Abstract
Gastrointestinal helminths can have a detrimental effect on the fitness of wild ungulates. Arctic and Subarctic ecosystems are ideal for the study of host-parasite interactions due to the comparatively simple ecological interactions and limited confounding factors. We used a unique dataset assembled in the early seventies to study the diversity of gastrointestinal helminths and their effect on fitness indicators of Dall’s sheep, Ovis dalli dalli, in the Mackenzie Mountains, Northwest Territories, Canada. Parasite diversity included nine species, among which the abomasal nematode Marshallagia marshalli occurred with the highest prevalence and infection intensity. The intensity of M. marshalli increased with age and was negatively associated with body condition and pregnancy status in Dall’s sheep across all the analyses performed. The intensity of the intestinal whipworm, Trichuris schumakovitschi, decreased with age. No other parasites were significantly associated with age, body condition, or pregnancy. Our study suggests that M. marshalli might negatively influence fitness of adult female Dall’s sheep.
Introduction
It is widely accepted that nematode parasites can have a subtle but significant detrimental effects on host fitness[1–3]. In wild ungulates, for instance, experimental and observational work has shown that gastrointestinal helminths can negatively influence pregnancy rates[4,5], body condition[5–8], offspring survival[9] and mortality[9–11]. The study of these effects in wild ungulates from Arctic and Subarctic regions is important for a variety of reasons: i) human communities in these regions depend on wild ungulates as a source of food, as economic drivers through sport hunting and tourism, and as a focus for cultural activities[12], ii) Arctic and Subarctic regions are among the areas with least human disturbance globally, however, the current and predicted climate change is more prominent in high latitudes causing rapid and deep ecological changes and altering host-parasite dynamics[13–15], iii) wild animal and parasite communities are comparatively more simple than in temperate and tropical systems decreasing the number and complexity of potential confounding factors, and thus, serving as an ideal model for testing ecological mechanisms[4,16]
The Dall’s sheep (Ovis dalli dalli) is a wild ungulate endemic to the arctic and subarctic regions of North America, including Alaska (AK, United States), Yukon (YT, Canada), and Northwest Territories (NT, Canada)[17]. It breeds in late November and early December with births occurring the following April-May. During the short summer, ewes seek solitude and protection in rugged terrain for growth and development of lambs and to recover their body reserves[18,19]. Dall’s sheep are an important subsistence species for northern indigenous people as well as a valuable sport hunting prize, appreciated for their high-quality meat and large horn size[20,21].
The parasitological information on Dall’s sheep is limited to serological surveys of microparasites like Toxoplasma gondii[22], description of Eimeria spp.[23], detailed taxonomic and ecological studies on the protostrongylid nematodes Parelaphostrongylus odocoilei and Protostrongylus stilesi[24–26], and broad-based fecal and gastrointestinal parasite surveys[27–30]. The nematode Marshallagia marshalli has been documented as the gastrointestinal macroparasite with the highest infection intensities and prevalence throughout Dall’s sheep distribution range[28–30]. The pathophysiological changes caused by M. marshalli include a marked loss of functional parietal cells in the abomasum of the host resulting in an increase of abomasal pH, and an increase of both serum pepsinogen and serum gastrin concentrations[31,32]. The clinical presentation is similar to other gastrointestinal parasites including appetite depression, weight loss, constipation and diarrhea[31,33]. Based on field observations in Alaska, Neilsen and Neiland[29] suggested that M. marshalli might chronically weaken adult sheep and alter the digestion capacity of crude protein and dry matter in lambs during their first winter. Despite the importance of Dall’s sheep for the local economy and subsistence, plus the mounting evidence on the detrimental effect of parasites on wild ungulates in arctic and subarctic regions[4,5,34], little is known about the impacts of gastrointestinal helminths and particularly M. marshalli on Dall’s sheep.
The objectives of this study were to describe the diversity of gastrointestinal parasites in Dall’s sheep and to determine the relationship between parasite intensity and fitness indicators. To do this, we used an unprecedented historical collection of parasites and associated data in Dall’s sheep collected from the Mackenzie Mountains, Canada in 1971–1972. We had three specific objectives; to (i) describe the diversity and intensity of gastrointestinal helminths, and their relationship with age classes and sex, (ii) determine the association between M. marshalli intensity and body condition indicators, and finally, (iii) determine the association between infection intensity of M. marshalli and pregnancy status in adult females Dall’s sheep.
