Abstract
As part of our studies on the regulation of polyamine biosynthesis in Saccharomyces cerevisiae, we have investigated the effect of spermidine on the degradation of ornithine decarboxylase in this organism. We have found that in S. cerevisiae, as in other eukaryotic cells, the rate of degradation of ornithine decarboxylase, measured either enzymatically or immunologically, is increased by the addition of spermidine to a yeast culture. It is noteworthy that this effect of added spermidine is found even when the experiments are conducted with strains in which the ornithine decarboxylase is overexpressed several hundred-fold more than the wild-type level. The effect of added spermidine in the overexpressed SPE1 strains is best seen in spe2 mutants in which the initial intracellular spermidine is very low or absent. Experiments with cycloheximide show that new protein synthesis is required to effect the breakdown of the ornithine decarboxylase. These results indicate that S. cerevisiae contains an antizyme-like mechanism for the control of the level of ornithine decarboxylase by spermidine, even though, as contrasted with other eukaryotic cells, no specific antizyme homologue has been detected either in in vitro experiments or in the S. cerevisiae genome.
The regulation of ornithine decarboxylase has been studied extensively in a variety of cells and has been shown to involve a very unusual regulatory mechanism. In this system, a specific and unusual protein, called antizyme, is synthesized in response to spermidine/spermine administration. This protein not only inhibits ornithine decarboxylase but also is important in preparing the ornithine decarboxylase protein for degradation by proteasomes without ubiquitination (reviewed in refs. 1–3). A similar protein has been shown recently to be synthesized in Schizosaccharomyces pombe after the administration of spermidine (4, 5). However, no direct evidence for the presence of an antizyme-like homologue has been demonstrated in Saccharomyces cerevisiae, despite numerous attempts by a number of investigators, nor has an antizyme homologue been found in the sequence of the S. cerevisiae genome (6). In view of this difference, we have been interested in extending our studies on the factors involved in the regulation of ornithine decarboxylase by spermidine in S. cerevisiae, and, in particular, on the factors involved in the degradation of the enzyme, especially because in S. cerevisiae, regulation of the rate of degradation of ornithine decarboxylase seems to be more important than regulation of its biosynthesis (7, 8).
Materials and Methods
Subcloning of the Yeast SPE1 Gene into a Yeast Constitutive Expression Vector.
To obtain a yeast strain containing a larger amount of ornithine decarboxylase, the SPE1 gene was inserted into plasmid pVT101U obtained from T. Vernet (9). For this construction, we digested the 2,955-bp fragment from pSPE1U (10) with SstI and HindIII (partial) and inserted the resultant fragment into the multicloning site of plasmid pVT101U. With this plasmid (pVT101U/SPE1), the transcription of the SPE1 gene is driven by an alcohol dehydrogenase promoter, resulting in a 20- to 200-fold overexpression of ornithine decarboxylase “constitutively” (9). The resultant plasmid was used to transform S. cerevisiae strain BJ1991 and strain Y362 to give strains Y387 and Y465, respectively. We thank W. A. Fonzi for the pSPE1 plasmid that was used to construct the pSPE1U strain.
Culture Conditions.
The strains used in these experiments are listed in Table 1; they were maintained on yeast extract–peptone-dextrose or synthetic dextrose minimal medium (SD) medium (11) containing the necessary nutrients plus 10−4 M spermidine. All incubations were at 30°C and were in polyamine-free SD medium, unless otherwise indicated.
Table 1.
Strains
| S. cerevisiae strain | Characteristics | Source or reference |
|---|---|---|
| BJ1991 | prb1-1122 pep 4-3 leu2 trp1 | E. W. Jones |
| SPE1SPE2 | ||
| 28-8D | SPE1 spe2-4 | American type culture collection no. 42295, ref. 17 |
| Y362 | ura3-52 leu2Δspe1∷LEU2 Δspe2∷LEU2 | Ref. 18 |
| Y387 | prb1-1122 pep 4-3 leu2 trp1 | BJ1991/pVT101U/SPE1 |
| SPE1SPE2 pVT101U/SPE1 | ||
| Y465* | ura3-52 leu2Δspe1∷LEU2 | Y362/pVT101U/SPE1 |
| Δspe2∷LEU2/pVT101U/SPE1 |
This construct is similar to that described by Balasundaram, et al. (19), except for different auxotrophic markers in the host strain (Y450) used in the latter paper. Y362 is ura3-52 leu2 Δspe1∷LEU2 Δspe2∷LEU2, while Y450 is arg4 ura3-52 trp1-289 thr1 leu2 Δspe1∷LEU2 Δspe2∷LEU2.
