Abstract
Purpose
An examination of multiple primary cancers can provide insight into the etiologic role of genes, the environment, and prior cancer treatment on a cancer patient’s risk of developing a subsequent cancer. Different rules for registering multiple primary cancers (MP) are used by cancer registries throughout the world making data comparisons difficult.
Methods
We evaluated the effect of SEER and IARC/IACR rules on cancer incidence rates and trends using data from the SEER Program. We estimated age-standardized incidence rate (ASIR) and trends (1975–2011) for the top 26 cancer categories using joinpoint regression analysis.
Results
ASIRs were higher using SEER compared to IARC/IACR rules for all cancers combined (3 %) and, in rank order, melanoma (9 %), female breast (7 %), urinary bladder (6 %), colon (4 %), kidney and renal pelvis (4 %), oral cavity and pharynx (3 %), lung and bronchus (2 %), and non-Hodgkin lymphoma (2 %). ASIR differences were largest for patients aged 65+ years. Trends were similar using both MP rules with the exception of cancers of the urinary bladder, and kidney and renal pelvis.
Conclusions
The choice of multiple primary coding rules effects incidence rates and trends. Compared to SEER MP coding rules, IARC/IACR rules are less complex, have not changed over time, and report fewer multiple primary cancers, particularly cancers that occur in paired organs, at the same anatomic site and with the same or related histologic type. Cancer registries collecting incidence data using SEER rules may want to consider including incidence rates and trends using IARC/IACR rules to facilitate international data comparisons.
Keywords: Incidence rates, Trends, Multiple primary cancers, Population-based cancer registry, SEER, IARC, IACR
Introduction
According to the International Agency for Research on Cancer (IARC), over 14 million new cases of cancer were diagnosed worldwide in 2012, the most recent year for which data are available [1]. The most commonly diagnosed cancers were those of the lung and bronchus, female breast, colorectum, and prostate. Incident cases worldwide are projected to increase to over 22.2 million by 2030 due primarily to rapid population growth and aging [2]. Cancer control strategies are urgently needed to address the growing cancer burden. To this end, population-based cancer registries serve a vital role by providing information necessary for directing and monitoring cancer control activities and health policy initiatives [3].
Much time and effort have been spent on standardizing the collection, consolidation, analysis, and reporting of cancer surveillance data to ensure that these data are high quality, complete, and suitable for comparisons within the USA [4, 5], North America [6], among countries worldwide [7–11]. In the mid-1970s, around the start of the Surveillance, Epidemiology, and End Results (SEER) Program [12], coding rules were developed to enumerate primary cancers including differentiating a new primary cancer from a distant metastasis or a recurrent cancer [13]. These rules have been revised over time with the most recent changes implemented in 2007 and are used by cancer registries throughout North America. In 2004, a working group comprised of individuals representing IARC, the International Association of Cancer Registries (IACR), and the European Network of Cancer Registries updated and published multiple primary coding rules previously developed by IACR [14]. The IARC/IACR rules are predominately used by cancer registries outside North America and have not been modified since their publication in 2004. Compared to SEER multiple primary coding rules, IARC/IACR rules are less complex, have not changed over time, and report fewer multiple primary cancers, particularly cancers that occur in paired organs, at the same anatomic site and with the same or related histologic type [15].
To address issues of comparability, IARC developed an algorithm that can be used to identify multiple primary cancers defined according to IARC/IACR rules among primary cancer cases whose data were originally collected and consolidated using less conservative rules such as the SEER rules [16]. This program is used to process cancer data from North America for inclusion in Cancer Incidence in Five Continents [10], GLOBOCAN [11], and for the inclusion of survival data in CONCORD studies [7, 8] and the International Cancer Benchmarking Partnership [9].
Differences in coding practices used to collect and consolidate multiple primary cancers can result in differences in incidence rates and trends [17, 18]. The importance of this issue is likely to grow as increasingly more cancer patients are living longer following a diagnosis of cancer [5] and are thus at greater risk of being diagnosed with a subsequent cancer [19]. To date, variations in breast cancer incident counts have been examined using SEER and IARC/IACR multiple primary coding rules [20] and the impact of these rules has been assessed on cancer survival [21]. In this paper, we examine the effect of these two widely used multiple primary coding rules on incidence rates and trends for leading cancers using a common dataset.
Materials and methods
Source of data
Cancer incidence data for patients diagnosed 1975 through 2011 were obtained from nine population-based cancer registries participating in the SEER Program in five states (Connecticut, Hawaii, Iowa, New Mexico, Utah) and four metropolitan areas (Atlanta, Detroit, San Francisco-Oakland, Seattle) covering approximately 10 % of the US population [5]. These registries were selected because they have been in operation for more than 35 years and will have more information (i.e., past cancer diagnoses) with which to correctly identify a prior cancer than registries that have been in operation for fewer years [22]. Data were collected and reported using standard codes and procedures [23] and according to the SEER multiple primary coding rules in use at the time of diagnosis [13]. The primary site and histology of each cancer were coded according to the International Classification of Diseases for Oncology (ICD-O) edition in use at the time of diagnosis, converted to the third edition, and classified according to the SEER site recodes [24]. In situ urinary bladder cancers were considered invasive for the purpose of reporting incidence data in the SEER Program [25].
In order to compare case counts, incidence rates and trends by using different multiple primary cancer coding rules, we looked at first primary cancers [only primary cancer diagnosed in a patient (sequence number: 00) or first primary cancer of multiple primary cancers diagnosed in a patient (sequence number: 01)] according to SEER rules and the additional number (if any) of primary cancers diagnosed in a patient (sequence number: 02+) according to SEER and IARC/IACR rules, respectively.
Analysis
Age-standardized incidence rates (ASIR) per 100,000 persons adjusted to the 2000 the US standard population [26] and corresponding 95 % confidence intervals (95 % CI) were generated for all sites combined and for 26 leading site categories using SEER*Stat software (version 8.0.4) [27]. This version of the software contains a feature whereby invasive multiple primary cancers collected using SEER multiple primary rules can be re-classified according to IARC/IACR rules.
ASIR ratios (SEER: IARC/IACR) were generated for cases diagnosed 2005–2009. We restricted cases to this 5-year time period to maximize the operational length of the registries (to identify multiple primary cancers) while allowing at least 3 years between the time a patient was diagnosed and when their data were submitted to the SEER Program to maximize case ascertainment (and mitigate reporting delays) [28]. As there is no formal statistical test to compare incidence rates on the same population by using different case definitions methods, ratios were noted where the 5-year point estimate for the IARC/IACR rate was not contained within the corresponding 95 % CI of the SEER rate.
