Abstract
The semicircular canal (SC) system of the inner ear detects head angular accelerations and is essential for navigation and spatial awareness in vertebrates. Because the bony labyrinth encloses the membranous labyrinth SCs, it can be used as a proxy for animal behavior. The bony labyrinth of dicynodonts, a clade of herbivorous non-mammalian synapsids, has only been described in a handful of individuals and remains particularly obscure. Here we describe the bony labyrinth anatomy of three Endothiodon cf. bathystoma specimens from Mozambique based on digital reconstructions from propagation phase-contrast synchrotron micro-computed tomography. We compare these findings with the bony labyrinth anatomy of their close relative Niassodon. The bony labyrinths of Endothiodon and Niassodon are relatively similar and show only differences in the shape of the horizontal SCs and the orientation of the vertical SCs. When compared to extant mammals, Endothiodon and Niassodon have highly eccentric SCs. In addition, the Endothiodon SCs are nearly orthogonal. An eccentric and orthogonal SC morphology is consistent with a specialization in rapid head movements, which are typical of foraging or feeding behaviors. Furthermore, we estimate the body mass of these Endothiodon specimens at ~116 to 182 kg, based on the average SC radii calculated using a linear regression model optimized by the Amemiya Prediction Criterion. Our findings provide novel insights into the paleobiology of Endothiodon which are consistent with the peculiar feeding mechanism among dicynodonts presumed from their multiple postcanine toothrows.
Introduction
The semicircular canal (SC) system of the inner ear has a fundamental role in proprioception by encoding rotations of the head [1–5]. The development of computed tomography has significantly increased our knowledge of the anatomy of the bony labyrinth in extinct and extant taxa, from reptilians to synapsids. It is currently accepted that the three-dimensional morphology of the bony labyrinth provides an ecomorphological signal, because it reflects how sensitive the inner ear is to angular motion of the head [6–11]. Thus, the analysis of fossilized inner ears can provide important insights into the lifestyle of extinct species (e.g., [12–14]). Furthermore, labyrinth anatomy and morphometrics are also useful for systematic purposes [15, 16].
Although a vast amount of information on mammaliaform labyrinth morphological diversity has accumulated over recent years (e.g., [17–23]), few studies have addressed the vestibular anatomy of non-mammaliaform synapsids (e.g., [24–27]). For instance, dicynodont osseous labyrinths have only been described in a small number of specimens [27–38] representing a minor subset of the enormous diversity of more than 120 dicynodont species currently known [39]. Furthermore, as most of these anatomical descriptions relied on imprecise reconstructions from serial grinding, rigorous morphological comparisons of non-mammaliaform synapsid bony labyrinths have remained challenging. This technical limitation has also hindered comparisons with extant species to address ecomorphological questions, for instance. Contrary to the view that the inner ear has conserved morphology, significant variations have been reported in some extant species, even at the intraspecific level (e.g., [40, 41]). This morphological diversity provides information about the animals’ lifestyles.
In this study, we used synchrotron radiation-based micro-computed tomography to examine the anatomy of the bony labyrinth of three rare specimens of the extinct Mozambican Endothiodon cf. bathystoma [42] and Niassodon [35] collected from the K5 Formation [43, 44] of the Metangula Graben, Niassa Province, Mozambique. Our results revealed unusually high SC eccentricity in Endothiodon and Niassodon, when compared to extant animals of the synapsid lineage. Interestingly, the unusual SC morphology of these Endothiodontia allowed us to explore a previously poorly known region of the bony labyrinth morphospace [45]. Furthermore, we found low intraspecific variation in the SC system among these Endothiodon specimens. Finally, we used the dimensions of the SC radii to estimate the body mass for Endothiodon with a highly-significant linear regression model.
Materials and methods
Institutional acronyms
MTA/ACL, Academia das Ciências de Lisboa, Lisbon, Portugal; AMNH, American Museum of Natural History, New York, United States of America; GPIT/RE, Institut und Museum für Geologie und Paläontologie, Tübingen, Germany; MB.R, Museum für Naturkunde, Leibniz-Institut für Evolutions- und Biodiversitätsforschung, Berlin, Germany; SAM-PK, Iziko South African Museum, Cape Town, Republic of South Africa.
Materials
We examined three rare and fragile Endothiodon cf. bathystoma specimens that preserve partial occipital and basicranial regions, namely MTA/ACL001 (Fig 1), MTA/ACL002 (Fig 2), MTA/ACL003 (Fig 3) [42]. The Endothiodon specimens can be consulted at the Academia de Ciências de Lisboa. The specimens were all collected from the K5 Formation [43, 44] of the Metangula Graben, Niassa Province, Mozambique. Niassodon is permanently deposited in the Museu Nacional de Geologia (Mozambique) collections. A comprehensive review of the history of the specimens and the collection of fossil vertebrates from Mozambique is described in the Supporting Information.
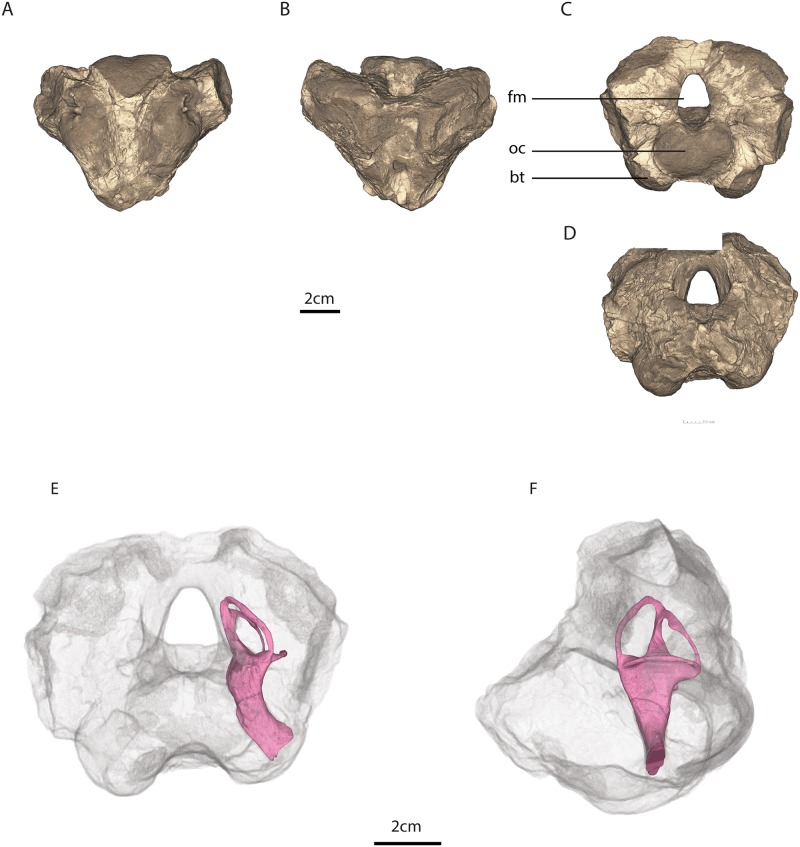
Fig 1. MTA/ACL001 basicranium.
A, ventral, B, dorsal, C, posterior, and E, anterior views. Osseous labyrinth within the basicranium in E, anterior and F, left lateral views. Legend: bt, basal tubera, fm, foramen magnum, oc, occipital condyle.
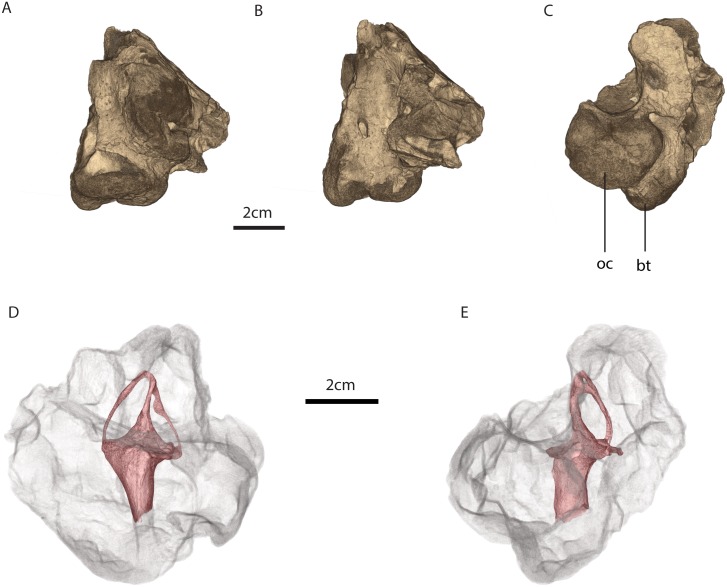
Fig 2. MTA/ACL002 basicranium.
A, ventral view, B, dorsal view, C, posterior view, D, medial view with the osseous labyrinth, E, posterior view with the osseous labyrinth. Legend: bt, basal tubera, oc, occipital condyle.
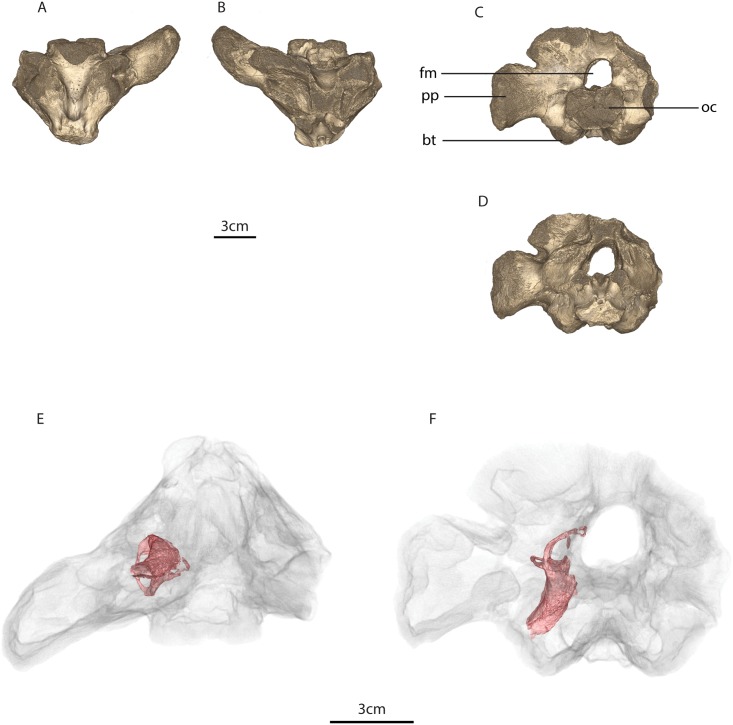
Fig 3. MTA/ACL003 basicranium.
A, ventral view, B, dorsal view, C, posterior view, D, anterior view. Osseous labyrinth within the basicranium in E, ventral view, F, anterior view. Legend: bt, basal tubera, fm, foramen magnum, oc, occipital condyle, pp, paroccipital process.
MTA/ACL001 is composed of the ventral portion of the supraoccipital enclosing the foramen magnum, the basioccipital fused to the exoccipitals with the occipital condyle eroded, the posterior portion of the basisphenoid with relatively intact basisphenoid tubera, and only the medial portions of the opisthotic (Fig 1).
