Abstract
Purpose
Environmental stressors are disturbing our ecosystem at an accelerating rate. An increasingly relevant stressor are air pollutants, whose levels are increasing worldwide with threats to human health. These air pollutants are associated with increased mortality and morbidity from cardiovascular diseases. In this review we discuss environmental stressors focusing mainly on the various types of air pollutants, their short-term and long-term cardiovascular effects, and providing the epidemiological evidence associated with adverse cardiovascular outcomes. Direct and indirect pathophysiological mechanisms are also linked with cardiovascular complications such as thrombosis, fibrinolysis, hypertension, ischemic heart diseases and arrhythmias.
RESULTS
Evidence to date suggests that humans are constantly being exposed to unhealthy levels of environmental toxicants with the potential of serious health conditions. Environmental stressors adversely affect the cardiovascular system and pose an increased risk for cardiovascular diseases for those who reside in highly polluted areas.
CONCLUSION
People with existing risk factors and those with established cardiovascular disease have increased susceptibility to environmental stressors. The literature reviewed in this article thus support public health policies aimed at reducing pollutant exposure to benefit public health.
Keywords: Particulate matter, environmental stress, heart, oxidative stress, inflammation, cardiovascular mortality
Introduction
Pollution levels continue to remain a major public issue, resulting in approximately 200,000 deaths each year in the United States alone. This is due in large part to emissions from transportation and power generation but also multiple industrial sectors and seasonal fluctuations. Moreover, studies showed that premature mortality will increase due to rises in these sectors on a global scale. Despite global efforts to address air pollution, such as the Paris Climate Accords, over 5.5 million deaths a year have still been reported [1].
The World Health Organization models that 92% of the world’s population live in areas that exceed recommended limits for air pollution allowing it to be linked to one out of every nine deaths worldwide [2]. Air pollution has both short and long-term effects and has been implicated in many non-communicable and congenital diseases all over the world. Current lines of research aim to understand the pathophysiological consequences and mechanisms of air pollution and its epidemiology in efforts to refine environmental policies and recommendations. There are numerous studies and reviews that have documented the role of particulate matter (PM) pollution in the pathogenesis of cardiac diseases, which has considerable morbidity and mortality and continues to be the leading cause of death in the world. However, few studies explore the underlying mechanisms and pathophysiology of air pollutant-induced cardiac diseases. Therefore, the purpose of this article is to review the pathophysiology of the cardiovascular system as a result of particulate matter exposure.
Environmental Stressors
Ambient air pollution is commonly recognized as a heterogeneous mixture of gas, liquid, and solid particles. Among the harmful components of air pollution, here, we focus mainly on PM since it is associated widely with the incidence of cardiopulmonary diseases. PM refers to the solid particles and liquid droplets of varying size found in air pollution that result from both human and natural activities [3]. It assembles into the air once emitted from both mobile and stationary sources. Based on differing sources of emission, PM is subdivided into primary and secondary particles. Primary particles are directly emitted into the air via automobiles, industrial equipment, forest fires, and burning other related materials. Crustal material, which is emitted by various construction and industrial operations, is a commonly found component of primary particles (U.S. EPA 2014). Secondary particles are those dispersed into the air via reactions involving precursor chemicals such as sulfates, nitrates, and carbon. The carbon of secondary particles is found in the form of a reactive organic gas emitted from many of the same sources of primary particles (U.S. EPA 2014). These secondary particles can potentially have both synergistic and antagonistic effects that are not completely understood. PM components are further classified according to their aerodynamic diameters: coarse (PM10), fine (PM2.5), and ultrafine (PM0.1).
PM10, known as coarse particles, have a diameter between 2.5 and 10 μm. Human activities that lead to PM10 production include road and agricultural dust, tire wear emissions, and construction sites. PM10 can also be naturally emitted by wildfires and movement of dust. After inhalation, the coarse fraction cannot travel beyond the bronchi. Fine particulate matter (PM2.5) includes particles with a diameter less than 2.5 μm that is predominantly found in traffic and industry fuel combustion from power plants and oil refineries [3]. Compared to the larger PM10, a significant amount of research has proven that PM2.5 can travel further along the respiratory tract into the pulmonary endothelium and blood stream leading to more adverse health effects [4]. Ultrafine particulate matter (PM0.1) are particles that are less than 0.1 μm in diameter [3]. PM0.1 is commonly found in the mobile emissions via the tailpipe and can further penetrate through the respiratory tract and can even incorporate into remote organs [3]. Recent evidence has shown that PM0.1 can aggregate into larger particles about the size of PM2.5.
