Abstract
Background
Symptoms of psychosis in schizophrenia (SZ) reflect disturbances in sense of agency (SoA)—difficulty distinguishing internally from externally generated sensory and perceptual experiences. One theory attributes these anomalies to a disruption in corollary discharge (CD), an internal copy of generated motor commands used to distinguish self-movement generated sensations from externally-generated stimulation.
Methods
We used a trans-saccadic shift detection paradigm to examine possible deficits in CD and SoA based on the ability to perceive visual changes in 31 SZ patients (SZP) and 31 healthy controls (HC). We derived perceptual measures based on manual responses indicating the trans-saccadic target shift direction. We also developed a distance-from-unity-line measure to quantify use of CD versus purely sensory (visual) information in evaluating visual changes in the environment following an eye movement.
Results
SZP had higher perceptual thresholds in detecting shift of target location than HC, regardless of movement direction or amplitude. Despite producing similar hypometric saccades, HC overestimated target location, whereas SZP relied more on the experienced visual error and consequently underestimated the target position. We show that in SZP the post-saccadic judgment of the initial target location was largely aligned with the measure based only on visual error, suggesting a deficit in utilization of CD. This CD deficit also correlated with positive SZ symptoms and disturbances in SoA.
Conclusions
These results provide a novel approach in quantifying abnormal utilization of CD in SZP and provide a framework to distinguish deficits in sensory processing versus defects in the internal CD-based monitoring of movement.
Keywords: Schizophrenia, saccade, corollary discharge, positive symptoms, visual perception, sense of agency
INTRODUCTION
Schizophrenia (SZ) is associated with positive psychotic symptoms, negative symptoms and cognitive impairments. Psychotic symptoms (e.g. hallucinations and delusions) are marked by sensory perception disturbances and difficulty distinguishing between origins of endogenous and exogenous stimuli. Patients with schizophrenia (SZP) may sense that their actions and thoughts are controlled by external forces or may ascribe ownership to external events. Reports have often described inaccurate agency judgments in SZP (1–6) but neurobiological mechanisms underlying these debilitating symptoms remain unclear.
It is suggested that predictive coding mechanisms (e.g. action-based corollary discharge, CD) mediating sensory cortex modulation may be compromised in SZ. A purported copy of issued motor commands prepares the sensory cortex for inputs resulting from self-generated motor acts, and distinguishes sensations resulting from endogenous actions from those externally generated by comparing the actual and predicted sensory consequences of motion (7–10). This comparator mechanism is thought to support evaluation of action awareness—directly guiding subjective sense of agency (SoA, i.e., recognition of action ownership, 3,11). Based on this framework, delusions of influence, hallucinations and misattribution of agency may ensue from inadequacies in CD (12–17).
Although CD deficits in SZP have been studied within the auditory (18,19,21,22,23) and somatosensory systems (4–6,24–26), the saccadic eye-movement system is advantageous in probing SoA defects due to a comprehensive understanding of movement generation (27). Specifically, the saccadic CD signal can be used to update knowledge about eye position and allow for distinction between endogenous and exogenous visual scene changes (28–34,38). Thus, the saccadic system provides a tractable approach to study SoA.
Eye movement CD impairment in SZP has been previously demonstrated in various paradigms (saccade-based tasks: 39,40,41,42,43; smooth pursuit based tasks: 44–49). We build off this previous work by examining CD dysfunction within the comparator model framework, directly quantifying a measure that specifically distinguishes perception based on sensory feedback from that based on CD-derived predicted feedback, and demonstrating a strong link of this deficit to positive symptoms and SoA. We used a task in which subjects make a saccade to an initial target location and then evaluate the direction of target location changes that occurred during the movement (trans-saccadic shifts). To study localization, we remove the target while the saccade is in flight and after a blank period it reappears at a shifted location. In this task, proprioception from the eye muscles is not likely to play a significant role as it has been shown that these signals are likely to be too slow to be effective, and performance is not affected when these signals are eliminated (50–52). Due to lack of visual references and imprecise proprioceptive information, subjects must make a perceptual judgment based on the CD vector of the initial movement. Thus, this task serves as a sensitive assay of CD utilization in visual perception. We assess the role of CD by quantifying where subjects perceptually estimate initial target location. An ideal eye movement would land on the initial target location and shifts would be judged relative to this eye position. However, eye movements typically undershoot the target resulting in a discrepancy between saccade landing point and target. If the CD of the saccade is impaired and the discrepancy is not accounted for, estimation of initial target location would rely on experienced visual error rather than the CD-based predicted error resulting from the inaccurate saccade. This task allows separation of visual error processing disturbances from internal monitoring deficits by comparing target location estimation based on visual error (VE only, 53) versus location estimation derived from the perceptual reports (VE+CD, 53,54). Since we hypothesize that CD is impaired in SZP, we expect that patients will base location estimation on post-movement sensory information (visual error) and display a perceptual bias closely aligned to that derived from the visual error. In order to quantify this alliance and utilization of CD, we formulated a 'distance-from-unity-line' measure - the difference between the VE-only and VE+CD based bias. Our aim is to examine CD disruption and dissociate components of the comparator mechanism that are intact from those that are impaired in SZ. Our data demonstrate that reliance on visual error feedback and a corresponding decrease in CD-based estimation is correlated with impaired SoA and psychotic symptoms, collectively providing compelling support for the hypothesis that CD and comparator model deficits underlie perceptual abnormalities in SZ.
METHODS AND MATERIALS
Subjects
Demographic information is presented in Table 1. Sixty-two veterans between 24–70 years of age were recruited from the Washington DC Veterans Affairs Medical Center (DCVAMC). Thirty-one subjects met DSM-IV criteria for schizophrenia (N=19) or schizoaffective disorder (N = 12) confirmed using the Structured Clinical Interview for DSM IV and chart review. They were outpatients receiving stable doses of either typical (N=1) or atypical (N=29) antipsychotic medication, or an antidepressant (N=1) for at least three months prior to testing. Thirty-one age, gender and education-matched subjects with no history of psychiatric or substance abuse disorder and no first-degree relative with mental illness were enrolled in the control group (HC). Subjects were naïve to the purpose of the study and were compensated for participation. Exclusion criteria were: significant medical/neurological disorder, history of loss of consciousness for > five minutes, substance use disorder within the three months prior to the study, and history of eye surgery or impaired vision. The DCVAMC Institutional Review Board approved the study and informed consent was obtained from subjects.
Table 1.
Participant demographics. Means (SD) are displayed, along with statistical values for comparison between groups. We based premordbid IQ on word reading from the WRAT 4 (Wide Range Achievement Test). CPZ, Chlorpromazine; PANSS, Positive and Negative Syndrome Scale.
| HC, N=31 | SZP, N=31 | |||
|---|---|---|---|---|
|
| ||||
| Mean (SD) | Mean (SD) | Statistic | p | |
| Age | 54.81(9.01) | 56.25 (9.84) | t = 0.06 | 0.95 |
| Gender | 3 F /28 M | 2 F /29 M | φ =0.22 | 0.65 |
| Years of education | 14.89 (0.33) | 13.66 (0.41) | t = 2.35 | 0.07 |
| Standard WRAT Scores (Estimation of IQ)* | 100.19 (8.82) | 93.75 (12.80) | t = 3.12 | 0.03 |
| Handedness | 3 L /28 R | 6 L /25 R | φ =1.17 | 0.28 |
| Duration of illness | 28.11(10.04) | |||
| CPZ Equivalent Dose | 454.37 (277.51) | |||
| PANSS Total | 57.14 (11.54) | |||
| PANSS Positive | 16.42 (5.43) | |||
| PANSS Negative | 15.67 (6.49) | |||
| PANSS General | 26.29 (6.51) | |||
Clinical Measures
The Positive and Negative Syndrome Scale (PANSS) (55) was used to assess severity of positive, negative and general symptoms in SZP. To assess abnormal experiences of agency in both groups, we used the Sense of Agency Scale (SOAS) (56–58, Supplement) having three subscales: 1) Mental SoA-sense of agency involving thoughts, mental activity or perception, 2) Physical SoA-sense of agency involving somatic experiences, and 3) Social SoA-sense of agency involving social activities. Subjects reported how often the items applied to their daily life. High scores indicated unstable sense of agency.