MTA/ACL002 contains of a small fragment of the right portion of the supraoccipital and the most medial portion of the right opisthotic perforated by the right jugular foramen. MTA/ACL002 also contains the basioccipital, which forms together with the exoccipitals a tripartite occipital condyle, the latter being pierced by two roots of the hypoglossal foramina (Fig 2).
MTA/ACL003 is composed of the right opisthotic, the ventral portion of the supraoccipital, and the basioccipital, although the occipital condyle is eroded. In MTA/ACL003 the exoccipitals are fused to the basioccipital and there is a portion of the basisphenoid whose basisphenoidal tubera was largely eroded. In all specimens due to modern-day erosion an outer layer of the bone surface was significantly demineralized.
AMNH6156 natural cast of the inner ear was found to possess similar morphology to the Endothiodon inner ear endocasts, therefore, we incorporated a description and comparisons in S1 Text.
Propagation phase-contrast synchrotron micro-computed tomography and segmentation
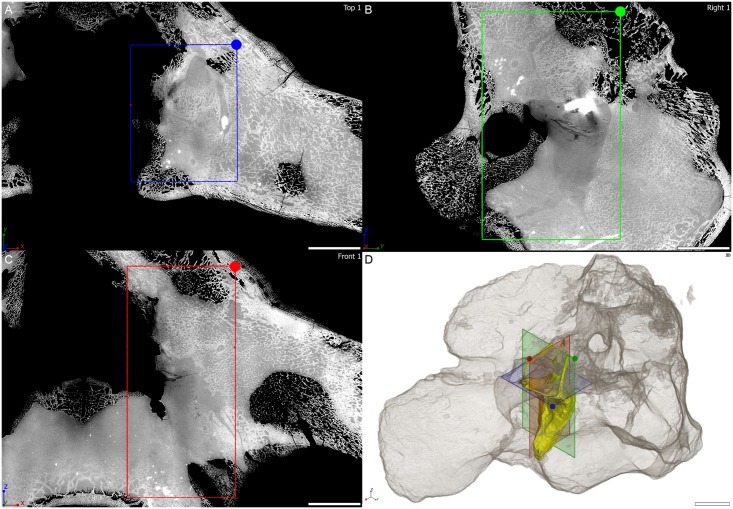
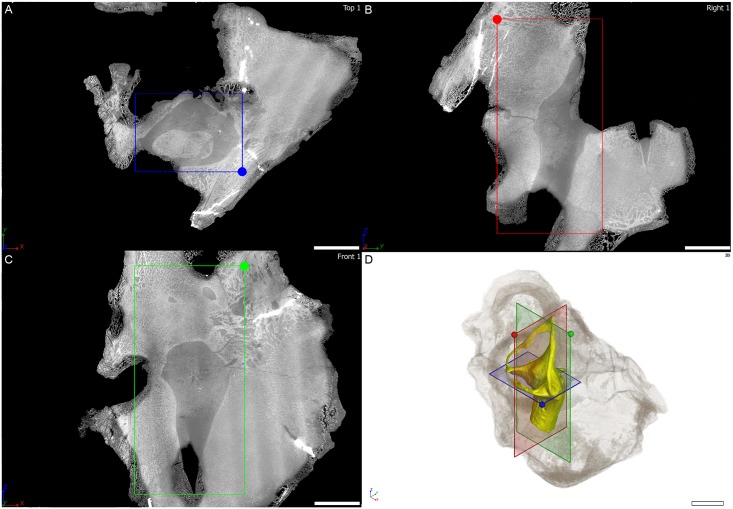
The Endothiodon specimens were scanned at the beamline ID17 of the European Synchrotron Radiation Facility (ESRF, Grenoble, France), using Propagation Phase Contrast Synchrotron micro-Computed Tomography (PPC-SRμCT). The set up consisted of a 130 keV monochromatic beam (bent double Laue), a tapered scintillating fiber-optic, a 0.5x set of lenses and a FReLoN-2K camera resulting in an isotropic voxel size of 45.98 μm in reconstructed data. The data acquisition comprised 3100 projections of 0.1 s each over 360°, and laterally shifted center of rotation to increase the reconstructed horizontal field of view (i.e., half-acquisition protocol [46]). Consecutive scans had an approximate 35% vertical overlap to compensate for the vertical beam profile. To produce Figs 4, 5 and 6 three orthogonal virtual thin sections are oriented based on the plane containing the horizontal semi-circular canal. All virtual thin sections result from the maximum intensity projection of three adjacent tomograms in order to reduce the density gradient present near the edge of the specimen. The contrast on the images has been adjusted setting the background to 0 and reaching saturation for the denser inclusion in the specimen.
Fig 4. Virtual thin section through the osseous labyrinth of MTA/ACL001 specimen.
Orthogonal virtual thin sections in A, horizontal plane, B, coronal plane, C, sagittal plane through the right osseous labyrinth of MTA/ACL001 specimen. D, three-dimensional rendering of the right osseous labyrinth with semi-transparent outline of the whole specimen in anterolaterodorsal view. Scale bar: 10 mm.
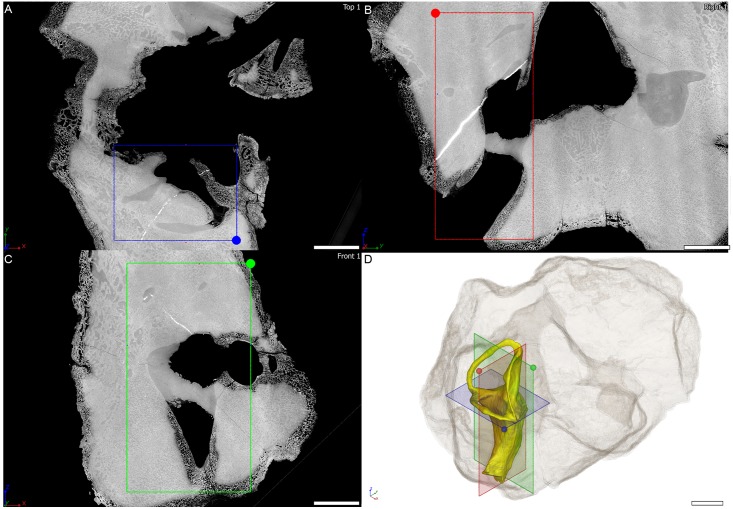
Fig 5. Virtual thin section through the osseous labyrinth of MTA/ACL002 specimen.
Orthogonal virtual thin sections in A, horizontal plane, B, coronal plane, C, sagittal plane through the right osseous labyrinth of MTA/ACL002 specimen. D, three-dimensional rendering of the right osseous labyrinth with semi-transparent outline of the whole specimen in anterolaterodorsal view. Scale bar: 10 mm.
Fig 6. Virtual thin section through the osseous labyrinth of MTA/ACL003 specimen.
Orthogonal virtual thin sections in A, horizontal plane, B, coronal plane, C, sagittal plane through the left osseous labyrinth of MTA/ACL003 specimen. D, three-dimensional rendering of the left osseous labyrinth with semi-transparent outline of the whole specimen in posterolaterodorsal view. Scale bar: 10 mm.
The Niassodon specimen was imaged at the ID19 beamline of the European Synchrotron Radiation Facility (ESRF, Grenoble, France). The setup consisted of a FReLoN-2k camera, a 0.475x magnification set of lenses, a 750 μm LuAG scintillator, white beam from a W150 wiggler (gap 58 mm) filtered with Al 2 mm and Cu 4 mm (detected total integrated energy at 97.8 keV) and a sample-detector distance of 16 m to perform Propagation Phase Contrast Synchrotron micro Computed Tomography (PPC-SRμCT). The tomography was computed based on 6000 projections of 0.1 s each over 360 degrees resulting in data with a 27.85 μm isotropic voxel size. Additionally, the center of rotation was shifted by ~18 mm to increase the horizontal field of view in the reconstructed data (i.e., half acquisition protocol).
Both tomographic reconstructions were performed using the single distance phase retrieval approach of the software PyHST2 [47,48]. For this purpose, a range of ð/ß values were tested first (500–2000, steps of 250) from which a value of 1000 was selected as it was giving best contrast on reconstructed slices. The resulting 32-bit data were converted to a stack of 16-bit tiff images using the minimum and maximum values excluding 0.001% of voxels on both sides of the 3D histogram generated by PyHST2.
Volume processing and rendering was undertaken using the software VGstudio MAX 2.1 (Volume Graphics, Heidelberg, Germany). The segmentation was carried out using semi-automatic 3D region growing tools. When this tool did not permit complete extraction (e.g., contrast too low between the bone and the matrix or elevated fracture level), missing parts were added slice by slice using manual segmentation as necessary.
Data accessibility
The tomographies described here are made accessible as.jpeg2000 stacks through the ESRF Paleontological Database (paleo.esrf.eu).
Measurements and statistics
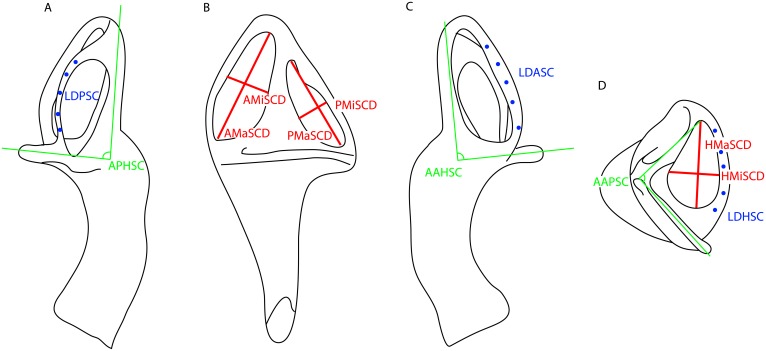
The measurements were performed in Amira (FEI, Hillsboro, Oregon, USA) using the 2D angle measurement tool and the 3D linear measurement tool following the scheme of Fig 7 (Tables 1, 2, 3, S1 and S2 Tables). To calculate the elliptical eccentricity of the vertical semicircular canals we used the formula , where a is the major axis, and b the minor axis of the SC. We used the surface thickness computation module in Amira to perform the lumen diameter measurements of the various semicircular canals. Each measurement was performed five times at different occasions to ensure repeatability and to test intra-observer variability (Tables 2, 3, S1 and S2 Tables). The five measurements of lumen diameter were performed in relatively equidistant points along the semicircular canal. Summary statistics were calculated for the linear measurements of the lumen diameter of the SCs (Table 2), whereas circular statistics [49] were used to treat the angular measurements between the three SCs for each specimen (Table 3). We used the t-distribution with two tails and n-1 degrees of freedom. We also calculated the relative and absolute Technical Error of Measurement (TEM) and performed repeated measures ANOVA’s for the angles between SC’s because these are truly repeated measurements (S2 Table); the lumen diameters are not made in the same homologous points (see Fig 7). Therefore, variations through the SCC are expected, so that the TEM cannot be calculated. Also, because the TEM is designed for 2 repeated measurements, not 5, we calculated the TEM for all pairwise combinations between the 5 repeated measurements and then averaged the calculated TEM for all 10 pairwise combinations.
Fig 7. Diagrammatic scheme of the linear measurements performed.
