Abstract
We sought to determine the significance of minimal residual disease (MRD) relapse in patients with ALL after achieving MRD negative status following induction and consolidation therapy. Between January 2003 and September 2014, 647 newly diagnosed patients were treated [Hyper-CVAD-based (n=531); Augmented BFM (n=116)]. Six hundred and one (93%) achieved complete remission (CR), and 546 (91%) became MRD negative. Fifty-five patients [HyperCVAD-based (n=49); Augmented BFM (n=6)] developed recurrence of MRD while still in morphological CR and are the subjects of this study. MRD was assessed by 6-color (4-color prior to 2009) multi-parameter flow cytometry (MFC) at CR and multiple time points thereafter. Their median age was 44 years (range, 18–72 years), median WBC at initial presentation was 7.3 K/μL−1 (range, 0.6–303.8 K/μL−1) and median bone marrow blast percentage 88% (range, 26–98%). The median time to MRD relapse was 14 months (range 3–58 months). Forty-four (80%) patients subsequently developed morphological relapse after median of 3 months (range, <1–33 months) from detection of MRD recurrence. Treatments received after MRD positivity and prior to morphological relapse: 16 continued maintenance chemotherapy; 15 received late intensification; 9 allogeneic stem cell transplant, 9 changed chemotherapy, 6 no further therapy. Only six remain alive and in CR1 and nine are alive after morphological relapse. MRD relapse detected by MFC at any time after achieving CR is associated with a high risk for morphological relapse. SCT can result in long-term remission in some patients. Prospective studies of long-term MRD assessments, together with less toxic treatment strategies to eradicate MRD, are warranted.
1 INTRODUCTION
The assessment of minimal residual disease (MRD) at the end of induction chemotherapy has been established as one of the most important independent predictors of patient outcomes in pediatric and adult ALL, and is becoming a key component of therapeutic decision making in modern ALL treatment regimens.1–5 New techniques for the detection of MRD in various leukemia subtypes have been developed and are being refined and validated. These techniques are aimed at recognition of submicroscopic disease persistence or recurrence and for potential early therapeutic intervention.6–11 Multi-parameter flow cytometry (MFC) has been used extensively in pediatric and, more recently, adult ALL studies, to determine the likelihood of relapse and overall success of therapy.6–8,12,13 From 2004, we have routinely and systematically evaluated the presence of MRD using flow cytometry in bone marrow specimens obtained from patients with ALL treated on various clinical trials at our institution.3,14,15 While the role of MRD assessment and significance of achieving MRD negativity as assessed specifically by MFC at the end of induction and consolidation have been evaluated in previous studies including the adult ALL population,3,5 there is limited existing data on the significance of MRD recurrence in the absence of morphological relapse and whether it is universally associated with a poor outcome. Herein, we aim to determine the significance of MRD relapse, as detected by MFC, in adult patients with ALL after achieving MRD negativity, following induction and consolidation therapy.
2 METHODS
2.1 Patient data collection
We conducted a retrospective, single-institution analysis of patients with ALL (including T- and B-ALL and excluding Burkitt’s) treated at our institution who were ≥18 years and had MRD evaluation during the course of their therapy. Among 647 patients diagnosed with ALL (January 2003-September 2014), 546 (91%) became MRD negative; 55 patients (9% of the total population) developed recurrence of MRD while still in morphological complete remission (CR) and are the focus of this analysis. Figure 1 depicts the study flowchart for patient disposition. Two chemotherapy backbone regimens were used: HyperCVAD-based and Augmented BFM, with the specific details of each program reported previously.16,17 Table 1 describes pre-treatment patient characteristics and presents a comparison of the patients in the study group (n=55) versus those with persistent MRD negativity CR (n=491). There were no significant differences found among the two groups in terms of pretreatment characteristics. Table 2 lists the specific chemotherapy regimens received (Supporting Information File, S1). All patients signed informed consent as applicable for clinical trial enrollment. All research was performed in accordance with the Declaration of Helsinki.