For assays of ornithine decarboxylase in polyamine-deficient cells and for testing the effect of cycloheximide and/or spermidine on these levels, it was first necessary to grow the strains on polyamine-free SD medium to decrease the levels of endogenous intracellular polyamines. For experiments with the spe2–4 strain (28–8D), 60 μl of a fresh overnight yeast extract–peptone–dextrose culture was added to 3 ml of SD medium, and the culture was grown overnight. This culture was then diluted to an OD at 600 nm of 0.05 and a volume of 300–400 ml. Growth was continued until the OD reached 0.3–0.4.
The culture conditions were somewhat different for the Δspe1Δspe2 strain (Y465), because this strain cannot grow with no added spermidine. Therefore, this strain was grown for several days in SD medium containing 10−9 M spermidine and was used when the OD at 600 nm reached 0.3–0.4.
Preparation of Cell Lysates.
At each of the times indicated in Figs. 1–3, 50–100 ml of culture was harvested by centrifugation at 5,000 rpm for 10 min at 4°C. Each cell pellet was suspended in 0.8 ml of a yeast disruption buffer (20 mM Tris⋅HCl (pH 8.0)/10 mM MgCl2/1 mM EDTA/5% glycerol/1 mM dithiothreitol (DTT)/1 mM phenylmethylsulfonylfluoride). An equal volume of acid-washed ice-cold glass beads (diameter; 0.4–0.6 mm) was added. The tubes were shaken in a bead beater for 3 min at 4°C. The lysate was centrifuged, and the supernatant solution was collected; then 80 μl of 0.01 M pyridoxal phosphate and 8 μl of 0.1 M DTT were added to each extract.
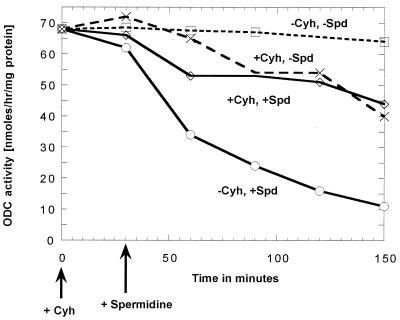
Figure 1.
Effect of spermidine (Spd) and cycloheximide (Cyh) additions on the ornithine decarboxylase activity of a polyamine-deficient spe2–4 mutant (strain 28-8D) that is derepressed for ornithine decarboxylase. At 0 time, cycloheximide (final concentration 200 μg/ml) was added to the incubations labeled “+Cyh.” At 30 min, spermidine (final concentration 100 μM) was added to the incubations labeled “+Spd” (solid lines).
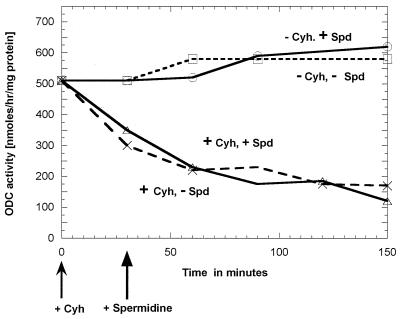
Figure 3.
Effect of spermidine (Spd) and cycloheximide (Cyh) additions on the ornithine decarboxylase activity of a strain (Y387) of S. cerevisiae containing a high intracellular polyamine level resulting from the presence of an overexpression SPE1 plasmid. At 0 time, cycloheximide (final concentration 200 μg/ml) was added to the incubations labeled “+Cyh.” At 30 min, spermidine (final concentration 100 μM) was added to the incubations labeled “+Spd.”
