Abstract
High uric acid (UA) levels have been correlated with a reduced risk of many neurodegenerative diseases through mechanisms involving chelating Fenton reaction transitional metals, antioxidant quenching of superoxide and hydroxyl free radicals, and as an electron donor that increases antioxidant enzyme activity (e.g. SOD). However, the clinical usefulness of UA is limited by its’ low water solubility and propensity to form inflammatory crystals at hyperuricemic levels. This review focuses on the role of UA in neuroprotection, as well as potential strategies aimed at increasing UA levels in the soluble range, and the potential therapeutic use of more water-soluble methyl-UA derivatives from the natural catabolic end-products of dietary caffeine, theophylline, and theobromine.
Keywords: Aging, Antioxidant, Caffeine, Neurodegeneration, Theobromine, Urate Oxidase, Uric acid
Graphical abstract
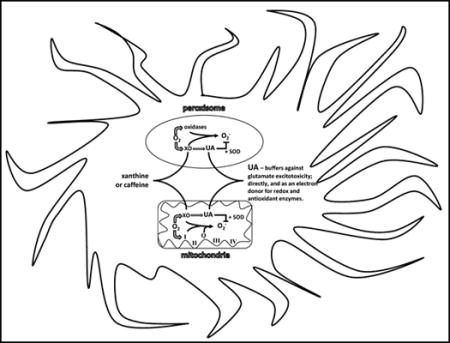
1. INTRODUCTION
Increased blood levels of uric acid (UA) have been correlated with longevity between species, and a reduced risk of many neurodegenerative diseases including Multiple Sclerosis (MS), Amyotropic Lateral Sclerosis (ALS), Parkinson’s (PD), and Alzheimer’s (AD) among humans [1–5]. Most studies on the mechanism for neuroprotection by UA has focused on it’s antioxidant properties, which has been found to be dependent on the presence of astroglia in most models tested [6–7]. However, a vast number of clinical studies have reported a positive association of UA with circulating levels of triglyceride, cholesterol, and glucose, which has been indicated as a possible independent risk factor for developing lipidemia and associated diseases (e.g. hypertension, cardiovascular disease, type 2 diabetes mellitus, metabolic syndrome, and gout) [8–9]. The possitive correlation between circulating UA and triglycerides, cholesterol, and glucose levels is strongest between species [10]. One hypothesis for this proposed correlation is that UA modulates metabolic efficiency by acting as a sensitizer of de novo postprandial lipemic response via inhibition of AMP-activated protein kinase (AMPK), the major negative regulator of SREBP-1c [8, 11]. However, no clinical studies have been published to support these findings [12].
Uric acid is mainly derived from de novo catabolism of ATP into adenosine during exercise, RNA turnover, and DNA catabolism from cell death and mitochondria turnover, with approximately 30% coming from the diet [13–14]. Local and systemic levels of UA acutely spike as a result of released purines caused by tissue damage (e.g. physical injury, exercise), inflammation, chemotherapy and starvation catabolysis [14]. Because UA is produced quickly at the site of metabolic stress and injury indicates a potential for its’ use as a first response factor to buffer damage (i.e. antioxidant and nitric oxide), as an immune homing and repair signal, and at higher levels as an inducer of apoptosis [15]. Previous mouse studies have shown roles for purine-mediated signaling (e.g. adenosine, guanosine) in the repair and apoptosis responses from ischemia or trauma, but little work has been done in animal models with inactive urate oxidase [16].
The urate oxidase (Uox) gene is found in all living organisms, from bacteria to apes. It is involved in purine catabolism, which utilizes oxygen and water to catalyze UA into allantoin and hydrogen peroxide. However, in apes, birds, bats, and some reptiles Uox, which is mainly expressed in liver peroxisomes and mitochondria, has accumulated multiple mutations resulting in decreasing enzymatic activity, and eventual loss of transcription thereby making UA the final end product of purine catabolism [17–18]. Humans have also evolved a relatively high renal reabsorption (63%) of UA compared to other animals, due to differences in levels of URAT1, GLUT9, and OAT1, 3, & 4, suggesting that it may be biologically viewed as a beneficial factor, rather than as a waste product [19]. To protect against over-production and gout, humans have repressed both xanthine oxidase transcription and core promoter activity, resulting in lower production of UA, in addition to having higher albumin levels that increase UA solubility [20].
1.1. Loss of Urate Oxidase in Hominoids
The range of circulating UA in most mammals is relatively low (0.5–2.0 mg/dL). However, humans and higher primates (Great Apes: Orangutan, Chimpanzee, Bonobo, Gorilla; Lesser Apes: Gibbons) have higher levels of UA (0.2–7.0 mg/dL) than most mammals due to the silencing of the Uox gene through a series of mutations that occurred in our ancestors during the Miocene period [21]. Uox expression was also lost via independent mutationsin some species of New World monkeys, while in most Old World monkeys UOX enzyme activity is strongly reduced [22]. The fact that the inactivation of UOX occurred over millions of years rather that in a single step, strongly suggests the need for the co-evolution of adaptive compensatory mechanisms that allowed our ancestors to overcome the negative impact of large increases of circulating UA levels (e.g. acute uratenephropathy). However, since in humans and closely related primates 90% of filtered UA is reabsorbed in the kidney rather than excreted [23], the evolutionary advantages provided by the gradual rise of UA levels must have overcome its potential harmfulness. Several hypotheses have been suggested over the years to explain what benefits UA may have provided to early hominoids. Because UA stimulates the renin-angiotensin system [24], promoting salt retention and increasing blood pressure, it was proposed that UA might have allowed hominoids to stabilize their blood pressure during the transition to an erect posture [24]. UA is one of the most important antioxidants in plasma accounting for more than two-thirds of all its free radical scavenging capacity [25]. The increased antioxidant capacity would have led to decreased age-specific cancer rates and lengthening of life span [25]. In this scenario, the inactivation of Uox became necessary to substitute for the lost ability of higher primates to synthesize ascorbate due to aprior mutation in L-Gulonolactone oxidase [25].