FIGURE 1.
Patient disposition: study flowchart. [Color figure can be viewed at wileyonlinelibrary.com]
TABLE 1.
Patient characteristics, MRD positive (n=55) versus MRD negative CR (n=491)
| Baseline characteristics | MRD relapse; N=55 | [Range] (%) | No MRD Relapse; N=491 | [Range] (%) | p value |
|---|---|---|---|---|---|
| Median age | 44 | [18–72] | 42 | [13–84] | 0.30 |
| Male | 32 | (58) | 273 | (56) | 0.72 |
| Median WBCa (K/μL−1) | 7.3 | [0.6–303.8] | 9.1 | [0.4–876] | 0.67 |
| Median hemoglobin(g/dL−1) | 9.2 | [4.9–16.4] | 9.6 | [3.5–16.1] | 0.10 |
| Median platelet (x109/L) | 41 | [4–661] | 45.5 | [3–626] | 0.13 |
| Median BMb blasts (%) | 88 | [26–98] | 85 | [20–100] | 0.11 |
| ALL Subtype | 0.56 | ||||
| B-ALL | 48 | (87%) | 441 | (90) | |
| T-ALL | 7 | (13%) | 50 | (10) | |
| Baseline cytogenetics: | 0.7 | ||||
| Diploid | 13 | (24) | 130 | (27) | |
| Philadelphiapositive | 16 | (29) | 153 | (31) | |
| Insufficient/not done | 6 | (11) | 50 | (10) | |
| Miscellaneous | 19 | (35) | 134 | (27) | |
| 11q23 rearrangement | 1 | (2) | 24 | (5) |
WBC=white blood cell count.
BM=bone marrow.
2.2 MRD assessments
Among 647 patients with ALL seen in the period of the study, 16 patients did not have MRD analysis or had insufficient analysis (16/647= 2%); 601 patients achieved CR, and 39 never became MRD negative. Among the 601 patients in morpholological CR, 546 became MRD negative and were included in the analysis. Fifty-five (10%) developed subsequent MRD recurrence while still in morphological CR and were the subjects of this study. All patients had assessment of MRD status by 6-color (4-color prior to 2009) MFC, according to recently published, institutionally standardized techniques detailed previously3 (for a complete listing of flow cytometry markers utilized, refer to Table 6, Supporting Information S7). Bone marrow specimens were obtained for MRD analysis at the time of CR, and approximately at 3-month intervals thereafter, and as clinically indicated. In our studies, MRD positivity was recognized as compared to normal antigen pattern expression of mature CD19+ B cells, with a clustering of at least 20 cells which demonstrated abnormal antigen expression constituting an abnormal population. The sensitivity of the MRD analysis was 0.01% (for both 4-color and 6-color techniques), with the expression of at least 2 aberrant antigens required for a positive MRD value to be recorded.3,18
2.3 Response definitions
CR was defined as <5% leukemic blasts in the bone marrow sample reviewed at the time of peripheral blood count recovery, absence of circulating peripheral blasts, absence of extramedullary disease, platelet count ≥100 × 109/L, and absolute neutrophil count ≥1.0 × 109/L. MRD relapse was defined as recurrence of detectable MRD (sensitivity for positive value ≥0.01%), despite persistence of clinical complete morphological remission. Morphological relapse was defined as presence of leukemic blasts in any extramedullary location, or in the bone marrow or peripheral blood at a level of >5%.
2.4 Statistical definitions
Overall survival (OS) was defined as the time interval from the time of MRD relapse and the date of death due to any cause. Patients who were alive were censored at the last follow-up date. Disease-free survival (DFS) was defined as the time interval between date of MRD relapse and the date of disease relapse, or death due to any cause, whichever event occurred first. Time to relapse was defined as the time interval between date of MRD relapse and the date of disease relapse (death before disease relapse was denoted as a censored event). The probabilities of OS, DFS, and time to relapse were estimated using the method of Kaplan and Meier,19 and the log-rank test20 was performed to assess differences between patients with MRD positive versus negative status. Statistical analyses were conducted in SAS 9.3 and Splus (SAS Institute, Cary, NC).
