [PubMed] [Google Scholar]
- 53.Bracaglia C, et al. Interferon-gamma in macrophage activation syndrome associated with systemic juvenile idiopathic arthritis: high levels in patients and a role in a murine MAS model. Presented at the 21st European Paediatric Rheumatology Congress; Belgrade. 2014. [Google Scholar]
- 54.Ibarra MF, et al. Serum neopterin levels as a diagnostic marker of hemophagocytic lymphohistiocytosis syndrome. Clin Vaccine Immunol. 2011;18:609–614. doi: 10.1128/CVI.00306-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bleesing J, et al. The diagnostic significance of soluble CD163 and soluble interleukin-2 receptor α-chain in macrophage activation syndrome and untreated new-onset systemic juvenile idiopathic arthritis. Arthritis Rheum. 2007;56:965–971. doi: 10.1002/art.22416. [DOI] [PubMed] [Google Scholar]
- 56.Henter JI, et al. Hypercytokinemia in familial hemophagocytic lymphohistiocytosis. Blood. 1991;78:2918–2922. [PubMed] [Google Scholar]
- 57.Henter JI, et al. Elevated circulating levels of interleukin-1 receptor antagonist but not IL-1 agonists in hemophagocytic lymphohistiocytosis. Med Pediatr Oncol. 1996;27:21–25. doi: 10.1002/(SICI)1096-911X(199607)27:1<21::AID-MPO5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 58.Sumegi J, et al. Gene expression profiling of peripheral blood mononuclear cells from children with active hemophagocytic lymphohistiocytosis. Blood. 2011;117:e151–e160. doi: 10.1182/blood-2010-08-300046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miettunen PM, et al. Successful treatment of severe paediatric rheumatic disease-associated macrophage activation syndrome with interleukin-1 inhibition following conventional immunosuppressive therapy: case series with 12 patients. Rheumatology (Oxford) 2011;50:417–419. doi: 10.1093/rheumatology/keq218. [DOI] [PubMed] [Google Scholar]
- 60.Durand M, Troyanov Y, Laflamme P, Gregoire G. Macrophage activation syndrome treated with anakinra. J Rheumatol. 2010;37:879–880. doi: 10.3899/jrheum.091046. [DOI] [PubMed] [Google Scholar]
- 61.Nigrovic PA, et al. Anakinra as first-line disease-modifying therapy in systemic juvenile idiopathic arthritis: report of forty-six patients from an international multicenter series. Arthritis Rheum. 2011;63:545–555. doi: 10.1002/art.30128. [DOI] [PubMed] [Google Scholar]
- 62.Zeft A, et al. Anakinra for systemic juvenile arthritis: the Rocky Mountain experience. J Clin Rheumatol. 2009;15:161–164. doi: 10.1097/RHU.0b013e3181a4f459. [DOI] [PubMed] [Google Scholar]
- 63.Grom AA, et al. Rate and clinical presentation of macrophage activation syndrome in patients with systemic juvenile idiopathic arthritis treated with canakinumab. Arthritis Rheumatol. 2016;68:218–228. doi: 10.1002/art.39407. [DOI] [PubMed] [Google Scholar]
- 64.Ilowite NT, et al. The RAndomized Placebo Phase Study Of Rilonacept in the Treatment of systemic juvenile idiopathic arthritis (RAPPORT) Arthritis Rheumatol. 2014;66:2570–2579. doi: 10.1002/art.38699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ravelli A, et al. Macrophage activation syndrome in patients with systemic juvenile idiopathic arthritis treated with tocilizumab [abstract] Arthritis Rheum. 2014;66(Suppl):S83–S84. [Google Scholar]
- 66.Yokota S, et al. Macrophage activation syndrome in patients with systemic juvenile idiopathic arthritis under treatment with tocilizumab. J Rheumatol. 2015;42:712–722. doi: 10.3899/jrheum.140288. [DOI] [PubMed] [Google Scholar]
- 67.Shimizu M, et al. Tocilizumab masks the clinical symptoms of systemic juvenile idiopathic arthritis-associated macrophage activation syndrome: the diagnostic significance of interleukin-18 and interleukin-6. Cytokine. 2012;58:287–294. doi: 10.1016/j.cyto.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 68.Kobayashi M, et al. Benefit and a possible risk of tocilizumab therapy for adult-onset Still’s disease accompanied by macrophage-activation syndrome. Mod Rheumatol. 2011;21:92–96. doi: 10.1007/s10165-010-0348-9. [DOI] [PubMed] [Google Scholar]