Another hypothesis is that UA may have a role in intelligence. The first observations that gout mainly affected the upper social classes date back to the first century when Gaius Suetonius Tranquillus called gout morbusdivitum. In 1683, Sydenham noted that gout was more frequent amongst the intelligentsia of society [26]. The possibility that UA may have contributed to the intellectual development of humans was proposed by Orowan in 1955 [27]. One possible mechanism by which UA might affect brain function is by stimulating neurons in a manner to structurally related purines such as caffeine and adenosine. In this view, UA would enhance neuronal network activity in the cerebral cortex during the evolution of higher primates, thereby contributing to their superior intellectual prowess. Although it is highly unlikely that the expansion of the cerebral cortex in hominoids is the direct result of the higher levels of UA, there is evidence that UA influences central nervous system functions. Several studies have found significant correlations between UA levels and higher intelligence in children and young adults [28–29], school performance [30], learning ability [31], achievement-driven behavior [32] and leadership [33]. In addition, UA levels correlate with increased exploratory behavior in mice and impulsivity in humans [34], as well as hyperactivity in children [35].
1.2. Uric Acid and Neurodegenerative Disorders
Hyperuricemia (>7mg/dL) may cause gout and acute and chronic nephropathy due to deposits of urate crystals. Furthermore, hyperuricemia is associated with hypertension, cardiovascular disease and metabolic syndrome [36–38]. While elevated levels of UA appear to negatively impact peripheral organs, the opposite may be true for the central nervous system. Low levels of UA have been linked to a variety of neurological disorders including MS, PD, ALS, and many forms of dementia including AD [1–5].
Compelling evidence has demonstrated a negative correlation between levels of UA and MS. An evaluation of the medical records of more than 20 million patients included in Medicare and Medicaid database revealed a lack of MS patients with gout [39] suggesting that UA may protect against MS [39]. Additional epidemiological studies confirmed this initial observation, and revealed that UA levels fluctuate during active (lower) and remission (higher) phases of MS [2, 40]. Administration of UA either before or after the symptoms of experimental allergic encephalomyelitis promoted long-term survival of the mice [39] suggesting a potential therapeutic benefit of UA-elevating treatments in MS. Recent pilot clinical trials showed that MS patients treated with inosine, a precursor of UA, had a decreased relapse rate, reduced number of gadnolinium-enhanced lesions and better neurological scores than untreated MS patients [41, 42]. ALS is a progressive fatal neurodegenerative disorder affecting lower and upper motor neurons. The evidence that UA may influence the progression of ALS came from recent epidemiological studies which first showed decreased levels of UA in ALS patients compared to matched controls [4], and subsequently linked higher UA baseline levels with slower progression and prolonged survival [43–45]. Similarly to ALS, the functional decline in Huntington’s disease is negatively correlated with baseline UA levels [46]. Postmortem and epidemiological studies found lower UA levels in serum and substantia nigra of PD patients compared to age-matched controls [1, 47]. Increased levels of UA are associated with a lower risk of PD [47] and predict the clinical outcome of PD. Higher blood and/or cerebrospinal fluid UA levels are associated with a slower progression [48] and less cognitive impairment in PD patients [49]. Furthermore, it was also observed that diets with a high purine content are associated with a lower risk of PD and slower progression of the symptoms [50], while mutations in the transporter SLC2A9 that decrease UA levels predict earlier age at onset and rate of PD progression [51–52]. Preliminary trials with inosine supplementation in PD patients revealed an inverse relationship between long-term increases in serum UA concentrations and rate of clinical progression in women but not in men [53]. Patients with AD and dementia have significantly reduced levels of UA and other antioxidants [3, 54, 55]. A recent population-based study showed that gout is inversely correlated with AD [56], and high UA associates with a slower progression and lower cognitive impairment [57–58].
The role-played by UA in prevention and post neurological damage from stroke has been more controversial. Several studies have shown that exogenous administration of UA or UA analogs is beneficial in animal stroke models [59–61]. However, in humans both positive and negative associations have been observed. Supportive of a beneficial role for increased UA in acute ischemic stroke are the results of a recent small clinical trial where in addition to thrombolytic therapy patients received UA or placebo. Although the overall difference between the UA and placebo groups in the primary end-point did not reach statistical significance (p=0.09), likely because of the low power of the study, a clear increase of an excellent clinical outcome at 90 days was observed in women (13%) but not in men [62]. The gender differences highlighted by both PD and acute stroke trials are likely a reflection of the lower basal levels of UA in women versus men. They also warrant consideration of sex differences in the design, as well as the analysis and interpretation of future trials of UA-elevating interventions in neurological disorders.
1.3. Relevance for New Drug Targets in the Prevention of Age-Related Neurodegenerative Diseases
The protective properties of UA against age-related diseases has been reported to utilize several different mechanisms, including acting as a primary antioxidant by chelating Fenton reaction transitional metals secondary antioxidant in quenching superoxide and hydroxyl free radicals, and; electron donor that increases antioxidant enzyme activity (e.g. SOD) [19]. Although UA levels in brain tissue per weight has been reported to be a magnitude lower than blood levels, recent data indicates high levels are present in areas of high metabolic stress such as synaptic terminals [63]. Many reports from epidemiological and lab model data has indicated a neuroprotective function for UA [19], however, mechanisms to increase UA in the brain and its’ beneficial limits requires further exploration [53]. One of the most exciting areas of research has been in the neuroprotective properties of dietary methylxanthines (i.e. caffeine, theobromine, theophylline) that crosses the blood brain barrier and is then metabolized into very water-soluble methyl UA derivatives [60, 64–65]. Future research is needed to test the transitional metal chelating, antioxidant quenching, and electron donor to antioxidant enzyme, properties of these water-soluble methyl UA derivatives (e.g. 1,7-dimethyl-UA) vs. UA.