Measurement of Ornithine Decarboxylase Activity and Protein Concentration.
Ornithine decarboxylase activity was measured essentially as previously described (12) by measuring the 14CO2 formed in 20 min at 37°C by decarboxylation of [1-14C]-ornithine. Protein concentration was estimated by the method of Bradford (13). Specific activity is defined as nanomoles of CO2 formed per hour per milligram of protein.
Western Blot Analysis.
For these studies, yeast ornithine decarboxylase was purified to homogeneity from an Escherichia coli strain containing an overexpression plasmid containing the yeast SPE1 gene, coding ornithine decarboxylase (unpublished data). Antibodies against this protein were raised in rabbits by the Berkeley Antibody Company, Berkeley, CA. For the experiment presented in Fig. 2, aliquots of the various cell lysates, containing equal amounts of protein, were applied to a 10% SDS polyacrylamide gel, transferred after electrophoresis onto poly(vinylidene difluoride) (Hybond-P, Amersham Pharmacia) membranes, and developed with the polyclonal antibody specific for ornithine decarboxylase. The antigen–antibody complex was visualized on photographic film after treatment with the Chemiluminescence ECL kit (horseradish peroxidase-labeled anti-rabbit antibody) from Amersham Pharmacia. The bands were quantitated on a scanner.
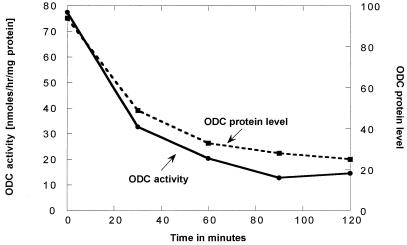
Figure 2.
Loss of ornithine decarboxylase activity after spermidine addition to a spe2–4 culture (strain 28-8D) is accompanied by a parallel loss in the amount of ornithine decarboxylase protein (measured immunologically). The conditions for this experiment were essentially the same as those used for the experiment presented in Fig. 1; namely, the culture was grown on a minimal SD medium, and 100 μM spermidine (final concentration) was added at the position indicated as 0. The values for the ornithine decarboxylase protein represent the scanned values (in arbitrary units) for the Western blot analysis.
Results
Ornithine Decarboxylase Activity in Different Strains.
Line 1 (Table 2) shows the ornithine decarboxylase activity in a SPE1 SPE2 strain (BJ1991). Line 2 shows that, as expected, a considerably higher level of ornithine decarboxylase was found in a spe2–4 mutant (28–8D). This strain has a normal SPE1 gene, but it contains no detectable spermidine or spermine because of a point mutation in the SPE2 gene that codes for S-adenosylmethionine decarboxylase. The spe2–4 deficiency is presumably not complete, because this strain still grows at a very slow rate even when completely deprived of added polyamines.
Table 2.
Ornithine decarboxylase activity in extracts of different strains
| S. cerevisiae strain* | Chromosomal genotype | Plasmid | Ornithine decarboxylase activity nmol/hr/mg protein |
|---|---|---|---|
| BJ1991 | SPE1SPE2 | — | 10 |
| 28-8D | spe2-4 | — | 80† |
| Y387 | SPE1SPE2 | pVT101U/SPE1 | 200–500 |
| Y465 | Δspe1∷LEU2 | pVT101U/SPE1 | 2,000–6,000‡ |
| Δspe2∷LEU2 |
See Table 1.
After growth in amine-deficient SD media for ≈66 hr. At this time, the cultures still showed logarithmic growth, but with a doubling time of about 18–20 hr.
After growth in 5 × 10−5 M to 5 × 10−8 M spermidine. [Because yeast ornithine decarboxylase, purified to homogeneity from an E. coli strain with an overexpression plasmid, has an activity of 164,000 nmol/hr/mg protein (unpublished data), we estimate that approximately 2–4% of the soluble protein in the Y465 extract is ornithine decarboxylase.]