Trends in ASIRs were analyzed using joinpoint regression which involved fitting a series of joined straight lines on a logarithmic scale to the trends in the annual ASIRs [29]. A maximum of five line segments were allowed in the models for the period 1975 through 2011. We described the resulting trends by the slope of each line segment as the annual percent change (APC), using t tests (two-sided, p < 0.05) to assess whether the APCs were statistically significantly different from zero [30]. We used the terms increase or decrease to describe significant trends and stable to describe nonsignificant trends.
Results
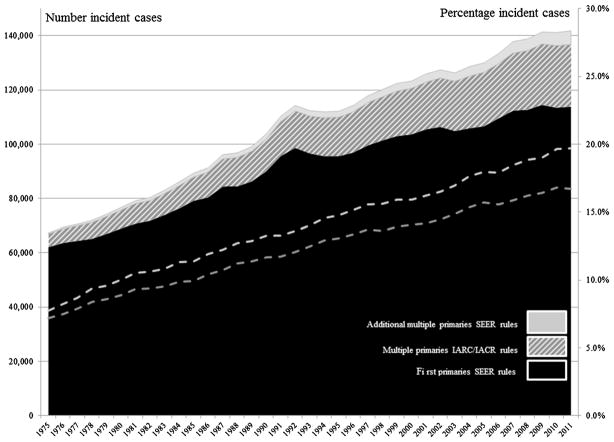
Figure 1 shows the number of primary cancers that were reported as a first primary cancer according to SEER multiple primary coding rules and the number and percentage of additional cases reported as multiple primary cancers according to IARC/IACR and SEER rules between 1975 and 2011. In 1975, 62,136 cases of first primary cancer were reported. An additional 4,805 (7.2 %) cases were reported as multiple primary cancers according to IARC/IACR rules and 5,222 (7.8 %), or an additional 422 cases, according to SEER rules. By 2011, 16.7 % of cases were reported as multiple primary cancers according to IARC/IACR rules and 19.7 %, or an additional 5,059 cases, according to SEER rules.
Fig. 1.
Number of cancers reported as a first primary cancer and number and percentage of cancers reported according to SEER and IACR/IACR multiple primary (MP) coding rules, by year of diagnosis (SEER: 1975–2011)
ASIRs for patients diagnosed 2005 through 2009 (Table 1), as shown by the rate ratio, were higher using SEER rules compared to incidence rates using IACR rules for all cancer sites combined (ASIR 1.03 %, or 3 %) and eight site-specific cancers: in rank order, melanoma (9 %), female breast (7 %), urinary bladder (6 %), colon (4 %), kidney and renal pelvis (4 %), oral cavity and pharynx (3 %), lung and bronchus (2 %), and non-Hodgkin lymphoma (2 %). Rate ratios increased with increasing age at diagnosis and were largest for patients 65 years of age and older: in rank order, melanoma (13 %), female breast (10 %), urinary bladder (6 %), colon (5 %), kidney and renal pelvis (5 %), oral cavity and pharynx (5 %), lung and bronchus (3 %), and non-Hodgkin lymphoma (3 %). ASIRs did not differ for all ages combined or by age group for Hodgkin lymphoma, myeloma, leukemia, mesothelioma, or Kaposi sarcoma, or for cancers of the esophagus, rectum and rectosigmoid junction, stomach, liver and intrahepatic bile duct, pancreas, larynx, cervix uteri, corpus uterine, ovary, prostate, testis, brain and other nervous system, or thyroid.
Table 1.
Age-standardized cancer incidence rates (ASIR) and rate ratios by SEER and IARC/IACR multiple primary coding rules and age at diagnosis of leading cancers (SEER: 2005–2009
| Site | Sex | ASIR | ASIR ratios (SEER: IARC/IACR) | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| SEER | IARC/IACR | All ages | 0–19 | 20–44 | 45–64 | 65+ | ||
| All sites | Both | 475.3 (474.1, 476.4) | 461.1 | 1.03* | 1.00 | 1.02* | 1.02* | 1.03* |
| Oral cavity and pharynx | Both | 10.9 (10.7, 11.0) | 10.5 | 1.03* | 1.00 | 1.01 | 1.02 | 1.05* |
| Esophagus | Both | 4.7 (4.6, 4.8) | 4.6 | 1.00 | 1.00 | 1.01 | 1.00 | 1.00 |
| Stomach | Both | 7.4 (7.3, 7.5) | 7.4 | 1.01 | 1.00 | 1.00 | 1.01 | 1.01 |
| Colon excluding rectum | Both | 33.0 (32.7, 33.3) | 31.5 | 1.04* | 1.04 | 1.02 | 1.03* | 1.05* |
| Rectum and rectosigmoid junction | Both | 12.8 (12.7, 13.0) | 12.7 | 1.01 | 1.00 | 1.01 | 1.01 | 1.01 |
| Liver and intrahepatic bile duct | Both | 7.5 (7.3, 7.6) | 7.4 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Pancreas | Both | 12.6 (12.4, 12.8) | 12.6 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Larynx | Both | 3.3 (3.2, 3.4) | 3.2 | 1.01 | 1.01 | 1.01 | 1.01 | 1.01 |
| Lung and bronchus | Both | 61.4 (61.9, 61.8) | 60.0 | 1.02* | 1.00 | 1.01 | 1.01 | 1.03* |
| Melanoma | Both | 22.5 (22.3, 22.7) | 20.8 | 1.09* | 1.00 | 1.04 | 1.08* | 1.13* |
| Breast | F | 127.9 (127.1, 128.7) | 117.1 | 1.07* | 1.00 | 1.04* | 1.06* | 1.10* |
| Cervix uteri | F | 6.8 (6.6, 7.0) | 6.8 | 1.00 | 1.00 | 1.00 | 1.00 | 1.01 |
| Corpus uterine | F | 25.1 (24.8, 25.5) | 25.1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Ovary | F | 13.0 (12.7, 13.2) | 12.9 | 1.00 | 1.00 | 1.01 | 1.00 | 1.00 |
| Prostate | M | 162.9 (161.9, 163.9) | 162.9 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Testis | M | 6.0 (5.8, 6.2) | 5.8 | 1.03 | 1.00 | 1.03 | 1.03 | 1.00 |
| Urinary bladder | Both | 21.5 (21.2, 21.7) | 20.3 | 1.06* | 1.00 | 1.02 | 1.03* | 1.06* |
| Kidney and renal pelvis | Both | 15.1 (14.9, 15.3) | 14.5 | 1.04* | 1.03 | 1.03 | 1.03* | 1.05* |
| Brain and other nervous system | Both | 6.6 (6.5, 6.8) | 6.6 | 1.01 | 1.01 | 1.02 | 1.01 | 1.00 |
| Thyroid | Both | 12.4 (12.3, 12.7) | 12.4 | 1.01 | 1.00 | 1.00 | 1.01 | 1.01 |
| Hodgkin lymphoma | Both | 3.0 (2.9, 3.1) | 2.8 | 1.00 | 1.00 | 1.00 | 1.00 | 1.01 |
| Non-Hodgkin lymphoma | Both | 20.8 (20.5, 21.0) | 20.3 | 1.02* | 1.00 | 1.01 | 1.02 | 1.03* |
| Myeloma | Both | 6.2 (6.0, 6.3) | 6.1 | 1.02 | 1.00 | 1.01 | 1.01 | 1.02 |
| Leukemia | Both | 13.5 (13.3, 13.7) | 13.3 | 1.01 | 1.00 | 1.01 | 1.01 | 1.02 |
| Mesothelioma | Both | 1.0 (0.9, 1.0) | 1.0 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Kaposi sarcoma | Both | 0.7 (0.6, 0.7) | 0.7 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
Rate differences between SEER MP and IARC/IACR MP data sets where the 5-year IARC/IACR rate estimate was not contained within the corresponding 95 % CIs from the SEER MP rate estimate
Table 2 shows the trends in ASIRs between 1975 and 2011 by cancer site for first primary cancers and for all cancers combined (first and multiple primary cancers) using the SEER and IARC/IACR rules, respectively. The cancer sites selected for comparison were all cancers combined and the eight cancer site groups noted in Table 1 to have different ASIRs according to SEER versus IARC/IACR rules.