3 RESULTS
Among 647 patients diagnosed with ALL during the study period (January 2003–September 2014), 601 (93%) achieved CR. Among them, 546 (91%) achieved an MRD negative bone marrow exam. Fifty-five patients ultimately experienced recurrence of MFC detectable disease while still in morphological CR and were the focus of this analysis. The overall median value for MRD positivity by flow cytometry for the 55 patients of the study group was 0.07%, with range of 0.01–11%. For those patients who experienced subsequent morphologic relapse, the median flow cytometry value was 0.09%, with range (0.01–11%); there was no significant difference found between the levels of MRD values for the overall group versus those with subsequent relapse.
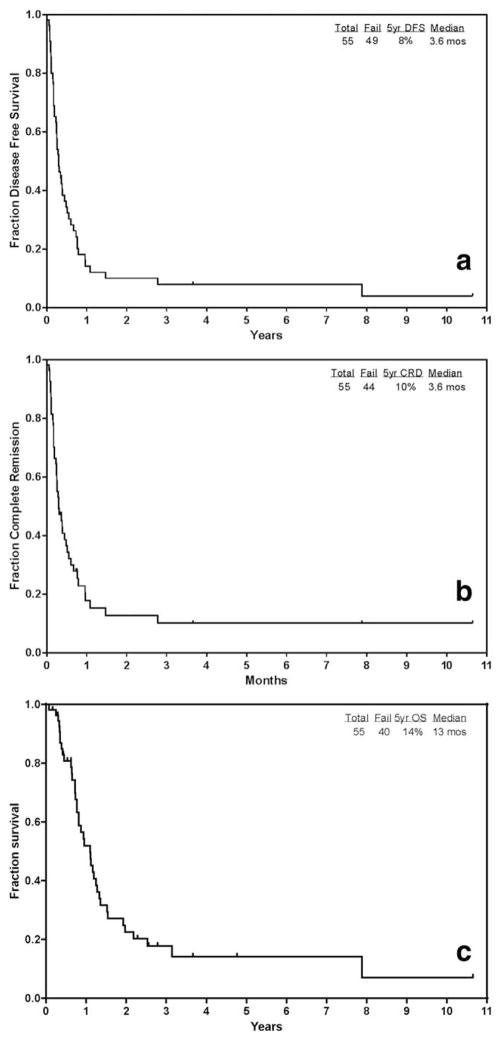
The median disease-free survival (DFS) for the group (n=55) from the time of MRD relapse was 3.6 months (range, <1–128 months) (Figure 2a). Median CR duration from the time of MRD relapse was 3.6 months (range, <1–128 months) (Figure 2b). The median overall survival (OS) from the time of MRD relapse was 13 months (range, <1–128 months) (Figure 2c). The 5-year CR duration and 5-year OS were 10 and 14%, respectively. The median follow-up time from time of MRD relapse for the group was 7.5 months (range, 2–128 months).
FIGURE 2.
(a) Disease-free survival (DFS) in patients with MRD relapse (n=55) from the time of MRD relapse. (b) Complete remission duration (CRD) in patients with MRD relapse (n=55) from the time of MRD relapse. (c) Overall survival (OS) in patients with MRD relapse (n=55) from the time of MRD relapse
For comparison, 5-year CRD and 5-year OS was evaluated between the n=55 study group versus the n=491 patients who had persistent MRD negative CR. There was a significant difference in both CRD and OS favoring the MRD negative group (Supporting Information S4a, S4b).