Particulate Matter Exposure and Cardiovascular Morbidity and Mortality
Short-term effects: Short-term exposure to PM results in acute effects such as changes in heart rate (HR) and heart rate variability (HRV), blood pressure, vascular tone, and blood coagulability. A recent report demonstrated that short-term exposure to increased levels of air pollutants increases the hospital admissions for heart and respiratory diseases [5]. Liang et al. showed that for every 10μg/m3 increase in PM2.5 lead to a 0.371% and 0.199% increase in cardiovascular mortality for men and women, respectively [6]. Furthermore, a recent study conducted in Yazd, Iran demonstrated that 4.988% of all deaths were due to short term exposure to increased PM concentrations [7]. PM exposure can also act as a trigger for MI as it was demonstrated that short term exposure to high levels of PM2.5 and PM10 were associated with death from MI [8]. Long-term effects: Prolonged exposure (long-term) to PM, on the other hand, increases the concentration of particles in systemic circulation leading to serious conditions such as atherosclerosis. Various cohort studies provide useful information on serious adverse effects of PM exposure. Increased morbidity is well-established after prolonged exposure to increased levels of air pollutants [9]. There is an escalated increase in cardiovascular mortality from heart failure, ischemic heart disease (IHD), and cardiac arrest after long term exposure to PM [10]. Regardless of duration of exposure, clinical and pre-clinical studies undeniably indicate that air pollution is major factor for increased morbidity and mortality in the general population.
Particulate Matter Exposure and Cardiovascular Events
Exposure to ambient particulate matter leads to increases in systolic pressure, arterial BP, and hypertension [11]. It has also been shown that an increase in PM10 leads to an increased risk of acute heart failure [5]. A meta-analysis concluded that there was a positive association between short-term increases in PM and an increased risk of hospitalization or death from congestive heart failure [5]. Recent studies have connected PM2.5 exposure with risk for STEMI (ST-segment elevation myocardial infarction), but not NSTEMI (non-ST-segment elevation myocardial infarction) [8]. Long term exposure was correlated with higher incidences of myocardial infarction, coronary artery disease (CAD), and atherosclerosis [12]. Short term exposure to PM10 leads to increases in coronary artery calcification and progression of atherosclerosis [13]. All of these studies undeniably indicate that air pollution poses a large threat to human cardiovascular health.
Pathophysiological Mechanisms of PM-induced Adverse Effects
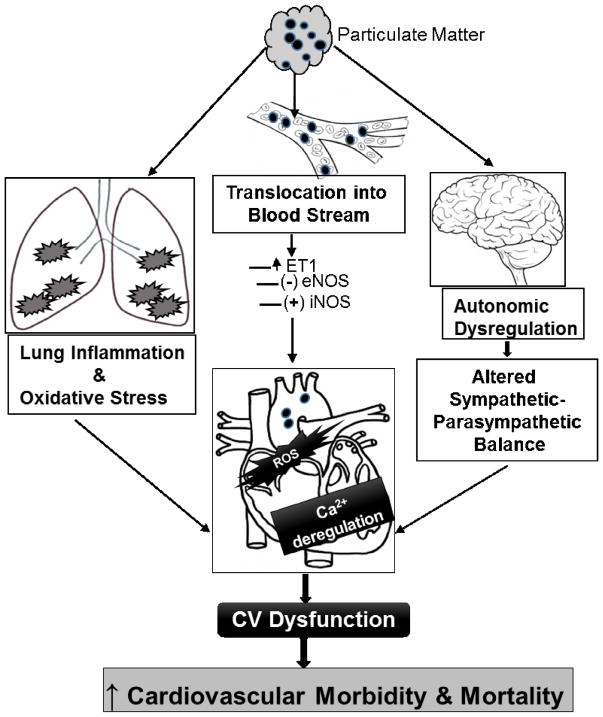
Upon inhalation, PM particles translocate into the blood stream and travel to remote organs, thus eliciting “direct” pathophysiological mechanisms. On the other hand, PM particles deposited into lung alveoli also trigger an “indirect” cascade of inflammation, oxidative stress and interaction with autonomic nervous system, which in turn exert effects on remote organs including the heart (Figure 1).