Apparatus
Data were collected using a SMI eye tracker (Sampling rate 240 Hz, iView v2.2.4, SensoMotoric Instruments GmbH). E-Primev2 software (Psychology Software Tools, Inc.) was used to present stimuli onto an Acer AL1715 monitor with a 75 Hz refresh rate. The eye tracker system had a spatial resolution of 0.01° and proces sing latency of < 0.5 ms. Subjects were seated 56 cm from the computer screen in a headrest containing the camera. A chin and forehead rest stabilized the participant’s head with a Plexiglas molded notch holding the nose in place. To eliminate effects of visual cues on localization, the task was performed in a dimly lit room. The fixation cross and circular targets were white with a luminance of 56 cd/m2 viewed against a black background of luminance of < 0.1 cd/m2. At the start of each session, a 9-point gaze calibration was performed.
Design and Procedure
We modified a task previously used in our studies on trans-saccadic perception (53,54,59–62 Figure 1A). Trials began with a variable fixation period, after which an initial saccade target appeared at one of two amplitudes (4º or 8º) and two directions (leftward or rightward) from the fixation cross. When eye position was detected beyond a virtual square window around the fixation cross, the target was extinguished, followed by a 250 ms blank period. The target reappeared at a shifted position (± 0.5º collinear increments) between ± 3.5º. Target shifts were randomly drawn from a Gaussian distribution centered at 0º, with smaller shifts sampled with greater frequency than larger, more detectable shifts. Subjects judged the direction of target shift with a manual response. Each subject completed 384 trials in one session that included a break. On each trial the initial target amplitude and direction were randomly selected.
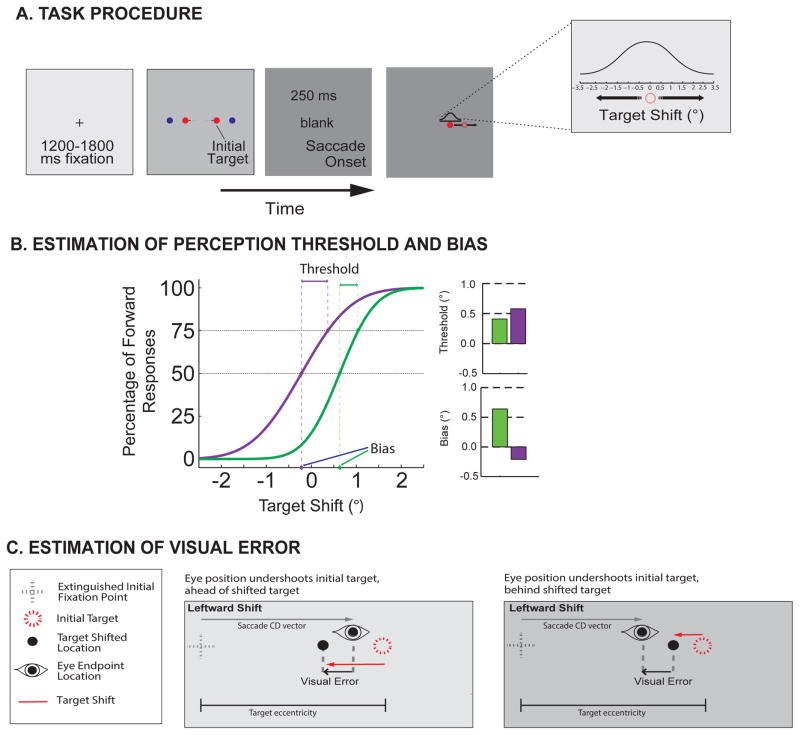
Figure 1. Trans-saccadic shift-detection task.
(A) Task procedure. Each trial began with a central fixation cross (0.3o in extent) appearing for a variable period (random duration between 1200 and 1800 ms), followed by the appearance of an initial target that could appear randomly at any one of the two amplitudes (4° or 8°) and two directions (leftward or rightward). Subjects were required to make a saccadic eye movement toward this initial target, and upon online detection (when eye position exceeded a virtual window, 3.2° in extent) the target was displaced during the saccade, reappearing at a new location after a 250 ms blank period. This blank period was used to measure properties of the saccade CD rather than the effect of saccadic suppression on perception—an effect studied when the target immediately reappears at the shifted location. Previous results suggest that differences in perception due to CD are observable during this blank target condition (32,57). The displaced target, T2, appeared randomly at shifted positions according the illustrated Gaussian distribution ranging from -3.5° to 3.5° shifts. After the reappearance of the target a t the shifted location, subjects were required to indicate the direction of the displacement (backward or forward) using the left or right mouse buttons. A successful trial required a response to occur within 3000 ms of the shifted target’s reappearance, but no instructions or feedback were given regarding reaction time and accuracy. (B) The percentage of forward responses on the y-axis is plotted as a function of target displacement on the abscissa. The manual response data were fitted with a cumulative Gaussian distribution to determine the psychometric function and measures of perceptual bias and threshold. Two example psychometric curves are shown with different slopes. Two perceptual measures are derived: perceptual threshold and bias. The threshold, top right, is computed as the difference in target displacement between the 50% and the 75% points of the sample psychometric curves. The perceptual bias, bottom right, was taken as the displacement from 0 (x-axis) at the point where the forward and backward responses were equal to 50% (y-axis). Each example curve yields a different threshold and bias. (C)The visual error was quantified by taking the vector magnitude, in visual angle degrees, between the eye position at the time when the shifted target appeared and the location of the shifted target. The examples show rightward saccades that undershoot the target, and leftward target shifts. Upon presaccadic target appearance the corollary discharge (CD) vector is used to predict the saccadic landing position. In this example, the saccade falls short of the target and the postsaccadic target is to the left of the presaccadic target, efficient CD-driven remapping would predict that subjects will perceive the shift as backward. In the left panel the large target shift and visual error are aligned, resulting in the correct ‘backward’ response. In the right panel the small target shift is in the opposite direction of the resulting visual error since the landing site is ‘behind’ the shifted target, with no CD vector information, it would result in an incorrect ‘forward’ response. These visual errors were used to derive hypothetical responses for target localization and psychometric curves based solely on the experienced sensory information.
Eye movement recording and analysis
Eye movements were analyzed offline using MATLABv8.1.0 (Mathworks,MA,USA). During the task, saccade initiation was detected when the saccade left a 3.2° square fixation window. Due to system limitations, this online detection may have occurred with some minimal lag. Details on saccade durations and offset are provided in Figure S4. For offline analysis, saccades were identified as follows: (1) occurring ≥ 75 ms after the initial target appeared, (2) velocity ≥ 75°/s and acceleration ≥ 2000°/s 2. Primary saccades included in the analyses were required to be initiated within the fixation window, with a distance exceeding 1/3 of initial target amplitude, and endpoint within the average eye position ± 2 SD (79.4 ± 3.2% of trials for SZP and 83.4 ± 2.2% for HC were included).
Analysis
Saccade Measures
We analyzed saccade characteristics (amplitude, latency, variability (SD)) and manual response reaction times to assess any group differences, or asymmetry based on target amplitude or direction. Endpoints of the primary saccade were determined per target amplitude and direction. To assess whether the primary saccade endpoint influenced shift detection, percent gain of initial saccade amplitude was also quantified as the ratio between the mean saccade endpoint and initial target position.
Perceptual Performance Data
CD and Sensory-Information Based
Consistent with our previous work (28,53,54), we derived post-saccadic estimates of initial target location and quantified the difficulty in detecting target shifts. These measures were based on psychometric curves (inferential models that relate subject performance to a physical quantity of a stimulus). These curves were fitted to the proportion of manual responses and specified the relationship between the probability of forward responses and the magnitude of the target shift. Psychometric functions from manual responses are assumed to be based on visual error + extraretinal information (VE+CD), because post-saccadic visual error as well as extraretinal information, such as the CD signal, are available to make perceptual judgments (32,53,54). Perceptual bias, inferred as the post-saccadic estimation of the location of the pre-saccadic (initial) target, was quantified as shift from 0 at the point where the percentage of forward responses was 50% (28,53,54). Since CD (see CD vector, Figure 1C) provides information about saccade metrics (hypo or hyper), this is purportedly used to make a perceptual estimation about presaccadic target location with respect to eye movement, and this estimation is quantified by the bias measure. A positive bias indicated that the initial target location was perceived to be ahead of its actual position; a negative bias indicated that initial target location was perceived to be behind the actual position. (Note that when there is a lack of CD utilization, saccade end-point errors could be used for this perceptual decision; the postsaccadic errors resulting from hypometric saccades would appear forward of the saccade endpoint more frequently. Thus, more frequent forward reports would shift the psychometric function to the left, resulting in a negative bias.) As done previously (53,54), we quantified the difference between the perceptual estimate (the bias) and actual target location as a percent error of the target location: bias divided by the initial target amplitude scaled by 100. This was done to compare over or underestimaton of initial target amplitude to the percent gain of initial saccade amplitude (Figure 2). Perceptual threshold, calculated as difference in shift size between the 50% and 75% points on the psychometric curve, quantified perceptual sensitivity in detecting target shifts; larger thresholds represent increased difficulty in perceiving trans-saccadic shifts (Figure 1B).