Osseous labyrinth in A, posterior view. B, lateral view. C, anterior view. D, dorsal view. Abbreviations: APHSC, angle between the posterior and horizontal semicircular canal, AAHSC, angle between the anterior and horizontal semicircular canal, AAPSC, angle between the anterior and posterior semicircular canal. LDASC, lumen diameter of the anterior semicircular canal. LDPSC, lumen diameter of the posterior semicircular canal. LDHSC, lumen diameter of the anterior semicircular canal. AMaSCD, anterior major axis of the semicircular canal diameter. AMiSCD, anterior minor axis of the semicircular canal diameter. PMaSCD, posterior major axis of the semicircular canal diameter. PMiSCD, posterior minor axis of the semicircular canal diameter. HMaSCD, horizontal major axis of the semicircular canal diameter. HMiSCD, horizontal minor axis of the semicircular canal diameter.
Table 1. Vertical semicircular canal elliptical eccentricity measurements for the mammalian sampled taxa, the Endothiodon specimens and Niassodon.
Abbreviations: AMiSCD, anterior semicircular canal minor axis diameter, AMaSCD, anterior semicircular canal major axis diameter, PMiSCD, posterior semicircular canal minor axis diameter, PMaSCD, posterior semicircular canal major axis diameter, HMiSCD, horizontal semicircular canal minor axis diameter, HMaSCD, horizontal semicircular canal major axis diameter, LogBM, log10 body mass, asterisk refers to estimated body mass calculated using the linear regression model. See also S3 Table.
| Genus | Specimen | AMaSCD | AMiSCD | PMaSCD | PMiSCD | HMaSCD | HMiSCD | Log10 BM | e |
|---|---|---|---|---|---|---|---|---|---|
| Atelerix | unvouchered | 1.33 | 1.22 | 1.23 | 1.08 | 0.96 | 0.89 | 2.52 | 0.44 |
| Canis | TMMM-150 | 1.73 | 1.72 | 1.42 | 1.42 | 1.50 | 1.46 | 4.60 | 0.10 |
| Cavia | TMM-M-7283 | 2.15 | 1.53 | 1.46 | 1.44 | 1.90 | 1.16 | 2.86 | 0.57 |
| Chrysochloris | AMNH82372 | 1.20 | 0.78 | 0.68 | 0.48 | 0.67 | 0.58 | 1.67 | 0.75 |
| Cynocephalus | AMNH187859 | 2.09 | 1.95 | 1.64 | 1.53 | 1.49 | 1.37 | 3.10 | 0.36 |
| Dasypus | TMM-M-152 | 1.90 | 1.50 | 1.63 | 1.62 | 1.45 | 1.38 | 3.60 | 0.47 |
| Dasypus | TMM-M-1065 | 1.96 | 1.66 | 1.80 | 1.72 | 1.44 | 1.40 | 3.60 | 0.44 |
| Dasypus | TMM-M-1880 | 2.06 | 1.31 | 1.88 | 1.72 | 1.58 | 1.54 | 3.60 | 0.64 |
| Dasypus | TMM-M-1885 | 2.15 | 1.31 | 1.94 | 1.93 | 1.48 | 1.37 | 3.60 | 0.61 |
| Didelphis | TMM-M-2517 | 1.47 | 1.40 | 1.11 | 1.10 | 0.92 | 0.84 | 3.39 | 0.25 |
| Equus | TMM-M-171 | 3.53 | 3.43 | 3.49 | 3.20 | 3.10 | 3.04 | 5.61 | 0.33 |
| Eumetopias | unvouchered | 3.32 | 2.38 | 2.83 | 2.32 | 2.59 | 1.91 | 5.58 | 0.64 |
| Felis | TMM-M-968 | 1.99 | 1.67 | 1.84 | 1.73 | 1.73 | 1.53 | 3.46 | 0.46 |
| Hemicentetes | AMNH161535 | 1.13 | 0.95 | 0.79 | 0.65 | 0.69 | 0.58 | 2.13 | 0.55 |
| Homo | UTO-HS01 | 1.99 | 1.83 | 2.83 | 2.38 | 2.72 | 2.51 | 4.77 | 0.49 |
| Macaca | TMM-M-5987 | 2.68 | 1.34 | 2.36 | 2.27 | 2.36 | 2.02 | 5.67 | 0.70 |
| Macroscelides | AMNH161535 | 1.19 | 1.16 | 1.07 | 0.73 | 1.17 | 0.76 | 1.59 | 0.55 |
| Manis | AMNH53896 | 1.40 | 1.13 | 1.40 | 1.15 | 0.82 | 0.78 | 3.19 | 0.58 |
| Monodelphis | TMM-M-7599 | 1.02 | 1.00 | 0.94 | 0.87 | 0.74 | 0.69 | 1.97 | 0.29 |
| Mus | TMM-M-3196 | 0.86 | 0.58 | 0.71 | 0.47 | 0.59 | 0.52 | 1.29 | 0.74 |
| Nycteris | AMNH268369 | 0.99 | 0.85 | 0.81 | 0.70 | 0.96 | 0.61 | 1.47 | 0.50 |
| Orycteropus | AMNH51909 | 3.36 | 2.86 | 4.07 | 2.95 | 3.44 | 2.88 | 4.75 | 0.62 |
| Procavia | TMM-M-4351 | 2.15 | 1.57 | 2.30 | 1.72 | 2.03 | 1.48 | 3.47 | 0.67 |
| Pteropus | AMNH237593 | 1.54 | 1.36 | 1.45 | 1.15 | 1.28 | 1.25 | 2.50 | 0.54 |
| Rhinolophus | AMNH245591 | 0.87 | 0.70 | 0.74 | 0.66 | 0.92 | 0.77 | 1.35 | 0.53 |
| Sorex | unvouchered | 0.78 | 0.41 | 0.70 | 0.43 | 0.44 | 0.44 | 0.84 | 0.83 |
| Sus | TMM-M-2689 | 2.46 | 2.00 | 2.20 | 1.53 | 1.88 | 1.56 | 4.93 | 0.65 |
| Sylvilagus | TMM-M-2689 | 1.75 | 1.69 | 1.42 | 1.28 | 1.27 | 1.18 | 3.08 | 0.34 |
| Tadarida | TMM-M-3030 | 0.83 | 0.73 | 0.69 | 0.61 | 0.89 | 0.61 | 1.10 | 0.48 |
| Trichechus | MSW03156 | 4.07 | 4.06 | 3.72 | 3.59 | 4.53 | 4.27 | 5.67 | 0.19 |
| Tupaia | TMM-M-2256 | 1.91 | 1.61 | 1.67 | 1.26 | 1.84 | 1.20 | 2.12 | 0.60 |
| Tursiops | SDNHM21212 | 1.08 | 0.96 | 0.80 | 0.68 | 1.29 | 1.27 | 5.45 | 0.49 |
| Endothiodon | MTA-ACL-002 | 5.24 | 2.00 | 4.61 | 1.81 | 3.24 | 1.74 | 5.15* | 0.92 |
| Endothiodon | MTA-ACL-003 | 4.93 | 2.74 | 3.91 | 1.87 | 2.97 | 1.62 | 5.06* | 0.85 |
| Endothiodon | MTA-ACL-001 | 5.15 | 2.64 | 4.24 | 1.96 | 3.47 | 2.09 | 5.26* | 0.87 |
| Niassodon | ML1620 | 2.4 | 1.68 | 2.08 | 1.08 | 3.46 | 2.93 | 2.69* | 0.79 |
Table 2. Linear measurements for each Endothiodon cf. bathystoma specimen.
Each variable for the three specimens was measured at five different loci of each SC. Abbreviations: SE, standard error, σ, variance, conf. int. confidence interval, LDASC Lumen diameter of the anterior semicircular canal, LDPSC Lumen diameter of the posterior semicircular canal, LDHSC Lumen diameter of the horizontal semicircular canal.
| LDASC | LDPSC | LDHSC | |
|---|---|---|---|
| MTA-ACL-001 | 0.75 | 0.42 | 0.57 |
| 0.80 | 0.35 | 0.58 | |
| 0.80 | 0.40 | 1.02 | |
| 0.84 | 0.42 | 0.88 | |
| 0.76 | 0.39 | 0.66 | |
| Mean | 0.79 | 0.40 | 0.74 |
| σ | 0.04 | 0.03 | 0.20 |
| St error | 0.02 | 0.01 | 0.09 |
| Lower bound 95% conf. int. | 0.75 | 0.36 | 0.49 |
| Upper bound 95% conf. int. | 0.83 | 0.43 | 0.99 |
| MTA-ACL-002 | 0.69 | 0.43 | 0.76 |
| 0.81 | 0.38 | 0.77 | |
| 0.94 | 0.44 | 0.73 | |
| 0.59 | 0.44 | 0.88 | |
| 0.53 | 0.42 | 0.66 | |
| Mean | 0.71 | 0.42 | 0.76 |
| σ | 0.17 | 0.02 | 0.08 |
| St error | 0.07 | 0.01 | 0.04 |
| Lower bound 95% conf. int. | 0.51 | 0.39 | 0.66 |
| Upper bound 95% conf. int. | 0.92 | 0.45 | 0.86 |
| MTA-ACL-003 | 0.61 | 0.41 | 0.41 |
| 0.68 | 0.36 | 0.62 | |
| 0.64 | 0.40 | 0.47 | |
| 0.70 | 0.42 | 0.77 | |
| 0.71 | 0.41 | 0.51 | |
| Mean | 0.67 | 0.40 | 0.56 |
| σ | 0.04 | 0.02 | 0.14 |
| St error | 0.02 | 0.01 | 0.06 |
| Lower bound 95% conf. int. | 0.62 | 0.37 | 0.38 |
| Upper bound 95% conf. int. | 0.72 | 0.43 | 0.73 |
Table 3. Angular measurements and respective descriptive circular statistics for each Endothiodon cf. bathystoma specimen.
Five repeated measurements of the angle between the three SCs was measured for the three sampled specimens. Abbreviations: conf. int., confidence interval, AAHSC, Angle between the anterior and horizontal semicircular canal, APHSC, Angle between the posterior and horizontal semicircular canal, AAPSC, Angle between the anterior and posterior semicircular canal.
| AAHSC | APHSC | AAPSC | |
|---|---|---|---|
| MTA-ACL-001 | 91.7 | 90.4 | 89.7 |
| 92 | 90.5 | 89.4 | |
| 90.6 | 90.1 | 88.8 | |
| 91.5 | 89.2 | 89.6 | |
| 89.6 | 90.1 | 90.9 | |
| Circular mean | 91.08 | 90.06 | 89.68 |
| Circular standard deviation | 0.84 | 0.25 | 0.37 |
| Standard error | 0.38 | 0.11 | 0.16 |
| Lower bound 95% conf int | 90.03 | 89.75 | 89.23 |
| Upper bound 95% conf int | 92.13 | 90.37 | 90.13 |
| MTA-ACL-002 | 91.2 | 91.7 | 88.6 |
| 92.2 | 91.2 | 90.1 | |
| 92.2 | 92.0 | 89 | |
| 92.5 | 89.2 | 89.0 | |
| 92.1 | 89.8 | 89.7 | |
| Circular mean | 92.04 | 90.78 | 89.28 |
| Circular standard deviation | 1.8 | 0.67 | 0.66 |
| Standard error | 0.8 | 0.3 | 0.29 |
| Lower bound 95% conf int | 89.81 | 89.95 | 88.46 |
| Upper bound 95% conf int | 94.27 | 91.61 | 90.1 |
| MTA-ACL-003 | 90.5 | 89.5 | 87 |
| 91.4 | 90.4 | 88 | |
| 91.5 | 90.2 | 90.3 | |
| 90.7 | 90.4 | 90.4 | |
| 88.9 | 89.6 | 89.6 | |
| Circular mean | 90.08 | 89.91 | 87.44 |
| Circular standard deviation | 91.12 | 90.13 | 90.68 |
| Standard error | 0.19 | 0.04 | 0.58 |
| Lower bound 95% conf int | 90.22 | 89.94 | 87.89 |
| Upper bound 95% conf int | 90.98 | 90.1 | 90.23 |
Results
Anatomical description of the Endothiodon bony labyrinth
The Endothiodon osseous labyrinths are exquisitely preserved (Figs 8, 9, 10, S1, S2 and S3 Figs) except for small dorsal portions of the anterior and posterior SCs as well as a part of the crus communis in MTA/ACL003 (Fig 10).