A higher ornithine decarboxylase activity was found in a SPE1 SPE2 strain carrying a multicopy plasmid (pVT101U/pSPE1) that contained the SPE1 gene downstream of an ADH1 (alcohol dehydrogenase) promoter (line 3). An even higher ornithine decarboxylase activity was found if the host for the plasmid had a deletion in the SPE2 gene (line 4). This strain (Y465) contains a large amount of putrescine but has no spermidine or spermine because of the absence of S-adenosylmethionine decarboxylase.
Decrease in Ornithine Decarboxylase Activity After Addition of Spermidine to the Cultures.
As shown in Fig. 1, the addition of 10−4 M spermidine to the cultures causes a relatively rapid fall in the ornithine decarboxylase levels of the spe2–4 strain. This loss of ornithine decarboxylase activity was caused by a loss of the enzyme protein, as measured immunologically (Fig. 2).
We also tested the effect of spermidine addition to a strain containing a multicopy plasmid because both Fonzi (7) and Toth and Coffino (8) reported that the degradation of ornithine decarboxylase in strains containing a multicopy plasmid is not affected by spermidine addition. Because the host for their experiments with a multicopy plasmid was SPE2+, we assumed that the intracellular spermidine concentration must have been high, and we felt it was not surprising that added spermidine had no additional effect. Therefore, we tested the effect of spermidine addition on the ornithine decarboxylase levels of our strains that contained the multicopy plasmid (pVT101U/SPE1) in either SPE2+ (Y387) or spe2 − backgrounds. The SPE2+ strain would have a high intracellular spermidine content and, as shown in Fig. 3, we found, as expected, that addition of exogenous spermidine to the SPE2+ cells had no effect on the ornithine decarboxylase level. In contrast (Table 3), when spermidine was added to the strain containing the multicopy plasmid in a spe2− strain, there was a fall in the level of ornithine decarboxylase.
Table 3.
Decrease in ornithine decarboxylase activity after addition of spermidine to a Δspe1Δspe2 culture containing the pVT101U/SPE1 plasmid (Y465)
| Specific activity* | |
|---|---|
| No spermidine addition | 3,200 |
| 1.5 hr after spermidine addition | 1,900 |
| 3.3 hr after spermidine addition | 1,900 |
nmol CO2 formed/hr/mg protein.
Y465 was grown in SD medium containing 10−9 M spermidine, as described in Materials and Methods. The doubling time in 10−9 M spermidine was approximately 5 hr. At zero time, spermidine was added to a final concentration of 10−4 M. Only a small increase in optical density was observed during the 3.3 hr after the spermidine addition.
In addition, we show in Table 4 that the presence of 5 × 10−6 M or higher concentrations of spermidine in the growth medium of Y465 resulted in a considerable decrease in the ornithine decarboxylase level. In both sets of experiments, one would not expect the spermidine addition to affect the ADH1 promoter, and hence the effect observed was probably because of an effect on the degradation of the enzyme. Also, in both sets of experiments, it is not surprising that the loss of ornithine decarboxylase after spermidine addition was not complete, because any degradation of the enzyme would be continually offset by the synthesis of new enzyme.
Table 4.
Effect of spermidine concentration in the growth medium on the level of ornithine decarboxylase in Δspe1Δspe2 cells containing the pVT101U/pSPE1 plasmid (Y465) and on the degradation of ornithine decarboxylase after cycloheximide treatment
| Spermidine concentration in medium | 5 × 10−4 M | 5 × 10−5 M | 5 × 10−6 M | 5 × 10−7 M | 5 × 10−8 M | 1 × 10−8 M |
|---|---|---|---|---|---|---|
| Doubling time, hr* | 1.7 | 1.8 | 1.8 | 2.1 | 2.7 | 3.2 |
| ODC activity† before cycloheximide | 830 | 560 | 780 | 2,300 | 2,050 | 1,710 |
| ODC activity 90 min after cycloheximide‡ | 290 (35%) | 270 (48%) | 280 (36%) | 1,590 (69%) | 2,110 (103%) | 1,570 (92%) |
Determined in a parallel run in the absence of cycloheximide. The doubling time in 1 × 10−9 M spermidine was 4.4 hr. Without any added spermidine, the doubling time of the deprived cells was >20 hr.
nmol CO2 formed/hr/mg protein.