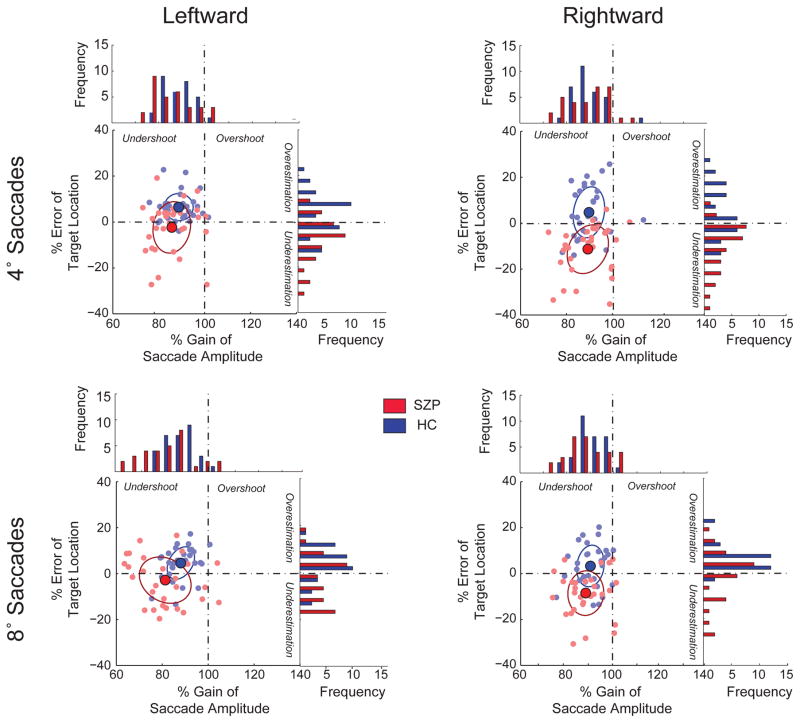
Figure 2. Percent gain of saccade amplitude and percent error estimation of target location.
We plot the percent error of the estimated target location per amplitude and direction as a function of percent gain in the mean saccade amplitude. Percent gain is the saccade amplitude as a percentage of the required movement amplitude. A percentage above 100 signifies that the mean saccade amplitude exceeded the target amplitude (overshoot); a percentage below 100 signifies that the mean saccade amplitude fell short of the target amplitude (undershoot). Percent error was perceptual bias as a percentage of the target amplitude. A percentage above 0 signified that the perceptual estimate exceeded the target amplitude (overestimation); a negative percentage signified that the perceptual estimate was lower than the target amplitude (underestimation). Each filled circle represents the mean values for each subject (Red: SZP, Blue:HC). Each panel shows the result for the respective saccade amplitude and direction. The larger solid circles represent the respective mean percent gains in saccade amplitude and mean percent errors in target location estimation, with the corresponding ellipses representing the 95% confidence interval. The histograms to the right and above each panel are the respective distributions of the percent error and the percent gain.
Solely Sensory-Information Based
We derived hypothetical psychometric functions if the perceptual decision was driven by only the visual error (VE) (53). We assume that VE represents shifted target direction, and that the CD-based estimate of eye position is not utilized. On every trial we determined the difference between the eye position at time of target reappearance and the shifted target location (VE) (Figure 1C) and direction of the resultant error vector was used as the basis for the simulated target shift judgment. Percentage of these VE-based forward judgments was plotted as a function of target shift to obtain a hypothetical psychometric function. Our assumption in the “VE-based” condition is that subjects have no extraretinal/CD-based information about the saccade metrics; only post-saccadic VE information is available for the perceptual judgment. This is a simplification, but provides a baseline under experimental conditions to determine how actual perceptual performance (utilizing extraretinal information, specifically CD) is superior to the limited VE-based situation.
Statistical Analyses
We assessed group differences in saccade metrics using separate mixed-design ANOVAs, and a Group by Direction by Amplitude ANOVA was performed on psychophysical measures; Group (SZP vs HC) being a between-subject factor, and Direction (right or left) and Target Amplitude (4° or 8°) within-subject factors. Since we observe d group differences in Wide Range Achievement Test scores, we conducted ANCOVA using these scores as a covariate. We report ANOVA results, as covariate analyses yielded the same results. We performed correlation tests between perceptual performance and clinical measures. Statistical analyses were performed using MATLAB and SPSS software (IBM SPSS Statistics, Version 20.0. Armonk, NY: IBM Corp). Significance level was set at 0.05.
RESULTS
Saccade and Response Measures
Table 2 shows the saccade metrics that we compared between groups. Although SZP made shorter saccades than HC, this was not significant [F(1,60)=1.13,P=0.293]. SZP had more variable saccadic endpoints, but there were no significant effects of group or direction for saccade variability (SD). The variability was higher for saccades to 8° targets [F(1,60)=9.61, P=0.004]. Sac cadic latencies did not differ by direction [F(1,60)=0.42, P=0.53], or group [F(1,60)=1.78, P=0.175], but were longer for 8 degree saccades [F(1,60)=21.74,P<0.001]. Also, the frequency of corrective saccades was larger for 8° targets [F(1,60)=12.65,P<0.001], with no direction [F(1,60)=1.38, P=0.32] or group effect [F(1,60)=0.56, P=0.27]. Manual response reaction time (RT) did not differ by direction, [F(1,60)=0.04, P=0.84], but RT was shorter for 4° t han for 8° targets [F(1,60)=7.226, P=0.01] and SZP took longer to respond [F(1,60)=10.01, P=0.003]. As reported in the Supplement, due to hardware/software system limitations, online detection may have occurred with some system lag, and on a small portion of trials saccades may have landed prior to target offset. We compared the percentage of correct responses on trials in which the target was still illuminated to the accuracy when the saccade was still in flight. The percent correct was not significantly different between the two groups (SZP and HC) or saccade amplitudes (all P > 0.23 in all cases) and there was no interaction effects (P > 0.44 in all cases).
Table 2. Saccade Kinematics.
Saccade and manual response metrics. Means and SDs for each measure are displayed, separated by group, target amplitude and direction.
| Leftward | Rightward | ||||
|---|---|---|---|---|---|
|
| |||||
| 4° | 8° | 4° | 8° | ||
|
| |||||
| Saccade Amplitude | HC | 3.54 (0.25) | 7.03 (0.48) | 3.56 (0.21) | 7.22 (0.5) |
| SZ | 3.43 (0.33) | 6.89 (0.67) | 3.36 (0.38) | 7.07 (0.63) | |
|
| |||||
| Saccade Variability (SD) | HC | 0.70 (0.03) | 1.16 (0.12) | 0.71 (0.11) | 1.17 (0.13) |
| SZ | 0.79 (0.12) | 1.19 (0.18) | 0.81 (0.09) | 1.24 (0.16) | |
|
| |||||
| Saccade Latency (ms) | HC | 242.58 (34.6) | 257.64 (37.84) | 246.3(35.28) | 260.63 (38.2) |
| SZ | 265.79 (46.24) | 286.27 (38.77) | 262.59 (52.2) | 275.45 (43.62) | |
|
| |||||
| Manual Response RT (ms) | HC | 559.16 (152.42) | 578.82 (159.42) | 546.12 (153.28) | 572.46 (154.71) |
| SZ | 714.93 (226.94) | 761.76 (261.1) | 720.55 (227.73) | 769.85 (256.99) | |
|
| |||||
| Corrective Saccades (% of trials) | HC | 42.28 (2.29) | 44.33 (2.02) | 42.97 (2.10) | 44.59 (2.16) |
| SZ | 38.02 (2.54) | 42.95 (3.82) | 40.84 (2.91) | 43.78 (2.86) | |
Perceptual Performance
We investigated the detection of trans-saccadic shifts of visual targets and found that SZP, consistent with previous studies (40,41), had greater difficulty in detecting trans-saccadic shifts than HC (higher perceptual thresholds) [F(1,60)=21.71, P<0.001]. Thresholds increased with target amplitude for both groups [F(1,60)=13.29,P<0.001].