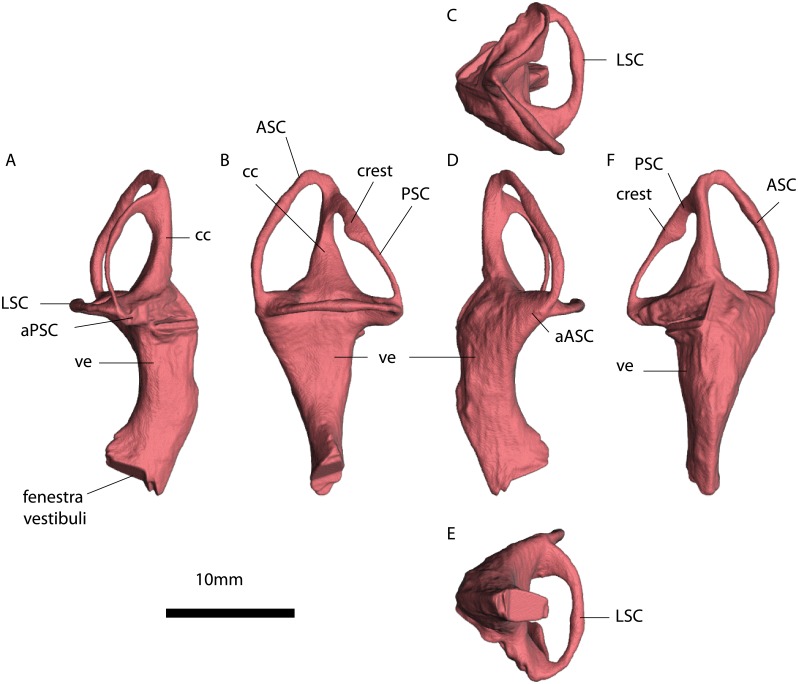
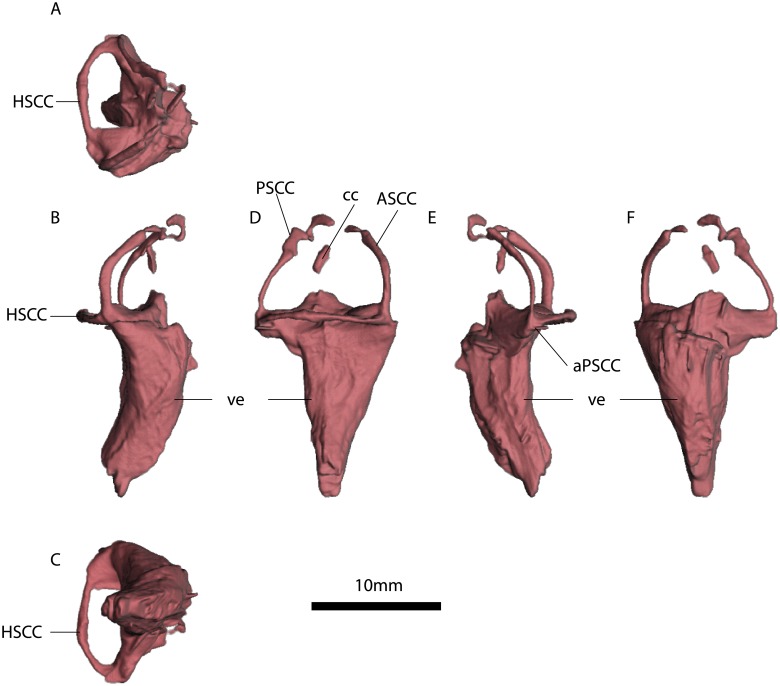
Fig 8. MTA/ACL001 left inner ear.
A, anterior view. B, lateral view. C, dorsal view. D, anterior view. E, ventral view. F, medial view. Abbreviations: ASC, Anterior semicircular canal. PSC, Posterior semicircular canal. HSC, horizontal semicircular canal. ve, vestibulus. cc, crus communis. aASC, Ampulla of the anterior semicircular canal. aPSC, Ampulla of the posterior semicircular canal, “*”, estimates based on the average radius of the semicircular canal. See also S1 Fig.
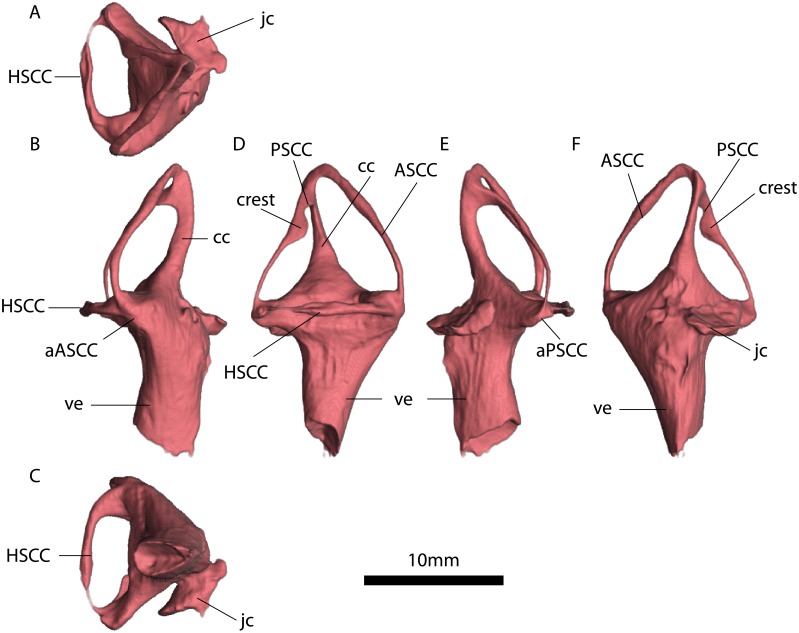
Fig 9. MTA/ACL002 right inner ear.
A, dorsal view. B, anterior view. C, ventral view. D, lateral view. E, posterior view. F, medial view. Abbreviations: ASCC, Anterior semicircular canal. PSCC, Posterior semicircular canal. HSCC horizontal semicircular canal. ve. vestibulus. cc. crus communis. aASCC, Ampulla of the anterior semicircular canal. aPSCC, Ampulla of the posterior semicircular canal. jc. jugular canal. See also S1 Fig.
Fig 10. MTA/ACL003 right inner ear.
A, dorsal view. B, posterior view. C, ventral view. D, lateral view. E, anterior view. F, medial view. Abbreviations: ASCC, Anterior semicircular canal. PSCC, Posterior semicircular canal. HSCC, Horizontal semicircular canal. ve, vestibulus. cc, crus communis. aPSCC, Ampulla of the posterior semicircular canal. See also S3 Fig.
The fenestrae vestibuli and lagena are not clearly distinguishable. The bone surrounding the fenestra vestibuli is not completely preserved, missing the lateralmost portions of the basisphenoid and opisthotic. The vestibule is slightly curved, with a medial curvature convexity (Figs 8, 9 and 10, S1, S2 and S3 Figs). The vestibule is a broad chamber, which is subtriangular in cross section. It develops the cochlea ventrally as a straight canal. The major axis radius of the anterior SC is slightly longer (average = 9.47 mm) than the posterior SC (average = 8.47 mm); see Table 1. The anterior SC major axis is 1.37 to 2.49 times longer than the minor axis, whereas the posterior SC is slightly more eccentric (2.20 to 2.56; Table 1). At the level of the fenestra vestibuli a horizontal SC develops laterally. The horizontal SC is ellipsoidal in shape, with a major axis ranging from 8.03 to 8.48 mm, and the minor axis from 3.05 to 3.50 mm (Figs 8, 9 and 10). The horizontal SC is broad near the vestibulum but it becomes abruptly thin laterally. Similarly, the vertical SCs are thin and dorsoventrally elongated. The SCs are suborthogonally-oriented relative to each other (Table 3, Figs 11, 12). However, the angle between the anterior and horizontal SC is consistently around ~91° in the different specimens, and the angle between the anterior and posterior SC is consistently around ~89° (Fig 12A).
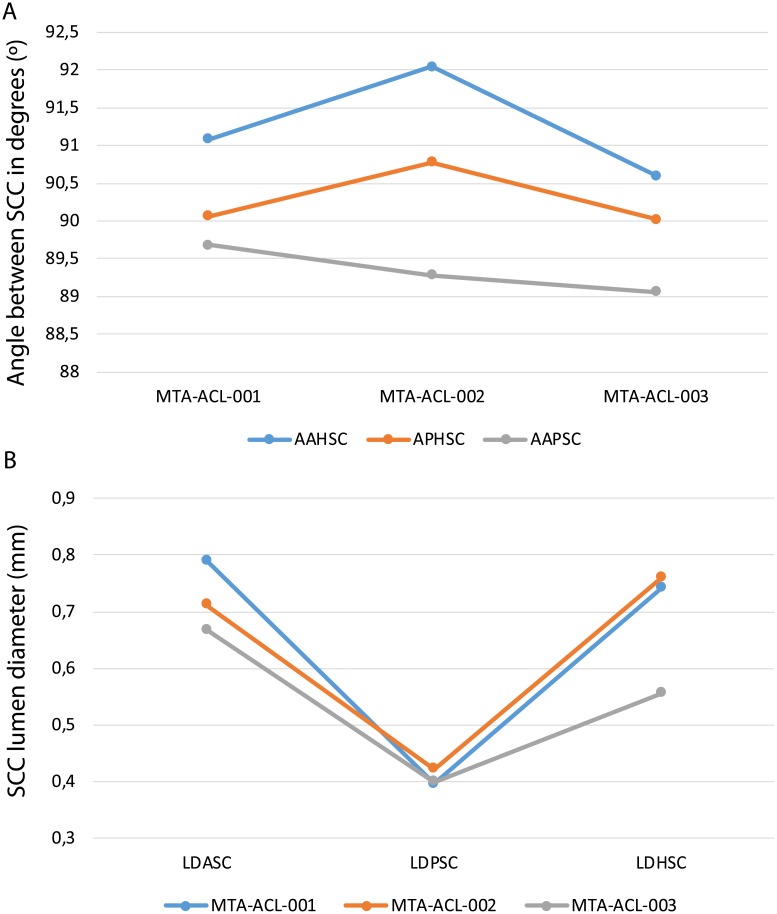
Fig 11. Comparative charts between the various semicircular canals among the different Endothiodon cf. bathystoma specimens.