Table 2.
Annual percent change (APC) for select cancer reported as a first or only cancer and for all cancers according to SEER and IARC/IACR multiple primary (MP) coding rules (SEER: 1975–2011)
| Site | Sex | Multiple primary rules |
Start year |
Segment 1 | Segment 2 | Segment 3 | Segment 4 | Segment 5 | Segment 6 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||||||
| APC | Change point |
APC | Change point |
APC | Change point |
APC | Change point |
APC | Change point |
APC | ||||
| All sites | All | SEER | 1975 | 1.2* | 1989 | 2.8 | 1992 | −2.4 | 1995 | 1.1 | 1998 | −0.3* | 2009 | −2.0 |
| IARC/IACR | 1975 | 1.2* | 1989 | 2.9 | 1992 | −2.5 | 1995 | 1.1 | 1998 | −0.4* | 2009 | −2.2 | ||
| First | 1975 | 0.8* | 1989 | 2.6 | 1992 | −2.9 | 1995 | 0.6 | 1999 | −1.0* | – | – | ||
| Oral cavity and pharynx | All | SEER | 1975 | 0.7 | 1981 | −1.1* | 2003 | 0.6 | ||||||
| IARC/IACR | 1975 | 1.3 | 1979 | −1.1* | 2003 | 0.3 | ||||||||
| First | 1975 | −0.6 | 1984 | −1.7* | 2001 | −0.2 | ||||||||
| Colon excluding rectum | All | SEER | 1975 | 1.2* | 1985 | −1.7* | 1995 | 1.2 | 1998 | −2.4* | 2008 | −5.3* | ||
| IARC/IACR | 1975 | 1.0* | 1985 | −1.7* | 1995 | 0.1 | 2000 | −2.7* | 2008 | −4.9* | ||||
| First | 1975 | 0.6* | 1985 | −2.1* | 1995 | −0.4 | 2001 | −3.4* | ||||||
| Lung and bronchus | All | SEER | 1975 | 2.5* | 1982 | 0.9* | 1991 | −0.7* | 2007 | −2.6* | ||||
| IARC/IACR | 1975 | 2.5* | 1982 | 0.9* | 1991 | −0.7* | 2007 | −2.8* | ||||||
| First | 1975 | 1.7* | 1984 | 0.1 | 1991 | −1.4* | 2007 | −3.7* | ||||||
| Melanoma | All | SEER | 1975 | 5.7* | 1981 | 2.9* | 2005 | 1.3* | ||||||
| IARC/IACR | 1975 | 5.7* | 1981 | 2.7* | 2006 | 0.5 | ||||||||
| First | 1975 | 4.4* | 1985 | 1.8* | ||||||||||
| Breast | F | SEER | 1975 | −0.5 | 1980 | 4.0* | 1987 | −0.2 | 1994 | 1.8* | 1999 | −2.3* | 2004 | 0.2 |
| IARC/IACR | 1975 | −0.8 | 1980 | 3.7* | 1987 | −0.2 | 1994 | 1.6* | 1999 | −2.3* | 2005 | 0.3 | ||
| First | 1975 | −0.8 | 1980 | 3.6* | 1987 | −0.4 | 1994 | 1.6* | 1999 | −2.5 | 2005 | 0.1 | ||
| Urinary bladder | All | SEER | 1975 | 0.7* | 1986 | 0.2* | 2007 | −1.6* | ||||||
| IARC/IACR | 1975 | 0.7* | 1987 | −0.1 | 2008 | −2.0 | ||||||||
| First | 1975 | 0.2 | 1987 | −0.7* | 2008 | −2.7* | ||||||||
| Kidney and renal pelvis | All | SEER | 1975 | 2.3* | 1994 | −0.5 | 1997 | 3.1* | 2008 | −1.4 | ||||
| IARC/IACR | 1975 | 1.9* | 2002 | 3.3* | 2008 | −1.4 | ||||||||
| First | 1975 | 1.7* | 1994 | −1.2 | 1997 | 2.7* | 2008 | −1.7 | ||||||
| Non-Hodgkin lymphoma | All | SEER | 1975 | 3.7* | 1991 | 0.5* | ||||||||
| IARC/IACR | 1975 | 3.6* | 1991 | 0.6* | 2007 | −1.8* | ||||||||
| First | 1975 | 3.2* | 1991 | 0.1 | 2009 | −4.4* | – | – | ||||||
APC is statistically different from zero (two-sided t test, p < 0.05) [30]
Incidence rates for all cancers combined increased (1.2 % per year) between 1975 and 1989, were stable between 1989 and 1998, declined (0.3 and 0.4 % per year) between 1998 and 2009, and were stable through 2011, using SEER and IARC/IACR rules. Rates of first cancers (all sites combined) increased (0.8 % per year) between 1975 and 1989, were stable between 1989 and 1999, and declined (1.0 % per year) through 2011.