2. NEUROPROTECTIVE MECHANISMS OF URIC ACID
Given its high metabolic rate (20% of the daily oxygen intake), and elevated content of unsaturated fatty acids, the brain is particularly susceptible to oxidative damage. The dysfunction of antioxidant defense mechanisms in quenching inflammation has been reported to be involved in most age-related neurodegenerative disorders [66]. Cytoplasm and mitochondrial superoxide dismutase (SOD1 and 2) have been shown to be abundant cellular components in cell bodies, dendrites and axons in motor neurons, astroglia, and central nervous system neurons, including pyramidal cells in the cerebral cortex and sectors CA3–4 of the hippocampus [67]. Whereas, extracellular superoxide dismutase (SOD3) has been shown to be secreted and bound to heparin sulfate on the cell surface in the hilar region of the hippocampus, suprachiasmatic nuclei of the hypothalamus, and the lateral habenular nucleus of the thalamus, as well as neurons throughout the cortex and striatum [68]. Although SOD degrades the superoxide radical at a rate that is 4 to 5 times faster than antioxidants such as vitamin C or E, it can be deactivated by physiological levels of hydrogen peroxide during oxidative stress. Hink, and colleagues show that UA can not only protect SOD from inhibition by hydrogen peroxide, but at physiological levels acts to increase enzymatic activity 2 to 3 fold, likely as an electron donor [69]. The distribution of cells and regions with a high abundance of SOD3 matches up with those containing the neuronal isoform of nitric oxide synthase, indicating a mechanism for preservation and buffering of nitric oxide bioactivity [69]. Peaks of nitric oxide can cause degeneration of neurons via inactivation of protein disulfide isomerase and S-nitrosylation mediated neuropeptide misfolding [70, 71]. An increase in levels of carbon dioxide during metabolic stress (e.g. exercise or ischemia) catalytically enhances peroxynitrite anion nitration of proteins (e.g. tyrosine), for which UA has an unusually high specific activity to block this reaction [72]. Buffering nitric oxide levels has been shown to reduce ischemic reperfusion damage in mouse models [73]. Uric acid reacts with superoxide, nitric oxide, and peroxynitrite to produce allantoin, 6-aminouracil, and triuret respectively [74, 75]. Removing UOX also has the benefit of sparing the utilization of 1-oxygen and 1-water that could then be used for respiration, and in sparing the production of 1-hydrogen peroxide and 1-carbon dioxide that would have otherwise acted to decrease energy efficiency [25,67]. In addition, by limiting the evolution of superoxide prevents its’ reaction with nitric oxide and thus the formation of peroxynitrite. Peroxynitrite has been shown to be a strong activator of AMP-activated protein Kinase (AMPK), which is a master regulator of cellular energy homeostasis by turning off anabolic pathways and turning on catabolic energy producing pathways [76]. During metabolic stress, lower blood glucose and increased peroxynitrite can activate AMPK thereby blocking liver anabolism of lactate, amino acids, and glycerol into glucose (i.e. gluconeogenesis) [77]. The use of UA in buffering AMPK mediated shut down of gluconeogenesis (e.g. liver and kidneys) during times of acute metabolic stress as a means to maintain blood and brain glucose levels is hypothesized by the authors as possibly being a major driver for the loss of UOX in hominoids.
Because of its’ intrinsic antioxidant properties, the neuroprotective effects elicited by UA have mostly been considered the result of a direct decrease in ROS-dependent damage. However, recent studies suggest that UA may have additional modalities of action. For example, it was shown that UA modulates glutathione (GSH) synthesis; Aoyama and colleagues [78] demonstrated that administration of UA or caffeine could promote cysteine uptake and GSH synthesis in hippocampal neurons. Similarly, in dopaminergic cell line protection against oxidative damage by UA have been shown to require UA-dependent activation of Nrf2, which, in turn, increased the expression of heme-oxygenase-1 and of the rate-limiting enzyme for the synthesis of the GSH (i.e. gamma-glutamyl-cysteine ligase) [79]. In addition to the ability to regulate GSH synthesis, it was also recently shown that UA treatment induces the up-regulation of the glial glutamate transporter EAAT-1, and that blockage of EAATs nullify UA neuroprotection against glutamate toxicity [63]. This effect on the glial glutamate transporter is particularly interesting given the great number of neurodegenerative disorders involving glutamate excitotoxicity [66].
2.1. Strategies to Increase Uric Acid Levels
The association of UA with risk of cardiovascular disease indicates a J shaped curve, with the lowest risk for males at 3.5 mg/dl and females 5.0 mg/dl [80]. However, the clinical usefulness of UA for the treatment of neurodegenerative disorders is limited by the fact that it is not orally bioavailable is poorly soluble in water at physiological pH levels, and readily crystallizes under saturating conditions. The fact that UA is the end-product of purine catabolism could be used as a potential strategy to overcome its reduced bioavailability. Dietary supplementation with UA precursors can boost its endogenous levels. To date the best-characterized supplement to increase UA levels within the normal range is inosine. Inosine is used to treat a variety of autoimmune diseases [81], and following the discoveries linking UA levels with neurodegenerative disorders is now being investigated in clinical trials for PD [82] and ALS (https://clinicaltrials.gov/ct2/show/NCT02288091). An alternative to inosine could be using ATP supplements. ATP supplements have been developed and marketed to enhance physical performance in athletes. While the supplements are safe when tested in scientific settings, they have failed to change circulating levels of ATP but have consistently lead to increased UA levels [83, 84].