For the purpose of this report, we focus our analyses on perceptual bias as it provides a direct assay of perceptual estimation as related to eye movements. Detailed perceptual threshold results (and associated correlations) are provided in Table S1. For perceptual bias, there was a significant direction by group interaction, [F(1,60)=12.23,P=0.001]. SZP displayed more negative biases (−0.18 ± 0.75) than HC (0.32 ± 0.46), [F(1,60)=29.69, P<0.001], indicating SZP mostly underestimated whereas HC overestimated the pre-saccadic target location. Note that the average bias value does not reveal the overall distribution of the negative and positive bias values within each group. In the supplementary materials we provide the median and spread of the bias values to give information on the collective perceptual accuracy (Figure S1).
CD Utilization for Perceptual Judgments
To assess group similarities in saccade metrics, but differences in perceptual judgments, we compared the percent gain in saccade amplitude and percent error in target location (Figure 2). For both groups, the majority of eye movements were hypometric (91% in SZP and 97% in HC). In HC, despite movement undershoot, subjects largely (in 72.6% of cases) overestimated target location, whereas SZP overestimated target location in only 38.7% of cases. In addition, to determine whether the saccadic end-point error influenced shift detection judgments, we plotted the percent of forward responses as a function of saccade error (Figure S3). For HC, we see that perception is independent of saccade error, whereas for SZP, the forward perceptual report is influenced by the magnitude of this end point error.
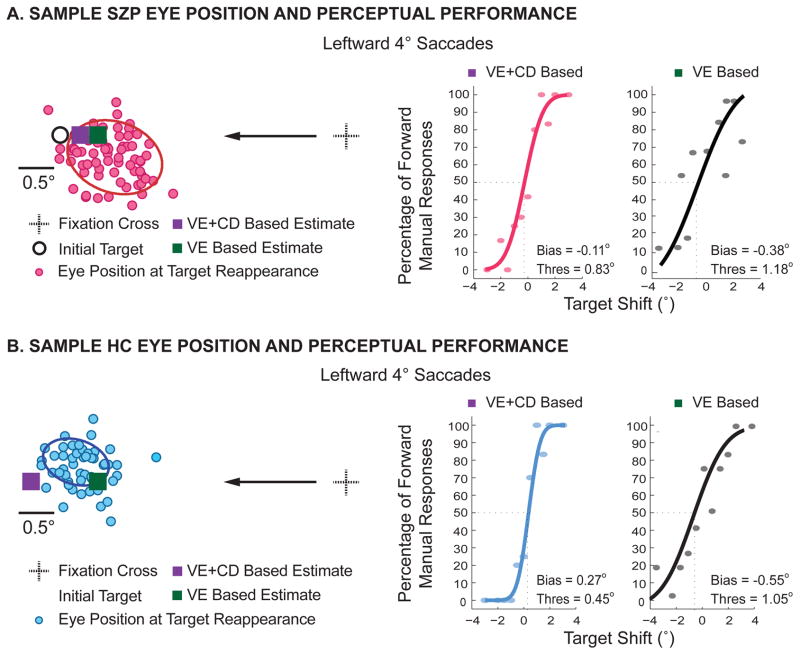
Figure 3 shows eye positions, VE-based and actual psychometric functions (VE+CD-based) for a sample SZP (Figure 3A) and HC (Figure 3B). For simplicity, we only show results for saccades to the leftward 4o target, but the results are consistent for the other amplitude and direction. For the SZP, post-saccade estimation of the pre-saccade target based on actual manual responses (purple square) is closely aligned to the estimate based solely on experienced visual error (green square). Importantly, both measures underestimate the true initial target location. This is not the case for the HC; while the VE-based measure underestimates the target location, the VE+CD measure based on the actual manual responses overestimates the location.
Figure 3. Example psychometric curves for leftward 4° saccades.
Eye positions of an example subject from each group for saccades to a leftward target at 4°. Eye position at the time the target reappeared are displayed as filled red (A, SZP) and blue (B, HC) circles, respectively. The initial target is marked by a white circle with a black outline, and the solid ellipses represent the spatial extent of the 1 SD confidence interval of eye position variability. (Note that we could not depict the shifted target because this displacement varied on each trial.) Purple and green squares represent the perceptual (Visual Error + CD-based) and hyopothetical Visual Error-based estimates of the target location, respectively. Visual Error-based and Visual Error + CD-based psychometric functions of perceived target shift are shown next to the corresponding eye movements. The corresponding values for bias and threshold are given in each plot.
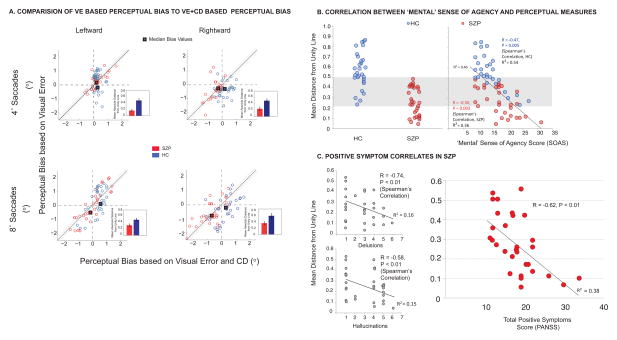
With respect to bias, the focus of the analysis was to assess differential CD utilization between the groups. Figure 4A displays perceptual biases derived from actual psychometric functions (based on VE+CD) on the abscissa, with biases derived from hypothetical VE curves on the ordinate. We found a significant interaction effect of group by condition (VE vs. VE+CD) by direction [F(1,60)=8.7, P=0.005], as well as a main effect of condition [F(1,60)= 5.974, P=0.018], and a condition by group effect [F(1,60)=12.778, P=0.001]. For HC, we found significant differences between VE-based and VE+CD-based biases (P<0.05 for all conditions) whereas in SZP, paired t-tests did not show any significant difference between VE-based biases and those based on VE+CD (Figure S5A). We further probed this relationship by examining dispersion of biases. We obtained a ‘distance-from-unity-line’ measure as a sensitive quantification of CD deficits to assay how the two perceptual bias derivations (VE+CD and VE only) were associated. Figure 4A shows that for patients, there is less dispersion and the majority of points lie close to the unity line, whereas for controls, points are more dispersed (Main effect of Group on ‘distance-from-unity-line’ measure [F(1,60)=32.02, P<0.001], more so for 8o saccades [F(1,60)=6.47, P=0.04]).
Figure 4. Clinical correaltions to behavioral measures.
(A) Individual data points (light circles, red for SZP and blue for HC) for perceptual bias. Estimates for visual error-based (ordinate) vs. visual error + CD-based (abscissa) are plotted for each target amplitude and direction. Square symbols with the thick black outline represent the mean values across the respective groups. We obtain a distance from the unity line measure as an assay of how closely the two estimates of perceptual bias matched. Points along the unity line designate subjects for whom the two biases are closely related, thus indicating the lack of CD utilization in the estimate of the pre-saccadic target location and an incorrect reliance on post-saccade sensory information. The distance of points lying above or below the line indicate the magnitude of misalignment between the two measures. The bar charts show mean absolute values indicating the magnitude of the distance from the unity line across the respective group. (B) Scatter plot showing correlations between mean distance from the unity line (across the two directions and movement amplitudes) and “Mental” SoA for SZP (Red circles) and HC (Blue circles), respectively with Spearman’s R and p-values displayed, as well as the R2 value for the regression line over the entire sample (SZP and HC combined). The grey shaded area shows overlap between HC and SZP subjects—across groups these subjects had similar mean distance-from-unity-line values. The HC within this range demonstarted perceptual judgments that were closely related to the hypothetical judgments derived from the experienced visual error, similar to most SZP. Thus, these HC had lower values for distance-from-unity-line measure and also demonstrated a disturbed subjective sense of mental agency. (C) Scatter plots showing significant correlation between mean distance from the unity line and Hallucinations, Delusions and Total PANSS score.
Clinical Correlates
Considering the postulated link between CD impairment and aberrant perception leading to symptoms of psychosis, we assessed the relationship between psychophysical perceptual measures and PANSS positive symptoms in SZP, and associations with SoA measures in both groups. We observed various associations as seen in Figures 4B and C.