Forty-four (80%) patients subsequently developed morphological relapse [36 bone marrow (BM), three BM with central nervous system (CNS) involvement, three isolated CNS involvement, and two non-CNS extramedullary sites of relapse], after a median of 3 months (range, <1–33 months) from detection of MRD recurrence. Details for the outcomes regarding these five patients who developed extramedullary disease are listed in Supporting Information S5. Among these 44 patients, treatments received after the discovery of MRD positivity and prior to their morphological relapse included: 16 continued their maintenance chemotherapy regimen according to the specified treatment protocol; 13 received late intensification chemotherapy, as specified according to the individual treatment protocol; six did not receive further therapy; seven were initiated on salvage therapy with nelarabine, blinatumumab, or rituximab, and two went directly to allogeneic stem cell transplant (SCT). Median DFS from the time of MRD relapse for this group (n=44) was 3 months (range, <1–33 months), and median OS was 13 months (range, 3–57 months). Notably, treatment approaches varied among patients with recurrent MRD while still in morphologic remission, and was not determined by a pre-set treatment schema, in order to allow for individual patient-by-patient decision-making. Among the 55 patients, the breakdown by treatment regimens that were given to each patient after MRD detection can be divided into five major categories: n=16 maintenance chemotherapy with POMP; n=15 Intensification chemotherapy; n=9 stem cell transplant (SCT); n=9 change/other chemotherapy (including blinatumomab, rituximab, or nelarabine); n=6 no treatment. When comparing these treatment approaches after detection of MRD in this subset analysis, there was no significant difference found among the groups (although, each group is made up of a relatively small number of patients, Supporting Information S3).
Among the 55 patients in the overall study group, 16 (29%) were found to be Philadelphia-positive at baseline. Twelve of these 16 patients (75%) were in complete molecular remission by PCR prior to their MRD recurrence by MFC. At the time of MRD recurrence by MFC, the median quantitative real-time PCR value for BCR-ABL was 0.1731, range (0.0028–6.839), by international scale. For Table demonstrating flow cytometry values along with PCR value at the time of MRD detection for each of the Philadelphia-positive patients, please refer to Supporting Information S6.
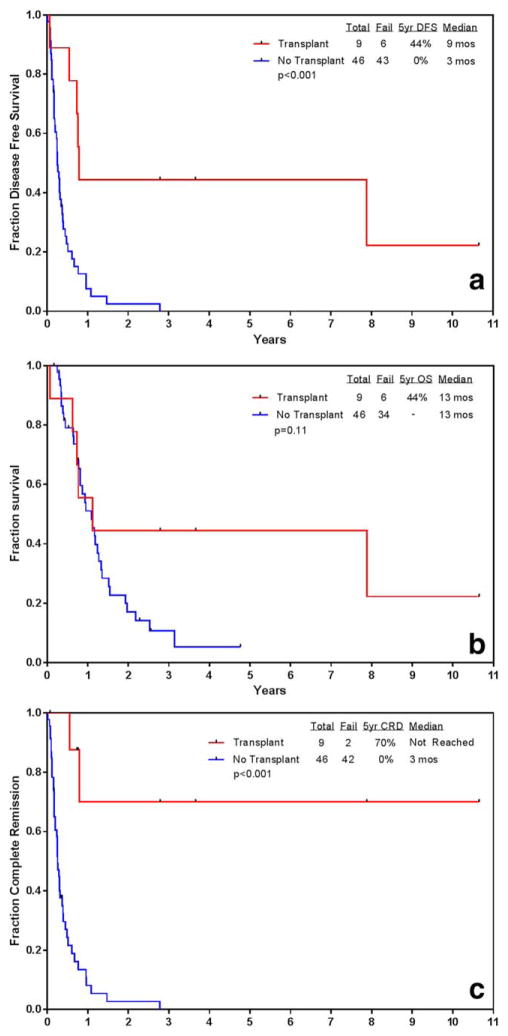
Nine out of 55 patients (16%) patients underwent SCT in the study group (overall post-SCT outcomes: 2 relapsed, 3 alive in CR, 4 died in CR). The median time from first MRD positivity to SCT was 14 weeks (range 2–39.4 weeks). Four of nine (44%) received intensification/consolidation chemotherapy regimens prior to SCT. Among these four patients, three (75%) achieved MRD negativity prior to SCT and two remain alive and in CR post-SCT. Notably, although there was a statistically significant difference favoring patients who underwent SCT for both median DFS and median CR duration, there was no statistically significant difference found in median OS between the patients who underwent SCT (n=9) versus those who did not (n=46) (Figure 3a–c).
