Abstract
An exuberant inflammatory response may exacerbate the primary tissue damage caused by injuries to the skin due to burns, surgery, excessive pressure, and other etiologies, thus increasing the time to heal. We hypothesized that application of factors that decrease inflammation would allow the skin to more quickly restore its barrier function, and promote the return to homeostasis. Resolvins are endogenous, pro-resolving lipid mediators derived from omega-3 fatty acids that serve to inhibit neutrophil migration and enhance macrophage phagocytosis, thus promoting the resolution of inflammation and the beginning of the proliferative phase of wound healing. Resolvins are derived either from docosahexaenoic (D-series) or eicosapentaenoic (E-series) acid. Herein, we compare the effects of resolvins D1 (RvD1), D2 (RvD2) and E1 (RvE1) on their abilities to inhibit neutrophil migration in vitro and to promote wound healing in vivo. In Transwell experiments, all resolvins inhibited neutrophil migration, with RvE1 being the most effective at a 2000nM concentration. In an in vivo murine excisional wound (1cm × 1cm) healing model, topically applied resolvins accelerated wound closure. RvE1-treated wounds healed by 19.4 ± 1.5 days post-wounding, which was significantly shorter than the RvD2-treated and RvD1-treated groups (p<0.05), which closed by an average of 22.8 ± 1.8 and 24.4 ± 2.2 days, respectively. Furthermore, all resolvin-treated groups healed faster than vehicle controls (p<0.05), which closed at 28.6 ± 1.5 days. There was a strong linear correlation (R2=0.9384) between each resolvin’s potency in inhibiting neutrophil migration in vitro versus accelerating wound healing in vivo. Furthermore, upon histological analysis, the RvE1-treated group exhibited more mature collagen organization and reepithelialization.
Keywords: Resolvins, Wound Healing, Inflammation, Skin
1. Introduction
Normal wound healing is a process that is highly dependent on the resolution of inflammation in order to successfully proceed towards completion. Several high-risk wounds that often exhibit persistent inflammation include surgical wounds, burn wounds, and pressure sores; in individuals who have impaired vascular functions (e.g. advanced diabetes), such wounds can become chronic. Wound care is a massive industry in the United States, amounting to approximately $20 billion dollars per year,1 due in part to costly, and often only partially effective treatments for chronic wounds. The extent of the problem is likely to worsen due to the increasing prevalence of obesity and diabetes. Thus, there is an urgent need to develop more effective wound healing therapies, as wounds that fail to close pose a major risk of invasive infection and limb amputation.
Before wound closure can occur, the inflammatory phase must be resolved. The abundance of proteases and phagocytes that sterilize the wound during the early inflammatory phase must be cleared in order to create an environment that can support cell and tissue growth during the proliferative phase. Resolvins are endogenous lipid mediators derived from omega-3 (ω-3) fatty acids that play a role in the resolution of inflammation. They are produced by neutrophils and macrophages in peripheral blood, which then travel to areas of inflammation to locally affect resolution.2,3 The fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are enzymatically converted to form E-series and D-series resolvins, respectively.4,5 Resolvins from DHA and EPA only occur at low levels during homeostasis, but they are significantly upregulated in inflammatory conditions in healthy individuals.5 However, chronic diseases, such as diabetes, result in decreased levels of resolvins due to impaired biosynthetic processes.5,6 Since the resolution of inflammation is a key feature of the proliferative phase of wound healing, it is reasonable to hypothesize that exogenously applied resolvins may hold potential as wound care therapeutics.
Resolvins have shown benefits in animal models of peritonitis, burn sepsis, bone regeneration, dry eye, and diabetes.5–10 Their main effects include reduced neutrophil migration and increased macrophage phagocytosis.2 One study used an acute inflammatory lung murine model induced by allergin aerosol airway challenge. Intravenous RvE1 decreased the infiltration of eosinophils, macrophages and lymphocytes in both periods of inflammatory initiation and resolution. Histologically, RvE1-treated mice had morphology similar to normal lung tissue.11 RvE1 used in a murine peritonitis model also decreased neutrophil infiltration and increased macrophage phagocytosis. When the resolvin biosynthetic pathway was inhibited, inflammation resolution was impaired, and could be rescued with exogenous RvE1.7
In the context of wound healing, intradermal injections of RvD1 into excisional wounds have been shown to accelerate wound closure in diabetic (db/db) mice. Furthermore, granulation tissue thickness increased and pro-inflammatory “M1” macrophage numbers decreased.6 Systemically administered RvD2 in mice with partial thickness burns decreased tissue necrosis, preserved the microvascular network, and also decreased the levels of inflammatory markers.12 While several studies report using resolvins RvD1, RvD2 and RvE1 in several inflammatory and injury settings, there is no head-to-head comparison of the potency of the different types of resolvins. Herein, we compare the inhibitory activities of RvD1, RvD2 and RvE1 on neutrophil migration in vitro, and determine whether this activity correlates with the propensity of the same resolvins to stimulate wound healing in vivo.
2. Methods
2.1. In vitro materials
RvD1, RvD2 and RvE1 were purchased from Cayman Laboratories a stock solutions containing 25µg in 250µl ethanol (266µM). Neutrophil migration studies were conducted in Corning HTS Transwell® 24 well plates with 8µm pore size membranes. Cells were cultured in Roswell Park Memorial Institute (RPMI) – 500ml supplemented with 8ml L-glutamine, 3g HEPES (Fisher Scientific), 10% (50ml) fetal bovine serum (FBS, Invitrogen), and 1% (5ml) Penicillin Streptomycin.
2.2 Cell culture
Neutrophil-like cells were derived from Human Promyelocytic Leukemia (HL-60) (American Type Cell Culture; Manassas, VA) cells through differentiation by culture in RPMI medium supplemented with 1.3% with dimethyl sulfoxide (DMSO). After 5 days, differentiated cells (dHL60) attached to the bottom of the flask. The cells were trypsinized, washed, counted, and re-suspended to obtain a concentration of at least 106 cells/mL of culture medium in preparation for the migration assay.