- 69.Shimizu M, et al. Distinct cytokine profiles of systemic-onset juvenile idiopathic arthritis-associated macrophage activation syndrome with particular emphasis on the role of interleukin-18 in its pathogenesis. Rheumatology (Oxford) 2010;49:1645–1653. doi: 10.1093/rheumatology/keq133. [DOI] [PubMed] [Google Scholar]
- 70.Maeno N, et al. Increased interleukin-18 expression in bone marrow of a patient with systemic juvenile idiopathic arthritis and unrecognized macrophage-activation syndrome. Arthritis Rheum. 2004;50:935–1938. doi: 10.1002/art.20268. [DOI] [PubMed] [Google Scholar]
- 71.Kawashima M, et al. Levels of interleukin-18 and its binding inhibitors in the blood circulation of patients with adult-onset Still’s disease. Arthritis Rheum. 2001;44:550–560. doi: 10.1002/1529-0131(200103)44:3<550::AID-ANR103>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 72.Novick D, et al. High circulating levels of free interleukin-18 in patients with active SLE in the presence of elevated levels of interleukin-18 binding protein. Cytokine. 2009;48:103–104. doi: 10.1016/j.jaut.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 73.Favilli F, et al. IL-18 activity in systemic lupus erythematosus. Ann NY Acad Sci. 2009;1173:301–309. doi: 10.1111/j.1749-6632.2009.04742.x. [DOI] [PubMed] [Google Scholar]
- 74.Mazodier K, et al. Severe imbalance of IL-18/IL-18BP in patients with secondary hemophagocytic syndrome. Blood. 2005;106:3483–3489. doi: 10.1182/blood-2005-05-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dinarello CA. Interleukin-18 and the pathogenesis of inflammatory diseases. Semin Nephrol. 2007;27:98–114. doi: 10.1016/j.semnephrol.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 76.Chiossone L, et al. Protection from inflammatory organ damage in a murine model of hemophagocytic lymphohistiocytosis using treatment with IL-18 binding protein. Front Immunol. 2012;3:239–249. doi: 10.3389/fimmu.2012.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Takada H, et al. Increased serum levels of interferon-γ-inducible protein 10 and monokine induced by gamma interferon in patients with haemophagocytic lymphohistiocytosis. Clin Exp Immunol. 2003;133:448–453. doi: 10.1046/j.1365-2249.2003.02237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.US National Library of Science. ClinicalTrials.gov [online] 2015 https://clinicaltrials.gov/ct2/show/NCT01818492?term=NCT01818492&rank=1.
- 79.Sikora KA, Fall N, Thornton S, Grom AA. The limited role of interferon-γ in systemic juvenile idiopathic arthritis cannot be explained by cellular hyporesponsiveness. Arthritis Rheum. 2012:3799–3808. doi: 10.1002/art.34604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ogilvie EM, et al. Specific gene expression profiles in systemic juvenile idiopathic arthritis. Arthritis Rheum. 2007;56:1954–1965. doi: 10.1002/art.22644. [DOI] [PubMed] [Google Scholar]
- 81.Ravelli A, et al. Preliminary diagnostic guidelines for macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. J Pediatr. 2005;146:598–604. doi: 10.1016/j.jpeds.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 82.Davi S, et al. An international consensus survey of diagnostic criteria for macrophage activation syndrome in systemic juvenile idiopathic arthritis. J Rheumatol. 2011;38:764–768. doi: 10.3899/jrheum.100996. [DOI] [PubMed] [Google Scholar]
- 83.Ravelli A, et al. Development and initial validation of classification criteria for macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. Arthritis Rheumatol. 2015 http://dx.doi.org/10.1002/art.39332.
- 84.Writing Group of the Histiocyte Society. Histiocytosis syndromes in children. Lancet. 1987;1:208–209. [PubMed] [Google Scholar]
- 85.Athreya BH. Is macrophage activation syndrome a new entity? Clin Exp Rheumatol. 2002;20:121–123. [PubMed] [Google Scholar]
- 86.Ramana AV, Baildam EM. Macrophage activation syndrome is hemophagocytic lymphohistiocytosis — need for the right terminology. J Rheumatol. 2002;29:1105. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.