FIGURE 3.
(a) Disease-free survival (DFS) in patients with MRD relapse from the time of MRD relapse (SCT n=9 vs. non-SCT n=46). (b) Complate remission duration (CRD) in patients with MRD relapse from the time of MRD relapse (SCT n-9 vs. non-sct n=46). (c) Overall survival (OS) in patients with MRD relapse from the time of MRD relapse (SCT n=9 vs. non-SCT n=46). [Color figure can be viewed at wileyonlinelibrary.com]
Overall, 11 patients (20%) did not have morphological relapse including five patients who died in complete morphological remission, and six patients who remain alive and in continued CR. Of the five patients who died prior to morphological relapse, four had undergone stem cell transplantation (SCT) (two matched-related donor, one cord blood, and one haplotype); three of these four died of post-SCT complications [one multi-organ failure, one septic shock, one progressive graft-versus-host-disease (GVHD) of GI tract].
Of the six patients (five B-ALL, one T-ALL,) remaining alive and in CR despite MRD recurrence, four had normal karyotype, one had Philadelphia chromosome positivity, and one had another cytogenetic abnormality (chromosome 8p11). After the finding of MRD positivity while in morphological remission: three (50%) underwent matched unrelated donor SCT, two were treated with blinatumomab, and one was treated with chemotherapy and a tyrosine kinase inhibitor (TKI) (twice daily fludarabine, cytarabine, vincristine, ponatinib), with both a median follow-up and OS from time of MRD relapse for these six patients of 1.6 years (range, 0.2–10.7 years).
To determine if there was an effect on outcome based on the time from CR to the time of MRD detection, patients were divided into two groups: <12 months and ≥12 months from CR to time of MRD recurrence. When OS was evaluated from treatment start to last follow-up, there was a significant difference favoring improved OS in those with longer time to detection of MRD recurrence (P value 0.0155, Figure 4, Supporting Information S2).
4 DISCUSSION
Although improvements in overall survival among all subgroups of adults with ALL have not yet matched those achieved in the pediatric ALL patient population,21 there have been several significant developments over the past decade leading to improved outcomes for adult patients.22 These include the introduction of novel targeted therapies, 22 expanding experience with the use of stem cell transplantation, 23,24 and better risk stratification and individualized therapeutic choices.13,22,25 Furthermore, the recent development of targeted monoclonal antibody-based strategies, has created new opportunities, including the potential for therapy in patients with MRD positive disease.26,27 Blinatumomab, a bi-specific T-cell engager antibody, which was initially studied in the MRD positive ALL setting, recently attained FDA approval in the United States for the treatment of patients with relapsed/refractory Philadelphia negative B-ALL.28
Because MRD detection is strongly associated with worse outcomes in patients with ALL, specific targeting for elimination of MRD remains an urgent, unmet need. Ample available data has established MRD detection at the end of induction and consolidation, as well as pre-and post-SCT, as an independent poor prognostic parameter among patients with ALL of all age groups, including pediatric, adolescent and young adult (AYA), and adult populations.3,5,6,12,17,29,30
In this analysis, among 546 patients with ALL who achieved an MRD negative CR, 55 (10%) were found to have recurrence of MRD while still maintaining morphological remission. With a median followup time from MRD relapse of 7.5 months (range, 2–128 months), the median CR duration was 3.6 months (range, <1–128 months) and the median overall survival (OS) from the time of MRD relapse was 13 months (range, <1–128 months). Forty-four (80%) patients subsequently experienced morphological relapse after a median of 3 months (<1–33 months) from detection of MRD recurrence. Nine (16%) patients were able to proceed to SCT in the study group; although limited numbers were available for definitive comparison in this analysis, there was no statistically significant difference in median OS between the patients who were able to proceed to SCT (n=9) versus those who did not (n=46).