2.3 Migration assay
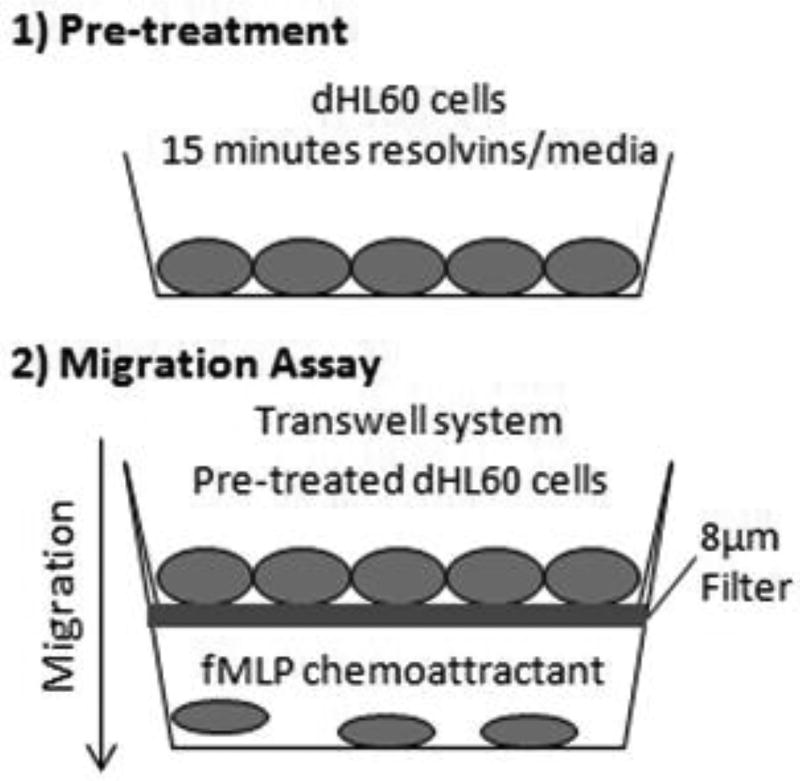
Figure 1 provides a schematic of the resolvin pre-treatment and neutrophil migration assay. RvD1, RvD2 and RvE1 were diluted to 500nM or 2000nM in RPMI medium. 5ml of dHL60 cells were exposed to either 5ml of RPMI medium with RvD1, RvD2 or RvE1 (500nM or 2000nM), or 5ml of RPMI medium vehicle (control) for 15 min at 37°C. 600µl of 100nM N-formyl-methionine-leucine-phenylalanine (fMLP), a strong neutrophil chemoattractant, were added to the bottom of each well of a 24-well plate. Each well received a Corning HTS Transwell® for 24 well plates with a 8µm filter. 300µl of 3×105 dHL60 cells that had been pre-treated with resolvins was added on top of the filters. Then, the plates were incubated at 37°C in a 5% CO2 atmosphere for two hours. After this, trypsin was added to the bottom wells in order to detach the cells and prepare them for counting using trypan blue and a hemocytometer.
Fig. 1.
Steps to the in vitro migration assay. HL60 cells were differentiated (dHL60) by culturing in 1.3% DMSO. 1) dHL60 cells were pre-treated with or without resolvins (500nM or 2000nM) for 15 minutes. 2) Then, these cells were trypsinized and transferred to the top chamber of a Transwell system with a 8µm filter. fMLP, a chemoattractant, was added to the bottom chamber. After 2 hours, migration of dHL60 cells from the top to bottom chambers was measured via cell counting with a hemocytometer.
2.4 In vivo materials
One week old wild type C57BL/6 mice (Charles River Laboratories; Wilmington, MA) were used in wound healing studies. Integra ® Dermal Regenerative Template (Integra LifeSciences, NJ) and Alloderm® Regenerative Tissue Matrix (LifeCell, NJ) skin grafts were used as skin substitutes to reduce healing by wound contraction (typical of murine healing) and promote re-epithelialization (typical of human healing). Additionally, Tegaderm™ (3M™ Company) wound dressing was used to cover the injury to prevent it from drying up and to create a physical barrier to protect from scratching.
2.5 Wounding procedure
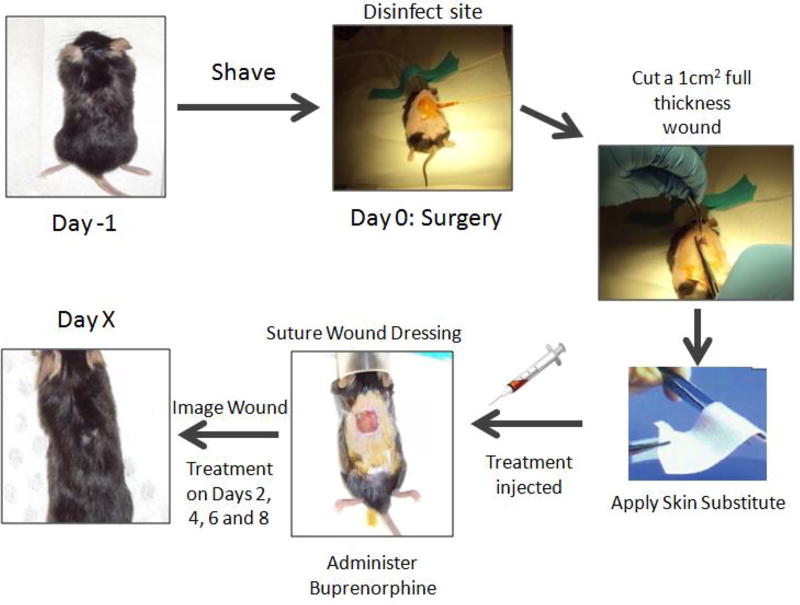
All procedures on animals were approved by the Rutgers Committee on Research Animal Care. Mice were housed four animals per cage before surgery in standard microisolator polycarbonate cages. Animal rooms were maintained at 64–79°F on a 12 hour light/dark cycle. Commercial rodent ration (LabDiet 5P00 Prolab RMH3000) was provided ad libitum along with acidified water. Figure 2 details the surgical process, beginning with shaving. One day before the surgery, the mice were: anesthetized with isoflurane, shaved dorsally, and dorsally depilated with Nair® (Church & Dwight Co., NJ). The mice were then caged individually on alpha dry bedding with access to food and water ad libitum.