Material and methods
The simmons collection
From 1968 to 1972 an extensive scientific collection of Dall’s sheep occurred as part of a demographic study of sheep in the Mackenzie Mountains, Northwest Territories (NWT), Canada[35]. The two largest collections occurred in the winters of 1971 and 1972 and, in addition to the physiological and reproductive data, gastrointestinal parasites were collected by Dr. Anne Currier, a veterinarian for the Canadian Wildlife Service, Fort Smith, NWT. After preliminary evaluation in the early 1970s, no further work was done on the parasite collection and its whereabouts were unknown until the spring of 2000, when it was located as an orphaned collection at the Canadian Museum of Nature (CMN) in Ottawa (Ontario, Canada). The specimens and associated documentation were recovered and evaluated by Susan Kutz along with Eric Hoberg and Alasdair Veitch, who determined that it was an extremely valuable, unprecedented intact dataset and parasite specimen collection for Dall’s sheep. It is now known as “The Simmons Collection”.
The collection consisted of four boxes containing approximately 100 vials of parasites each, as well as a small collection of specimens mounted on slides. All the documentation was stored in a single binder containing individual cards with the parasitological information from each sheep and an extensive scientific correspondence among the principal investigators involved in this research, including Dr. Norman Simmons (project leader, Fort Norman), Dr. Anne Currier (veterinarian, Fort Smith, post mortem examinations, isolation and quantification of helminths), Dr. Laurel P. E. Choquette (Ottawa, identification of helminths), Dr. Eric Broughton (veterinary pathologist, Ottawa, lung pathology), and Dr. G. G. Gibson (Ottawa, involved in the lungworm studies). In addition, Dr. Norman Simmons provided the original post mortem cards (Fig 1) and summary sheets with the information on physiological and reproductive parameters from each sheep collected in 1971 and 1972 (see below).
Fig 1. Original post mortem card.
Card used by Dr. Norman Simmons to record the individual physiological and reproductive information of Dall’s sheep captured in the Mackenzie Mountains during February 1971–1972.
Dall’s sheep captures and data collection
The Dall’s sheep collection occurred in February 1971 and 1972 from several independent subpopulations inhabiting the areas of Mountain and Moosehorn rivers in the north central Mackenzie Mountains (65 degrees N; 128 degrees W), Canada. The captures were performed with a high-powered rifle fired from a helicopter, including all sheep in the first group encountered except for those clearly identifiable as rams. The information on physiological and reproductive parameters on each sheep was recorded on individual post-mortem cards and summary sheets[35]. The post mortem cards included information on sex (i.e. male, female), age in years based on both counts of horn rings and tooth cementum annuli, reproductive status determined by the presence or absence of a foetus, body weight (Kg), several body measurements (e.g. total body length, chest circumference) and a body condition score based on the presence/absence of abdominal and back fat (Fig 1).
The gathering of specimens and data on gastrointestinal parasites was performed by Dr. Anne Currier. Adults worms from the abomasum, small intestine, large intestine and caeca of each sheep were isolated from each organ, provisionally identified, counted, and stored in 10% formalin. Between 2003–2005, S. Kutz, together with EP. Hoberg, retrieved these vials, rehydrated the specimens when needed, and then mounted them on microscope slides and cleared them in 5% glycerol for subsequent examination and identification at the species level according to [36–42].
Data analyses
Sheep body condition
We used two different approaches to assess body condition of the sheep: i) a categorical body condition score (BCS) based on the presence or absence of subcutaneous fat reserves, and ii) a non-destructive residual index based on mass and morphometric measurements (scale mass index, SMI)[43].
The BCS was determined in the field and recorded in the post mortem cards. Sheep with no abdominal or back fat were classified as ‘Lean’; sheep with abdominal but not back fat were classified as ‘Fair’; sheep with abdominal and some back fat were classified as ‘Good’, and sheep with much or good back fat were classified as ‘Very Good’.
The SMI is a recent innovation that standardizes body mass at a fixed value of a linear body measurement based on the scaling relationship between mass and length[43,44]. This is a versatile index as it is independent from body size, sex, and physiological status, and unlike most residual indices, it can be used to compare across populations[45]. The SMI was calculated according to [43] as shown in the following formula:
| (1) |
where Mi is the body mass and Li is the body length of individual i, the bSMA is the regression coefficient in the Standardized Major Axis regression of ln-transformed M on ln- transformed L, and L0 is an arbitrary value of L. Normally the arbitrary value used is the arithmetic mean of L for the sample under study. Finally, SMI is the predicted scale mass index for individual i. In the case of pregnant females, we corrected their weight by subtracting the weight of their fetus (mean fetuses weight: 0.236 kg, SE = 0.013) before calculating their SMI.