Figure 1.
Direct and indirect mechanisms of PM cardiovascular (CV) dysfunction
Direct Mechanisms
Preclinical studies have reported that PM can translocate into the blood stream and into specific organs causing cumulative toxicity. The circulating PM can directly cause cardiovascular dysfunction by: (1) oxidative stress and (2) deregulation of calcium levels/channels. Calcium regulation and reactive oxygen species (ROS) production are strictly interdependent. ROS is fundamental in reshaping local and global calcium signal amplitudes and kinetics in both physiological and pathological conditions.
Oxidative stress
An important role of cellular homeostasis is maintenance of the balance between ongoing ROS generation and antioxidant defense. The redox-signaling pathways which ensue following ROS production play a role in the expression of proinflammatory cytokines, chemokines, and adhesion molecules. Researchers proposed a hierarchical oxidative stress model in which incremental PM doses induce both protective and deleterious effects [14]. According to this model, PM exposure leads to activation of antioxidant response element (ARE) through Nrf2, resulting in the expression of antioxidant enzymes. An escalated increase in oxidative stress leads to activation of MAPK, cytokine/chemokine production, and inflammation. Alterations in the mitochondrial permeability transition pore (MPTP) and electron transfer chain occur at the maximum level of oxidative stress resulting in cell death. The two identified ROS involved are superoxide and hydrogen peroxide, the latter also affects sarcoplasmic reticulum calcium release.
Dysregulation of calcium levels/channels
ROS regulate cell function through redox modification of target proteins one being the L-type Ca2+ channel. Reports have demonstrated regulation of Ca2+ influx by local oxidative stress, thereby promoting enhanced L-type Ca2+ channel activity resulting in increased Ca2+ influx and contraction[15]. The activity of sarco/endoplasmic reticulum Ca2+-ATPase is also known to be modified by tyrosine nitration or cysteine oxidation by ROS generation [16]. Peroxides produced by PM can also affect calcium regulation of (Ca(2+))/calmodulin (CaM)-dependent protein kinase II (CaMKII) mediated oxidation and phosphorylation of regulatory proteins such as the Na+- Ca2+ exchanger [17]. The resulting imbalance in intracellular calcium concentration is known to mediate a variety of CVDs, such as contractile dysfunction, susceptibility to arrhythmogenic after depolarizations and ventricular polymorphic arrhythmias and heart failure. The increased Ca2+ levels also lead to the opening of the MPTP, resulting in reduced membrane depolarization [18]. Further, chemicals which are absorbed onto the surface of PM can affect the MPTP resulting in mitochondrial damage leading to cardiac dysfunction [19]. Together, both ROS and increased Ca2+ levels open the MPTP leading to deleterious effects on mitochondria such as membrane swelling and rupture, inhibition of oxidative phosphorylation and cytochrome c release [20].
Vascular Dysfunction
The endothelium is one of the major targets of oxidative stress. Impaired endothelial function in the vascular bed has been shown to be associated with an increased risk of acute cardiovascular events. Superoxide radicals combine with nitric oxide (NO) to form peroxynitrite, which inhibits nitric oxide synthase resulting in reduced NO bioavailability in the vessel wall and increased vasoconstriction, as demonstrated previously [21]. On the contrary, studies have also demonstrated an increase in the expression of endothelial NO synthase and plasma nitrate and nitrite concentrations in rat and humans [22], suggesting alterations in NO. Furthermore, superoxide inhibits the activity of guanylyl cyclase and cGMP-dependent protein kinase in vascular smooth muscle cells, resulting in reduced endothelium-dependent and -independent NO-mediated vasodilation [23]. Endothelin-1, produced by vascular endothelial cells, is a potent endogenous vasoconstrictor that has also shown to be increased after PM exposure, thereby contributing to vascular endothelial dysfunction [24].