Our primary variable of interest, the distance-from-unity-line measure is a novel quantification of CD deficit showing over-reliance on exogenous sensory feedback, rather than CD-generated internal monitoring in SZP. We observed robust associations (Figures 4B and C) between this measure and symptoms of psychosis (hallucinations and delusions) and PANSS Total Positive Symptoms in SZP and SoA measures in both SZP and HC. Subjects whose perceptual judgments were closely related to hypothetical judgments derived from the experienced visual error, having lower values for distance-from-unity-line, also demonstrated a disturbed subjective sense of mental agency. In HC, there were no correlations with Physical SoA (R=−0.14, P=0.45), nor with Social SoA (R=−0.013, P=0.95). For SZP, the distance-from-unity-line correlated with Physical SoA (R=−0.49, P=0.008), but not with Social SoA (R=0.20, P=0.28). Perceptual measures did not correlate with negative symptoms in SZP (R=0.12, P=0.42, Total PANSS Negative Symptoms).
DISCUSSION
We examined the ability of HC and SZP to detect trans-saccadic visual changes and show that for SZP, post-saccadic judgment of the initial target location was largely influenced by the experienced VE, suggesting a deficit in CD utilization. Consistent with previous studies (40,41), we provide evidence that SZP show impairments in the CD-based ability to remap visual targets following saccades and to make perceptual judgments of trans-saccadic target shifts. Similar to previous studies that related behavioral results to saccade metrics (38, 40), we obtained a sensitive measure to assess the type of information (retinal or extraretinal) subjects utilized to make perceptual judgments. We show that perceptual deficits in the task were selectively associated with positive symptoms in SZP, and demonstrate an association to SoA in both HC and SZP.
Behavior similarities between SZP and CD inactivation in nonhuman primates
Our results may relate to neural mechanisms involved in saccade CD. One of its transmission pathways (29,30,31,63,64) relays information pertaining to saccade metrics from the superior colliculus (SC) through the mediodorsal (MD) nucleus of the thalamus in order to update the receptive fields in frontal cortex (FEF). Recently, it has been shown that inactivating the MD relay alters perception in a similar trans-saccadic task (32). Additionally, thalamic lesion patients demonstrate deficits in making successive eye movements required for double-step saccades (34) and in perceptual performance in a trans-saccadic displacement task (38). These studies show that when the thalamic MD pathway is impaired, the ability to discriminate visual displacement becomes inaccurate. Cavanaugh and colleagues (32) showed that when the CD pathway was inactivated, there was a shift of the psychometric curve resulting in a perceptual bias that was more negative than the experimental control. This is the same difference we observe between our HC and SZP groups, suggesting that the inactivation resulted in an overreliance on the external sensory information, similar to the finding that SZP had more reliance on the VE in forming their perceptual decision. Trans-saccadic and similar tasks are an effective method for estimating properties of the CD signals that contribute to visual stability; however, our results do not necessarily insinuate visual instability in SZP, but rather an inefficient use of the CD in perception.
We can speculate that our findings in SZP are associated with disruptions to the SC-MD-FEF pathway, leading to imprecision of CD signal generation or relay. Additionally, there is evidence of neuroanatomical abnormalities in the MD of SZP. Young and colleagues (65) observed a post- reduced number of neurons and volume of MD in SZP. Reduction in glucose metabolism and size of MD in SZP (66–69) could disrupt the CD pathway (30,31,33,38), thus impeding the spatial updating required for visual stability. Studies have also shown compromised cortical-MD connectivity in SZP that is related to psychotic symptoms (70–72). Based on these neurobiological findings, it is possible that MD and associated pathway abnormalities underlie the CD impairments that contribute to symptoms of psychosis and disturbance of agency.
Sensory processing vs. impairments in internal monitoring of movement
Accurate anticipation of a self-generated action outcome (e.g., sensory consequences of a saccade) or perception of an environmental change (e.g., visual scene change) is based on internal motor predictions that guide SoA. It has been proposed that predictive or sensory feedback cues are weighted and integrated according to their relative efficiency, guiding the distinction between internally and externally generated stimuli (73,74). Such a context-dependent weighted integration of imprecise internal predictions and noisy external cues might explain misattributions of agency in SZP. Various visual perceptual (sensory processing) deficits have been found repeatedly in schizophrenia (for review see 75,76). Here, using quantitative, implicit measures we identify that the internal predictive information is likely responsible for these abnormalities, possibly ruling out impairment of sensory feedback processing. We deconstruct two elements of the comparator model that contribute to integration (internal prediction via CD signals, and sensory feedback via external, post-saccadic visual error), and demonstrate that SZP rely more on the sensory feedback (VE). This is corroborated by lack of significant group differences in saccade metrics. We observe that SZP are able to plan movement just as HC, but there is a possible breakdown in utilization of the CD for post-saccadic prediction of movement metrics. Whereas HC are able to implicitly make adjustments to perception, SZP appear to heavily rely on experienced post-saccadic VE, suggesting that sensory processing and saccade metrics of SZP are intact (within this paradigm), but a diminished internal prediction accompanying these movements drives the observed perceptual deficits.
Finally, we acknowledge that CD may not fully account for conscious experience about one’s actions. Previous studies (77,78) have shown, in certain patient groups, intact compensation for conflict between predicted sensory signals and outcomes. This may indicate that the CD, within the comparator/forward model has more of a role in awareness of the discrepancy between movement and its consequences, versus automatic compensation for conflict. Nonetheless, the current perceptual paradigm is an effective method for examining the role of movement-related CD signals in complex self-monitoring systems from which a sense of agency is derived.
Supplementary Material
Figure S1: For each direction and amplitude respectively, we show the spread of perceptual bias values as indicated by the boxplots and standard error as notches. We observe much higher variability in bias values for the VE-only condition, with the majority of the values falling below 0 (negative) for patients, while being less variable and more positive for controls.
Figure S2: Saccade amplitude over course of the experiment for each group (Red=SZP, Blue=HC), per amplitude, direction and shift direction. We observed no systematic change in saccade amplitude over the course of the experiment for each condition.
Figure S3: For each condition, we plot mean percentage of forward responses in each group as a function of primary saccade end point error. We binned saccade error into 0.5° bins ranging from −3° to 3° and derived the mean percentage of forward responses per bin for each subject group. A positive saccade error indicates that the target was ahead of the saccade end point, and a negative error indicates that the saccade was hypermetric and the target was behind the end point. Considering the comparator model and CD theory of predictive remapping, if subjects had impaired CD, and were relying on the sensory feedback from their saccadic end point position rather than the remapped pre-saccadic target location, they would show a higher percent of forward responses for increasingly positive saccadic error (More hypometric saccades). For HC, we found no significant association between saccade error and proportion of forward responses. (P > 0.1 in all cases). However, for patients, for all but the rightward 4 degree saccades, (P = 0.24), we see a significant positive association between saccade error and the percent of forward responses (P < 0.02 in all the remaining cases). Thus, for HC, perception is largely independent of eye movement variability /saccade error, whereas for SZP, the forward perceptual report is influenced by the magnitude of this end point error.
Figure S4: Due to hardware/software system limitations, there was some amount of variance in the online detection of saccades. We derived the duration for saccades meeting criteria (occurring ≥75 ms after the initial target appeared and velocity ≥75°/s and acceleration ≥2000°/s2) as the time the velocity first rose (onset) and then fell below 75°/s (offset). Of course this is a conservative estimate of the duration and it is likely that the saccade was still in flight at the time point we marked as its offset. We also obtained a software-generated timestamp for the corresponding saccade onset and offset. To determine whether saccade offset was prior to initial target offset, we calculated the difference between the saccade offset timestamp, and the timestamp of target offset. These timestamps did not take into account monitor lag. The trials in which the movement was completed before the target offset were identified as those for which this difference was negative (Target Offset Timestamp - Saccade offset Timestamp). A. The values in the top rows of the table are the mean saccade durations (standard error) in ms. The values in the bottom rows of the table are the mean percentage (standard error) of trials in which saccade offset occurred before target offset. There was a percentage of trials in which the movement was completed before the target was extinguished (~24% in 4 degree target trials and ~ 7% of cases for 8 degree trials). For the rest of the trials, for HC, the target disappeared 10.0 +/− 2.9 ms before saccade offset (13.8 +/− 4.2 ms for the 8 degree target) and for SZP, the target disappeared 9.7+/− 2.5 ms before saccade offset (10.9 +/− 3.4 ms for the 8 degree target). B. For the majority of trials (93.9 ± 5.8% for HC, 92.7 ± 3.7% for SZP) the difference between saccade and target offset was < 15 ms.