Bar, M et al, reported that MRD detection by MFC prior to myeloablative allogeneic SCT, was associated with a statistically significant higher rate of relapse and mortality; furthermore, this extended to the post-SCT setting, among 144 patients, in which MRD positive status indicated a significantly higher risk of relapse and death compared to MRD negative patients.12 According to this large retrospective analysis at a major SCT center, detection of MRD prior to SCT is a significant risk for transplant failure.12 These findings again highlight the need not only for serial assessment of MRD, but also the urgent need for novel, less toxic strategies, and enrollment on clinical trials specifically designed for patients with ALL and MRD persistence/recurrence.
One limitation of the current retrospective study is the lack of predetermined, standard time points during long-term follow-up and after induction and consolidation for uniform MRD assessments. Regardless, we demonstrate in this analysis that MRD recurrence at any time after induction and consolidation appears to be a harbinger of morphological relapse, and therefore therapy to eradicate MRD should be considered at the time of MRD relapse. Prospective MRD detection-focused strategies have been shown to be effective in improving both prognostication and even selection of pre-emptive therapeutic strategies in various other leukemia subtypes.9,10 In this regard, for patients with ALL and detectable MRD, therapy with blinatumomab has shown positive results and larger clinical trials to prospectively investigate the role of blinatumomab in this setting should be undertaken.31,32 Continued efforts to refine MFC detection, creation of rigorous criteria for standardization and reproducibility of MRD results, and use of novel assays for MRD monitoring may improve the sensitivity and specificity of MRD detection and help detect MRD at earlier time points.6
In this single institution retrospective analysis, the vast majority of patients with ALL who achieved MRD negative CR followed by MRD relapse, subsequently experienced morphological relapse, which suggests that MRD relapse at any time point during a patient’s follow-up is associated with a high risk for morphological relapse. Although allogeneic SCT rescued some patients with ongoing long-term remission, it was associated with mortality in others. Prospective studies of long-term, serial MRD assessments throughout a patient’s treatment course and follow-up, coupled with less toxic strategies designed to eradicate MRD represent an ongoing, urgent area of unmet medical need for adult patients with ALL.
Supplementary Material
Acknowledgments
This research is supported in part by the MD Anderson Cancer Center Support Grant P30 CA016672.
Footnotes
DISCLOSURES/CONFLICTS OF INTEREST
The authors have no conflicts of interest to disclose with regards to this manuscript.
AUTHORSHIP CONTRIBUTIONS
NP, HK, and FR designed the study. JLJ, SAW, and SK performed the pathology evaluations and minimal residual disease assessments. NP, HK, EJ, NJ, DT, SO, MK, TK, JC, and FR evaluated and treated the patients. NP, RG, SP, XW, XH, and FR conducted the data collection and statistical analysis. All authors provided critical analysis and approved the final manuscript.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article.