Indirect Mechanisms
The indirect mechanisms are initiated after prolonged inhalation of ambient particulate matter resulting in the activation of pulmonary inflammation and oxidative stress. Another indirect mechanism is known to be mediated through the autonomic nervous system (ANS).
Pulmonary Inflammation and oxidative stress
Upon inhalation, the airway epithelium is first exposed to PM and it has been shown that this exposure causes altered cytokine/chemokine gene expression and increased production of interleukin (IL)-1β, IL-6, IL-8 and tissue necrosis factor (TNF) α [25]. Next, PM enters the lung epithelium through alveolar epithelial lining and initiates ROS production leading to release of inflammatory mediators from respiratory macrophages. Surface proteins, such as toll-like receptor 4 (TLR4) and scavenger protein A found on macrophages, are known to be responsible for detecting foreign molecules. Once inhaled, PM can also oxidize the surfactant molecules in the alveoli which binds to TLR4 and amplifies the activation of respiratory macrophages [26].
Exposure of healthy individuals as well as those with coronary artery disease to PM has been shown to increase the serum levels of IL-6 and its transcriptional target, C-reactive protein [27]. IL-6 expression increases the transcriptional activity of HIF-1α by promoting its translocation to the nucleus [28]. Inside the nucleus, HIF-1α acts to decrease cell proliferation and increase the permeability of the vascular endothelium. Once the inflammatory cascade begins, production of ROS ensues. Circulating IL-1 triggers neutrophilic superoxide production. In the bronchial epithelial cells, ROS activation induces the expression of intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) in capillary endothelial cells [29].
This accelerates the further infiltration of leukocytes and lymphocytes into the airway system leading to exaggerated local and systemic inflammation. PM2.5 inhalation is associated with reduced oxygen saturation, which in turn, is associated with pulmonary and systemic inflammation.
Autonomic Nervous System
When stimulated (either mechanically or chemically), afferent fibers send signals to the central nervous system (CNS) resulting in either excitatory or inhibitory effects. Any perturbation in the functioning of the autonomic nervous system (ANS) contributes to the increased CVD risk and susceptibility.
HR and HRV are controlled by the ANS which influence the sympathetic and vagal input to the heart, thus affecting cardiac output. Mordukhovich et al. showed that PM exposure affects HRV suggesting that PM exposure and ANS functioning are closely related [30]. Studies also demonstrated that a decrease in HRV is due to a decrease in parasympathetic action on the heart [31] leading to compensatory activation of sympathetic input and increased cardiovascular morbidity and mortality. It has also been hypothesized that combined features of the CNS and ANS indirectly elicit cardiovascular responses. Furthermore, it can also be hypothesized that PM alters the ANS, which can lead to a predominance of sympathetic nerve activity (SNA). Oxidative stress and the activation of transient receptor potential cation channel subfamily V member 1 (TRPV1) receptors on vagal afferent C-fibers in the lungs are responsible for a reflex-increase in cardiac SNA in response to PM2.5 exposure. Further, transient receptor potential ankyrin 1 (TRPA1) receptors and autonomic imbalance characterized by a shift toward parasympathetic modulation is also shown to be involved in arrhythmic episodes and cardiac dysfunction [32].
Combined Effects of the Direct and Indirect Mechanisms of PM Exposure on the Heart
The involvement of direct and indirect mechanisms following inhalation of ambient PM particles is complex as it is difficult to elucidate whether they operate independently and in which order, or if it is a simultaneous involvement of both mechanisms. The following are the cardiovascular complications which occur following PM exposure due to activation of these mechanisms.
Thrombosis
Alveolar inflammation as a result of particle inhalation results in increased blood coagulation and accentuated risk of cardiovascular events. PM exposure also releases extracellular vesicle-packed miRNA associated with increased coagulation as reported by Pergoli and coworkers [33]. PM promotes clot formation shortly after entering systemic circulation whereas prolonged exposure promotes pulmonary inflammation, leading to thrombosis. In vivo intra-tracheal instillation of particulate matter increases arterial thrombosis associated with platelet activation [34]. Pope and colleagues suggested that cytokine release, macrophage activation and pulmonary inflammation are the necessary initial steps for PM-induced pro-thrombotic changes [35]. In addition to inflammatory reaction, particulate particles reach the circulation and directly enhance thrombus formation and increases cardiovascular mortality.