A, angle between the semicircular canals. B, lumen diameter of the various semicircular canals. Notice that all the semicircular canals are nearly orthogonal, but the AAHSC is consistently greater than 90°, and the AAPSC is consistently lower than 90°. Also, the horizontal and the anterior semicircular canals have consistently similar lumen diameters and the posterior semicircular canal is consistently the thinnest.
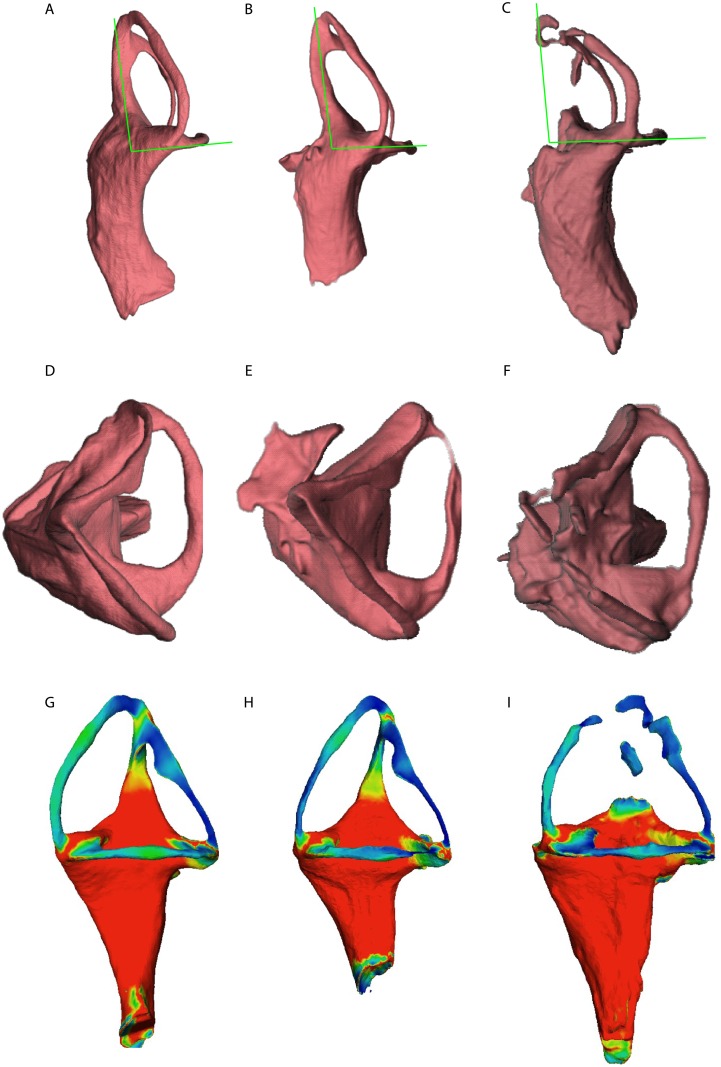
Fig 12. Comparison between the three Endothiodon cf. bathystoma specimens. Images are not at scale to facilitate comparisons.
A, MTA-ACL001 in anterior view. B, MTA-ACL002 in anterior view (reversed). C, MTA-ACL003 in anterior view (reversed). D, MTA-ACL001 in dorsal view. E, MTA-ACL002 in dorsal view (reversed). F, MTA-ACL003 in dorsal view (reversed). Variation of semicircular canal lumen diameter for: G, MTA-ACL001 in lateral view, H, MTA-ACL002 in lateral view (reversed), I, MTA-ACL003 in lateral view (reversed).
The lumina of the SCs are subcircular (i.e., elliptical eccentricity less than 0.15) to slightly elliptical in cross-section. Interestingly, the posterior SC possesses a crest close to the crus communis (Figs 8 and 9). The narrowest duct radius of the anterior SC ranges from 0.67 to 0.79 mm, and for the posterior SC from 0.40 to 0.42 mm (Table 2). The lumen diameter of the anterior and horizontal SCs are nearly the same (0.6–0.8 mm); however, the lumen of the posterior SC is consistently thinner (~0.4 mm; Fig 11B). Additionally, in dorsal view, the posterior SC is significantly more arched anteriorly than the horizontal and anterior SCs. The horizontal and anterior SCs are in-plane, i.e., there are no significant deviations from a planar toroid (Fig 12D, 12E and 12F). The osseous enclosure around the ampullae in all three SCs are poorly distinguishable from the slender portion of the canal. At the intersection of the anterior and horizontal SC there is an inflated portion, elliptical in cross section, which forms the secondary crus communis and houses the ampulla. The osseous enclosure around the ampulla of the posterior SC is slightly expanded ventrally, but also confounds with the posterior intersection of the horizontal SC. The crus communis is very broad at the base but becomes precipitously thin towards its dorsal portion, giving a subtriangular aspect. The cross-section of the crus communis changes dorsoventrally, being D-shaped ventrally with the convexity being laterally-oriented, subcircular at the midpoint and then becoming ellipsoidal dorsally. The jugular (vagal) canal meets the osseous labyrinth near the base of the posterior SC.
Anatomical description of the Niassodon bony labyrinth
Based on new segmentation derived from a PPC-SRμCT scan, we provide further details on the anatomy of Niassodon inner ear (Fig 13), a pivotal taxon related to Endothiodon according to the most recent phylogenetic analysis [50,51]. Indeed, the small-bodied Niassodon possesses relatively elongated SCs [35]. In Niassodon, the vestibular system is delimited by the supraoccipital, prootic and opisthotic. The supraoccipital delimits the posterior SC, the crus communis except its base and the posterior third of the anterior vertical SC. The prootic envelops the anterior two-thirds of the anterior vertical SC, the anterior part of the vestibule and the anterior portion of the horizontal SC, and the base of the crus communis. The lagena is delimited by the exoccipital, basisphenoid and basioccipital. The exoccipital envelops the posterior portion of the vestibule and a small dorsomedial portion of the lagena. The medial and posterior part of the lagena, as well as the dorsal part of the lateral aspect of the fenestra vestibuli is delimited by the basisphenoid. The basioccipital delimits the posterior aspect of the lagena.
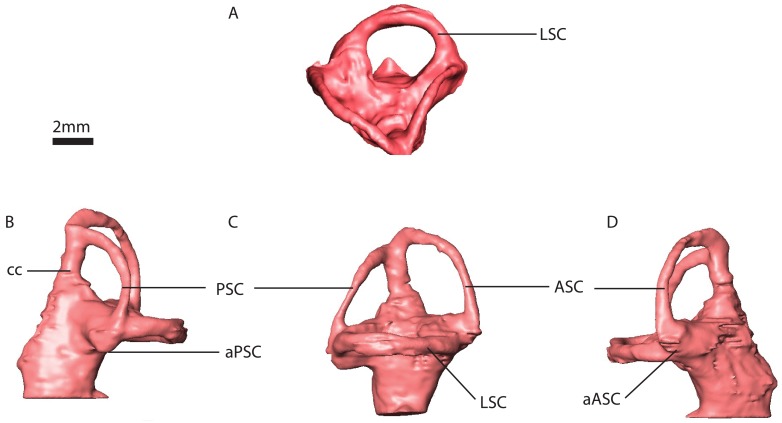
Fig 13. Niassodon mfumukasi (ML 1620) right inner ear.
A, dorsal view. B, posterior view. C, lateral view. D, anterior view. Abbreviations: ASC, Anterior semicircular canal. PSC, Posterior semicircular canal. LSC, horizontal semicircular canal. aPSC, ampulla posterior semicircular canal. aASC, ampulla anterior semicircular canal. cc, crus communis.
The SCs are ellipsoidal and orthogonally oriented with respect to each other (Fig 13). The anterior SC projects higher dorsally than the posterior SC. The least eccentric is the horizontal SC with 0.5 mm difference between the major and minor axis on average (Table 1). On the other hand, the posterior SC is the most eccentric with a 1.9 mm difference between major and minor axis (Table 1). The anterior SC is two and a half times more eccentric (1.25 mm) than the horizontal SC (Table 1).
Measured at the thinnest section of the canal, the horizontal SC is the thickest among the three with a diameter of averaging 0.61(±0.01) mm (Fig 13). The posterior SC is the thinnest (0.34±0.03 mm) and the anterior SC is about 0.47 mm diameter. Midway on the left anterior SC there seems to be a constriction (0.24 mm cross-section), but it appears to be an artifact of segmentation. The crus communis is nearly twice as thick as the vertical SCs, straight but somewhat expanded at the base (Fig 13).
The anterior ampulla is a projecting globular structure, detaching from the vestibule (Fig 13). The posterior ampulla is integrated with the vestibule, yet forming a ventral projection giving a somewhat rectangular appearance in posterolateral view. The ampulla of the horizontal canal cannot be differentiated from the vestibule (Fig 13).
The lagena projects ventrally from the SC system but it is truncated along the sagittal plane (Fig 13). Dorsally, near the vestibule, the lagena is a stout D-shaped cylinder with the apex of the curvature projecting posterolaterally, then the lagena tapers ventrally to an acute tip.
Intraspecific variation
The summary statistics for all of the morphometric variables allow us to understand measurement precision and by comparing the three specimens we have some information on intraspecific variation despite the small sample size. The lumen diameter of the anterior SC varies between specimens from 0.67–079 mm (we used the mean of the repeated measures for each variable to calculate the sample range) with a mean of 0.72 mm, likewise for the posterior SC varies from 0.40–0.42 mm averaging 0.41 mm, and the horizontal SC varies from 0.56–0.76 mm averaging 0.69 mm. The standard error for the lumen diameter of the anterior SC 0.03 mm, for the posterior SC 0.01 mm, and the horizontal SC 0.04 mm.
The angle between the horizontal and posterior SCs ranges from 90.02 to 90.78° averaging 90.29°, between the horizontal and anterior SCs ranges from 90.6 to 92.04° averaging 91.24°, and the angle between the anterior and posterior SCs ranges from 89.06 to 89.68° averaging 89.34°. The angle between the horizontal and posterior SCs has a standard error of 0.25°, for the angle between the horizontal and anterior SCs 0.18°, between the horizontal and posterior SCs is 0.25° and between the anterior and posterior SCs is 0.31°.
The repeated measures of the linear variables allow to check intra-canal variability. For the linear measurements, the standard error ranges from 0.01 mm for the posterior SC lumina diameters of all specimens and 0.09 mm for the lumina diameter of the horizontal SC in MTA/ACL001. Thus, linear measurements of the lumina diameters are relatively precise with errors not exceeding 14% of the measured quantities (Table 1). For the angular measurements, the repeated measurements allow to understand intra-observer variability. The average relative TEM is 0.82% for the angle between the posterior and horizontal SCs, 0.88% for the angle between the anterior and horizontal SCs, and 1.08% for the angle between the anterior and posterior SCs. Thus, angular measurements are very precise with errors not exceeding 1.08% of the measured quantities (Table 2, S2 Table). The overall average between angles for all SCs in all specimens is 90.29°.