Fig. 2.
Surgical steps in the murine excisional wound model. One day before surgery (Day −1) the hair on the dorsum is removed by shaving and treatment with depilatory cream. On Day 0, the mouse is anesthetized and the wound site is disinfected with ethanol and Betadine surgical scrub. A 1 cm × 1 cm full thickness wound is excised using sterile scissor and tweezers. A skin substitute is placed on top of the wound and the resolvin treatment is injected into it. Tegaderm wound dressing is sutured onto the back of the mouse and buprenorphine analgesic is administered. The wound is imaged on Day 0 and subsequent timepoints until the wound closes. Additional resolvin treatments are administered on Days 2, 4, 6 and 8.
On the day of the surgery (Day 0), mice were again anesthetized with isoflurane and the shaved dorsum region was treated with three alternating applications of Betadine surgical scrub and 70% alcohol to disinfect the surgery site. The dorsal area of the mouse skin was marked with a 1cm × 1cm square template. A full thickness wound was created by excising the marked area of skin (epidermis, dermis and panniculus carnosus) using sterile scissors and tweezers. An equivalent sized piece of skin substitute (Integra® or Alloderm®) was placed to conceal the exposed wound site. The corners of the substitute were tucked underneath the wound edges. Dermal substitutes were used here as biocompatible “sponges” to hold the treatment solutions; furthermore, these materials slow down the wound contraction process and promote reepithelialization. Tegaderm® wound dressing was placed over the wound site and sutured to the intact skin.
Next, 100µl of 2000nM RvD1, RvD2, RvE1 or saline vehicle (control) were pipetted into the skin substitute matrix. An image was taken so that initial wound size for each mouse could be measured. Prior to removal from anesthesia, mice were subcutaneously injected with analgesic (buprenorphine) at a dosage of 0.05mg/kg under the scruff region. The mice were then placed back into their cages until additional treatment or imaging on subsequent days. On Days 2, 4, 6 and 8, the wounds were imaged and pipetted with an additional 100µl of 2000nM Resolvin D1, D2, or E1, or saline vehicle as the mice were under anesthesia. Mice were sacrificed on Days 5, 10 or 30 by CO2 inhalation.
2.6 Wound closure analysis
Pictures of the wound site were taken regularly alongside a ruler (for scaling) to measure the rate of macroscopic wound closure. Images were taken using a Sony 10.1MP camera (optical lens f=5.8–23.2mm). For quantification of the wound area, the raw digital files were imported into NIH Image J software v1.40g (Image J, NIH, MD) for processing. The planimetry tool was used to calculate the wound area. Macroscopically, the wound edges were defined by dermal edges of exposed tissue or scab. Wound closure rate, in each individual case, was analyzed on the basis of the normalized wound area to Day 0. Percent wound closure (Wp) was calculated using Eq. (1):
| (1) |
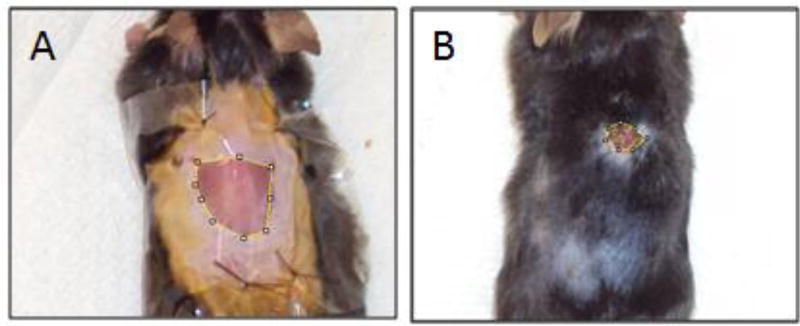
where Wx is the wound area on Day x and W0 is the initial area on Day 0. Figure 3 shows representative images of a saline-treated wound as it is open and in the inflammatory stage and then as it closes and heals.
Fig. 3.
Representative wound closure process in saline-treated mice. A) Wound appearance on Day 3, early in the inflammation stage. The skin has stretched such that the wound area is larger than the 1 cm × 1 cm excised area. Tegaderm ® wound dressing is sutured to keep the wound area moist and protected from scratching. B) Wound appearance on Day 18. The wound is mostly closed and significantly smaller compared to initial wound area. A small scab is present.
2.7 Histological analysis
In order to observe the microscopic differences between resolvin-treated and saline-treated wounds, a follow-up study was performed with mice treated with RvE1 or saline vehicle. Mice were wounded and treated in the same procedure as described previously. In this study, mice were sacrificed on Days 5, 10 or 30 (n=4 each)—these time points correspond approximately to the inflammatory, proliferative and remodeling stages of wound healing. At each time point, mice were euthanized with CO2 gas. Central wound cross sections were excised, fixed in 10% formalin, embedded in paraffin and sectioned in 5µm slices. Tissues were sectioned along a transverse plane through the center of each wound in order to capture the different skin and fascia layers in one section. Sections were stained following standard hematoxylin and eosin (H&E) and Masson’s Trichrome protocols. H&E stain was performed to characterize the cell/tissue distribution and architecture during different stages of healing. Masson’s Trichome stain was used to visualize collagen fibers, basement membrane, fibrin and hyaline. Lateral wound margins in the histological sections were determined by the presence of appendages (hair follicles, sweat glands), which were generally absent within the wound site, and organized epidermal and dermal layers. The digital images were obtained using an Eclipse 50I microscope (Nikon Instruments Inc.) with a 4× (Lens# CFI Plan N.A. 0.1) and 20× (Lens# CFI Plan Fluor N.A. 0.50) magnification.