Fibrinolysis
Tissue plasminogen activator (tPA) found on endothelial cells is a major enzyme responsible for clot breakdown and is a critical determinant of cardiovascular outcomes. Impaired fibrinolytic activity is shown to be associated with atherothrombosis and MI. Rückerl et al. have demonstrated associations between ambient air pollutants and markers of coagulation/fibrinolysis in susceptible populations which may aggravate atherosclerotic diseases and induce multi-organ damage [36].
The PM induced oxidative stress and inflammation have been observed in the arterial wall of animals causing augmented vasoconstriction and blunted vasorelaxation responses [37].
Atherosclerosis
There is strong evidence that PM-induced systemic inflammation can accelerate atherosclerotic processes. Dai and colleagues demonstrated that exposure to environmentally relevant PM causes endothelial dysfunction and increases vascular plaque burden, thus accelerating the progression of atherosclerosis [38]. In addition, oxidative stress induced activation of IL-12 cytokine family is shown to be involved in the regulation of regulatory T cells (Tregs) in the process of atherogenesis [39]. These events also result in atheromatous plaque destabilization and rupture. As a result of ROS generation, low-density lipoproteins (LDLs) also get oxidized.
Subendothelial macrophages phagocytize these oxidized LDLs and become foam cells that contribute to plaque instability [40]. These events increase the risk of stroke and MI, which has been observed in populations residing in high PM areas [3].
Hypertension
Data from epidemiological studies demonstrate an association between short-term exposures to PM with elevated blood pressure (BP). Lu and colleagues put forward the mechanistic link between Nox4-induced ROS/RhoA/ROCK pathway and hypertension [41]. It has been shown that PM affects blood pressure by modifying the expression of miRNAs [42]. IL-13 and IL-17A are also reported to be inducers of inflammation and associated hypertension in response to PM inhalation [43]. In addition, PDGF signaling has been demonstrated to be a major mediator of vascular remodeling responsible for causing pulmonary arterial hypertension in rodents [44].
Ischemic heart disease
Ischemic heart disease is a condition associated with narrowing of the coronary vessels, which leads to reduced blood supply to the heart. Systemic inflammation, endothelial dysfunction, and thrombosis are key mechanisms by which PM causes ischemic injury [3]. Further, it can be hypothesized that because PM is linked with hemodynamic and hemostatic alterations, there is a possibility this association might trigger ischemic events. The link between ambient particulate levels and mortality and morbidity due to IHD is also established by Pope et al. by demonstrating an increased risk of ST segment elevation in subjects with existing seriously diseased coronary arteries [45].
Arrhythmias
Numerous studies have linked air pollution to cardiac arrhythmias and sudden cardiovascular mortality. Carll and colleagues demonstrated prolonged PR-interval, heart rate variability (HRV) parameters of parasympathetic tone and atrioventricular block arrhythmias after post PM exposure [46]. In aged patients, PM exposure alters the duration of the QT interval [47]. In addition, exposure to individual pollutants or the combination increases T-wave Alternans (TWA) [48]. TWA provides information on repolarizing currents (specifically calcium cycling) and is considered an index of arrhythmic susceptibility. Dysregulation in cardiac sodium channels and carotid body sensitivity is also found to mediate PM-induced pathophysiology of cardiac arrhythmias [49]. Another study by Kim et al suggested that oxidative stress and CaMKII activation caused by PM induces APD prolongation, EAD and ventricular arrhythmia [50].
Conclusion
This article summarizes data obtained from epidemiological, clinical and preclinical studies on the effects of ambient PM on cardiovascular complications. There is still much unknown about harmful effects of ambient PM and the mechanistic pathways responsible for triggering adverse cardiovascular health effects. Necessary interventions to limit air pollution should be adopted to prevent harmful effects. Besides interventions, studying the epidemiology and understanding of mechanisms by which air pollution imparts adverse effects on the cardiovascular system is equally important. The caveats in existing knowledge needs to be fulfilled in order to increase the effectiveness of environmental research across the globe.