Final concentration, 200 μg/ml.
Effect of Cycloheximide on the Level of Ornithine Decarboxylase.
As shown in Fig. 1, addition of cycloheximide had little effect on the level of ornithine decarboxylase if added to a culture of cells that had very low levels of spermidine because of a spe2 mutation. On the contrary, as shown in Fig. 3, addition of cycloheximide to cells that have a high intracellular spermidine level caused a rapid loss of ornithine decarboxylase activity.
The same dependence of the cycloheximide effect on the intracellular spermidine levels is shown in Table 4, in which the effect of cycloheximide was measured in the cells grown at different concentrations of spermidine. Thus, cycloheximide had little effect on the ornithine decarboxylase activity of cells grown in 10−8 M spermidine but caused a relatively rapid decrease when added to cultures grown in 5 × 10−4 M spermidine. These experiments are consistent with the postulation that cells with high levels of intracellular spermidine contain an already induced antizyme-like activity that results in a marked decrease in the levels of ornithine decarboxylase when new ornithine decarboxylase synthesis is inhibited by the addition of cycloheximide, and that such antizyme-like activity is not present in cells with low levels of intracellular spermidine. These experiments also are consistent with the postulation that administration of spermidine to polyamine-deficient cells induces the formation of such an antizyme-like protein, i.e., resulting in the degradation of the existing ornithine decarboxylase.
Cycloheximide Pretreatment Prevents the Loss of Ornithine Decarboxylase Resulting from the Addition of Spermidine (Fig. 1).
As mentioned above, the data in Fig. 1 show that added spermidine (“−Cyh, +Spd”) results in a relatively rapid loss in ornithine decarboxylase activity. In contrast (“+Cyh, +Spd”), added spermidine did not result in any decrease in ornithine decarboxylase activity if the cells had been pretreated with cycloheximide 30 min before the addition of spermidine. Indeed, the fall in enzyme activity was no greater than that found with cycloheximide alone (“+Cyh, −Spd”). If the spermidine was added 30 min before the cycloheximide, the addition of the cycloheximide did not affect the fall in enzyme activity (data not shown).
Discussion
The regulation of ornithine decarboxylase in yeast is of special interest because of the many papers describing the rapid turnover of ornithine decarboxylase in mammalian cells. In the present studies, we have shown that the level of ornithine decarboxylase in yeast depends very much on the level of intracellular spermidine, supplied either exogenously or endogenously. spe2 mutants that contain little or no intracellular spermidine contain levels of ornithine decarboxylase 10 times greater than that found in a SPE2+ strain. When a spe2Δ strain bears a SPE1-containing plasmid under the control of an ADH1 promoter, the ornithine decarboxylase levels are 200- to 600-fold higher than the wild-type levels. Studies with the latter organism were of particular interest, because they showed that spermidine still had a regulatory effect even in the presence of a markedly increased level of the enzyme. This strain (Y465) has the added advantage that the SPE1 gene is under the control only of the ADH1 promoter, and this promoter would not be affected by any specific effect of spermidine added to the medium.
The time course of the loss of ornithine decarboxylase activity after the addition of spermidine to the culture indicates that the major control of the ornithine decarboxylase levels in these experiments is by degradation of the enzyme. The immunological experiments (ref. 8 and Fig. 2) show that both ornithine decarboxylase activity and the ornithine decarboxylase protein decrease after the addition of spermidine to the culture. The time course of these measurements shows that the activity and the amount of ornithine decarboxylase protein decrease at the same rate.
As shown in Fig. 1 and Table 4 in this paper and in ref. 14, the loss of ornithine decarboxylase that is induced by the addition of spermidine requires the synthesis of new protein, as it did not occur if cycloheximide had been added to the culture before the addition of spermidine. The latter observation could not have been because of an effect of cycloheximide on spermidine uptake, because in comparable studies, we have found (unpublished work) that cycloheximide at the concentrations used in these experiments does not interfere with the uptake of the spermidine for at least 1 hr. This observation that new protein synthesis is required for the degradation of ornithine decarboxylase strongly suggests that S. cerevisiae contains a system similar to that of the antizyme system described in other biological systems, i.e., that, in response to the spermidine addition, the cells synthesize some type of proteolytic system responsible for ornithine decarboxylase breakdown. The specific nature of this proteolytic system is probably different in detail from the usual antizyme system in view of the inability to demonstrate antizyme activity in vitro and the lack of any homologous sequences in S. cerevisiae. Although proteasomes are probably involved in the overall degradation (8, 15, 16), our studies do not address the specific role of the proteasomes in the response to spermidine administration.