2.8 Statistical analysis
Results are expressed as mean ± standard deviation. One way Analysis of Variance (ANOVA) was performed using Tukey’s post-hoc analysis. A p-value < 0.05 was considered significant. Because no difference in healing was seen between Alloderm® and Integra®, in vivo data using both were aggregated when performing comparisons among groups.
3. Results
3.1 Neutrophil migration assay
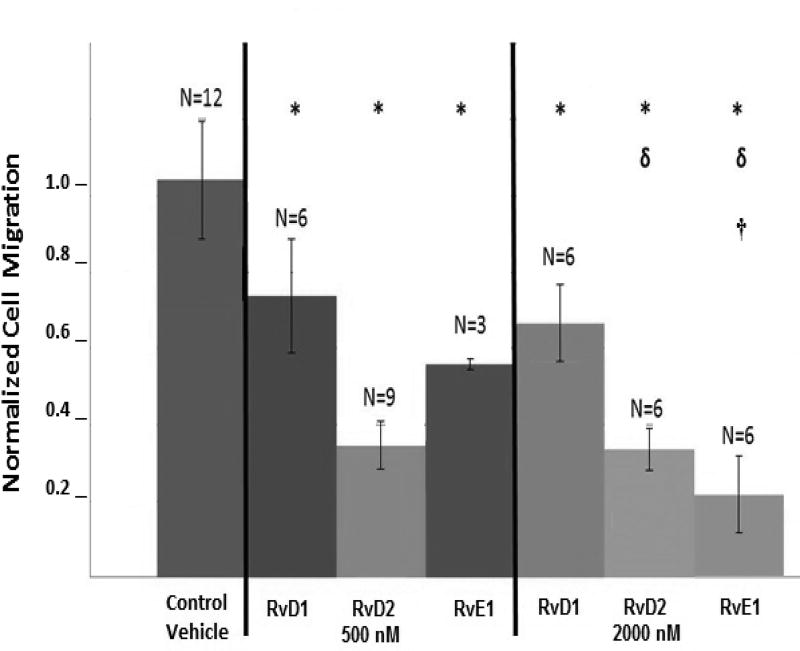
All resolvin conditions significantly inhibited migration compared to the vehicle controls (p<0.05) (Figure 4). At 500 nM, resolvins decreased dHL60 migration by 30–70%. At 2000 nM, RvE1 decreased migration by 80%, the highest inhibition level observed in these experiments, which was significantly more than at 500nM (with only about 50% migration reduction). This dose-dependent behavior is consistent with the previously reported effects of RvE1 on neutrophils.13 Conversely, increasing RvD1 and RvD2 doses from 500 nM to 2000 nM did not result in further inhibition of neutrophil migration. Since overall the 2000 nM dose was as effective, if not more effective, than the respective 500 nM dose for each resolvin type at inhibiting neutrophil migration, this concentration was chosen for the following in vivo studies.
Fig. 4.
Resolvins inhibit dHL60 migration to fMLP in vitro. dHL60 cells were pretreated with 500nM or 2000nM of RvD1, RvD2 or RvE1 for 15 minutes. The cells were then transferred to the top chamber of a Transwell and allowed to migrate to the bottom chamber towards the chemoattractant fMLP. After 2 hours, dHL60 cells that had migrated to the bottom chamber were counted. Data are the average of n = 3–12 replicates per group ± SD, normalized to the control vehicle group. *: significant compared to control vehicle. δ: significant compared to RvD1 - 2000 nM. †: significant compared to RvE1 – 500 nM.
3.2 Wound healing studies
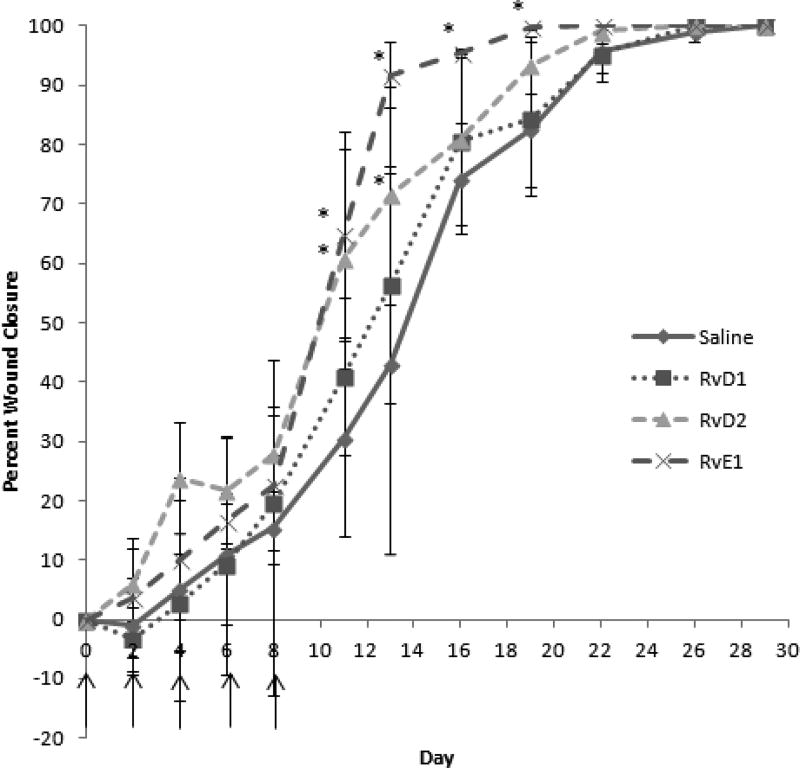
Wounds were macroscopically evaluated post-surgery from Day 0 until Day 30. Images were taken at each timepoint and percent wound closure was calculated using Eq. (1). In addition to RvE1 achieving the earliest wound closure overall at 19 days, this group also demonstrated accelerated healing beginning at Day 11 (Figure 5). For example, on Day 11, RvE1 wounds were approximately 65% closed vs. 25% for the saline groups. Furthermore, on Day 13, RvE1 wounds were nearly closed (90%), whereas saline-treated wounds were only 30% closed. RvD2 also achieved significantly accelerated wound closure compared to saline on days 11 and 13. This group had the second fastest rate of wound closure overall. Earlier in the wound healing process, from Days 0–8, RvD2 treated wounds were slightly more closed (non-significant) compared to the other groups, until RvE1 groups became dominant from Day 8 on.