Acknowledgments
Funding
This work was supported in part by grants from the National Institute of Health [R01ES019923, R01HL139348, R01AG057046 and R01NR012618] to L.E. Wold.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Collaborators, G.B.D.R.F. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1659–1724. doi: 10.1016/S0140-6736(16)31679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brauer M, et al. Ambient Air Pollution Exposure Estimation for the Global Burden of Disease 2013. Environ Sci Technol. 2016;50(1):79–88. doi: 10.1021/acs.est.5b03709. [DOI] [PubMed] [Google Scholar]
- 3.Du Y, et al. Air particulate matter and cardiovascular disease: the epidemiological, biomedical and clinical evidence. J Thorac Dis. 2016;8(1):E8–E19. doi: 10.3978/j.issn.2072-1439.2015.11.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schulze F, et al. Air Quality Effects on Human Health and Approaches for Its Assessment through Microfluidic Chips. Genes (Basel) 2017;8(10) doi: 10.3390/genes8100244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaduganathan M, et al. Risk of Cardiovascular Hospitalizations from Exposure to Coarse Particulate Matter (PM10) Below the European Union Safety Threshold. Am J Cardiol. 2016;117(8):1231–5. doi: 10.1016/j.amjcard.2016.01.041. [DOI] [PubMed] [Google Scholar]
- **6.Liang RM, et al. Acute effect of fine particulate matters on daily cardiovascular disease mortality in seven cities of China. Zhonghua Liu Xing Bing Xue Za Zhi. 2017;38(3):283–289. doi: 10.3760/cma.j.issn.0254-6450.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Miri M, et al. Human health impact assessment of exposure to particulate matter: an AirQ software modeling. Environ Sci Pollut Res Int. 2017;24(19):16513–16519. doi: 10.1007/s11356-017-9189-9. [DOI] [PubMed] [Google Scholar]
- 8.Argacha JF, et al. Air pollution and ST-elevation myocardial infarction: A case-crossover study of the Belgian STEMI registry 2009–2013. Int J Cardiol. 2016;223:300–305. doi: 10.1016/j.ijcard.2016.07.191. [DOI] [PubMed] [Google Scholar]
- 9.Makar M, et al. Estimating the Causal Effect of Low Levels of Fine Particulate Matter on Hospitalization. Epidemiology. 2017;28(5):627–634. doi: 10.1097/EDE.0000000000000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **10.Dehbi HM, et al. Air pollution and cardiovascular mortality with over 25years follow-up: A combined analysis of two British cohorts. Environ Int. 2017;99:275–281. doi: 10.1016/j.envint.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Byrd JB, et al. Acute increase in blood pressure during inhalation of coarse particulate matter air pollution from an urban location. J Am Soc Hypertens. 2016;10(2):133–139e4. doi: 10.1016/j.jash.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 12.McGuinn LA, et al. Association between satellite-based estimates of long-term PM2.5 exposure and coronary artery disease. Environ Res. 2016;145:9–17. doi: 10.1016/j.envres.2015.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaufman JD, et al. Association between air pollution and coronary artery calcification within six metropolitan areas in the USA (the Multi-Ethnic Study of Atherosclerosis and Air Pollution): a longitudinal cohort study. Lancet. 2016;388(10045):696–704. doi: 10.1016/S0140-6736(16)00378-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *14.Lawal AO. Air particulate matter induced oxidative stress and inflammation in cardiovascular disease and atherosclerosis: The role of Nrf2 and AhR-mediated pathways. Toxicol Lett. 2017;270:88–95. doi: 10.1016/j.toxlet.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 15.Muralidharan P, et al. The cardiac L-type calcium channel alpha subunit is a target for direct redox modification during oxidative stress-the role of cysteine residues in the alpha interacting domain. Clin Exp Pharmacol Physiol. 2017 doi: 10.1111/1440-1681.12750. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe Y, Cohen RA, Matsui R. Redox Regulation of Ischemic Angiogenesis- Another Aspect of Reactive Oxygen Species. Circ J. 2016;80(6):1278–84. doi: 10.1253/circj.CJ-16-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foteinou PT, Greenstein JL, Winslow RL. Mechanistic Investigation of the Arrhythmogenic Role of Oxidized CaMKII in the Heart. Biophys J. 2015;109(4):838–49. doi: 10.1016/j.bpj.2015.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *18.Fink BD, et al. Regulation of ATP production: dependence on calcium concentration and respiratory state. Am J Physiol Cell Physiol. 2017;313(2):C146–C153. doi: 10.1152/ajpcell.00086.2017. [DOI] [PubMed] [Google Scholar]