One approach that has been used to circumvent the solubility problems is the development of analogs that maintain high antioxidant activity together with increased solubility. For example, 1,7-dimethyl UA and 6,8-dithio UA have been shown to ameliorate focal ischemic brain injury in mice [60]. Interestingly, 1,7-dimethyl UA is actually the major and natural metabolite of caffeine, which can cross the blood brain barrier. There have been numerous reports of the age-related neuroprotection and cognitive benefits of dietary consumption of methylxanthines (i.e. caffeine, theophylline, and theobromine) contained in coffee, tea, and cocoa [83–84].
CONCLUSION
As the result of mutations of the Uox gene that occurred during late primate evolution, circulating UA levels are much greater in apes and humans than lower mammalian species. The adaptive value of elevated UA levels is poorly understood, but may involve increased antioxidant capacity to support endurance, and neurostimulatory effects in the brain. The results of epidemiological studies suggest that individuals with low UA levels are more vulnerable to neuronal dysfunction and degeneration in age-related brain disorders including PD, AD and stroke. Experiments using animal models, and preliminary clinical trials in humans, suggest that interventions that increase UA levels can protect neurons against degeneration and improve the clinical outcome in PD and stroke. Randomized controlled clinical trials of UA-elevating agents (e.g., inosine), more soluble UA analogs, and natural metabolites of UA (e.g. 1,7-dimethyl UA) will be required to determine efficacy of UA-based approaches to the prevention and treatment of neurodegenerative disorders. Future studies aimed at understanding how UA and its metabolites affect cellular signaling pathways involved in energy metabolism and neuroplasticity will likely reveal previously unappreciated biological activities of UA.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute on Aging.
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
References
- 1.Church WH, Ward VL. Uric acid is reduced in the substantia nigra in Parkinson’s disease: effect of dopamine oxidation. Brain Res Bull. 1994;33:419–425. doi: 10.1016/0361-9230(94)90285-2. [DOI] [PubMed] [Google Scholar]
- 2.Toncev G, Millicic B, Toncev S, Samardzic G. Serum uric acid in multiple sclerosis patients correlate with activity of disease and blood-brain barrier dysfunction. Eur J Neuro. 2002;9:221–226. doi: 10.1046/j.1468-1331.2002.00384.x. [DOI] [PubMed] [Google Scholar]
- 3.Kim TS, Pae CU, Yoon SJ, Jang WY, Lee NJ, Kim JJ, Lee SJ, Lee C, Paik IH, Lee CU. Decreased plasma antioxidants in patients with Alzheimer’s disease. Int J Geriatr Psychiatry. 2006;21:344–348. doi: 10.1002/gps.1469. [DOI] [PubMed] [Google Scholar]
- 4.Keizman D, Ish-Shalom M, Berliner S, Maimon N, Vered Y, Artamonov I, Tsehori J, Nefussy B, Drory VE. Low uric acid levels in serum of patients with ALS: further evidence for oxidative stress? J Neurol Sci. 2009;285:95–99. doi: 10.1016/j.jns.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Euser SM, Hofman A, Westendorp RG, Breteler MM. Serum uric acid and cognitive function and dementia. Brain. 2009;132:377–382. doi: 10.1093/brain/awn316. [DOI] [PubMed] [Google Scholar]
- 6.Du Y, Chen CP, Tseng CY, Eisenberg Y, Firestein BL. Astroglia-mediated effects of uric acid to protect spinal cord neurons from glutamate toxicity. Glia. 2007;55(5):463–472. doi: 10.1002/glia.20472. [DOI] [PubMed] [Google Scholar]
- 7.Cipriani S, Desjardins CA, Burdett TC, Xu Y, Xu K, Schwarzschild MA. Protection of dopaminergic cells by urate requires its accumulation in astrocytes. J Neurochem. 2012;123(1):172–181. doi: 10.1111/j.1471-4159.2012.07820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakagawa T, Hu H, Zharikov S, Tuttle KR, Short RA, Glushakova O, Ouyang X, Feig DI, Block ER, Herrera-Acosta J, Patel JM, Johnson RJ. A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Renal Physiol. 2006;290(3):F625–631. doi: 10.1152/ajprenal.00140.2005. [DOI] [PubMed] [Google Scholar]
- 9.Soltani Z, Rasheed K, Kapusta DR, Reisin E. Potential role of uric acid in metabolic syndrome, hypertension, kidney injury, and cardiovascular diseases: is it time for reappraisal? Curr Hypertens Rep. 2013;15(3):175–181. doi: 10.1007/s11906-013-0344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao G, Huang L, Song M, Song Y. Baseline serum uric acid level as a predictor of cardiovascular disease related mortality and all-cause mortality: a meta-analysis of prospective studies. Atherosclerosis. 2013;231(1):61–68. doi: 10.1016/j.atherosclerosis.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 11.Lin J, Yang R, Tarr PT, Wu PH, Handschin C, Li S, Yang W, Pei L, Uldry M, Tontonoz P, Newgard CB, Spiegelman BM. Hyperlipidemic effects of dietary saturated fats mediated through PGC-1beta coactivation of SREBP. Cell. 2005;120(2):261–273. doi: 10.1016/j.cell.2004.11.043. [DOI] [PubMed] [Google Scholar]