Fig. 5.
Resolvins accelerate wound closure dynamics in mice. 2000nM of RvD1, RvD2 or RvE1, or saline was applied to full-thickness excisional wounds on the backs of mice on Day 0, 2, 4 and 8 (treatment times denoted by arrows). Images of wounds were taken over time order to measure wound size and calculate percent wound closure. Data shown are the average ± SD of the percentage of wound closure vs. time for n=5 mice per group. Resolvin-treated wounds particularly accelerate closure between Day 8–13, as the wound is overcoming the inflammatory phase. *: significant compared to saline.
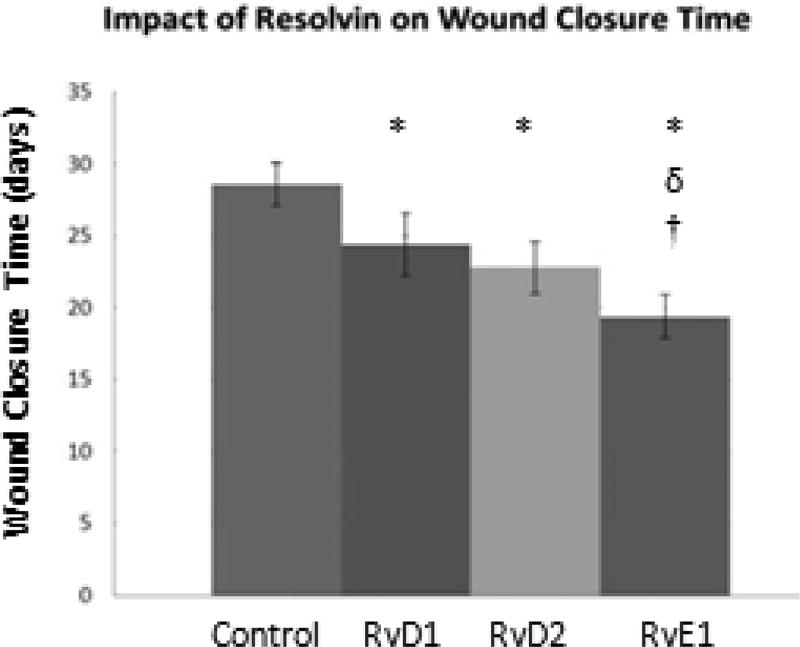
Complete closure and re-epithelialization was deemed to have occurred when the wound surface appeared free of redness/inflammation, and overall appeared similar in color to that of the surrounding uninjured skin. Wounds treated with saline vehicle demonstrated complete re-epithelialization by an average of 28.6 ± 1.5 days (Figure 6). Wounds treated with resolvins, regardless of type, healed significantly faster (p<0.05). RvE1-treated wounds closed the fastest, by 19.4 ± 1.5 days post-wounding. This was also significant compared to the RvD2 and RvD1 groups (p<0.05), which closed by 22.8 ± 1.8 and 24.4 ± 2.2 days, respectively.
Fig. 6.
Resolvins decrease time to complete wound closure. 2000nM of RvD1, RvD2 or RvE1, or saline was applied to full-thickness excisional wounds on the backs of mice on Day 0, 2, 4 and 8. Images of wounds were taken over time to evaluate wound closure. The wound was considered closed when it was no longer red, there was no presence of a scab, and otherwise appeared similar to the surrounding, uninjured skin. Data shown are the average ± SD of the time for complete wound closure for n = 5 mice per group. *: significant compared to control. δ: significant compared to RvD1. †: significant compared to RvD2.
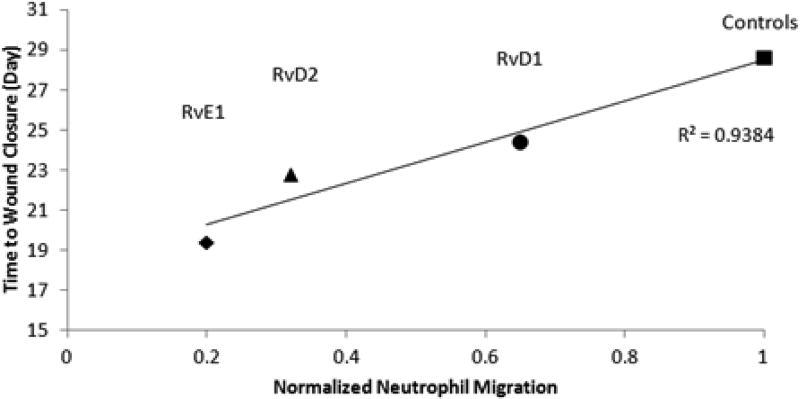
Comparing the results of the in vivo and in vitro studies, the time to wound closure for each resolvin was proportional to its neutrophil migration inhibitory effect (R2=0.9384) (Figure 7). The resolvins that have a greater inhibitory effect on neutrophil migration result in accelerated wound closure. For example, RvE1, which slowed neutrophil migration to the greatest extent in vitro, also resulted in the fastest wound closure in vivo. In contrast, control groups had the greatest neutrophil migration and the longest time to wound closure.
Fig. 7.
Neutrophil migration in vitro is directly proportional to time to wound closure in vivo. Resolvins (particularly RvE1) that slowed neutrophil migration to greater extents in vitro, resulted in shorter time to wound closure in vivo.
Histological analysis was performed on mice sacrificed on Days 5, 10 and 30, in order to sample wounds at stages of inflammation, proliferation, and remodeling. This study was limited to the RvE1 and saline vehicle groups, which showed the widest difference in wound closure between each other. Figures 8 and 9 contain histology slides for saline-treated and RvE1-treated wounds, respectively. Columns A and B, in both figures, show the H&E and Masson's Trichrome sections, respectively.