Body mass estimation
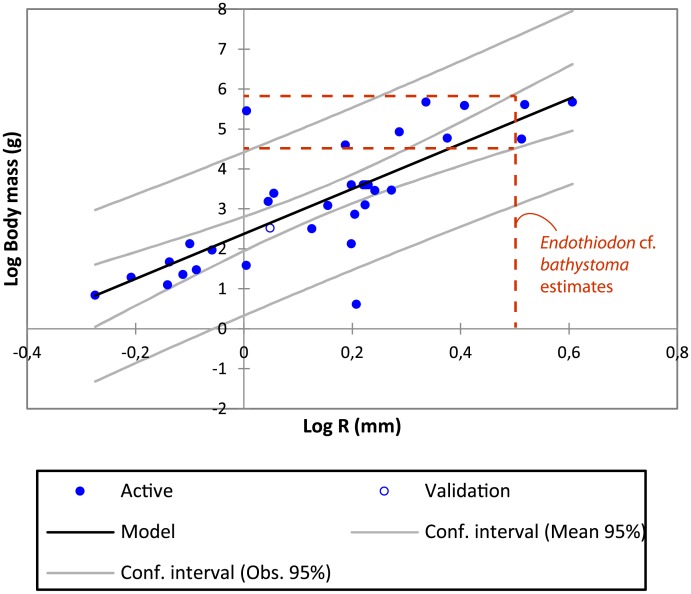
We used the average of all SC radii to estimate Endothiodon body mass (Fig 14), which has given statistically significant results by previous authors for other taxa [12,15]. The resulting linear regression equation, optimized for Amemiya Prediction Criterion, is highly significant (correlation coefficient = 0.787, , P<0.0001, α = 0.05, see Fig 14). Significant deviations of the SC radius have so far only been detected in highly specialized taxa (e.g., [12]). The resulting linear regression equation estimates that Endothiodon weighed between ~116 to 182 kg. MTA/ACL002 is the Endothiodon specimen closest to the average (140 kg).
Fig 14. Linear regression of the log10 average semicircular canal radius to estimate the log10 body mass.
The estimates for the Endothiodon cf. bathystoma specimens range from ~116 to 182 kg. The correlation coefficient between the two variables is 0.787. The extant mammal measurements can be found in S3 Table and from [15].
Discussion
Intraspecific variation of the semicircular canal system
The summary statistics on the Endothiodon cf. bathystoma angular and linear measurements indicate low intraspecific variability, despite the small sample. However, as there is currently limited information on dicynodont inner ear morphology in the literature, we cannot conclude at this stage whether low intraspecific variability is a common trend among dicynodonts. It is crucial to investigate, when possible, more than one specimen of the same dicynodont taxon. High intraspecific variability in the vestibular system has been linked to relaxed selective pressures resulting, for instance, from slow and infrequent movements [40]. However, although our results tend to suggest that Endothiodon cf. bathystoma was not a slow-moving organism, we cannot perform the Levene’s heteroscedasticity test due to the small sample size. In addition to this possible low variability in the SCs of Endothiodon cf. bathystoma, we found several other conservative morphological characters among specimens (see below).
Our results show that the SCs of Endothiodon are nearly orthogonal, as the sum of the squared differences from orthogonality for all specimens only amounts to 7.8 (Table 3, S2 Table). Malinzak et al. [4] suggest that small deviations from SC orthogonality are associated with agility in strepsirrhine primates, and this rationale has been applied to various other mammalian synapsids (e.g., [21]). However, it is most likely that Endothiodon was not as agile as an arboreal primate, but that instead performed rapid head movements, which would also explain our results.
The pig, Sus, which are animals of comparable body mass to Endothiodon, do fast head movements while foraging and ingesting food [52] and have elevated SC eccentricity (e~0.65) and near orthogonal SCs. Sus SCs are quasi-orthogonal too (AAHSC is 86°, APHSC is 89.7° and AAPSC is 90.8°). Endothiodon could have employed a similar foraging and food processing behavior. Based on the occipital index, previous authors [53] suggest that Endothiodon performed a significant degree of lateral head movements, and the evidence here provided is consistent with such behavior.
Comparative anatomy and evolution of Endothiodontia inner ears
Niassodon has been recovered as the sister-taxon of Endothiodon in recent phylogenetic analyses [50,51], and hence comparisons between the taxa are relevant, as they both belong to Endothiodontia. The vertical SCs of Endothiodon (n = 3) are substantially more eccentric (e~0.88) than those of Niassodon (e~0.79, n = 1). Additionally, the anterior and posterior SCs of Endothiodon are more vertically positioned than those of Niassodon. In Niassodon the SCs are elliptical but rotated sub-horizontally whereas in Endothiodon they are vertically oriented (Figs 8–10 and 13). These results suggest that within the Endothiodontia lineage there was a re-orientation and elongation of the vertical SCs, leading to changes in sensitivity of the vestibular system [5,45]. The surprisingly eccentric Endothiodon SCs may be related to the specialized feeding apparatus of this genus [53], which could require particular head movements for foraging, processing or ingesting food. Based on the anatomy of the oral apparatus, Cox and Angielczyk [53] described the food processing cycle of Endothiodon in detail and demonstrated that it was capable of cropping food items with a uniquely peculiar ‘hare lip’. Furthermore, the occipital region suggests Endothiodon had a significant degree of lateral head movements [53]. Despite the differences between the SCs of Endothiodon and Niassodon, both taxa have strikingly higher SC eccentricity than other known dicynodonts [27–38]. However, whereas this feature is unusual in the mammalian lineage (e.g., [15, 45]), it is premature to conclude that SC eccentricity was widespread among dicynodonts. Some authors [54] have speculated that the elongation of the anterior SC in dinosaurs may be linked to bipedalism, because humans also have similar SC elongation [55]. It was also suggested that horizontal SC elongation could be related to quick and powerful neck lateroflexion in tyrannosaurs [54]. Nevertheless, these hypotheses remain untested with robust biomechanical modelling.
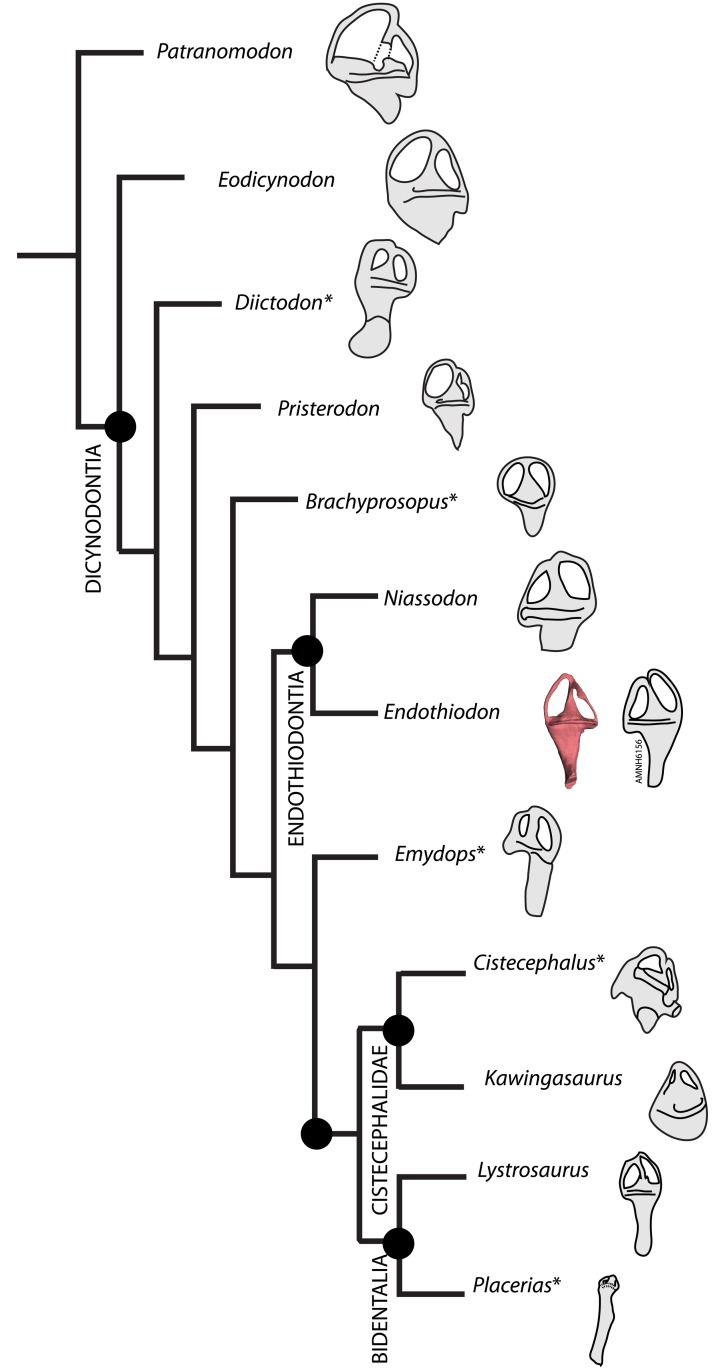
The Endothiodon inner ear can be readily distinguished from that of Eodicynodon based on a posteriorly tilted crus communis (Fig 15). Pristerodon can be distinguished from Endothiodon because its horizontal SC is subcircular and the crus communis is not smoothly dorsally tapering (Fig 15). Lystrosaurus has a strongly ellipsoidal duct cross-section, contrary to the Endothiodon condition (Fig 15). Due to its inflated vestibule morphology [35], Kawingasaurus clearly contrasts with Endothiodon (Fig 15). Some features seem to be shared by other dicynodonts, such as a ventrally broad crus communis, a significantly shorter posterior SC compared to the anterior SC, the absence of well-delimited ampullae, and a certain degree of eccentricity of the SCs [27, 35] (Fig 15). However, Endothiodon seems to have considerably more eccentric canals than any other dicynodont published so far [27–38].
Fig 15. Simplified cladogram of published dicynodont bony labyrinths.
Phylogeny based on the most recent analysis [51]. Patranomodon, Eodicynodon, Pristerodon and Lystrosaurus from [27]; Diictodon from [29]; Brachyprosopus from [38]; Emydops from [34]; Cistecephalus from [33]; Kawingasaurus from [35] and Placerias from [31]. Asterisks indicate bony labyrinths that did not result from computed tomography renderings.
Other clades exhibit elevated eccentricity. For instance, caecilian amphibians have horizontally elongated SCs (e.g., [56]), squamates have obliquely-oriented elongated SCs (e.g., [7]), and some large-bodied dinosaurs have highly eccentric canals (e.g., [57, 58]). Additional studies outside Dicynodontia could elucidate broader evolutionary patterns of the SCs, namely a trend toward more circular canals across synapsid history, with some reversals in taxa with specific biomechanical demands, such as in Endothiodontia. Nevertheless, regardless of the possible direct causal functions for SC elongation, it appears that complex biomechanical stimuli (such as foraging habits, locomotion type, ecology, etc.) are related to the evolution of unusual eccentric morphologies.
Another important character in Endothiodon cf. bathystoma is the crest on the anterior SC, which is absent in Niassodon. This morphological feature has not been previously described and it is not an artifact of segmentation because it is consistently present in the individuals that could be measured (not in MTA/ACL003 because this section of the SCs is not preserved). Apart from these more striking differences, Endothiodon and Niassodon have relatively similar bony labyrinths. For instance, the SCs have a comparable development of the ampullae, both possess a triangular vestibule, the angles between SCs are similar, and the vertical SCs do not follow the same plane but are slightly deflected toward each other, particularly the posterior SC (see Anatomical Description). The current phylogenetic position of Niassodon and Endothiodon as sister taxa indicates that these characters are synapomorphies of Endothiodontia. Additional knowledge on the vestibular system of E. tolani [53] and E. uniseries [59] may reinforce these results. Based on the anatomical characters here provided and the natural cast of the AMNH6156 bony labyrinth, it seems that this specimen is an Endothiodon as well (S1 Text).