Figure S5 A. Perceptual Bias Post ANOVA Paired T-test P-values for full and saccade-offset filtered datasets. B and C. We filtered out trials in which saccade offset occurred prior to target offset and obtained identical measures as for the main full dataset. For the Distance-From-Unity-Line measure, we observe similar results to the full dataset; there is less dispersion (lower absolute distance-from-unity-line magnitude) for SZP, whereas for HC, there is more dispersion. Main effect of group on distance-from-unity-line measure [F(1,38)=9.91,P=0.003]. For 8° saccades, there is more dispersion (main effect of amplitude on distance-from-unity-line measure [F(1,38)=28.56,P<0.001]). D and E. We show correlations between distance-from-unity-line measure (from saccade offset-filtered dataset) and SoA ‘Mental’ agency and Positive symptoms in SZP.
Figure S6: Association between perceptual deficits and positive symptoms in SZP.
Table S1: Perceptual Threshold Values
Acknowledgments
This work was supported by a grant from the National Eye Institute (R00 EY021252) to WMJ.
Footnotes
Financial Disclosures: All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Haggard P, Martin F, Taylor-Clarke M, Jeannerod M, Franck N. Awareness of action in schizophrenia. Neuroreport. 2003;14(7):1081–1085. doi: 10.1097/01.wnr.0000073684.00308.c0. [DOI] [PubMed] [Google Scholar]
- 2.Keefe RSE, Arnold MC, Bayen UJ, McEvoy JP, Wilson WH. Source-monitoring deficits for self-generated stimuli in schizophrenia: Multinomial modeling of data from three sources. Schizophrenia Research. 2002;57:51–67. doi: 10.1016/s0920-9964(01)00306-1. [DOI] [PubMed] [Google Scholar]
- 3.Gallagher S. Neurocognitive models of schizophrenia: a neurophenomenological critique. Psychopathology. 2004;37(1):8–19. doi: 10.1159/000077014. [DOI] [PubMed] [Google Scholar]
- 4.Maeda T, Kato M, Muramatsu T, Iwashita S, Mimura M, Kashima H. Aberrant sense of agency in patients with schizophrenia: Forward and backward over-attribution of temporal causality during intentional action. Psychiatry research. 2012;198(1):1–6. doi: 10.1016/j.psychres.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 5.Maeda T, Takahata K, Muramatsu T, Okimura T, Koreki A, Iwashita S, Kato M. Reduced sense of agency in chronic schizophrenia with predominant negative symptoms. Psychiatry research. 2013;209(3):386–392. doi: 10.1016/j.psychres.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 6.Werner JD, Trapp K, Wüstenberg T, Voss M. Self-attribution bias during continuous action-effect monitoring in patients with schizophrenia. Schizophrenia Research. 2014;152(1):33–40. doi: 10.1016/j.schres.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 7.Helmholtz HV. Concerning the perceptions in general. Treatise on physiological optics. 1866:3. [Google Scholar]
- 8.Held R, Freedman SJ. Plasticity in human sensorimotor control. Science. 1963;142(3591):455–462. doi: 10.1126/science.142.3591.455. [DOI] [PubMed] [Google Scholar]
- 9.Sperry RW. Neural basis of the spontaneous optokinetic response produced by visual inversion. Journal of Computational Physiology Psychology. 1950;43(6):482. doi: 10.1037/h0055479. [DOI] [PubMed] [Google Scholar]
- 10.Holst E, Mittelstaedt H. Das reafferenzprinzip. Naturwissenschaften. 1950;37(20):464–476. [Google Scholar]
- 11.Gallagher S. Philosophical conceptions of the self: implications for cognitive science. Trends in Cognitive Sciences. 2000;4:14–21. doi: 10.1016/s1364-6613(99)01417-5. [DOI] [PubMed] [Google Scholar]
- 12.Frith CD, Blakemore S, Wolpert DM. Explaining the symptom of schizophrenia Abnormalities in the awareness of action. Brain Research Brain Research Reviews. 2000;31:357–363. doi: 10.1016/s0165-0173(99)00052-1. [DOI] [PubMed] [Google Scholar]
- 13.Feinberg I. Efference copy and corollary discharge. Schizophrenia bulletin. 1978;4(4):636–640. doi: 10.1093/schbul/4.4.636. [DOI] [PubMed] [Google Scholar]
- 14.Blakemore SJ, Wolpert DM, Frith CD. Abnormalities in the awareness of action. Trends in cognitive sciences. 2002;6(6):237–242. doi: 10.1016/s1364-6613(02)01907-1. [DOI] [PubMed] [Google Scholar]
- 15.Fletcher PC, Frith CD. Perceiving is believing: a Bayesian approach to explaining the positive symptoms of schizophrenia. Nature Reviews Neuroscience. 2009;10(1):48–58. doi: 10.1038/nrn2536. [DOI] [PubMed] [Google Scholar]
- 16.Jeannerod M. The sense of agency and its disturbances in schizophrenia: a reappraisal. Experimental Brain Research. 2009;192(3):527–532. doi: 10.1007/s00221-008-1533-3. [DOI] [PubMed] [Google Scholar]
- 17.Frith CD. The cognitive neuropsychology of schizophrenia. Psychology Press; 1992. [Google Scholar]
- 18.Ford JM, Mathalon D, Kalba S, Whitfield S, Faustman W, Roth W. Cortical responsiveness during inner speech in schizophrenia: an event-related potential study. American Journal of Psychiatry. 2001;158:1914–1916. doi: 10.1176/appi.ajp.158.11.1914. [DOI] [PubMed] [Google Scholar]
- 19.Ford JM, Mathalon DH, Whitfield S, Faustman WO, Roth WT. Reduced communication between frontal and temporal lobes during talking in schizophrenia. Biological Psychiatry. 2002;21:485–492. doi: 10.1016/s0006-3223(01)01335-x. [DOI] [PubMed] [Google Scholar]
- 20.Ford JM, Mathalon DH. Corollary discharge dysfunction in schizophrenia: can it explain auditory hallucinations? International Journal of Psychophysiology. 2005;58(2–3):179–89. doi: 10.1016/j.ijpsycho.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 21.Ford JM, Mathalon DH. Electrophysiological evidence of corollary discharge dysfunction in schizophrenia during talking and thinking. Journal of Psychiatry Research. 2004;38(1):37–46. doi: 10.1016/s0022-3956(03)00095-5. [DOI] [PubMed] [Google Scholar]
- 22.Ford JM, Mathalon DH, Kalba S, Whitfield S, Faustman WO, Roth WT. Cortical responsiveness during talking and listening in schizophrenia: an event-related brain potential study. Biological Psychiatry. 2001;50(7):540–549. doi: 10.1016/s0006-3223(01)01166-0. [DOI] [PubMed] [Google Scholar]
- 23.Ford JM, Gray M, Faustman WO, Heinks TH, Mathalon DH. Reduced gamma-band coherence to distorted feedback during speech when what you say is not what you hear. International Journal of Psychophysiology. 2005;57(2):143–150. doi: 10.1016/j.ijpsycho.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Blakemore SJ, Wolpert DM, Frith CD. The cerebellum contributes to somatosensory cortical activity during self-produced tactile stimulation. Neuroimage. 1999;10(4):448–459. doi: 10.1006/nimg.1999.0478. [DOI] [PubMed] [Google Scholar]
- 25.Shergill SS, Bays PM, Frith CD, Wolpert DM. Two eyes for an eye: the neuroscience of force escalation. Science. 2003;301(5630):187–187. doi: 10.1126/science.1085327. [DOI] [PubMed] [Google Scholar]
- 26.Shergill SS, Samson G, Bays PM, Frith CD, Wolpert DM. Evidence for sensory prediction deficits in schizophrenia. American Journal of Psychiatry. 2014;162(12):2384–2386. doi: 10.1176/appi.ajp.162.12.2384. [DOI] [PubMed] [Google Scholar]
- 27.Leigh RJ, Zee DS. The neurology of eye movements. Vol. 90. Oxford University Press; USA: 2015. [Google Scholar]
- 28.Joiner WM, Cavanaugh J, FitzGibbon EJ, Wurtz RH. Corollary discharge contributes to perceived eye location in monkeys. Journal of Neurophysiology. 2013;110(10):2402–2413. doi: 10.1152/jn.00362.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sommer MA, Wurtz RH. A pathway in primate brain for internal monitoring of movements. Science. 2002;296(5572):1480–1482. doi: 10.1126/science.1069590. [DOI] [PubMed] [Google Scholar]
- 30.Sommer MA, Wurtz RH. What the brain stem tells the frontal cortex. I. Oculomotor signals sent from superior colliculus to frontal eye field via mediodorsal thalamus. Journal of Neurophysiology. 2004a;91(3):1381–1402. doi: 10.1152/jn.00738.2003. [DOI] [PubMed] [Google Scholar]
- 31.Sommer MA, Wurtz RH. What the brain stem tells the frontal cortex. II. Role of the SC-MD-FEF pathway in corollary discharge. Journal of Neurophysiology. 2004b;91(3):1403–1423. doi: 10.1152/jn.00740.2003. [DOI] [PubMed] [Google Scholar]