Abbreviation
- SD
synthetic dextrose minimal medium
Footnotes
An initial report of this study was presented at the annual meeting of the American Society for Biochemistry and Molecular Biology, May 20, 1998, Washington, DC (Fed. Proc. 12, A1435).
References
- 1.Hayashi S, Murakami Y, Matsufuji S. Trends Biochem Sci. 1996;21:27–30. [PubMed] [Google Scholar]
- 2.Coffino P. Nat Rev Mol Cell Biol. 2001;2:187–194. doi: 10.1038/35056508. [DOI] [PubMed] [Google Scholar]
- 3.Cohen S S. A Guide to the Polyamines. New York: Oxford Univ. Press; 1998. [Google Scholar]
- 4.Chattopadhyay M K, Murakami Y, Matsufuji S. J Biol Chem. 2001;276:21235–21241. doi: 10.1074/jbc.M010643200. [DOI] [PubMed] [Google Scholar]
- 5.Ivanov I P, Matsufuji S, Murakami Y, Gesteland R F, Atkins J F. EMBO J. 2000;19:1907–1917. doi: 10.1093/emboj/19.8.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu C, Karplus K, Grate L, Coffino P. Bioinformatics. 2000;16:478–481. doi: 10.1093/bioinformatics/16.5.478. [DOI] [PubMed] [Google Scholar]
- 7.Fonzi W A. J Biol Chem. 1989;264:18110–18118. [PubMed] [Google Scholar]
- 8.Toth C, Coffino P. J Biol Chem. 1999;274:25921–25926. doi: 10.1074/jbc.274.36.25921. [DOI] [PubMed] [Google Scholar]
- 9.Vernet T, Dignard D, Thomas D Y. Gene. 1987;52:225–233. doi: 10.1016/0378-1119(87)90049-7. [DOI] [PubMed] [Google Scholar]
- 10.Xie Q-W, Tabor C W, Tabor H. Yeast. 1990;6:455–460. doi: 10.1002/yea.320060602. [DOI] [PubMed] [Google Scholar]
- 11.Wickner R B. In: Saccharomyces. Tuite M F, Oliver S G, editors. Vol. 4. New York: Plenum; 1991. pp. 101–147. [Google Scholar]
- 12.Tyagi A K, Tabor C W, Tabor H. Methods Enzymol. 1983;94:135–139. doi: 10.1016/s0076-6879(83)94021-1. [DOI] [PubMed] [Google Scholar]
- 13.Bradford M M. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 14.Tyagi A K, Tabor C W, Tabor H. J Biol Chem. 1981;256:12156–12163. [PubMed] [Google Scholar]
- 15.Elias S, Bercovich B, Kahana C, Coffino P, Fischer M, Hilt W, Wolf D H, Ciechanover A. Eur J Biochem. 1995;229:276–283. [PubMed] [Google Scholar]
- 16.Mamroud-Kidron E, Kahana C. FEBS Lett. 1994;356:162–164. doi: 10.1016/0014-5793(94)01260-1. [DOI] [PubMed] [Google Scholar]
- 17.Cohn M S, Tabor C W, Tabor H. J Bacteriol. 1978;134:208–213. doi: 10.1128/jb.134.1.208-213.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balasundaram D, Xie Q W, Tabor C W, Tabor H. J Bacteriol. 1994;176:6407–6409. doi: 10.1128/jb.176.20.6407-6409.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balasundaram D, Dinman J D, Tabor C W, Tabor H. J Bacteriol. 1994;176:7126–7128. doi: 10.1128/jb.176.22.7126-7128.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]