Endothiodon body mass
Body mass is intimately related to various aspects of ecology and physiology [60–62]. In dicynodonts, body mass estimates are rare. We attempted to calculate body mass in Endothiodon based on SC dimensions, rather than skull length, because our specimens do not have complete skulls. Whereas several proxies based on specific skeletal elements such as postcranial bones have been used to estimate body mass in extinct taxa, body mass also has a significant correlation with SC dimension (e.g., [63]). We used an extant mammalian dataset [15] of osseous labyrinths as a baseline to calculate body mass estimates for the three Endothiodon specimens here described (Fig 14). Our results indicate that these Endothiodon specimens ranged between 116 kg and 182 kg.
SAM-PK-K11271 is a complete Endothiodon specimen whose propodial dimensions can be used to compare with the SC dimension estimates here provided. Other authors [64] derived equations to estimate body mass based on the femur and humerus circumference. SAM-PK-K11271 has a humeral and femoral narrowest midshaft circumference of 17.5 cm and 13.0 cm, respectively. Body mass estimates based on these estimates range from ~309 to 556 kg, using Campione and Evans equations [64]. In contrast, our body mass estimates for the Mozambican Endothiodon specimens based on SC dimensions ranged from 116–182 kg (see above). Although they are in the same order of magnitude, the body mass estimates of SAM-PK-K11271 are more than triple our estimates for the Mozambican specimens. This difference may be due to the larger size of SAM-PK-K11271 when compared to the Mozambican Endothiodon specimens. Indeed, the measurement of the basioccipital condyle width (~6 cm) and of the width between the basal tubera (~9.5 cm) for SAM-PK-K11271 (Roger Smith personal communication) reveals that this specimen is significantly larger than the Mozambican specimens here described (~4 cm basioccipital condyle width and ~6 cm basal tubera width).
In the Late Permian of Mozambique, Endothiodon is among the most abundant and largest fossil taxa discovered to date. Thus, Endothiodon was likely a large-bodied herbivorous element of the late Permian terrestrial fauna occupying an ecological role similar to grazing mammals in African savannas today.
Conclusions
Our study gives important new insights into dicynodont bony labyrinth anatomy. These findings raise new interesting questions about the biomechanics and functional morphology of the SCs. As CT-scanning technology becomes a widespread resource for paleontological research, more of the anatomy that was previously unclear due to classical preparation techniques is now becoming available. Thus, the morphology and variation of the non-mammalian synapsid inner ear is expected to shed light into the ecomorphology and systematic utility of this organ. The main conclusions of the present contribution are:
Endothiodon has low intraspecific variability of various biophysically relevant morphometric features, namely lumen diameter and angle between the SCs.
Niassodon and Endothiodon have highly eccentric semicircular canals when compared to a wide range of extant synapsids.
Within Endothiodontia, Endothiodon has unique vertically oriented SCs and an ellipsoidal horizontal SC.
Endothiodon was probably capable of performing fast head movements, probably during foraging and food processing.
Body mass estimates using inner ear morphology in dicynodonts may be more widely applicable than other proxies because: (i) it offers a proxy that can be sampled in a broader array of dicynodont taxa when compared to other methods (as dicynodont postcranial material is rarer); and (ii) there is a wide range of available information on the inner ear of mammalian taxa; (iii) the equations for estimating body mass based on SC dimension are very robust for modern mammals.
Given its abundance in the Mozambican Karoo deposits, Endothiodon must have been one of the large (>100 kg) dominant herbivorous members of the late Permian fauna from Mozambique.
Supporting information
The various important moments of Mozambican Karoo vertebrate paleontology are outlined starting 1949 with Domingos da Rocha to the 1980’s by Brigadas de Cartografia Geológica da Bacia carbonífera de Metangula.
(DOCX)
(XLSX)
(XLSX)
(XLSX)
(STL)
(STL)
(STL)
Acknowledgments
We thank Jessie Maisano and Stig Walsh for letting us access data on other osseous labyrinths from various other extant species. Also, we thank Gabriel G. Martins for imaging software support and Luís Costa Júnior as well as Dino Milisse and Nelson Nhamutole for continuous support on the PalNiassa Project. Rodolfo Salas-Gismondi for kindly providing photographs of the AMNH6156 specimen and Isabel L. Torres for perusing and editing the manuscript. The authors wish also to thank Southern Methodist University library for continuous support in finding rare articles for this study. The experiments were performed on beamline ID17 and ID19 at the European Synchrotron Radiation Facility (ESRF), Grenoble, France. We also thank Roger Smith for the SAM specimen measurements. R. Araújo is grateful to the support by Pierre-Olivier Antoine and Laurent Marivaux, as well as Maëva Orliac for discussions on the anatomy of the bony labyrinth morphology and function, and to Christiaan Katelaar for help with circular statistics. R. Araújo and R. Castanhinha are grateful to Élio Sucena and Joaquín Rodriguez-Léon for all the continuous scientific support. We would also like to thank the academic editor, Gabriela Sobral and two other anonymous reviewers for valuable suggestions that improved the manuscript. The publication of this article was funded by the Open Access Fund of the Leibniz Association.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by Portuguese national funding through FCT - Fundação para a Ciência e a Tecnologia within the framework of the project EXPL/BIA-EVF/0665/2013 (PALEOTECH) and by the European Synchrotron Radiation Facility (ESRF), Grenoble, France through experiment IH-LS-1541. R. Araújo and R. M.S. Martins gratefully acknowledge FCT/MCTES for a postdoctoral fellowship (SFRH/BPD/96205/2013) and a contract under IF2014 Programme. (IF/00036/2014/CP1214/CT0009), respectively. R. Castanhinha was funded by a doctoral scholarship (SFRH/BD/51184/2010) and a postdoctoral fellowship (PALEOTECH - EXPL/BIA-EVF/0665/2013), the latter R.M.S. Martins was the principal investigator. J. Fröbisch was financially supported by a Sofja Kovalevskaja Award of the Alexander von Humboldt Foundation donated by the German Federal Ministry for Education and Research. The publication of this article was funded by the Open Access Fund of the Leibniz Association.
References
- 1.Rabbitt RD, Damiano ER, Grant JW. Biomechanics of the semicircular canals and otolith organs In: Highstein SM, Fay RR, Popper AN, editors. Springer: The vestibular system; 2004. pp. 153–201. [Google Scholar]
- 2.Highstein SM, Fay RR, Popper AN. Springer: The vestibular system; 2004. [Google Scholar]
- 3.Ifediba MA, Rajguru SM, Hullar TE, Rabbitt RD. The role of 3-canal biomechanics in angular motion transduction by the human vestibular labyrinth. Ann Biomed Eng. 2007; 35:1247–63. 10.1007/s10439-007-9277-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malinzak MD, Kay RF, Hullar TE. Locomotor head movements and semicircular canal morphology in primates. Proc Nat Acad Sci. 2012; 109:17914–9. 10.1073/pnas.1206139109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pfaff C, Martin T, Ruf I. Bony labyrinth morphometry indicates locomotor adaptations in the squirrel-related clade (Rodentia, Mammalia). Proc R Soc B. 2015; 282: 20150744. 10.1098/rspb.2015.0744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alonso PD, Milner AC, Ketcham RA, Cookson MJ, Rowe TB. The avian nature of the brain and inner ear of Archaeopteryx. Nature. 2004; 430:666–9. 10.1038/nature02706 [DOI] [PubMed] [Google Scholar]
- 7.Boistel R, Herrel A, Daghfous G, Libourel PA, Boller E, Tafforeau P, Bels V. Assisted walking in Malagasy dwarf chamaeleons. Biol Lett. 2010; 12: rsbl20100322. 10.1098/rsbl.2010.0322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yi H, Norell MA. The burrowing origin of modern snakes. Sci Adv. 2015; 1:e1500743 10.1126/sciadv.1500743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sobral G, Sookias RB, Bhullar BA, Smith R, Butler RJ, Müller J. New information on the braincase and inner ear of Euparkeria capensis Broom: implications for diapsid and archosaur evolution. R Soc Open Sci. 2016: 3:160072 10.1098/rsos.160072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azuma Y, Xu X, Shibata M, Kawabe S, Miyata K, Imai T. A bizarre theropod from the Early Cretaceous of Japan highlighting mosaic evolution among coelurosaurians. Sci Rep. 2016; 6: 20478 10.1038/srep20478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costeur L, Mennecart B, Müller B, Schulz G. Prenatal growth stages show the development of the ruminant bony labyrinth and petrosal bone. J Anat. 2017; 230: 347–353. 10.1111/joa.12549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spoor F, Bajpal S, Hussaim ST, Kumar K, Thewissen JGM. Vestibular evidence for the evolution of aquatic behaviour in early cetaceans. Nature. 2002; 417: 163–166. 10.1038/417163a [DOI] [PubMed] [Google Scholar]
- 13.Cox CB. A new digging dicynodont from the Upper Permian of Tanzania In: Joysey KA, Kemp TS, editors. Studies in Vertebrate Evolution. Edinburgh: Oliver and Boyd; 1972. pp. 173–189. [Google Scholar]
- 14.Fröbisch J, Reisz RR. The Late Permian herbivore Suminia and the early evolution of arboreality in terrestrial vertebrate ecosystems. Proc R Soc Lond B: Biol Sci. 2009; 276: 3611–3618. 10.1098/rspb.2009.0911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ekdale EG. Comparative anatomy of the bony labyrinth (inner ear) of placental mammals. PLoS One 2013. 2013; 8: e66624 10.1371/journal.pone.0066624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boistel R, Herrel A, Lebrun R, Daghfous G, Tafforeau P, Losos JB, Vanhooydonck B. Shake rattle and roll: the bony labyrinth and aerial descent in squamates. Int Comp Biol. 2011; 51: 957–968. 10.1093/icb/icr034 [DOI] [PubMed] [Google Scholar]
- 17.Meng J, Fox RC. Osseous inner ear structures and hearing in early marsupials and placentals. Zool J Linn Soc. 1995; 115: 47–71. 10.1111/j.1096-3642.1995.tb02323.x [DOI] [Google Scholar]