- 12.Lanaspa MA, Cicerchi C, Garcia G, Li N, Roncal-Jimenez CA, Rivard CJ, Hunter B, Andrés-Hernando A, Ishimoto T, Sánchez-Lozada LG, Thomas J, Hodges RS, Mant CT, Johnson RJ. Counteracting roles of AMP deaminase and AMP kinase in the development of fatty liver. PLoS One. 2012;7(11):e48801. doi: 10.1371/journal.pone.0048801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nieto FJ, Iribarren C, Gross MD, Comstock GW, Cutler RG. Uric acid and serum antioxidant capacity: a reaction to atherosclerosis? Atherosclerosis. 2000;148(1):131–139. doi: 10.1016/s0021-9150(99)00214-2. [DOI] [PubMed] [Google Scholar]
- 14.Rock KL, Kataoka H, Lai JJ. Uric acid as a danger signal in gout and its comorbidities. Nat Rev Rheumatol. 2013;9(1):13–23. doi: 10.1038/nrrheum.2012.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pardo M, Budick-Harmelin N, Tirosh B, Tirosh O. Antioxidant defense in hepatic ischemia-reperfusion injury is regulated by damage-associated molecular pattern signal molecules. Free Radic Biol Med. 2008;45(8):1073–1083. doi: 10.1016/j.freeradbiomed.2008.06.029. [DOI] [PubMed] [Google Scholar]
- 16.Parkinson FE, Sinclair CJ, Othman T, Haughey NJ, Geiger JD. Differences between rat primary cortical neurons and astrocytes in purine release evoked by ischemic conditions. Neuropharmacology. 2002;43(5):836–846. doi: 10.1016/s0028-3908(02)00083-7. [DOI] [PubMed] [Google Scholar]
- 17.Hayashi S, Fujiwara S, Noguchi T. Evolution of urate-degrading enzymes in animal peroxisomes. Cell Biochem Biophys. 2000;32:123–129. doi: 10.1385/cbb:32:1-3:123. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura Y, Ogura M, Ogura K, Tanaka D, Inagaki N. SIRT5 deacetylates and activates urate oxidase in liver mitochondria of mice. FEBS Lett. 2012;586:4076–4081. doi: 10.1016/j.febslet.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 19.Alvarez-Lario B, Macarrón-Vicente J. Is there anything good in uric acid? QJM. 2011;104(12):1015–1024. doi: 10.1093/qjmed/hcr159. [DOI] [PubMed] [Google Scholar]
- 20.Xu P, LaVallee PA, Lin JJ, Hoidal JR. Characterization of proteins binding to E-box/Ku86 sites and function of Ku86 in transcriptional regulation of the human xanthine oxidoreductase gene. J Biol Chem. 2004;279(16):16057–16063. doi: 10.1074/jbc.M305856200. [DOI] [PubMed] [Google Scholar]
- 21.Oda M, Satta Y, Takenaka O, Takahata N. Loss of urate oxidase activity in hominoids and its evolutionary implications. Mol Biol Evol. 2002;19:640–653. doi: 10.1093/oxfordjournals.molbev.a004123. [DOI] [PubMed] [Google Scholar]
- 22.Wu X, Muzny DM, Lee CC, Caskey CT. Two independent mutational events in the loss of urate oxidase during hominoid evolution. J Mol Evol. 1992;34:78–84. doi: 10.1007/BF00163854. [DOI] [PubMed] [Google Scholar]
- 23.Bobulescu IA, Moe OW. Renal transport of uric acid: evolving concepts and uncertainties. Adv Chronic Kidney Dis. 2012;19:358–371. doi: 10.1053/j.ackd.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watanabe S, Kang DH, Feng L, Nakagawa T, Kanellis J, Lan H, Mazzali M, Johnson RJ. Uric acid, hominoid evolution and the pathogenesis of salt-sensitivity. Hypertension. 2002;40:355–360. doi: 10.1161/01.hyp.0000028589.66335.aa. [DOI] [PubMed] [Google Scholar]
- 25.Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in human against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci USA. 1981;78:6858–6862. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sydenham T. Health and Disease. G Kettilby; London, England: 1683. Tractatus de Podagra et Hydrope. Early History of Medicine. [Google Scholar]
- 27.Orowan E. The origin of Man. Nature. 1955;175:683–684. doi: 10.1038/175683a0. [DOI] [PubMed] [Google Scholar]
- 28.Stetten D, Jr, Hearon JZ. Intellectual level measured by army classification battery and serum uric acid concentration. Science. 1959;129:1737. doi: 10.1126/science.129.3365.1737. [DOI] [PubMed] [Google Scholar]
- 29.Inouye E, Park KS, Asaka A. Blood uric acid level and IQ: a study in twin families. Acta Genet Med Gemellol. 1984;33:237–242. doi: 10.1017/s0001566000007273. [DOI] [PubMed] [Google Scholar]
- 30.Bloch S, Brackenridge CJ. Psychological, performance and biochemical factors in medical students under examination stress. J Psychosom Res. 1972;16:25–33. doi: 10.1016/0022-3999(72)90020-7. [DOI] [PubMed] [Google Scholar]
- 31.Stevens HA, Cropley AJ, Blatter DP. Intellect and serum uric acid: an optimal concentration of serum urate for human learning? Soc Biol. 1975;22:229–234. doi: 10.1080/19485565.1975.9988171. [DOI] [PubMed] [Google Scholar]
- 32.Kasl SV, Brooks GW, Rodgers WL. Serum uric acid and cholesterol in achievement behavior and motivation. I. The relationship to ability, grades, test performance and motivation. JAMA. 1970;213:1158–1164. [PubMed] [Google Scholar]
- 33.Brooks GW, Mueller E. Serum urate concentrations among university professors relation to drive, achievement, and leadership. JAMA. 1966;195:415–418. [PubMed] [Google Scholar]