- 19.Holland NA, et al. Ultrafine Particulate Matter Increases Cardiac Ischemia/Reperfusion Injury via Mitochondrial Permeability Transition Pore. Cardiovasc Toxicol. 2017 doi: 10.1007/s12012-017-9402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biasutto L, et al. The mitochondrial permeability transition pore in AD 2016: An update. Biochim Biophys Acta. 2016;1863(10):2515–30. doi: 10.1016/j.bbamcr.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 21.Barrier M, et al. Involvement of Heme Oxygenase-1 in particulate matter-induced impairment of NO-dependent relaxation in rat intralobar pulmonary arteries. Toxicol In Vitro. 2016;32:205–11. doi: 10.1016/j.tiv.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Langrish JP, et al. Cardiovascular effects of particulate air pollution exposure: time course and underlying mechanisms. J Intern Med. 2012;272(3):224–39. doi: 10.1111/j.1365-2796.2012.02566.x. [DOI] [PubMed] [Google Scholar]
- 23.Ritchie RH, et al. The opposing roles of NO and oxidative stress in cardiovascular disease. Pharmacol Res. 2017;116:57–69. doi: 10.1016/j.phrs.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 24.Chan EA, et al. The heart as an extravascular target of endothelin-1 in particulate matter-induced cardiac dysfunction. Pharmacol Ther. 2016;165:63–78. doi: 10.1016/j.pharmthera.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *25.Meng K, et al. Immunity-Related Protein Expression and Pathological Lung Damage in Mice Poststimulation with Ambient Particulate Matter from Live Bird Markets. Front Immunol. 2016;7:252. doi: 10.3389/fimmu.2016.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cevallos VM, Diaz V, Sirois CM. Particulate matter air pollution from the city of Quito, Ecuador, activates inflammatory signaling pathways in vitro. Innate Immun. 2017;23(4):392–400. doi: 10.1177/1753425917699864. [DOI] [PubMed] [Google Scholar]
- 27.Li W, et al. Short-Term Exposure to Ambient Air Pollution and Biomarkers of Systemic Inflammation: The Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2017;37(9):1793–1800. doi: 10.1161/ATVBAHA.117.309799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dai J, et al. Exposure to concentrated ambient fine particulate matter disrupts vascular endothelial cell barrier function via the IL-6/HIF-1alpha signaling pathway. FEBS Open Bio. 2016;6(7):720–8. doi: 10.1002/2211-5463.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelly FJ, Fussell JC. Linking ambient particulate matter pollution effects with oxidative biology and immune responses. Ann N Y Acad Sci. 2015;1340:84–94. doi: 10.1111/nyas.12720. [DOI] [PubMed] [Google Scholar]
- *30.Mordukhovich I, et al. Exposure to sub-chronic and long-term particulate air pollution and heart rate variability in an elderly cohort: the Normative Aging Study. Environ Health. 2015;14:87. doi: 10.1186/s12940-015-0074-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rich DQ, et al. Ambient and Controlled Particle Exposures as Triggers for Acute ECG Changes. Res Rep Health Eff Inst. 2016;(186):5–75. [PubMed] [Google Scholar]
- 32.Kurhanewicz N, et al. TRPA1 mediates changes in heart rate variability and cardiac mechanical function in mice exposed to acrolein. Toxicol Appl Pharmacol. 2017;324:51–60. doi: 10.1016/j.taap.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pergoli L, et al. Extracellular vesicle-packaged miRNA release after short-term exposure to particulate matter is associated with increased coagulation. Part Fibre Toxicol. 2017;14(1):32. doi: 10.1186/s12989-017-0214-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tabor CM, et al. Platelet activation independent of pulmonary inflammation contributes to diesel exhaust particulate-induced promotion of arterial thrombosis. Part Fibre Toxicol. 