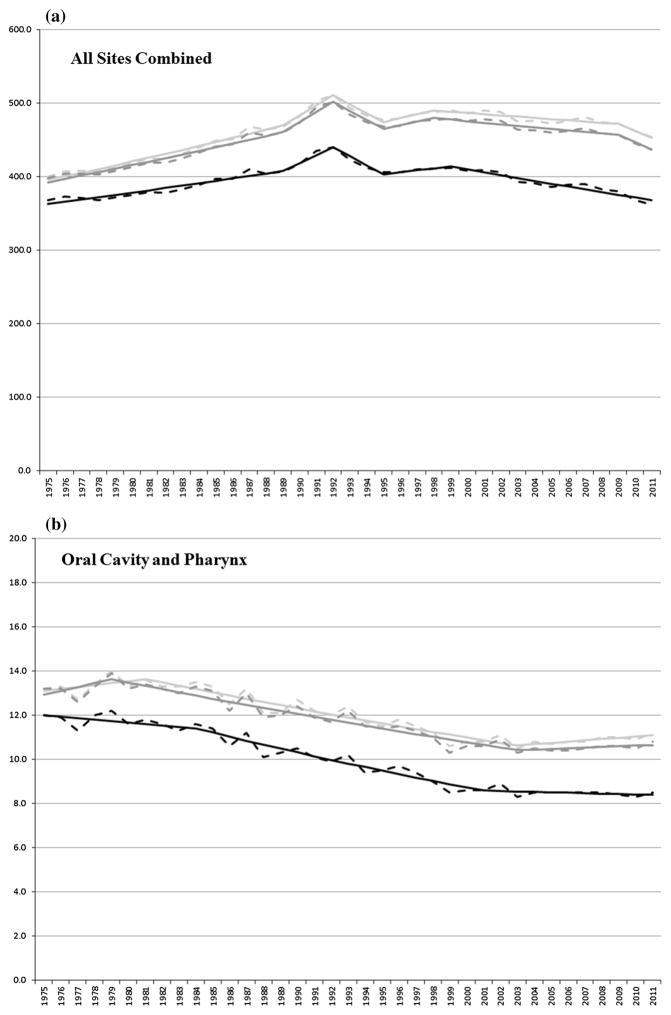
Incidence rates for oral cavity and pharynx cancers were stable between 1975 and 1979/1981, decreased (1.1 % per year) through 2003, and were stable through 2011, using SEER and IARC/IACR rules. Incidence rates for first oral cancers were stable between 1975 and 1984, decreased (1.7 % per year) through 2001, and were stable through 2011.
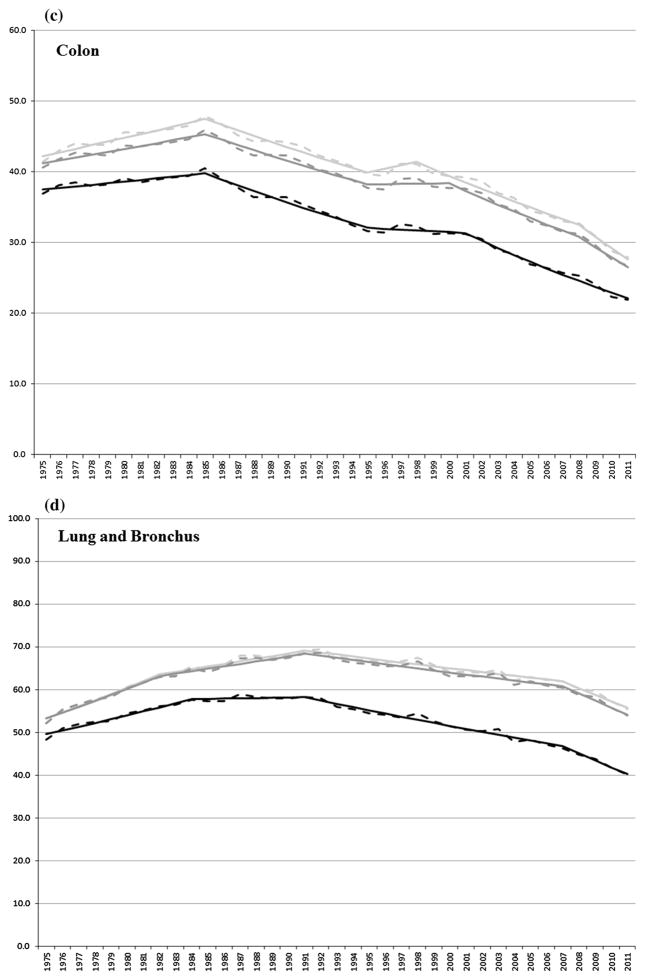
Incidence rates of colon cancer increased (1.2 % and 1.0 % per year) between 1975 and 1985, decreased (1.7 % per year) between 1985 and 1995, were stable between 1995 and 1998/2000, and decreased (2.4 or 2.7 and 5.3 or 4.9 % per year, respectively) through 2011, using SEER and IARC/IACR rules. Incidence rates of first colon cancers increased (0.6 % per year) between 1975 and 1985, decreased (2.1 % per year) through 1995, were stable through 2001, and declined (3.4 % per year) through 2011.
Incidence rates for cancers of the lung and bronchus increased (2.5 and 0.9 % per year) between 1975 and 1991 and declined (0.7 and 2.6 or 2.8 % per year, respectively) through 2011, using SEER and IARC/IACR rules. Incidence rates for first cancers of the lung and bronchus increased (1.7 % per year) between 1975 and 1984, were stable between 1984 and 1991, and decreased (1.4 and 3.7 % per year) through 2011.
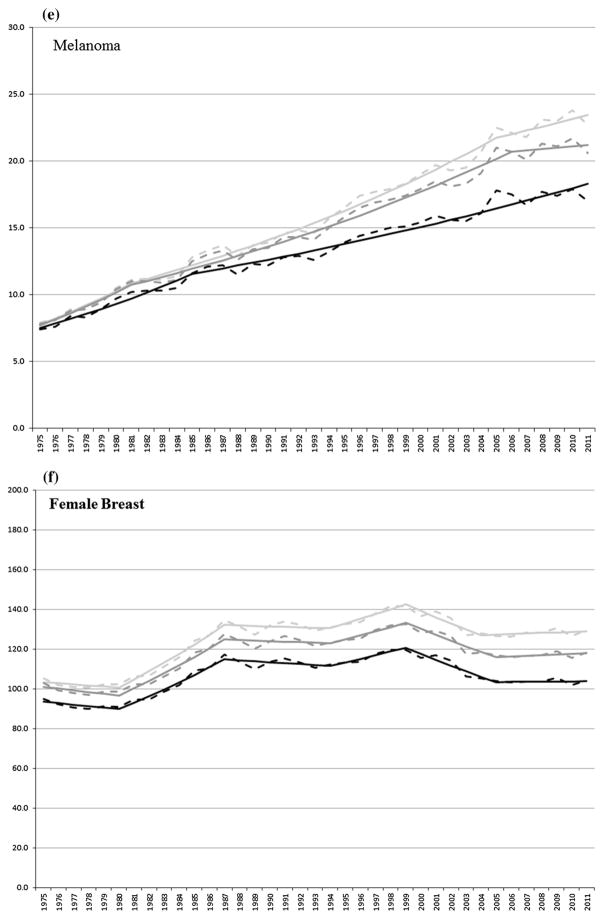
Melanoma incidence rates increased (5.7 and 2.9 or 2.7 % per year, respectively) between 1975 and 2005/2006 using SEER and IARC/IACR rules. Between 2005 and 2011, melanoma incidence rates increased (1.3 % per year) using SEER rules and were stable between 2006 and 2011 using IARC/IACR rules. Between 1975 and 2011, incidence rates of first melanoma cancers increased (4.4 and 1.8 % per year).