Fig. 8.
A) H&E and B) Masson-Trichrome stains of saline-treated murine wound cross-sections over time.
Fig. 9.
A) H&E and B) Masson-Trichrome stains of RvE1-treated murine wound cross-sections over time.
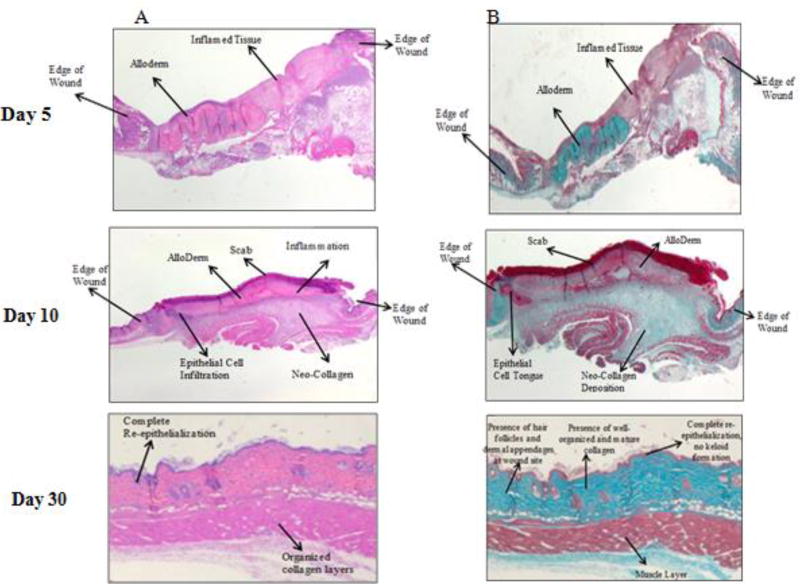
On Day 5, wounds were in the early/mid-inflammatory stage. The wound area was still large, red and inflamed with substantial exudate. Comparing saline-treated and RvE1-treated mice, the wounds were similar in size and appearance on the macro scale. In the H&E stained sections, both groups exhibited inflammatory infiltrates; however, more granulation tissue was seen in the RvE1 group, which contained fibroblasts, granulocytes and loosely organized extracellular matrix. The wound edges were clearly identifiable due to the sudden disappearance of skin appendages in the transition zone between normal skin and the wound area. At this early stage, the wound edge also lacked epidermis. The skin substitute was also clearly noticed as a blue stained material in the Masson's Trichrome sections. On Day 10, wounds began to resolve the inflammation and moved on to the proliferative phase of healing. Scabs formed over the wounds to create a physical barrier against the outside environment. At this point, RvE1-treated wounds were much smaller than saline-treated wounds. Histologically, in the RvE1-treated group, epithelial cell infiltration and an epithelial cell tongue can be identified nearby a thick, intact scab. Masson's Trichrome staining of the same wounds revealed regions with lighter color that could be easily distinguished from the collagen of the skin substitute. This lighter staining suggests neo-collagen deposition, which was likely secreted from adjacent fibroblasts. By the same criteria, saline-treated wounds exhibited less new collagen formation.
By Day 30, the RvE1 groups show more complete healing at the microscopic level. The control wounds had nearly closed, but a scab layer was still present, on top of a thick, non-layered epithelial region. Underneath the epithelium, there was a loose collagen layer without any apparent directional organization. This suggests that remodeling was still needed in order to increase collagen maturation and strength. In contrast, the RvE1 wound exhibited complete re-epithelialization and denser collagen layers that were better organized. These observations are consistent with wounds at a more advanced stage of healing and maturation, which is in line with the fact that such wounds had already closed 1 week earlier at this point.
4. Discussion and Conclusions
In this study we compared the ability of resolvins D1, D2, and E2 to inhibit neutrophil migration in vitro and promote wound closure in vivo. The data show that while all resolvins tested qualitatively exhibited similar effects, RvE1 was the most effective in both of these assays. On average, RvE1 at 2000nM inhibited neutrophil migration to the greatest extent as compared to controls and RvD1. RvD2 allowed more cell migration than RvE1, however, the difference was not significant. RvE1 showed a stronger effect by increasing the dose from 500nM to 2000nM, while RvD1 and RvD2 did not show more activity by increasing the dose beyond 500 nM.
Overall, all resolvins tested significantly inhibited neutrophil migration to varying degrees, with RvE1 having the strongest effect. RvE1 also resulted in the shortest wound closure time, approximately 19 days post-wounding, compared to 23, 24, and 28 days for RvD2, RvD1, and saline vehicle, respectively. When the normalized in vitro neutrophil migration was plotted against the in vivo time to wound closure for each resolvin, the two factors demonstrated a linear relationship. Greater inhibition of neutrophil migration by each resolvin resulted in faster wound closure in vivo. Although there are several other factors that could affect wound healing in the in vivo study that are not recapitulated in the in vitro model, this correlation suggests that inhibiting neutrophil migration may be a key factor that aids in faster healing. Future studies will observe neutrophil population in response to topical resolvin treatment in vivo. In accordance to the macroscopic observations, RvE1 wounds also exhibited more advanced healing characteristics on a microscopic level. Histological images revealed that RvE1 wounds overcame inflammation quickly and generated more neo-collagen and epidermal layering at earlier times than saline-treated wounds. By Day 30, RvE1 wounds resembled normal skin: the epidermal layer was complete and intact, with well-organized underlying collagen. In contrast, saline wounds still possessed a scab and the neo-collagen was loose and unorganized. Overall, RvE1 treatments yielded better wound healing results in the in vivo murine wound model.