- 18.Ruf I, Luo ZX, Wible JR, Martin T. Petrosal anatomy and inner ear structures of the Late Jurassic Henkelotherium (Mammalia, Cladotheria, Dryolestoidea): insight into the early evolution of the ear region in cladotherian mammals. J Anat. 2009; 214: 679–93. 10.1111/j.1469-7580.2009.01059.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo ZX, Ruf I, Schultz JA, Martin T. Fossil evidence on evolution of inner ear cochlea in Jurassic mammals. Proc R Soc Lond B: Biol Sci. 2011; 278:28–34. 10.1098/rspb.2010.1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodrigues PG, Ruf I, Schultz CL. Digital reconstruction of the otic region and inner ear of the non-mammalian cynodont Brasilitherium riograndensis (Late Triassic, Brazil) and its relevance to the evolution of the mammalian ear. J Mamm Evol. 2013; 20:291–307. 10.1007/s10914-012-9221-2 [DOI] [Google Scholar]
- 21.Ruf I, Volpato V, Rose KD, Billet G, de Muizon C, Lehmann T. Digital reconstruction of the inner ear of Leptictidium auderiense (Leptictida, Mammalia) and North American leptictids reveals new insight into leptictidan locomotor agility. Paläontol Z. 2016; 90: 153–71. 10.1007/s12542-015-0276-2 [DOI] [Google Scholar]
- 22.Orliac M, Araújo R, Lihoreau F. The petrosal and inner ear of Diplobune minor, an enigmatic Artiodactyla from the Oligocene of Western Europe. J Morphol. 278: 1168–1184. 10.1002/jmor.20702 [DOI] [PubMed] [Google Scholar]
- 23.Ekdale EG. Form and function of the mammalian inner ear. Journal of anatomy. 2016; 228:324–37. 10.1111/joa.12308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olson EC. The occipital, otic, basicranial and pterygoid regions of the Gorgonopsia. J Morph 1938; 62:141–175. 10.1002/jmor.1050620202 [DOI] [Google Scholar]
- 25.Olson EC. The origin of mammals based upon the cranial morphology of the therapsid suborders. Spec Pap Geol Soc Am 1944; 55:1–95. 10.1130/SPE55-p1 [DOI] [Google Scholar]
- 26.Araújo R, Fernandez V, Polcyn MJ, Fröbisch J, Martins RM. Aspects of the gorgonopsian paleobiology: insights from the basicranium, occiput, osseous labyrinth, vasculature and neuroanatomy. PeerJ. 5: e3119 10.7717/peerj.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benoit J, Manger PR, Fernandez V, Rubidge BS The bony labyrinth of late Permian Biarmosuchia: palaeobiology and diversity in non-mammalian Therapsida. 2017; 52: 58–77. [Google Scholar]
- 28.Cox CB. A natural cast of the inner ear of a dicynodont. Am Mus Novit. 1962; 2116:1–6. url: http://hdl.handle.net/2246/3417 [Google Scholar]
- 29.Sollas IB, Sollas WJ. A study of the skull of a Dicynodon by means of serial sections. Phil Trans R Soc B. 1914; 204: 201–225. [Google Scholar]
- 30.Pearson HS. The skull of the dicynodont reptile Kannemeyeria. Proc Zool Soc. 1924; 94: 793–826. 10.1111/j.1096-3642.1924.tb03316.x [DOI] [Google Scholar]
- 31.Camp CL, Welles SP. Triassic dicynodont reptiles. Part I The North American genus Placerias. Berkeley: University of California Press; 1956. [Google Scholar]
- 32.Barry TH. The cranial morphology of the Permo-Triassic anomodont Pristerodon buffaloensis with special reference to the neural endocranium and visceral arch skeleton. Annals S Afr Mus. 1967; 50: 131–161 [Google Scholar]
- 33.Keyser AW. A preliminary study of the type area of the Cistecephalus zone of the Beaufort Series, and a revision of the anomodont family Cistecephalidae. S Afr Geol Surv Memoirs. 1973; 62: 1–71 [Google Scholar]
- 34.Fourie H. A detailed description of the internal structure of the skull of Emydops (Therapsida: Dicynodontia). Palaeontologia Africana. 1993; 30: 103–111 [Google Scholar]
- 35.Castanhinha R, Araújo R, Júnior LC, Angielczyk KD, Martins GG, Martins RMS, Chaouiya C, Beckmann F, Wilde F. Bringing dicynodonts back to life: paleobiology and anatomy of a new emydopoid genus from the Upper Permian of Mozambique. PLoS One. 2013; 8: e80974 10.1371/journal.pone.0080974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laaß M. Bone‐conduction hearing and seismic sensitivity of the Late Permian anomodont Kawingasaurus fossilis. J Morphol. 2015; 276: 121–143. 10.1002/jmor.20325 [DOI] [PubMed] [Google Scholar]
- 37.Laaß M. The origins of the cochlea and impedance matching hearing in synapsids. Acta Palaeontol Pol. 2016; 61: 267–280. 10.4202/app.00140.2014 [DOI] [Google Scholar]
- 38.Angielczyk KD, Rubidge BS, Day MO, Lin F. A reevaluation of Brachyprosopus broomi and Chelydontops altidentalis, dicynodonts (Therapsida, Anomodontia) from the middle Permian Tapinocephalus Assemblage Zone of the Karoo Basin, South Africa. J Vertebr Paleontol. 2016; 36: e1078342 10.1080/02724634.2016.1078342 [DOI] [Google Scholar]
- 39.Fröbisch J. Composition and similarity of global anomodont-bearing tetrapod faunas. Earth-Sci Rev. 2009; 95: 119–157. 10.1016/j.earscirev.2009.04.001 [DOI] [Google Scholar]
- 40.Billet G, Hautier L, Asher RJ, Schwarz C, Crumpton N, Martin T, Ruf I. High morphological variation of vestibular system accompanies slow and infrequent locomotion in three-toed sloths. Proc R Soc B: Biol Sci. 2012; 279: rspb20121212. 10.1098/rspb.2012.1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ekdale EG. Morphological variation in the ear region of Pleistocene Elephantimorpha (Mammalia, Proboscidea) from central Texas. J Morphol. 2011; 272: 452–464. 10.1002/jmor.10924 [DOI] [PubMed] [Google Scholar]
- 42.Antunes MT. Sur quelques reptiles du Karroo de Maniamba, Mocambique. Colloq Int Cent Nat Rech Sci. 1975; 218: 371–378. [Google Scholar]
- 43.Jourdan PP, Verniers J. 1983. Relatório final do Karroo da mancha de Metangula (Maniamba). Relatório da Direcçao Nacional de Geologia do Moçambique (Maputo) (= Professional papers of the Geological Survey of Mozambique), 81 p.
- 44.Verniers J, Jourdan PP, Paulis RV, Frasca-Spada L, De Bock FR. The Karroo Graben of Metangula Northern Mozambique. J Afr Earth Sci. 1989; 9: 137–158. 10.1016/0899-5362(89)90016-X [DOI] [Google Scholar]
- 45.Cox PG, Jeffrey N. Semicircular canals and agility: the influence of size and shape measures. J Anat. 2010; 216: 37–47. 10.1111/j.1469-7580.2009.01172.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carlson KJ, Stout D, Jashashvili T, de Ruiter DJ, Tafforeau P, Carlson K, Berger LR. The endocast of MH1, Australopithecus sediba. Science. 2011; 333: 1402–1407. 10.1126/science.1203922 [DOI] [PubMed] [Google Scholar]
- 47.Paganin D, Mayo S, Gureyev TE, Miller PR, Wilkins SW. Simultaneous phase and amplitude extraction from a single defocused image of a homogeneous object. J Microscopy. 2002; 206: 33–40. 10.1046/j.1365-2818.2002.01010.x [DOI] [PubMed] [Google Scholar]
- 48.Mirone A, Brun E, Gouillart E, Tafforeau P, Kieffer J. The PyHST2 hybrid distributed code for high speed tomographic reconstruction with iterative reconstruction and a priori knowledge capabilities. Nucl Instr Meth Phys Res. 2014; 324: 41–48. 10.1016/j.nimb.2013.09.030 [DOI] [Google Scholar]
- 49.Mardia K. V., Jupp P. E. Directional statistics. 2nd ed West Sussex: John Wiley and Sons Ld.; 2000. [Google Scholar]
- 50.Boos ADS, Kammerer CF, Schultz CL, Soares MB, Ilha ALR. A New Dicynodont (Therapsida: Anomodontia) from the Permian of Southern Brazil and its implications for Bidentalian origins. PLoS One. 2016; 11: e0155000 10.1371/journal.pone.0155000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Angielczyk KD, Kammerer CF. The cranial morphology, phylogenetic position and biogeography of the upper Permian dicynodont Compsodon helmoedi van Hoepen (Therapsida, Anomodontia). Papers in Palaeontology. 2017, 1–33 10.1002/spp2.1087
- 52.Herring SW, Scapino RP. Physiology of feeding in miniature pigs. J Morphol. 1973; 141:427–60. 10.1002/jmor.1051410405 [DOI] [PubMed] [Google Scholar]
- 53.Cox CB, Angielczyk KD. A new endothiodont dicynodont (Therapsida, Anomodontia) from the Permian Ruhuhu Formation (Songea Group) of Tanzania and its feeding system. J Vertebr Paleontol. 2015; 35:e935388 10.1080/02724634.2014.935388 [DOI] [Google Scholar]
- 54.Witmer LM, Ridgely RC. New insights into the brain, braincase, and ear region of tyrannosaurs (Dinosauria, Theropoda), with implications for sensory organization and behavior. Anat Rec. 2009; 292: 1266–1296. 10.1002/ar.20983 [DOI] [PubMed] [Google Scholar]
- 55.Spoor F, Wood B, Zonneveld F. Implications of early hominid labyrinthine morphology for evolution of human bipedal locomotion. Nature. 1994; 369: 645–648. 10.1038/369645a0 [DOI] [PubMed] [Google Scholar]
- 56.Maddin HC, Sherratt E. Influence of fossoriality on inner ear morphology: insights from caecilian amphibians. J Anat. 2014; 225: 83–93. 10.1111/joa.12190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clarke AH. On the vestibular labyrinth of Brachiosaurus brancai. J Vestibular Res. 2005; 15: 65–71. [PubMed] [Google Scholar]
- 58.Knoll F, Witmer LM, Ortega F, Ridgely RC, Schwarz-Wings D. The braincase of the basal sauropod dinosaur Spinophorosaurus and 3D reconstructions of the cranial endocast and inner ear. PloS One. 2012; 7: p.e30060 10.1371/journal.pone.0030060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ray S. Endothiodont dicynodonts from the Late Permian Kundaram Formation, India. Palaeontology. 2000; 43:375–405. 10.1111/1475-4983.00132 [DOI] [Google Scholar]
- 60.Peters RH. 1983. The Ecological Implications of Body Size New York: Cambridge University Press. [Google Scholar]
- 61.Calder WAI. 1984. Size, Function, and Life History Cambridge. MA: Harvard University Press. [Google Scholar]
- 62.Brown JH, Marquet PA, Taper ML. Evolution of body size: consequences of an energetic definition of fitness. Am Naturalist. 1993; 142, 573–584. 10.1086/285558 [DOI] [PubMed] [Google Scholar]
- 63.Jones GM, Spells KE. A theoretical and comparative study of the functional dependence of the semicircular canal upon its physical dimensions. Proc R Soc B. 1963; 157, 403–419. 10.1098/rspb.1963.0019 [DOI] [PubMed] [Google Scholar]
- 64.Campione NE, Evans DC. A universal scaling relationship between body mass and proximal limb bone dimensions in quadrupedal terrestrial tetrapods. BMC Biol. 2012; 10: 60 10.1186/1741-7007-10-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The various important moments of Mozambican Karoo vertebrate paleontology are outlined starting 1949 with Domingos da Rocha to the 1980’s by Brigadas de Cartografia Geológica da Bacia carbonífera de Metangula.
(DOCX)
(XLSX)
(XLSX)
(XLSX)
(STL)
(STL)
(STL)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.
The tomographies described here are made accessible as.jpeg2000 stacks through the ESRF Paleontological Database (paleo.esrf.eu).