- 34.Sutin AR, Cutler RG, Camandola S, Uda M, Feldman NH, Cucca F, Zonderman AB, Mattson MP, Ferrucci L, Schlessinger D, Terracciano A. Impulsivity is associated with uric acid: evidence from humans and mice. Biol Psychiatry. 2014;75:31–37. doi: 10.1016/j.biopsych.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barrera CM, Ruiz ZR, Dunlap WP. Uric acid: a participating factor in the symptoms of hyperactivity. Biol Psychiatry. 1988;24:344–347. doi: 10.1016/0006-3223(88)90205-3. [DOI] [PubMed] [Google Scholar]
- 36.Jossa F, Farinaro E, Panico S, Krogh V, Celentano E, Galasso R, Mancini M, Trevisan M. Serum uric acid and hypertension: the Olivetti heart study. J Hum Hypertens. 1994;8:677–681. [PubMed] [Google Scholar]
- 37.Freedman DS, Williamson DF, Gunter EW, Byers T. Relation of uric acid to mortality and ischemic heart disease. The NHANES I epidemiologic follow-up study. Am J Epidemiol. 1995;141:637–644. doi: 10.1093/oxfordjournals.aje.a117479. [DOI] [PubMed] [Google Scholar]
- 38.Heinig M, Johnson RJ. Role of uric acid in hypertension, renal disease and metabolic syndrome. Cleve Clin J Med. 2006;73:1059–1064. doi: 10.3949/ccjm.73.12.1059. [DOI] [PubMed] [Google Scholar]
- 39.Hooper DC, Spitsin S, Kean RB, Champion JM, Dickson GM, Chaudhry I, Koprowski H. Uric acid, a natural scavenger of peroxynitrite, in experimental allergic encephalomyelitis and multiple sclerosis. Proc Natl Acad Sci U S A. 1998;95:675–680. doi: 10.1073/pnas.95.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drulović J, Dujmović I, Stojsavljević N, Mesaros S, Andjelković S, Miljković D, Perić V, Dragutinović G, Marinković J, Lević Z, Mostarica Stojković M. Uric acid levels in sera from patients with multiple sclerosis. J Neurol. 2001;248:121–126. doi: 10.1007/s004150170246. [DOI] [PubMed] [Google Scholar]
- 41.Toncev G. Therapeutic value of serum uric acid levels increasing in the treatment of multiple sclerosis. Vojnosanit Pregl. 2006;63:879–882. doi: 10.2298/vsp0610879t. [DOI] [PubMed] [Google Scholar]
- 42.Markowitz CE, Spitsin S, Zimmerman V, Jacobs D, Udupa JK, Hooper DC, Koprowski H. The treatment of multiple sclerosis with inosine. J Altern Complement Med. 2009;15:619–625. doi: 10.1089/acm.2008.0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paganoni S, Zhang M, Quiroz Zárate A, Jaffa M, Yu H, Cudkowicz ME, Wills AM. Uric acid levels predict survival in men with amyotrophic lateral sclerosis. J Neurol. 2012;259:1923–1928. doi: 10.1007/s00415-012-6440-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kataoka H, Kiriyama T, Kobayashi Y, Horikawa H, Ueno S. Clinical outcomes and serum uric acid levels in elderly patients with amyotrophic lateral sclerosis aged ≥ 70 years. Am J Neurodegener Dis. 2013;2:140–144. [PMC free article] [PubMed] [Google Scholar]
- 45.Atassi N, Berry J, Shui A, Zach N, Sherman A, Sinani E, Walker J, Katsovskiy I, Schoenfeld D, Cudkowicz M, Leitner M. The PRO-ACT database: design, initial analyses, and predictive features. Neurology. 2014;83:1719–1725. doi: 10.1212/WNL.0000000000000951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Auinger P, Kieburtz K, McDermott MP. The relationship between uric acid levels and Huntington’s disease progression. Mov Disord. 2010;25:224–228. doi: 10.1002/mds.22907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Lau LM, Koudstaal PJ, Hofman A, Breteler MM. Serum uric acid levels and the risk of Parkinson disease. Ann, Neurol. 2005;58:797–800. doi: 10.1002/ana.20663. [DOI] [PubMed] [Google Scholar]
- 48.Ascherio A, LeWitt PA, Xu K, Eberly S, Watts A, Matson WR, Marras C, Kieburtz K, Rudolph A, Bogdanov MB, Schwid SR, Tennis M, Tanner CM, Beal MF, Lang AE, Oakes D, Fahn S, Shoulson I, Schwarzschild MA, Parkinson Study Group DATATOP Investigators Urate as a predictor of the rate of clinical decline in Parkinson disease. Arch, Neurol. 2009;66:1460–1468. doi: 10.1001/archneurol.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Annanmaki T, Pessala-Driver A, Hokkanen L, Murros K. Uric acid associates with cognition in Parkinson’s disease. Parkinsonism Relat Disord. 2008;14:576–578. doi: 10.1016/j.parkreldis.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 50.Gao X, Chen H, Choi HK, Curhan G, Schwarzschild MA, Ascherio A. Diet, urate, and Parkinson’s disease risk in men. Am J Epidemiol. 2008;167:831–838. doi: 10.1093/aje/kwm385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Facheris MF, Hicks AA, Minelli C, Hagenah JM, Kostic V, Campbell S, Hayward C, Volpato CB, Pattaro C, Vitart V, Wright A, Campbell H, Klein C, Pramstaller PP. Variation in the uric acid transporter gene SLC2A9 and its association with AAO of Parkinson’s disease. J Mol Neurosci. 2011;43:246–250. doi: 10.1007/s12031-010-9409-y. [DOI] [PubMed] [Google Scholar]