Parasite intensity, prevalence, and species dominance
We determined the intensity of infection (i.e. median number of worms) and prevalence (i.e. percentage of positive cases + 95% confidence intervals) for each species of gastrointestinal helminth by sex, pregnancy status and year of collection. In the case of infection intensity and prevalence by sheep age, we used two different approaches: i) to compare juveniles with adults we used two age categories; juveniles (<24 months of age) and adults (> 24 months of age), and ii) to determine the age pattern of infection for the six most abundant parasite species we used five age categories: lambs (<1 year), yearlings (1 year old), young adults (2 to <6 years), mature adults (6 to <11 years) and old adults (11 years and older). In all the analyses, the differences in infection intensity between categories were explored using a t-test with Welch Correction to account for differences in variance. In the cases where the assumption of normality was not met a permutation t-test with 10,000 permutations was used. The differences in parasite prevalence among categories were determined using chi-squared test with Monte-Carlo significance test procedures (2000 replicates) to account for low cell counts (<5) in the contingency tables. To test differences in parasite infection intensity by BCS we used a one-way ANOVA and log+1 transformed parasite intensity to account for normally distributed residuals. The Tukey HDS test was used for pairwise comparisons. In the univariate analyses described above, all the observations available for each species were used.
The species dominance among gastrointestinal parasites was determined by using two approaches: a Dominance Index (DI) and a Dominance Value (DV). The DI, originally proposed by [46], considers species prevalence and is calculated by multiplying the total number of species i by the number of hosts infected with species i and divided by the total number of hosts examined squared. In this index, a dominant species was defined as one in which the DI is greater than 1, and a co-dominant species the one which the DI value was between 0.01 and 0.99. The DV, originally proposed by [47], considers the total number of parasites in the entire host sample. DV is calculated simply by dividing the total number of species i in the entire host sample by the total number of gastrointestinal parasites including all the species in the host sample, and multiplying the result by one hundred. Only the sheep with complete information for all the parasite species were included in the calculation of both approaches in order to obtain comparable results among species.
Fitness indicators and gastrointestinal parasites
The relationship between fitness indicators in the host (i.e. BCS, SMI and pregnancy), and the infection intensity of gastrointestinal helminths was explored using two approaches: i) Generalized Linear Models (GLM), this approach provide a flexible framework to study the association of continuous (i.e. SMI) and discrete (i.e. BCS and pregnancy status) outcomes and a set of independent variables. However, the number of predictor variables in the candidate model is directly limited by, among other factors, sample size (e.g. >10 outcome events per predictor variable) [48]. The models with too few outcome events relative to the number of predictor variables might produce biased estimates of regression coefficients [49], and ii) Partial Least Squares Regression (PLS-R) provides a flexible framework to study the association of continuous variables (i.e. SMI) and a set of independent variables. This approach is particularly useful when the number of sample units is low with respect to the number of measured independent variables [50]. Specifically, the PLS-R was used to investigate the simultaneous effect of helminths co-infection in host fitness.
The GLMs were fitted as follows: in the case of BCS we used ordinal logistic regression to account for the order on the categories in the outcome variable. Proportional odds ratios were obtained by the exponentiation of the coefficients from the independent variables in the final model. In order to meet the sample size requirements per predictor variable, the parameters included were reduced to: infection intensity of only the helminth species negatively associated with fitness indicators in the univariate analyses (i.e. M. marshalli and T. schumakovitschi), infection intensity of all the intestinal parasite species combined, age in years and pregnancy status. The SMI was analyzed using a GLM with a Gaussian error structure and a log link function and the parameters included in the models were the same as in the analyses of BCS. Finally, a GLM with binomial errors structure and a log link function was used to analyze pregnancy status in adult female sheep using the same parameters included in the analyses of BCS plus body condition (i.e. SMI). In order to facilitate the interpretation of coefficients and proportional odds ratios in all the models fitted, parasite intensity was divided by one hundred before being included in the models. By doing this we re-scaled the independent variable to a more meaningful unit from one parasite to one hundred parasites. In order to include pregnancy status as an independent variable, only adult females were included in the analyses. In all the cases when GLMs were used the model comparison was done using Akaike Information Criterion (AIC)[51].