- 32.Cavanaugh J, Berman RA, Joiner WM, Wurtz RH. Saccadic corollary discharge underlies stable visual perception. Journal of Neuroscience. 2016;36(1):31–42. doi: 10.1523/JNEUROSCI.2054-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bellebaum C, Daum I, Koch B, Schwarz M, Hoffmann KP. The role of the human thalamus in processing corollary discharge. Brain. 2005;128(5):1139–1154. doi: 10.1093/brain/awh474. [DOI] [PubMed] [Google Scholar]
- 34.Bellebaum C, Hoffmann KP, Koch B, Schwarz M, Daum I. Altered processing of corollary discharge in thalamic lesion patients. European Journal of Neuroscience. 2006;24(8):2375–2388. doi: 10.1111/j.1460-9568.2006.05114.x. [DOI] [PubMed] [Google Scholar]
- 35.Bridgeman B, Hendry D, Stark L. Failure to detect displacement of the visual world during saccadic eye movements. Visual Research. 1975;15:719–722. doi: 10.1016/0042-6989(75)90290-4. [DOI] [PubMed] [Google Scholar]
- 36.Duhamel JR, Colby CL, Goldberg ME. The updating of the representation of visual space in parietal cortex by intended eye movements. Science. 1992;255(5040):90. doi: 10.1126/science.1553535. [DOI] [PubMed] [Google Scholar]
- 37.Joiner WM, FitzGibbon EJ, Wurtz RH. Amplitudes and directions of individual saccades can be adjusted by corollary discharge. Journal of Vision. 2010;10(2):221–2212. doi: 10.1167/10.2.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ostendorf F, Liebermann D, Ploner CJ. Human thalamus contributes to perceptual stability across eye movements. Proceedings of the National Academy of Sciences. 2010;107(3):1229–1234. doi: 10.1073/pnas.0910742107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thakkar KN, Schall JD, Logan GD, Park S. Response inhibition and response monitoring in a saccadic double-step task in schizophrenia. Brain and cognition. 2015;95:90–98. doi: 10.1016/j.bandc.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rösler L, Rolfs M, Van der Stigchel S, Neggers SF, Cahn W, Kahn RS, Thakkar KN. Failure to use corollary discharge to remap visual target locations is associated with psychotic symptom severity in schizophrenia. Journal of Neurophysiology. 2015;114(2):1129–1136. doi: 10.1152/jn.00155.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Richard A, Churan J, Whitford V, O'Driscoll GA, Titone D, Pack CC. Perisaccadic perception of visual space in people with schizophrenia. The Journal of Neuroscience. 2014;34(14):4760–4765. doi: 10.1523/JNEUROSCI.4744-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coesmans M, Röder CH, Smit AE, Koekkoek SK, De Zeeuw CI, Frens MA, van der Geest JN. Cerebellar motor learning deficits in medicated and medication-free men with recent-onset schizophrenia. Journal of psychiatry & neuroscience: JPN. 2014;39(1):E3–E11. doi: 10.1503/jpn.120205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Picard H, Le Seac'h A, Amado I, Gaillard R, Krebs MO, Beauvillain C. Impaired saccadic adaptation in schizophrenic patients with high neurological soft sign scores. Psychiatry research. 2012;199(1):12–18. doi: 10.1016/j.psychres.2012.04.039. [DOI] [PubMed] [Google Scholar]
- 44.Thaker GK, Ross DE, Buchanan RW, Moran MJ, Lahti A, Kim C, Medoff D. Does pursuit abnormality in schizophrenia represent a deficit in the predictive mechanism? Psychiatry research. 1996;59(3):221–237. doi: 10.1016/0165-1781(95)02759-9. [DOI] [PubMed] [Google Scholar]
- 45.Hong LE, Avila MT, Adami H, Elliot A, Thaker GK. Components of the smooth pursuit function in deficit and nondeficit schizophrenia. Schizophrenia research. 2003;63(1):39–48. doi: 10.1016/s0920-9964(02)00388-2. [DOI] [PubMed] [Google Scholar]
- 46.Hong LE, Avila MT, Thaker GK. Response to unexpected target changes during sustained visual tracking in schizophrenic patients. Experimental brain research. 2005;165(1):125–131. doi: 10.1007/s00221-005-2276-z. [DOI] [PubMed] [Google Scholar]
- 47.Hong LE, Tagamets M, Avila M, Wonodi I, Holcomb H, Thaker GK. Specific motion processing pathway deficit during eye tracking in schizophrenia: a performance-matched functional magnetic resonance imaging study. Biological Psychiatry. 2005;57(7):726–732. doi: 10.1016/j.biopsych.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 48.Lindner A, Their P, Kircher TT, Haarmeier T, Leube DT. Disorders of agency in schizophrenia correlate with an inability to compensate for the sensory consequences of actions. Current Biology. 2005;15(12):1119–1124. doi: 10.1016/j.cub.2005.05.049. [DOI] [PubMed] [Google Scholar]
- 49.Spering M, Dias EC, Sanchez JL, Schütz AC, Javitt DC. Efference copy failure during smooth pursuit eye movements in schizophrenia. The Journal of Neuroscience. 2013;33(29):11779–11787. doi: 10.1523/JNEUROSCI.0578-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guthrie BL, Porter JD, Sparks DL. Corollary discharge provides accurate eye position information to the oculomotor system. Science. 1983;221(4616):1193–1195. doi: 10.1126/science.6612334. [DOI] [PubMed] [Google Scholar]
- 51.Lewis RF, Zee DS, Hayman MR, Tamargo RJ. Oculomotor function in the rhesus monkey after deafferentation of the extraocular muscles. Experimental Brain Research. 2001;141(3):349–358. doi: 10.1007/s002210100876. [DOI] [PubMed] [Google Scholar]
- 52.Wang X, Zhang M, Cohen IS, Goldberg ME. The proprioceptive representation of eye position in monkey primary somatosensory cortex. Nature Neuroscience. 2007;10(5):640–646. doi: 10.1038/nn1878. [DOI] [PubMed] [Google Scholar]
- 53.Bray LCJ, Bansal S, Joiner WM. Quantifying the spatial extent of the corollary discharge benefit to trans-saccadic visual perception. Journal of neurophysiology. 2016;15(3):1132–1145. doi: 10.1152/jn.00657.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bansal S, Bray LCJ, Peterson MS, Joiner WM. The effect of saccade metrics on the corollary discharge contribution to perceived eye location. Journal of neurophysiology. 2015;113(9):3312–3322. doi: 10.1152/jn.00771.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kay SR, Flszbein A, Opfer LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia bulletin. 1987;13(2):261. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 56.Asai T, Sugimori E, Tanno Y. Schizotypal personality traits and atypical lateralization in motor and language functions. Brain and Cognition. 2009a;71:26–37. doi: 10.1016/j.bandc.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 57.Asai T, Takano K, Sugimori E, Tanno Y. Development of the sense of agency scale and its factor structure. The Japanese Journal of Psychology. 2009b;80:414–421. doi: 10.4992/jjpsy.80.414. (in Japanese) [DOI] [PubMed] [Google Scholar]
- 58.Asai T, Sugimori E, Tanno Y. Auditory verbal hallucination in schizophrenic patients and the general population: the sense of agency in speech. Hallucinations: Types, Stages and Treatments. 2011:33–60. [Google Scholar]
- 59.Deubel H, Schneider W, Bridgeman B. Postsaccadic target blanking prevents saccadic suppression of image displacement. Vision research. 1996;36(7):985–996. doi: 10.1016/0042-6989(95)00203-0. [DOI] [PubMed] [Google Scholar]
- 60.Collins T, Rolfs M, Deubel H, Cavanagh P. Post-saccadic location judgments reveal remapping of saccade targets to non-foveal locations. Journal of vision. 2009;9(5):29–29. doi: 10.1167/9.5.29. [DOI] [PubMed] [Google Scholar]
- 61.Li W, Matin L. The influence of saccade length on the saccadic suppression of displacement detection. Perception & Psychophysics. 1990;48(5):453–458. doi: 10.3758/bf03211589. [DOI] [PubMed] [Google Scholar]
- 62.Li W, Matin L. Saccadic suppression of displacement: separate influences of saccade size and of target retinal eccentricity. Vision research. 1997;37(13):1779–1797. doi: 10.1016/s0042-6989(96)00301-x. [DOI] [PubMed] [Google Scholar]
- 63.Crapse TB, Sommer MA. Corollary discharge across the animal kingdom. Nature Reviews Neuroscience. 2008;9(8):587–600. doi: 10.1038/nrn2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sommer MA, Wurtz RH. Influence of the thalamus on spatial visual processing in frontal cortex. Nature. 2006;444(7117):374–7. doi: 10.1038/nature05279. [DOI] [PubMed] [Google Scholar]
- 65.Young KA, Manaye KF, Liang CL, Hicks PB, German DC. Reduced number of mediodorsal and anterior thalamic neurons in schizophrenia. Biological Psychiatry. 2000;47(11):944–53. doi: 10.1016/s0006-3223(00)00826-x. [DOI] [PubMed] [Google Scholar]