Female breast cancer incidence rates were stable between 1975 and 1981, increased (4.0 and 3.7 % per year, respectively) between 1980 and 1987, were stable between 1987 and 1994, increased (1.8 and 1.6 % per year, respectively) between 1994 and 1999, decreased (2.3 % per year) between 1999 and 2004/2005, and were stable through 2011 using SEER and IARC/IACR rules. First female breast cancers were stable between 1975 and 1980, increased (3.6 % per year) through 1987, were stable between 1987 and 1994, increased (1.6 % per year) between 1994 and 1999, and were stable through 2011.
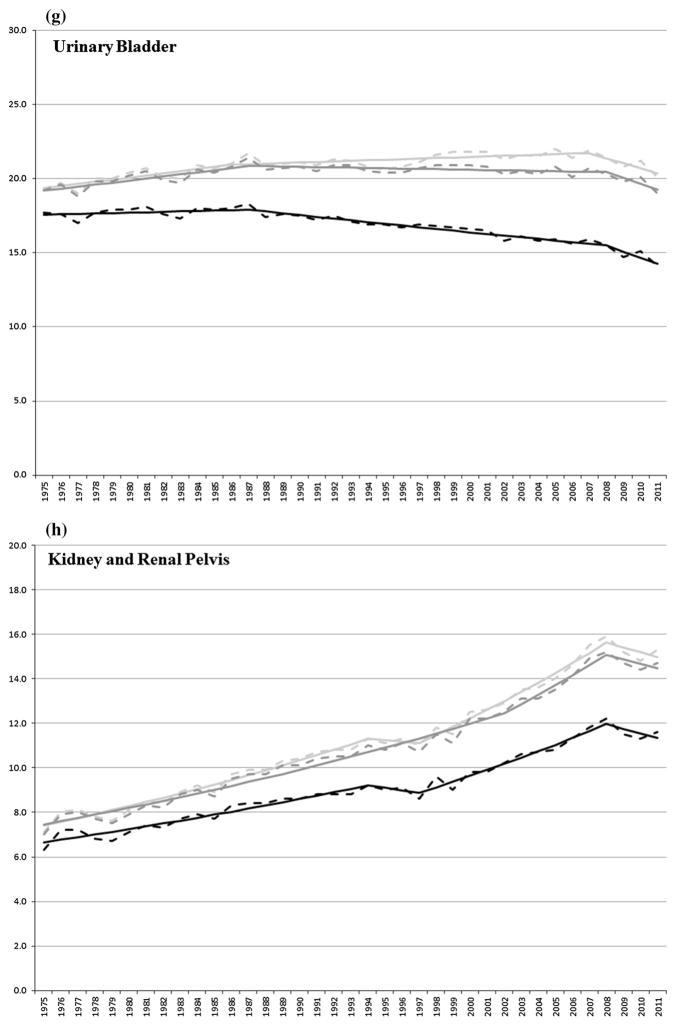
Urinary bladder incidence rates increased (0.7 % per year) between 1975 and 1986/1987 using SEER and IARC/IACR rules. Incidence increased (0.2 % per year) between 1986 and 2007 and decreased (1.6 % per year) through 2011 using SEER rules. Urinary bladder incidence rates were stable between 1987 and 2011 using IARC/IACR rules. The incidence of first urinary bladder cancer was stable between 1975 and 1987 and decreased (0.7 and 2.7 % per year) through 2011.
Kidney and renal pelvis cancer incidence rates increased (2.3 % per year) between 1975 and 1994, were stable between 1994 and 1997, increased (3.1 % per year) between 1997 and 2008, and were stable through 2011, using SEER rules. Kidney cancer incidence rates increased (1.9 and 3.3 % per year) between 1975 and 2008 and were stable through 2011 using IARC/IACR rules. Incidence rates of first kidney cancer increased (1.7 % per year) between 1975 and 1994, were stable between 1994 and 1997, increased (2.7 % per year) between 1997 and 2008, and were stable through 2011.
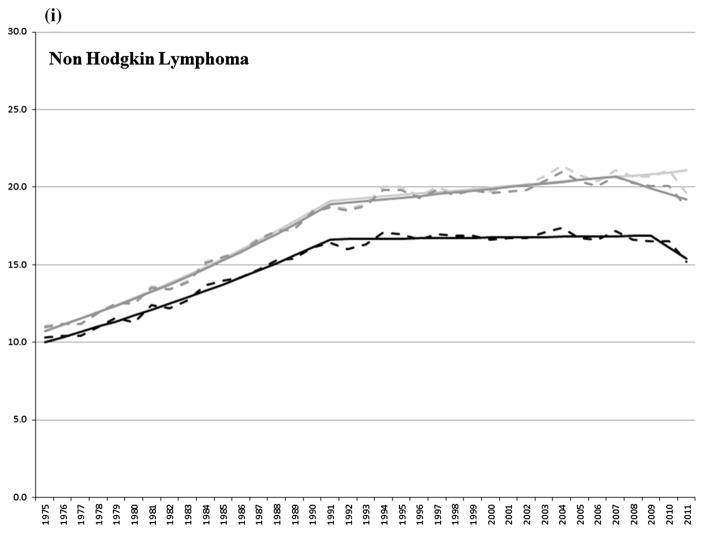
The incidence of non-Hodgkin lymphoma (NHL) increased (3.7 and 0.5 % per year) between 1975 and 2011 using SEER rules. Incidence increased (3.6 and 0.6 % per year) between 1975 and 2007 and decreased (1.8 % per year) between 2007 and 2011 using IACR rules. The incidence of first NHL increased (3.2 % per year) between 1975 and 1991, stabilized between 1991 and 2009, and decreased (4.4 % per year) through 2011.
For the cancers presented in Table 2, Fig. 2a–i shows the observed and joinpoint modeled ASIRs between 1975 and 2011 for first primary cancers and for all (first and multiple) primary cancers combined using SEER and IARC/IACR coding rules, respectively, for all sites combined and for select cancers.
Fig. 2.
a–i Observed (dashed line) and modeled (solid line) age-standardized incidence rates by first (black) primary cancer and by SEER (light gray) and IARC/IACR (dark gray) multiple primaries for select cancers
Discussion
This study provided a unique opportunity to evaluate the effect of two widely used multiple primary cancer coding rules on cancer incidence rates and trends using a common dataset. Incidence data from nine population-based cancer registries participating in the SEER Program were collected using the SEER multiple primary rules in use at the time of diagnosis and analyzed according to both SEER and IARC/IACR rules. The SEER 9 registries were selected because their annual data submissions must meet SEER Program standards with respect to the completeness and quality of their data and because the operational length of these registries affords the opportunity for more accurate and complete counting of subsequent primaries [5]. As has been noted [22], the proportion of cancer patients with a known prior cancer will depend on the length of operation of the cancer registry: Cancer registries that have been in operation for many years will have more information (i.e., past cancer diagnoses) with which to correctly identify a prior cancer than a cancer registry that has been in operation for fewer years. Thus, the differences between the age-standardized incidence rates and trends for a given cancer site reflect the differential impact that these coding rules have on enumerating the number of primary cancers diagnosed in a cancer patient.