In the case of the PLS-R, the independent variables included in the model were the intensity of each gastrointestinal parasite species (nine species), the species richness (i.e. number of parasite species per host), age in years, and pregnancy status, resulting in a total of 12 independent variables. The single dependent variable was SMI. In order to include pregnancy status as an independent variable, only adult females were included in the analysis. Both GLMs and PLS-R were performed with the R statistical software (R Development Core Team) and only sheep with complete information in all the independent variables were included. A significance level of p < 0.05 was used in all the analyses.
Results
General results
A total of 112 Dall’s sheep were collected from the Mackenzie Mountains during 1971 and 1972 [35]. However, The Simmons Collection at the CMN contained parasitological information only from 104 animals (23 from 1971 and 81 from 1972); 88 adult females (20 from 1971 and 68 from 1972), 6 yearling females (1 from 1971 and 5 from 1972), 8 yearling males (2 from 1971 and 8 from 1972) and two male lambs captured in 1972. It was not possible to determine the whereabouts of the information on the 8 remaining sheep.
Prevalence of gastrointestinal helminths was 100%. Eight different nematode species were identified; Marshallagia marshalli and Ostertagia gruehneri collected from the abomasum; Nematodirus andersoni, Nematodirus davtiani, Nematodirus oiratianus interruptus, Nematodirus spathiger, and Trichostrongylus sp. collected from the small intestine; and Trichuris schumakovitschi and Skrjabinema ovis collected from the large intestine (cecum and colon). In addition, the cestode Moniezia sp. was identified in the small intestine. There were no differences in parasite prevalence and intensity between 1971 and 1972 for any of the parasite species analyzed, total count of intestinal parasites or total gastrointestinal parasites (S1 Table). For this reason, the data in the rest of manuscript were analyzed and presented grouping both years. Information on abomasal helminths species was available only for 79 sheep. Total count of Nematodirus spp. was available for 102 sheep but Nematodirus species identification were only available for 54 sheep. Marshallagia marshalli was the most common parasite species among all sheep with the highest prevalence (100%, n = 79, 95% Confidence Interval [95% CI] = 94.1–100) and highest intensity of infection (median = 224, range 22–2457). Marshallagia marshalli is a dimorphic species with two distinct male forms [37]. The minor morphotype, designated as Marshallagia occidentalis, was not observed in the Simmons collection. Counts of M. marshalli reflected in the current paper would thus refer solely to the major morphotype. Nematodirus spp. were detected in 95% (n = 102, 95% CI = 88.2–98.1) of the sheep although with significantly lower intensity of infection (median = 98, range 1–1427) than M. marshalli (Permutation test, Z = 3.074, p-value<0.01). Among all Nematodirus spp., Nematodirus spathiger had the higher prevalence (94.4%, n = 54, 95%CI = 83.7–98.6) and infection intensity (median = 78, range 2–485). For more details see Table 1. Marshallagia marshalli was the species with the highest dominance index and dominance value, followed in both cases, by N. spathiger and N. oiratianus interruptus. These dominance indicators were several times higher in M. marshalli compared to any other species in both juvenile and adult sheep (Table 2).