2016;13:6. doi: 10.1186/s12989-016-0116-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pope CA, 3rd, et al. Exposure to Fine Particulate Air Pollution Is Associated With Endothelial Injury and Systemic Inflammation. Circ Res. 2016;119(11):1204–1214. doi: 10.1161/CIRCRESAHA.116.309279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruckerl R, et al. Associations between ambient air pollution and blood markers of inflammation and coagulation/fibrinolysis in susceptible populations. Environ Int. 2014;70:32–49. doi: 10.1016/j.envint.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 37.Moller P, et al. Atherosclerosis and vasomotor dysfunction in arteries of animals after exposure to combustion-derived particulate matter or nanomaterials. Crit Rev Toxicol. 2016;46(5):437–76. doi: 10.3109/10408444.2016.1149451. [DOI] [PubMed] [Google Scholar]
- 38.Dai J, et al. Exposure to Concentrated Ambient Fine Particulate Matter Induces Vascular Endothelial Dysfunction via miR-21. Int J Biol Sci. 2017;13(7):868–877. doi: 10.7150/ijbs.19868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chistiakov DA, Bobryshev YV, Orekhov AN. Heterogeneity of Tregs and the complexity in the IL-12 cytokine family signaling in driving T-cell immune responses in atherosclerotic vessels. Mol Immunol. 2015;65(1):133–8. doi: 10.1016/j.molimm.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 40.Chistiakov DA, Bobryshev YV, Orekhov AN. Macrophage-mediated cholesterol handling in atherosclerosis. J Cell Mol Med. 2016;20(1):17–28. doi: 10.1111/jcmm.12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu W, et al. The role of the Nox4-derived ROS-mediated RhoA/Rho kinase pathway in rat hypertension induced by chronic intermittent hypoxia. Sleep Breath. 2017 doi: 10.1007/s11325-016-1449-2. [DOI] [PubMed] [Google Scholar]
- 42.Lee HW, Park SH. Elevated microRNA-135a is associated with pulmonary arterial hypertension in experimental mouse model. Oncotarget. 2017;8(22):35609–35618. doi: 10.18632/oncotarget.16011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park SH, et al. Interleukin 13- and interleukin 17A-induced pulmonary hypertension phenotype due to inhalation of antigen and fine particles from air pollution. Pulm Circ. 2014;4(4):654–68. doi: 10.1086/678511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ten Freyhaus H, et al. Genetic Ablation of PDGF-Dependent Signaling Pathways Abolishes Vascular Remodeling and Experimental Pulmonary Hypertension. Arterioscler Thromb Vasc Biol. 2015;35(5):1236–45. doi: 10.1161/ATVBAHA.114.304864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pope CA, et al. Short-Term Exposure to Fine Particulate Matter Air Pollution Is Preferentially Associated With the Risk of ST-Segment Elevation Acute Coronary Events. J Am Heart Assoc. 2015;4(12) doi: 10.1161/JAHA.115.002506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carll AP, et al. Cardiomyopathy confers susceptibility to particulate matter-induced oxidative stress, vagal dominance, arrhythmia and pulmonary inflammation in heart failure-prone rats. Inhal Toxicol. 2015;27(2):100–12. doi: 10.3109/08958378.2014.995387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mordukhovich I, et al. Association Between Particulate Air Pollution and QT Interval Duration in an Elderly Cohort. Epidemiology. 2016;27(2):284–90. doi: 10.1097/EDE.0000000000000424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kusha M, et al. Controlled exposure study of air pollution and T-wave alternans in volunteers without cardiovascular disease. Environ Health Perspect. 2012;120(8):1157–61. doi: 10.1289/ehp.1104171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang T, et al. Particulate matter induces cardiac arrhythmias via dysregulation of carotid body sensitivity and cardiac sodium channels. Am J Respir Cell Mol Biol. 2012;46(4):524–31. doi: 10.1165/rcmb.2011-0213OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim JB, et al. Particulate air pollution induces arrhythmia via oxidative stress and calcium calmodulin kinase II activation. Toxicol Appl Pharmacol. 2012;259(1):66–73. doi: 10.1016/j.taap.2011.12.007. [DOI] [PubMed] [Google Scholar]