- 52.Simon KC, Eberly S, Gao X, Oakes D, Tanner CM, Shoulson I, Fahn S, Schwarzschild MA, Ascherio A, Parkinson Study Group Mendelian randomization of serum urate and parkinson disease progression. Ann Neurol. 2014;76:862–868. doi: 10.1002/ana.24281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schwarzschild MA, Macklin EA, Ascherio A, Parkinson Study Group SURE-PD Investigators Urate and neuroprotection trials. Lancet Neurol. 2014;13:758. doi: 10.1016/S1474-4422(14)70138-3. [DOI] [PubMed] [Google Scholar]
- 54.Maesaka JK, Wolf-Klein G, Piccione JM, Ma CM. Hypouricemia, abnormal renal tubular urate transport, and plasma natriuretic factor(s) in patients with Alzheimer’s disease. J Am Geriatr Soc. 1993;41:501–506. doi: 10.1111/j.1532-5415.1993.tb01885.x. [DOI] [PubMed] [Google Scholar]
- 55.Rinaldi P, Polidori MC, Metastasio A, Mariani E, Mattioli P, Cherubini A, Catani M, Cecchetti R, Senin U, Mecocci P. Plasma antioxidants are similarly depleted in mild cognitive impairment and in Alzheimer’s disease. Neurobiol Aging. 2003;24:915–919. doi: 10.1016/s0197-4580(03)00031-9. [DOI] [PubMed] [Google Scholar]
- 56.Lu N, Dubreuil M, Zhang Y, Neogi T, Rai SK, Ascherio A, Hernán MA, Choi HK. Gout and the risk of Alzheimer’s disease: a population-based, BMI-matched cohort study. Ann Rheum Dis. 2015 Mar 4; doi: 10.1136/annrheumdis-2014-206917. pii: annrheumdis-2014-206917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Euser SM, Hofman A, Westendorp RG, Breteler MM. Serum uric acid and cognitive function and dementia. Brain. 2009;132:377–382. doi: 10.1093/brain/awn316. [DOI] [PubMed] [Google Scholar]
- 58.Irizarry MC, Raman R, Schwarzschild MA, Becerra LM, Thomas RG, Peterson RC, Ascherio A, Aisen PS. Plasma urate and progression of mild cognitive impairment. Neurodegener Dis. 2009;6:23–28. doi: 10.1159/000170883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu ZF, Bruce-Keller AJ, Goodman Y, Mattson MP. Uric acid protects neurons against excitotoxic and metabolic insults in cell culture, and against focal ischemic brain injury in vivo. J Neurosci Res. 1998;53:613–625. doi: 10.1002/(SICI)1097-4547(19980901)53:5<613::AID-JNR11>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 60.Haberman F, Tang SC, Arumugam TV, Hyun DH, Yu QS, Cutler RG, Guo Z, Holloway HW, Greig NH, Mattson MP. Soluble neuroprotective antioxidant uric acid analogs ameliorate ischemic brain injury in mice. Neuromolecular Med. 2007;9:315–323. doi: 10.1007/s12017-007-8010-1. [DOI] [PubMed] [Google Scholar]
- 61.Romanos E, Planas AM, Amaro S, Chamorro A. Uric acid reduces brain damage and improves the benefits of rt-PA in a rat model of thromboembolic stroke. J Cereb Blood Flow Metab. 2007;27:14–20. doi: 10.1038/sj.jcbfm.9600312. [DOI] [PubMed] [Google Scholar]
- 62.Chamorro A, Amaro S, Castellanos M, Segura T, Arenillas J, Martí-Fábregas J, Gállego J, Krupinski J, Gomis M, Cánovas D, Carné X, Deulofeu R, Román LS, Oleaga L, Torres F, Planas AM, URICO-ICTUS Investigators Safety and efficacy of uric acid in patients with acute stroke (URICO-ICTUS): a randomized, double-blind phase 2b/3 trial. Lancet Neurol. 2014;13:453–460. doi: 10.1016/S1474-4422(14)70054-7. [DOI] [PubMed] [Google Scholar]
- 63.Du Y, Chen CP, Tseng CY, Eisenberg Y, Firestein BL. Astroglia-mediated effects of uric acid to protect spinal cord neurons from glutamate toxicity. Glia. 2007;55:463–472. doi: 10.1002/glia.20472. [DOI] [PubMed] [Google Scholar]
- 64.Beaudoin MS, Graham TE. Methylxanthines and human health: epidemiological and experimental evidence. Hand Exp Pharmacol. 2011;200:509–548. doi: 10.1007/978-3-642-13443-2_21. [DOI] [PubMed] [Google Scholar]
- 65.Franco R, Oñatibia-Astibia A, Martínez-Pinilla E. Health benefits of methylxanthines in cacao and chocolate. Nutrients. 2013;5:4159–4173. doi: 10.3390/nu5104159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mattson MP. Excitotoxic and excitoprotective mechanisms: abundant targets for the prevention and treatment of neurodegenerative disorders. Neuromolecular Med. 2003;3:65–94. doi: 10.1385/NMM:3:2:65. [DOI] [PubMed] [Google Scholar]
- 67.Pardo CA, Xu Z, Borchelt DR, Price DL, Sisodia SS, Cleveland DW. Superoxide dismutase is an abundant component in cell bodies, dendrites, and axons of motor neurons and in a subset of other neurons. Proc Natl Acad Sci U S A. 1995;92(4):954–958. doi: 10.1073/pnas.92.4.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oury TD, Card JP, Klann E. Localization of extracellular superoxide dismutasein adult mouse brain. Brain Res. 1999;850(1–2):96–103. doi: 10.1016/s0006-8993(99)02103-4. [DOI] [PubMed] [Google Scholar]
- 69.Hink HU, Santanam N, Dikalov S, McCann L, Nguyen AD, Parthasarathy S, Harrison DG, Fukai T. Peroxidase properties of extracellular superoxide dismutase: role of uric acid in modulating in vivo activity. Arterioscler Thromb Vasc Biol. 2002;22(9):1402–1408. doi: 10.1161/01.atv.0000027524.86752.02. [DOI] [PubMed] [Google Scholar]