Incidence rates using SEER multiple primary coding rules were higher compared to incidence rates using IARC/IACR rules for all cancers combined and for many of the leading cancers including the most commonly diagnosed cancers worldwide: female breast, lung and bronchus, and colon cancers. This reflects the fact that SEER rules generally allow more multiple cancers to be reported at the same anatomic site and with the same or related histologic type [15]. For example, under the SEER rules each subsite of the colon or each subsite of the skin for melanoma is considered its own separate primary site. In addition, the SEER rules consider the laterality of the tumor in the determination of multiple primary cancers and include a timing rule whereby a second cancer of the same histology in the same cancer site would be considered a second primary. While the IARC/IACR rules do make recommendations for incorporating laterality and cancer subsite in determining multiple primary cancers for colon and melanoma of the skin [14], the existing IARC/IACR algorithm does not take this into consideration [16] as many registries outside the USA do not follow these recommendations. The SEER rules thus typically result in the reporting of larger case counts and incidence rates for cancers occurring among paired organs (female breast, kidney, lung and bronchus), organs with large surface areas [skin (i.e., melanoma), urinary bladder, colon, lung and bronchus, oral cavity and pharynx, kidney and renal pelvis], and with the same or related histologic types (non-Hodgkin lymphoma).
Interpreting the effect of multiple primary coding rules on incidence trends is more difficult. Trends in incidence rates, which include all primary cancers regardless of the order in which they are diagnosed, have generally been interpreted as reflecting changes in etiologic risk factors operating at the population level or the impact of screening, rather than the choice of the rules used to enumerate primary cancers. For example, long term declines in incidence rates for cancers of the lung and bronchus and oral cavity and pharynx parallel to that of a long-term reduction in the use of tobacco [31] while the recent increase in oral cancer, primarily among white males, may be attributed to human papillomavirus infections [32]. Improved and/or expanded screening may partially explain the recent declines in colon cancer [33] and the long-term increase in female breast cancer incidence rates seen in the USA [34] and worldwide [35]. Because screening can advance the detection of a pre-invasive or preclinical cancer, screening can also alter the sequence in which two or more primary cancers are diagnosed in a patient or even if a cancer is diagnosed. The introduction of the PSA test in the late 1980s [36] may have led to the over diagnosis of prostate cancer in approximately 29 % of white men and 44 % of black men whose cancers were detected by PSA screening between 1988 and 1998 in the USA [37]. More recently, it has been estimated that as much as 20 % of female breast cancers detected through a population-based screening study in Ontario might never have caused clinical symptoms or death within the woman’s lifetime [38].
To help facilitate the interpretation of the effect of multiple primary coding rules on trends in incidence rates, we included trends for first primary cancers (i.e., first of multiple cancers or the only primary cancer diagnosed in a patient) as these trends tend to reflect the impact of changes in the underlying risk of being diagnosed with cancer. The present study showed that trends in incidence rates, which include all primary cancers, were similar for the most part whether using SEER or IARC/IACR multiple primary coding rules and tended to mirror the incidence of first cancers, thus reflecting the impact of either changes in underlying risk factors, diagnostic intensity or early detection related to screening. The choice of multiple primary coding rules appeared to have little impact on trends in incidence rates for all cancers combined and for the majority of cancers investigated herein with the exception of cancers of the urinary bladder, and kidney and renal pelvis. This is not surprising because the majority of multiple primary cancers tend to arise in separate organ systems and would thus be counted as separate primaries under either set of rules [19]. Cancers of the urinary system, on the other hand, tend to be more multifocal in nature and are therefore at greater susceptibility to differences in the way they are counted.
Both SEER and IARC/IACR rules include specific guidelines for designating cancer that should be considered as arising in the same anatomic site. According to SEER rules prior to 200 [7, 13], cancers of the renal pelvis and kidney should be considered one site, whereas cancers of urinary bladder should be considered a different site. However, according to the IARC/IACR rules, cancers of the renal pelvis and urinary bladder should be considered one site, whereas cancers of kidney should be considered a different site. In 2007, the SEER rules changed to group cancers of the bladder and renal pelvis together as they are both predominately made of transitional epithelial where multifocal tumors tend to occur and because it is thought there is a greater possibility of tumor spread from a single clone to several sites [39]. Further complicating a comparison of cancers of the urinary system is the fact that both sets of rules (SEER and IARC/IACR) incorporate cancer site differences into the multiple primary determination process. Assigning of the cancer sites therefore will depend on the order in which cancers of the urinary system are diagnosed. Consider the following three examples: If a patient had a first primary renal pelvis cancer followed by a urinary bladder cancer, the patient would be considered to have two primary cancers according to SEER rules prior to 2007, but only one cancer (renal pelvis) according to IARC/IACR rules and current SEER rules. If a second patient had a first primary urinary bladder cancer followed by a renal pelvis cancer, the patient would also be considered to have two primary cancers according to previous SEER rule, but one cancer (urinary bladder) according to IARC/IACR rules and current SEER rules. Finally, if a third patient had a renal pelvis cancer and a urinary bladder cancer diagnosed simultaneously, that patient would also have two primary cancers according to previous SEER rules, but only one cancer (urinary system, NOS) to according to IARC/IACR rules and current SEER rules and, therefore, not counted as either a kidney and renal pelvis cancer or a urinary bladder.
Unlike the IARC/IACR rules, SEER multiple primary coding rules have changed over time, including the introduction around 1979 of a time interval used to distinguish synchronous cancers (e.g., cancers diagnosed at the same time or within 2 months) and metachronous cancer (e.g., cancer diagnosed more than 2 months apart) which allowed for cancers of the same histologic type and occurring at the same anatomic site to be considered as two primaries, and the introduction around 2007 of terms (multifocal and multicentric) used to enumerate cancers occurring at the same anatomic site and in close proximity (i.e., urinary bladder and renal pelvis). Because SEER registries collected data according to the multiple primary rules in use at the time of diagnosis, a change in the rules for reporting multiple primary cancers could affect trends in incidence rates, including incidence rates for first cancers. This may help explain the decline in the incidence rate of first primary kidney and renal pelvis cancers seen around 2007.