References
- 1.Borowitz MJ, Devidas M, Hunger SP, et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: a Children’s Oncology Group study. Blood. 2008;111:5477–5485. doi: 10.1182/blood-2008-01-132837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dworzak MN, Froschl G, Printz D, et al. Prognostic significance and modalities of flow cytometric minimal residual disease detection in childhood acute lymphoblastic leukemia. Blood. 2002;99:1952–1958. doi: 10.1182/blood.v99.6.1952. [DOI] [PubMed] [Google Scholar]
- 3.Ravandi F, Jorgensen JL, O’brien SM, et al. Minimal residual disease assessed by multi-parameter flow cytometry is highly prognostic in adult patients with acute lymphoblastic leukaemia. Br J Haematol. 2016;172:392–400. doi: 10.1111/bjh.13834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campana D. Should minimal residual disease monitoring in acute lymphoblastic leukemia be standard of care? Curr Hematol Malig Rep. 2012;7:170–177. doi: 10.1007/s11899-012-0115-4. [DOI] [PubMed] [Google Scholar]
- 5.Ribera JM, Oriol A, Morgades M, et al. Treatment of high-risk Philadelphia chromosome-negative acute lymphoblastic leukemia in adolescents and adults according to early cytologic response and minimal residual disease after consolidation assessed by flow cytometry: final results of the PETHEMA ALL-AR-03 trial. J Clin Oncol. 2014;32:1595–1604. doi: 10.1200/JCO.2013.52.2425. [DOI] [PubMed] [Google Scholar]
- 6.van Dongen JJ, van der Velden VH, Bruggemann M, Orfao A. Minimal residual disease diagnostics in acute lymphoblastic leukemia: need for sensitive, fast, and standardized technologies. Blood. 2015;125:3996–4009. doi: 10.1182/blood-2015-03-580027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaipa G, Basso G, Biondi A, Campana D. Detection of minimal residual disease in pediatric acute lymphoblastic leukemia. Cytometry B Clin Cytom. 2013;84:359–369. doi: 10.1002/cyto.b.21101. [DOI] [PubMed] [Google Scholar]
- 8.Coustan-Smith E, Campana D. Immunologic minimal residual disease detection in acute lymphoblastic leukemia: a comparative approach to molecular testing. Best Pract Res Clin Haematol. 2010;23:347–358. doi: 10.1016/j.beha.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grimwade D, Jovanovic JV, Hills RK, et al. Prospective minimal residual disease monitoring to predict relapse of acute promyelocytic leukemia and to direct pre-emptive arsenic trioxide therapy. J Clin Oncol. 2009;27:3650–3658. doi: 10.1200/JCO.2008.20.1533. [DOI] [PubMed] [Google Scholar]
- 10.Ivey A, Hills RK, Simpson MA, et al. Assessment of minimal residual disease in standard-risk AML. N Engl J Med. 2016;374:422–433. doi: 10.1056/NEJMoa1507471. [DOI] [PubMed] [Google Scholar]
- 11.Jain P, Kantarjian H, Patel K, et al. Mutated NPM1 in patients with acute myeloid leukemia in remission and relapse. Leuk Lymphoma. 2014;55:1337–1344. doi: 10.3109/10428194.2013.840776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bar M, Wood BL, Radich JP, et al. Impact of minimal residual disease, detected by flow cytometry, on outcome of myeloablative hematopoietic cell transplantation for acute lymphoblastic leukemia. Leuk Res Treatment. 2014;2014:421723. doi: 10.1155/2014/421723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bassan R, Hoelzer D. Modern therapy of acute lymphoblastic leukemia. J Clin Oncol. 2011;29:532–543. doi: 10.1200/JCO.2010.30.1382. [DOI] [PubMed] [Google Scholar]
- 14.Ravandi F, Jorgensen JL, Thomas DA, et al. Detection of MRD may predict the outcome of patients with Philadelphia chromosome-positive ALL treated with tyrosine kinase inhibitors plus chemotherapy. Blood. 2013;122:1214–1221. doi: 10.1182/blood-2012-11-466482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Y, Slack R, Jorgensen JL, et al. The effect of peritransplant minimal residual disease in adults with acute lymphoblastic leukemia undergoing allogeneic hematopoietic stem cell transplantation. Clin Lymphoma Myeloma Leuk. 2014;14:319–326. doi: 10.1016/j.clml.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kantarjian HM, O’brien S, Smith TL, et al. Results of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia. J Clin Oncol. 2000;18:547–561. doi: 10.1200/JCO.2000.18.3.547. [DOI] [PubMed] [Google Scholar]