- 66.Buchsbaum MS, Someya T, Teng CY, Abel L, Chin S, Najafi A, et al. PET and MRI of the thalamus in never–medicated patients with schizophrenia. American Journal of Psychiatry. 1996;153(2):191–9. doi: 10.1176/ajp.153.2.191. [DOI] [PubMed] [Google Scholar]
- 67.Byne W, Buchsbaum MS, Kemether E, Hazlett EA, Shinwari A, Mitropoulou V, Siever LJ. Magnetic resonance imaging of the thalamic mediodorsal nucleus and pulvinar in schizophrenia and schizotypal personality disorder. Archives of General Psychiatry. 2001;58(2):133–40. doi: 10.1001/archpsyc.58.2.133. [DOI] [PubMed] [Google Scholar]
- 68.Byne W, Hazlett EA, Buchsbaum MS, Kemether E. The thalamus and schizophrenia: current status of research. Actaneuropathologica. 2009;117(4):347–368. doi: 10.1007/s00401-008-0404-0. [DOI] [PubMed] [Google Scholar]
- 69.Kemether EM, Buchsbaum MS, Byne W, Hazlett EA, Haznedar M, Brickman AM, et al. Magnetic resonance imaging of mediodorsal, pulvinar, and centromedian nuclei of the thalamus in patients with schizophrenia. Archives of general psychiatry. 2003;60(10):983–991. doi: 10.1001/archpsyc.60.9.983. [DOI] [PubMed] [Google Scholar]
- 70.Anticevic A, Yang G, Savic A, Murray JD, Cole MW, Repovs G, et al. Mediodorsal and visual thalamic connectivity differ in schizophrenia and bipolar disorder with and without psychosis history. Schizophrenia bulletin. 2014;40(6):1227–1243. doi: 10.1093/schbul/sbu100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shinn AK, Baker JT, Cohen BM, Öngür D. Functional connectivity of left Heschl's gyrus in vulnerability to auditory hallucinations in schizophrenia. Schizophrenia research. 2013;143(2):260–268. doi: 10.1016/j.schres.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Woodward ND, Karbasforoushan H, Heckers S. Thalamocortical dysconnectivity in schizophrenia. American Journal of Psychiatry. 2012;169(10):1092–1099. doi: 10.1176/appi.ajp.2012.12010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Frith CD. The cognitive neuropsychology of schizophrenia. Psychology Press; 2014. [Google Scholar]
- 74.Ondobaka S, de Lange FP, Wittmann M, Frith CD, Bekkering H. Interplay between conceptual expectations and movement predictions underlies action understanding. Cerebral Cortex. 2015;25(9):2566–2573. doi: 10.1093/cercor/bhu056. [DOI] [PubMed] [Google Scholar]
- 75.Silverstein SM, Keane BP. Perceptual organization impairment in schizophrenia and associated brain mechanisms: review of research from 2005 to 2010. Schizophrenia Bulletin. 2011a;37:690–699. doi: 10.1093/schbul/sbr052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Butler PD, Silverstein SM, Dakin SC. Visual perception and its impairment in schizophrenia. Biological Psychiatry. 2008;64:40–47. doi: 10.1016/j.biopsych.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fourneret P, Paillard J, Lamarre Y, Cole J, Jeannerod M. Lack of conscious recognition of one's own actions in a haptically deafferented patient. Neuroreport. 2002;13(4):541–7. doi: 10.1097/00001756-200203250-00036. [DOI] [PubMed] [Google Scholar]
- 78.Knoblich G, Stottmeister F, Kircher T. Self-monitoring in patients with schizophrenia. Psychological medicine. 2004;34(08):1561–9. doi: 10.1017/s0033291704002454. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: For each direction and amplitude respectively, we show the spread of perceptual bias values as indicated by the boxplots and standard error as notches. We observe much higher variability in bias values for the VE-only condition, with the majority of the values falling below 0 (negative) for patients, while being less variable and more positive for controls.
Figure S2: Saccade amplitude over course of the experiment for each group (Red=SZP, Blue=HC), per amplitude, direction and shift direction. We observed no systematic change in saccade amplitude over the course of the experiment for each condition.
Figure S3: For each condition, we plot mean percentage of forward responses in each group as a function of primary saccade end point error. We binned saccade error into 0.5° bins ranging from −3° to 3° and derived the mean percentage of forward responses per bin for each subject group. A positive saccade error indicates that the target was ahead of the saccade end point, and a negative error indicates that the saccade was hypermetric and the target was behind the end point. Considering the comparator model and CD theory of predictive remapping, if subjects had impaired CD, and were relying on the sensory feedback from their saccadic end point position rather than the remapped pre-saccadic target location, they would show a higher percent of forward responses for increasingly positive saccadic error (More hypometric saccades). For HC, we found no significant association between saccade error and proportion of forward responses. (P > 0.1 in all cases). However, for patients, for all but the rightward 4 degree saccades, (P = 0.24), we see a significant positive association between saccade error and the percent of forward responses (P < 0.02 in all the remaining cases). Thus, for HC, perception is largely independent of eye movement variability /saccade error, whereas for SZP, the forward perceptual report is influenced by the magnitude of this end point error.
Figure S4: Due to hardware/software system limitations, there was some amount of variance in the online detection of saccades. We derived the duration for saccades meeting criteria (occurring ≥75 ms after the initial target appeared and velocity ≥75°/s and acceleration ≥2000°/s2) as the time the velocity first rose (onset) and then fell below 75°/s (offset). Of course this is a conservative estimate of the duration and it is likely that the saccade was still in flight at the time point we marked as its offset. We also obtained a software-generated timestamp for the corresponding saccade onset and offset. To determine whether saccade offset was prior to initial target offset, we calculated the difference between the saccade offset timestamp, and the timestamp of target offset. These timestamps did not take into account monitor lag. The trials in which the movement was completed before the target offset were identified as those for which this difference was negative (Target Offset Timestamp - Saccade offset Timestamp). A. The values in the top rows of the table are the mean saccade durations (standard error) in ms. The values in the bottom rows of the table are the mean percentage (standard error) of trials in which saccade offset occurred before target offset. There was a percentage of trials in which the movement was completed before the target was extinguished (~24% in 4 degree target trials and ~ 7% of cases for 8 degree trials). For the rest of the trials, for HC, the target disappeared 10.0 +/− 2.9 ms before saccade offset (13.8 +/− 4.2 ms for the 8 degree target) and for SZP, the target disappeared 9.7+/− 2.5 ms before saccade offset (10.9 +/− 3.4 ms for the 8 degree target). B. For the majority of trials (93.9 ± 5.8% for HC, 92.7 ± 3.7% for SZP) the difference between saccade and target offset was < 15 ms.
Figure S5 A. Perceptual Bias Post ANOVA Paired T-test P-values for full and saccade-offset filtered datasets. B and C. We filtered out trials in which saccade offset occurred prior to target offset and obtained identical measures as for the main full dataset. For the Distance-From-Unity-Line measure, we observe similar results to the full dataset; there is less dispersion (lower absolute distance-from-unity-line magnitude) for SZP, whereas for HC, there is more dispersion. Main effect of group on distance-from-unity-line measure [F(1,38)=9.91,P=0.003]. For 8° saccades, there is more dispersion (main effect of amplitude on distance-from-unity-line measure [F(1,38)=28.56,P<0.001]). D and E. We show correlations between distance-from-unity-line measure (from saccade offset-filtered dataset) and SoA ‘Mental’ agency and Positive symptoms in SZP.
Figure S6: Association between perceptual deficits and positive symptoms in SZP.
Table S1: Perceptual Threshold Values