- 70.Davies KJ, Sevanian A, Muakkassah-Kelly SF, Hochstein P. Uric acid-iron ion complexes. A new aspect of the antioxidant functions of uric acid. Biochem J. 1986;235:747–754. doi: 10.1042/bj2350747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Obukuro K, Nobunaga M, Takigawa M, Morioka H, Hisatsune A, Isohama Y, Shimokawa H, Tsutsui M, Katsuki H. Nitric oxide mediates selective degeneration of hypothalamic orexin neurons through dysfunction of protein disulfideisomerase. J Neurosci. 2013;33(31):12557–12568. doi: 10.1523/JNEUROSCI.0595-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Squadrito GL, Cueto R, Splenser AE, Valavanidis A, Zhang H, Uppu RM, Pryor WA. Reaction of uric acid with peroxynitrite and implications for the mechanism of neuroprotection by uric acid. Arch Biochem Biophys. 2000;376(2):333–337. doi: 10.1006/abbi.2000.1721. [DOI] [PubMed] [Google Scholar]
- 73.Liu DH, Yuan FG, Hu SQ, Diao F, Wu YP, Zong YY, Song T, Li C, Zhang GY. Endogenous nitric oxide induces activation of apoptosis signal-regulating kinase 1 via S-nitrosylation in rat hippocampus during cerebral ischemia-reperfusion. Neuroscience. 2013;229:36–48. doi: 10.1016/j.neuroscience.2012.10.055. [DOI] [PubMed] [Google Scholar]
- 74.Kim KM, Henderson GN, Frye RF, Galloway CD, Brown NJ, Segal MS, Imaram W, Angerhofer A, Johnson RJ. Simultaneous determination of uric acid metabolites allantoin, 6-aminouracil, and triuret in human urine using liquid chromatography-mass spectrometry. Journal of chromatography B, Analytical technologies in the biomedical and life sciences. 2009;877(1–2):65–70. doi: 10.1016/j.jchromb.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Robinson KM, Morre JT, Beckman JS. Triuret: a novel product of peroxynitrite-mediated oxidation of urate. Archives of biochemistry and biophysics. 2004;423(1):213–217. doi: 10.1016/j.abb.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 76.Ruderman NB, Park H, Kaushik VK, Dean D, Constant S, Prentki M, Saha AK. AMPK as a metabolic switch in rat muscle, liver and adipose tissue after exercise. Acta physiologica Scandinavica. 2003;178(4):435–442. doi: 10.1046/j.1365-201X.2003.01164.x. [DOI] [PubMed] [Google Scholar]
- 77.Lanaspa MA, Sanchez-Lozada LG, Cicerchi C, Li N, Roncal-Jimenez CA, Ishimoto T, Le M, Garcia GE, Thomas JB, Rivard CJ, Andres-Hernando A, Hunter B, Schreiner G, Rodriguez-Iturbe B, Sautin YY, Johnson RJ. Uric acid stimulates fructokinase and accelerates fructose metabolism in the development of fatty liver. PLoS One. 2012;7(10):e47948. doi: 10.1371/journal.pone.0047948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aoyama K, Matsumura N, Watabe M, Wang F, Kikuchi-Utsumi K, Nakaki T. Caffeine and uric acid mediate glutathione synthesis for neuroprotection. Neuroscience. 2011;181:206–215. doi: 10.1016/j.neuroscience.2011.02.047. [DOI] [PubMed] [Google Scholar]
- 79.Zhang N, Shu HY, Huang T, Zhang QL, Li D, Zhang GQ, Peng XY, Liu CF, Luo WF, Hu LF. Nrf2 signaling contributes to the neuroprotective effects of urate against 6-OHDA toxicity. PLoS One. 2014;9:e100286. doi: 10.1371/journal.pone.0100286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Odden MC, Amadu AR, Smit E, Lo L, Peralta CA. Uric acid levels, kidney function, and cardiovascular mortality in US adults: National Health and Nutrition Examination Survey (NHANES) 1988–1994 and 1999–2002. Am J Kidney Dis. 2014;64(4):550–557. doi: 10.1053/j.ajkd.2014.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Haskó G, Sitkovsky MV, Szabó C. Immunomodulatory and neuroprotective effects of inosine. Trends Pharmacol Sci. 2004;25:152–157. doi: 10.1016/j.tips.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 82.Parkinson Study Group SURE-PD Investigators. Schwarzschild MA, Ascherio A, Beal MF, Cudkowicz ME, Curhan GC, Hare JM, Hooper DC, Kieburtz KD, Macklin EA, Oakes D, Rudolph A, Shoulson I, Tennis MK, Espay AJ, Gartner M, Hung A, Bwala G, Lenehan R, Encarnacion E, Ainslie M, Castillo R, Togasaki D, Barles G, Friedman JH, Niles L, Carter JH, Murray M, Goetz CG, Jagli J, Ahmed A, Russell DS, Cotto C, Goudrea JL, Russell D, Parashos SA, Ede P, Saint-Hilaire MH, Thomas CA, James R, Stacy MA, Johnson J, Gauger L, Antonelle de Marcaid J, Thurlow S, Isaacson SH, Carvajal L, Rao J, Cook M, Hope-Porche C, McClurg L, Grasso DL, Logan R, Orme C, Ross T, Brocht AF, Constantinescu R, Sharma S, Venuto C, Weber J, Eaton K. Inosine to increase serum and cerebrospinal fluid urate in Parkinson disease: a randomized clinical trial. JAMA Neurol. 2014;71:141–150. doi: 10.1001/jamaneurol.2013.5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Coolen EJ, Arts IC, Bekers O, Vervaet C, Bast A, Dagnelie PC. Oral bioavailability of ATP after prolonged administration. Br J Nutr. 2011;105:357–366. doi: 10.1017/S0007114510003570. [DOI] [PubMed] [Google Scholar]
- 84.Arts IC, Coolen EJ, Bours MJ, Huyghebaert N, Stuart MA, Bast A, Dagnelie PC. Adenosine 5′-triphosphate (ATP) supplements are not orally bioavailable: a randomized, placebo-controlled cross-over trial in healthy humans. J Int Soc Sports Nutr. 2012;9:16. doi: 10.1186/1550-2783-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]