- 17.Rytting ME, Thomas DA, O’brien SM, et al. Augmented Berlin–Frankfurt–Munster therapy in adolescents and young adults (AYAs) with acute lymphoblastic leukemia (ALL) Cancer. 2014;120:3660–3668. doi: 10.1002/cncr.28930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weir EG, Cowan K, LeBeau P, Borowitz MJ. A limited antibody panel can distinguish B-precursor acute lymphoblastic leukemia from normal B precursors with four color flow cytometry: implications for residual disease detection. Leukemia. 1999;13:558–567. doi: 10.1038/sj.leu.2401364. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan EaM P. Nonparametric estimator from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 20.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 21.Pui CH, Yang JJ, Hunger SP, et al. Childhood acute lymphoblastic leukemia: progress through collaboration. J Clin Oncol. 2015;33:2938–2948. doi: 10.1200/JCO.2014.59.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jabbour E, O’brien S, Konopleva M, Kantarjian H. New insights into the pathophysiology and therapy of adult acute lymphoblastic leukemia. Cancer. 2015;121:2517–2528. doi: 10.1002/cncr.29383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poon LM, Hamdi A, Saliba R, et al. Outcomes of adults with acute lymphoblastic leukemia relapsing after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19:1059–1064. doi: 10.1016/j.bbmt.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kebriaei P, Poon LM. The role of allogeneic hematopoietic stem cell transplantation in the therapy of patients with acute lymphoblastic leukemia. Curr Hematol Malig Rep. 2012;7:144–152. doi: 10.1007/s11899-012-0116-3. [DOI] [PubMed] [Google Scholar]
- 25.Alvarnas JC, Brown PA, Aoun P, et al. Acute lymphoblastic leukemia, Version 2.2015. J Natl Compr Canc Netw. 2015;13:1240–1279. doi: 10.6004/jnccn.2015.0153. [DOI] [PubMed] [Google Scholar]
- 26.Jabbour E, O’brien S, Ravandi F, Kantarjian H. Monoclonal antibodies in acute lymphoblastic leukemia. Blood. 2015;125:4010–4016. doi: 10.1182/blood-2014-08-596403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Batlevi CL, Matsuki E, Brentjens RJ, Younes A. Novel immunotherapies in lymphoid malignancies. Nat Rev Clin Oncol. 2016;13:25–40. doi: 10.1038/nrclinonc.2015.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Przepiorka D, Ko CW, Deisseroth A, et al. FDA approval: blinatumomab. Clin Cancer Res. 2015;21:4035–4039. doi: 10.1158/1078-0432.CCR-15-0612. [DOI] [PubMed] [Google Scholar]
- 29.van Dongen JJ, Seriu T, Panzer-Grumayer ER, et al. Prognostic value of minimal residual disease in acute lymphoblastic leukaemia in childhood. Lancet. 1998;352:1731–1738. doi: 10.1016/S0140-6736(98)04058-6. [DOI] [PubMed] [Google Scholar]
- 30.Stock W. Adolescents and young adults with acute lymphoblastic leukemia. Hematol Am Soc Hematol Educ Prog. 2010;2010:21–29. doi: 10.1182/asheducation-2010.1.21. [DOI] [PubMed] [Google Scholar]
- 31.Topp MS, Gokbuget N, Zugmaier G, et al. Long-term follow-up of hematologic relapse-free survival in a phase 2 study of blinatumomab in patients with MRD in B-lineage ALL. Blood. 2012;120:5185–5187. doi: 10.1182/blood-2012-07-441030. [DOI] [PubMed] [Google Scholar]
- 32.Kantarjian HM, Stein AS, Bargou RC, et al. Blinatumomab treatment of older adults with relapsed/refractory B-precursor acute lymphoblastic leukemia: results from 2 phase 2 studies. Cancer. 2016;122:2178–2185. doi: 10.1002/cncr.30031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.