All resolvins tested here inhibited neutrophil migration in vitro and promoted wound healing in vivo compared to the vehicle controls. Although RvE1 seemed to perform the best, RvD2 also performed well, and may be more potent, at least at lower concentrations. At 500nM, RvD2 reduced migration compared to controls by about 70%. This is close to the decrease in migration caused by 2000nM RvE1, which was 80% reduced. In the in vivo studies, RvD2 closed wounds the next fastest after RvE1. Also, in the beginning stages of healing, immediately following wounding, RvD2 wounds actually seemed to be closing slightly faster than the other groups, until RvE1 took over at Day 11. Similar to other studies, RvD2 had a greater effect on neutrophils and wound healing than RvD1.10 In fact, the effect of RvD1 in the wound healing study was not significant over saline control treatment at any of the time points.
Perhaps a combination of resolvins, particularly RvE1 and RvD2 may improve results, especially since they exhibited different wound closure patterns, with RvD2 dominating in early wound closure and RvE1 having the greatest effects later on. This may be partially explained due to the fact that different resolvins act on different receptors.14 Additionally, there are several other pro-resolution lipids that may yield other benefits. For example, RvD3 has recently been found to appear late in the resolution phase, suggesting that it may have a specific role that the other resolvins lack.2,15 RvD3 has also been found to have benefits in an acute lung injury model.16 RvE2, RvE3 and RvD4, RvD5 and RvD6 are other forms of resolvins on which there currently is a paucity of information, but they may warrant further investigation.2 Protectins, lipoxins and maresins are also derived from omega-3 fatty acids and also possess benefits in the resolution of inflammation.2
Further investigation and comparison of resolvins on their effects on other immune cells would help better understand their mechanism of action on the wound healing response. For example, resolvins act not only on neutrophils, but also on macrophages to increase their phagocytosis of microbes and dead cells.6,17 This enhanced phagocytotic activity may help prevent infection in chronic wounds and thereby lower associated complications.6,17 An interesting follow-up study would involve a dual wound/infection model, similar to that used in Ref. 10. In this study, the group found that RvD2 injections in burned mice improved their survival and protected against sepsis from the second insult. Repeating this study on the current excisional wound model, and comparing effects of D- and E-series resolvins could provide further insight into resolvins as prophylactic therapies. Other than pro-resolving and prophylactic properties, resolvins also possess analgesic properties, which could provide additional benefit as a treatment to painful burn and chronic wounds.4,18
Increasing the stability of therapeutic resolvins is critical for clinical application.4 Natural resolvins are rapidly metabolized and inactivated in vivo, and therefore efforts have been made to stabilize them so they can exhibit prolonged action. For example, RvE1 is inactivated by oxidation at the C-18 by dehydrogenase; in contrast, the synthetic derivative 19-(p-flurophenoxy)-RvE1 is resistant to this chemical conversion and functional inactivation.19 The stabilized form retained equipotent bioactivity in a murine peritonitis inflammatory model.
Additionally, a delivery system that promotes sustained release of resolvins would allow for longer treatment time before requiring reapplication and wound dressing changes. Nanoparticles formulated with encapsulated RvD1 and lipoxin A-4, in order to mimic endogenous neutrophil-derived, resolvin-containing microparticles, have been reported.20 The nanoparticle system was effective in reducing in vivo neutrophil infiltration in peritonitis and temporomandibular joint inflammatory models. Furthermore, the nanoparticles enhanced wound closure compared to free lipid mediators in an in vitro keratinocyte scratch model. More studies focused on chemical stabilization and on controlled delivery of resolvins will help make anti-inflammatory therapies more effective in the future.
Lastly, as resolvins serve to overcome inflammation and create a favorable environment for proliferative and remodeling phases, they may hold value in serving as initial, pro-resolution treatments, followed by methods that target later aspects of wound healing. Protein- and cell-based therapies, for example, have shown promising results in wound healing applications. It was shown that stromal cell-derived growth factor-1 (SDF-1) treatments, alone or in combination with granulocyte colony-stimulating factor, significantly decreased inflammatory cell infiltrate in an in vivo murine wound.21 SDF-1 exhibited additional wound healing benefits, by increasing the expression of α-smooth muscle actin (α-SMA) expression in contractile myofibroblasts during wound closure. Mesenchymal stromal cells (MSCs), which also increase fibroblast α-SMA,22 show great potential in wound healing applications as well. MSCs secrete a wealth of anti-inflammatory cytokines and growth factors that promote wound healing, including SDF-1, interleukin-10, vascular endothelial growth factor (VEGF) and many more.23–25 The drawback that remains with regards to protein and cell-based therapies is that the potent inflammatory environment present in challenging wounds is populated with proteases that degrade topically applied proteins and can negatively affect MSC viability and function. Therefore, as we learn how to use and optimize resolvins to overcome inflammation in wounds, a more favorable microenvironment will be created for subsequent protein or cell therapies.
Overall, as more studies comparing the effects of different types of resolvins are completed, better pro-resolution therapies may be developed for a wide range of inflammatory conditions. Not only might acute and chronic wounds benefit, but there are numerous potential applications in bone regeneration, asthma, and diabetes. In this study, we saw that 2000nM RvE1 inhibits neutrophil migration and accelerates wound healing to the greatest degree. However, there is still room for optimization of pro-resolution lipid therapies, and further understanding the unique role of each type of resolvin should allow for fine-tuning anti-inflammatory treatments.
Acknowledgments
Paulina Krzyszczyk is a recipient of a biotechnology training fellowship from the National Institute of General Medical Sciences (award number T32 GM008339) and a fellowship from the Department of Education Graduate Assistance in Areas of National Need (award number P200A150131)
Contributor Information
Riyesh Menon, Biomedical Engineering, Rutgers University, 599 Taylor Road Piscataway, NJ 08854, United States.
Paulina Krzyszczyk, Biomedical Engineering, Rutgers University, 599 Taylor Road Piscataway, NJ 08854, United States.
François Berthiaume, Biomedical Engineering, Rutgers University, 599 Taylor Road Piscataway, NJ 08854, United States.