The differential impact of multiple primary coding rules on incidence rates and trends is likely to grow as the number and percentage of multiple primary cancers continues to increase as shown in the SEER data (Fig. 1). This increase reflects the fact that the risk of being diagnosed with cancer generally increases with age, and over the past several decades, the US population, like many populations worldwide, has grown particularly in the older age groups [40]. In addition, survival following a diagnosis of many cancers has increased in recent years due to earlier detection and improved survival, and these cancer survivors remain at risk (sometimes elevated risk) of developing a subsequent cancer [19]. The prevalence of patients diagnosed with multiple cancers is expected to increase [41].
Cancer registries serve a unique and important role in surveillance research. An examination of patterns of excess risk of subsequent cancers, particularly for cancers that occur in different organ systems, can provide insight into potential causal mechanisms related to shared etiologies or genetic factors [19] or about excess risk related to prior cancer treatment [19, 42]. This type of research requires a large population base because events of interest may be relatively rare. For this reason, it is important that cancer registries adhere to a common protocol for enumerating primary cancers such that their data can be compared, combined, and examined over time. Clinicians may intuitively recognize a new primary cancer from that of a distant metastasis, extension, or recurrent cancer. However, it can be difficult to develop rules for identifying these cancers using routinely collected surveillance data. The identification of multiple primary cancers may reflect the quality and completeness of the surveillance data being reported to the cancer registry by hospitals, physicians’ offices, and laboratories; the training of hospital and registry staff to code the data and interpret the rules; and the follow-up of cancer patients to ascertain subsequent primary cancers. This may be particularly challenging for registries that use SEER rules as these rules are more complex, require more detailed information than IARC/IACR rules, and have changed over time.
Limitations
Cancer patients may move out of a registry’s catchment area before being diagnosed with a subsequent cancer. This may be more problematic for metropolitan area cancer registries and for some states more than others as the mobility and migration patterns of statewide populations in the USA are variable [43]. The two US federal cancer surveillance systems (the National Program of Cancer Registries [NPCR] [4] and SEER) are primarily case-based systems and registries do not report personal identifiers to their respective surveillance programs. Thus, it is difficult to identify inter-registry duplicate case reports or multiple primary cancer cases registered in the same cancer patient, but in different cancer registries [44]. This can result in over reporting of incidence cases and the under reporting of multiple primary cancers.
Acknowledgments
We would like to thank Ms. Jessica King for help with the joinpoint regression analyses of the SEER 9 data. There are no financial disclosures from any of the authors.
Abbreviations
- IACR
International Association of Cancer Registries
- IARC
International Agency for Research on Cancer
- SEER
Surveillance, Epidemiology, and End Results
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Forman D, Ferlay J. World cancer Report 2014. In: Stewart BW, Wild CP, editors; The global and regional burden of cancer. Vol. 2014. International Agency for Research on Cancer; Lyon, France: 2014. [Accessed 2 June 2015]. http://www.iarc.fr/en/publications/pdfs-online/wcr/ [Google Scholar]
- 2.Bray F, Jemal A, Grey N, et al. Global cancer transitions according to the Human Development Index (2008–2030): a population-based study. Lancet Oncol. 2012;13(8):790–801. doi: 10.1016/S1470-2045(12)70211-5. [DOI] [PubMed] [Google Scholar]
- 3.Brewster DH, Coebergh JW, Storm HH. Population-based cancer registries: the invisible key to cancer control. Lancet Oncol. 2005;6(4):193–195. doi: 10.1016/S1470-2045(05)70071-1. [DOI] [PubMed] [Google Scholar]
- 4.USCS: U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999–2011 Incidence and Mortality Web-based Report. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2015. [Accessed 2 June 2015]. 2014. www.cdc.gov/uscs. [Google Scholar]
- 5.Howlader N, Noone AM, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975–2011. National Cancer Institute; Bethesda, MD: 2015. [Accessed 2 June 2015]. http://seer.cancer.gov/csr/1975_2011/ [Google Scholar]
- 6.Copeland G, Lake A, Firth R, et al., editors. Volume One: Combined Cancer Incidence for the United States, Canada and North America. Springfield, IL: North American Association of Central Cancer Registries, Inc; 2014. May, [Accessed 2 June 2015]. Cancer in North America: 2007–2011. http://www.naaccr.org/DataandPublications/CINAPubs.aspx. [Google Scholar]
- 7.Coleman MP, Quaresma M, Berrino F, et al. Cancer survival in five continents: a worldwide population-based study (CONCORD) Lancet Oncol. 2008;9(8):730–756. doi: 10.1016/S1470-2045(08)70179-7. [DOI] [PubMed] [Google Scholar]
- 8.Allemani C, Weir HK, Carreira H, et al. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2) Lancet. 2015;385(9972):977–1010. doi: 10.1016/S0140-6736(14)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coleman MP, Forman D, Bryant H, et al. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995–2007 (the International Cancer Benchmarking Partnership): an analysis of population-based cancer registry data. Lancet. 2011;377(9760):127–138. doi: 10.1016/S0140-6736(10)62231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferlay J, Bray F, Steliarova-Foucher E, et al., editors. Cancer incidence in five continents, CI5plus: IARC CancerBase No. 9 [Internet] Lyon, France: International Agency for Research on Cancer; 2014. [Accessed 2 June 2015]. http://ci5.iarc.fr. [Google Scholar]
- 11.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 12.Hankey BF, Ries LA, Edwards BK. The surveillance, epidemiology, and end results program: a national resource. Cancer Epidemiol Biomarkers Prev. 1999;8(12):1117–1121. [PubMed] [Google Scholar]
- 13. [Accessed 9 Apr 2015];Multiple Primary and Histology Coding Rules. http://seer.cancer.gov/tools/mphrules/
- 14.Working Group R. International rules for multiple primary cancers (ICD-0 third edition) Eur J Cancer Prev. 2005;14(4):307–308. doi: 10.1097/00008469-200508000-00002. [DOI] [PubMed] [Google Scholar]
- 15. [Accessed 16 Apr 2015];A review of the definition for multiple primary cancers in the United States. http://prdupl02.ynet.co.il/forumfiles_2/19207389.pdf.