References
- 1.Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, Gottrup F, Gurtner GC, Longaker MT. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17(6):763–71. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510(7503):92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dalli J, Serhan CN. Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood. 2012;120(15):e60–72. doi: 10.1182/blood-2012-04-423525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ji RR, Xu ZZ, Strichartz G, Serhan CN. Emerging roles of resolvins in the resolution of inflammation and pain. Trends Neurosci. 2011;34(11):599–609. doi: 10.1016/j.tins.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hellmann J, Tang Y, Spite M. Proresolving lipid mediators and diabetic wound healing. Curr Opin Endocrinol Diabetes Obes. 2012;19(2):104–8. doi: 10.1097/MED.0b013e3283514e00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang Y, Zhang MJ, Hellmann J, Kosuri M, Bhatnagar A, Spite M. Proresolution therapy for the treatment of delayed healing of diabetic wounds. Diabetes. 2013;62(2):618–27. doi: 10.2337/db12-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447(7146):869–74. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao L, Faibish D, Fredman G, Herrera BS, Chiang N, Serhan CN, Van Dyke TE, Gyurko R. Resolvin E1 and chemokine-like receptor 1 mediate bone preservation. J Immunol. 2013;190(2):689–94. doi: 10.4049/jimmunol.1103688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li N, He J, Schwartz CE, Gjorstrup P, Bazan HE. Resolvin E1 improves tear production and decreases inflammation in a dry eye mouse model. J Ocul Pharmacol Ther. 2010;26(5):431–9. doi: 10.1089/jop.2010.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurihara T, Jones CN, Yu YM, Fischman AJ, Watada S, Tompkins RG, Fagan SP, Irimia D. Resolvin D2 restores neutrophil directionality and improves survival after burns. FASEB J. 2013;27(6):2270–81. doi: 10.1096/fj.12-219519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haworth O, Cernadas M, Yang R, Serhan CN, Levy BD. Resolvin E1 regulates interleukin 23, interferon-gamma and lipoxin A4 to promote the resolution of allergic airway inflammation. Nat Immunol. 2008;9(8):873–9. doi: 10.1038/ni.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bohr S, Patel SJ, Sarin D, Irimia D, Yarmush ML, Berthiaume F. Resolvin D2 prevents secondary thrombosis and necrosis in a mouse burn wound model. Wound Repair Regen. 2013;21(1):35–43. doi: 10.1111/j.1524-475X.2012.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haas-Stapleton EJ, Lu Y, Hong S, Arita M, Favoreto S, Nigam S, Serhan CN, Agabian N. Candida albicans modulates host defense by biosynthesizing the pro-resolving mediator resolvin E1. PLoS One. 2007;2(12):e1316. doi: 10.1371/journal.pone.0001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levy BD. Resolvin D1 and Resolvin E1 Promote the Resolution of Allergic Airway Inflammation via Shared and Distinct Molecular Counter-Regulatory Pathways. Front Immunol. 2012;3:390. doi: 10.3389/fimmu.2012.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalli J, Winkler JW, Colas RA, Arnardottir H, Cheng CY, Chiang N, Petasis NA, Serhan CN. Resolvin D3 and aspirin-triggered resolvin D3 are potent immunoresolvents. Chem Biol. 2013;20(2):188–201. doi: 10.1016/j.chembiol.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colby JK, Abdulnour RE, Sham HP, Dalli J, Colas RA, Winkler JW, Hellmann J, Wong B, Cui Y, El-Chemaly S, Petasis NA, Spite M, Serhan CN, Levy BD. Resolvin D3 and Aspirin-Triggered Resolvin D3 Are Protective for Injured Epithelia. Am J Pathol. 2016;186(7):1801–13. doi: 10.1016/j.ajpath.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, Flower RJ, Perretti M, Serhan CN. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461(7268):1287–91. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu ZZ, Zhang L, Liu T, Park JY, Berta T, Yang R, Serhan CN, Ji RR. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat Med. 2010;16(5):592–7. doi: 10.1038/nm.2123. 1p following 597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arita M, Oh SF, Chonan T, Hong S, Elangovan S, Sun YP, Uddin J, Petasis NA, Serhan CN. Metabolic inactivation of resolvin E1 and stabilization of its anti-inflammatory actions. J Biol Chem. 2006;281(32):22847–54. doi: 10.1074/jbc.M603766200. [DOI] [PubMed] [Google Scholar]
- 20.Norling LV, Spite M, Yang R, Flower RJ, Perretti M, Serhan CN. Cutting edge: Humanized nano-proresolving medicines mimic inflammation-resolution and enhance wound healing. J Immunol. 2011;186(10):5543–7. doi: 10.4049/jimmunol.1003865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarkar A, Tatlidede S, Scherer SS, Orgill DP, Berthiaume F. Combination of stromal cell-derived factor-1 and collagen-glycosaminoglycan scaffold delays contraction and accelerates reepithelialization of dermal wounds in wild-type mice. Wound Repair Regen. 2011;19(1):71–9. doi: 10.1111/j.1524-475X.2010.00646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faulknor RA, Olekson MA, Nativ NI, Ghodbane M, Gray AJ, Berthiaume F. Mesenchymal stromal cells reverse hypoxia-mediated suppression of alpha-smooth muscle actin expression in human dermal fibroblasts. Biochem Biophys Res Commun. 2015;458(1):8–13. doi: 10.1016/j.bbrc.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 23.Barminko J, Kim JH, Otsuka S, Gray A, Schloss R, Grumet M, Yarmush ML. Encapsulated mesenchymal stromal cells for in vivo transplantation. Biotechnol Bioeng. 2011;108(11):2747–58. doi: 10.1002/bit.23233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim JM, Kim J, Kim YH, Kim KT, Ryu SH, Lee TG, Suh PG. Comparative secretome analysis of human bone marrow-derived mesenchymal stem cells during osteogenesis. J Cell Physiol. 2013;228(1):216–24. doi: 10.1002/jcp.24123. [DOI] [PubMed] [Google Scholar]
- 25.Kolf CM, Cho E, Tuan RS. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res Ther. 2007;9(1):204. doi: 10.1186/ar2116. [DOI] [PMC free article] [PubMed] [Google Scholar]