- 16.Ferlay J, Burkhard C, Whelan S, Parkin DM. IACR Technical Report No. 42. Lyon: 2005. Check and conversion programs for cancer registries (IACR/IACR Tools for Cancer Registries) [Google Scholar]
- 17.Filali K, Hedelin G, Schaffer P, et al. Multiple primary cancers and estimation of the incidence rates and trends. Eur J Cancer. 1996;32A(4):683–690. doi: 10.1016/0959-8049(95)00621-4. [DOI] [PubMed] [Google Scholar]
- 18.Coyte A, Morrison DS, McLoone P. Second primary cancer risk: the impact of applying different definitions of multiple primaries—results from a retrospective population-based cancer registry study. BMC Cancer. 2014;14:272. doi: 10.1186/1471-2407-14-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fraumeni JF, Curtis RE, Edwards BK, Tucker MA. Chapter 1: Introduction. In: Curtis RE, Freedman DM, Ron E, Ries LAG, Hacker DG, Edwards BK, Tucker MA, Fraumeni JF Jr, editors. New Malignancies among Cancer Survivors: SEER Cancer Registries, 1973–2000. National Cancer Institute; Bethesda, MD: 2006. NIH Publ. No. 05-5302. [Google Scholar]
- 20.Hotes JL, Ellison LF, Howe HL, et al. Variation in breast cancer counts using SEER and IARC multiple primary coding rules. Cancer Causes Control. 2004;15(2):185–191. doi: 10.1023/B:CACO.0000019505.97836.7d. [DOI] [PubMed] [Google Scholar]
- 21.Weir HK, Johnson CJ, Thompson TD. The effect of multiple primary rules on population-based cancer survival. Cancer Causes Control. 2013;24(6):1231–1242. doi: 10.1007/s10552-013-0203-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brenner H, Hakulinen T. Patients with previous cancer should not be excluded in international comparative cancer survival studies. Int J Cancer. 2007;121(10):2274–2278. doi: 10.1002/ijc.22932. [DOI] [PubMed] [Google Scholar]
- 23.Thornton ML, editor. Standards for Cancer Registries Volume II: Data Standards and Data Dictionary, Record Layout Version 14. 18. Springfield, Ill: North American Association of Central Cancer Registries; Sep, 2013. [Accessed 2 June 2015]. revised November 2013. http://www.naaccr.org/StandardsandRegistryOperations/VolumeII.aspx. [Google Scholar]
- 24. [Accessed 2 Apr 2015];SEER Site Recode ICD-O-3 (1/27/2003) Definition. http://seer.cancer.gov/siterecode/icdo3_d01272003.
- 25.Hankey BF, Edwards BK, Ries LA, et al. Problems in cancer surveillance: delineating in situ and invasive bladder cancer. J Natl Cancer Inst. 1991;83(6):384–385. doi: 10.1093/jnci/83.6.384. [DOI] [PubMed] [Google Scholar]
- 26.Age-Adjustment Using the 2000 Projected U.S. Population. [Interne] National Center for Health Statistics, Centers for Disease Control and Prevention; 2001. [Accessed 15 May 2014]. http://www.cdc.gov/nchs/data/statnt/statnt20.pdf. [PubMed] [Google Scholar]
- 27. [Accessed 9 Apr 2015];SEER*stat software. http://seer.cancer.gov/seerstat/
- 28.Clegg LX, Feuer EJ, Midthune DN, et al. Impact of reporting delay and reporting error on cancer incidence rates and trends. J Natl Cancer Inst. 2002;94(20):1537–1545. doi: 10.1093/jnci/94.20.1537. [DOI] [PubMed] [Google Scholar]
- 29. [Accessed 9 Apr 2015];Joinpoint trend analysis software, version 4.1.0. http://surveillance.cancer.gov/joinpoint/
- 30.Clegg LX, Hankey BF, Tiwari R, et al. Estimating average annual per cent change in trend analysis. Stat Med. 2009;28(29):3670–3682. doi: 10.1002/sim.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jemal A, Thun MJ, Ries LA, et al. Annual report to the nation on the status of cancer, 1975–2005, featuring trends in lung cancer, tobacco use, and tobacco control. JNCI. 2008;100(23):1672–1694. doi: 10.1093/jnci/djn389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jemal A, Simard EP, Dorell C, et al. Annual Report to the Nation on the Status of Cancer, 1975–2009, featuring the burden and trends in human papillomavirus(HPV)-associated cancers and HPV vaccination coverage levels. JNCI. 2013;105(3):175–201. doi: 10.1093/jnci/djs491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116(3):544–573. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glass AG, Lacey JV, Jr, Carreon JD, et al. Breast cancer incidence, 1980–2006: combined roles of menopausal hormone therapy, screening mammography, and estrogen receptor status. JNCI. 2007;99(15):1152–1161. doi: 10.1093/jnci/djm059. [DOI] [PubMed] [Google Scholar]
- 35.Bray F, McCarron P, Parkin DM. The changing global patterns of female breast cancer incidence and mortality. Breast Cancer Res. 2004;6(6):229–239. doi: 10.1186/bcr932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baron JC, Peyret C, Leroy M, et al. Prostate-specific antigen in prostatic cancer. Am J Clin Oncol. 1988;11(Suppl 2):S75–S76. doi: 10.1097/00000421-198801102-00018. [DOI] [PubMed] [Google Scholar]
- 37.Etzioni R, Penson DF, Legler JM, et al. Overdiagnosis due to prostate-specific antigen screening: lessons from U.S. prostate cancer incidence trends. J Natl Cancer Inst. 2002;94(13):981–990. doi: 10.1093/jnci/94.13.981. [DOI] [PubMed] [Google Scholar]
- 38.Miller AB, Wall C, Baines CJ, et al. Twenty five year follow-up for breast cancer incidence and mortality of the Canadian National Breast Screening Study: randomised screening trial. BMJ. 2014;348:g366. doi: 10.1136/bmj.g366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Habuchi T. Origin of multifocal carcinomas of the bladder and upper urinary tract: molecular analysis and clinical implications. Int J Urol. 2005;12(8):709–716. doi: 10.1111/j.1442-2042.2005.01155.x. [DOI] [PubMed] [Google Scholar]
- 40.Vincent GK, Velkoff VA. Curr Popu Rep P25-1138. US Census Bureau; Washington, DC: 2010. [Accessed 2 June 2015]. The next four decades: the older population in the United States: 2010 to 2050. http://www.census.gov/prod/2010pubs/p25-1138.pdf. [Google Scholar]
- 41.Mariotto AB, Rowland JH, Ries LA, et al. Multiple cancer prevalence: a growing challenge in long-term survivorship. Cancer Epidemiol Biomarkers Prev. 2007;16(3):566–571. doi: 10.1158/1055-9965.EPI-06-0782. [DOI] [PubMed] [Google Scholar]
- 42.Travis LB. The epidemiology of second primary cancers. Cancer Epidemiol Biomarkers Prev. 2006;15(11):2020–2026. doi: 10.1158/1055-9965.EPI-06-0414. [DOI] [PubMed] [Google Scholar]
- 43.Ren Ping. Lifetime Mobility in the United States: 2010, American Community Survey Briefs. US Census Bureau; 2011. [Accessed 18 June 2014]. http://www.census.gov/prod/2011pubs/acsbr10-07.pdf. [Google Scholar]
- 44.Wohler B, Qiao B, Weir HK, et al. Using the National Death Index to identify duplicate cancer incident cases in Florida and New York, 1996–2005. Prev Chronic Dis. 2014;11:E167. doi: 10.5888/pcd11.140200. [DOI] [PMC free article] [PubMed] [Google Scholar]