Table 1. Prevalence and median infection intensity of gastrointestinal helminths in Dall´s sheep categorized as Juveniles (sheep younger than 24 months, including two lambs younger than 12 months) and adults (sheep 24 months and older).
| Juveniles | Adults | |||||||
|---|---|---|---|---|---|---|---|---|
| n | No. of infected | Prevalence % (95% CI) |
Median (Range) | n | No. of infected | Prevalence % (95% CI) | Median (Range) | |
| Abomasal parasites | ||||||||
| Ostertagia gruehneri | 13 | 0 | 0 (0–28.3) | NA | 66 | 1 | 1.5% (0–9.3) | 360 (1) |
| Marshallagia marshalli | 13 | 11 | 100 (67.8–100) | 252 (22–853) | 66 | 66 | 100 (93.1–100) | 223.5 (42–2457) |
| Small intestine parasites | ||||||||
| Moniezia sp. | 16 | 2 | 12.5 (2.2–39.6) | 1.5 (1–2) | 86 | 13 | 15.1 (8.6–24.8) | 1 (1–3) |
| Trichostrongylus spp. | 16 | 0 | 0 (0–24.1) | NA | 86 | 2 | 2.3 (0.4–8.9) | 2 (1–3) |
| Nematodirus andersoni | 12 | 7 | 58.3 (28.6–83.5) | 13 (3–66) | 42 | 21 | 50 (35.5–64.5) | 27 (2–189) |
| Nematodirus davtiani | 12 | 6 | 50 (25.3–74.6) | 29.5 (5–176) | 42 | 13 | 30.9 (18.1–47.2) | 19 (3–490) |
| Nematodirus oiratianus interrruptus | 12 | 9 | 75 (42.8–93.3) | 119 (20–431) | 42 | 25 | 59.5 (43.3–73.9) | 67 (4–469) |
| Nematodirus spathiger | 12 | 11 | 91.6 (59.8–99.6) | 109 (6–202) | 42 | 40 | 95.2 (82.6–99.2) | 73 (2–485) |
| Large intestine parasites | ||||||||
| Trichuris schumakovitschi | 16 | 16 | 100 (75.9–100) | 29.5 (5–210) | 86 | 75 | 87.2 (77.9–93.1) | 14 (1–131) * |
| Skrjabinema ovis | 16 | 6 | 37.5 (16.3–64.1) | 18.5 (1–68) | 86 | 55 | 63.9 (52.8–73.8) | 5 (1–45) * |
| Parasites grouped | ||||||||
| Total Nematodirus spp. | 16 | 16 | 100 (75.9–100) | 232.5 (4–630) | 86 | 81 | 94.1 (86.3–97.8) | 87 (1–1427) |
| Total Intestinal parasites | 16 | 16 | 100 (75.9–100) | 312 (45–643) | 86 | 86 | 100 (94.7–100) | 122.5 (2–1481) |
| Total Gastrointestinal | 13 | 13 | 100 (71.7–100) | 516 (173–1234) | 66 | 66 | 100 (93.1–100) | 414.5 (65–3715) |
Significant differences in intensity between age classes are indicated by * (p<0.05, Randomization test).
No differences in prevalence between age classes were found in any of the species or groups of parasites (p<0.05, chi-squared test).
Table 2. Species dominance of gastrointestinal helminths in Dall´s sheep categorized as Juveniles (sheep younger than 24 months, including two lambs younger than 12 months) and adults (24 months and older).
| Juveniles (n = 12) | Adults (n = 39) | |||
|---|---|---|---|---|
| Dominance Index | Dominance Value | Dominance Index | Dominance Value | |
| Abomasal parasites | ||||
| Ostertagia gruehneri | 0 | 0 | 0.0002 | 0.01 |
| Marshallagia marshalli | 280.1* | 47.9 | 478.5* | 61.9 |
| Small intestine parasites | ||||
| Moniezia sp. | 0.03 | 0.04 | 0.023 | 0.02 |
| Trichostrongylus spp. | 0 | 0 | 0.005 | 0.01 |
| Nematodirus andersoni | 7.8* | 2.3 | 14.7* | 3.7 |
| Nematodirus davtiani | 13.4* | 4.6 | 11.9* | 4.6 |
| Nematodirus oiratianus interruptus | 89.4* | 20.4 | 40.9* | 9.4 |
| Nematodirus spathiger | 96.9* | 16.6 | 124.7* | 17.0 |
| Large intestine parasites | ||||
| Trichuris schumakovitschi | 47.7* | 8.2 | 18.2* | 2.6 |
| Skrjabinema ovis | 0.15* | 0.09 | 2.24* | 0.6 |
Species classified as Dominant (Dominance Index >1) are indicated by *.
Distribution of gastrointestinal parasites by age and sex
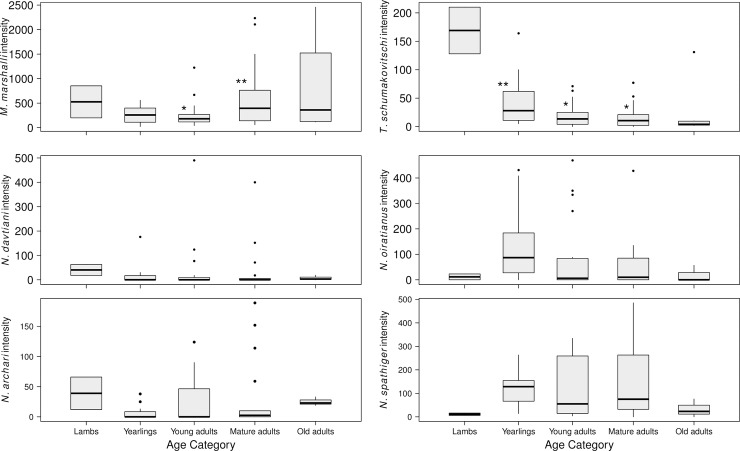
We analyzed the age distribution pattern of infection intensity for the six most abundant parasite species (i.e. five age classes of the host): M. marshalli, N. andersoni, N. davtiani, N. oiratianus interruptus, N. spathiger and T. schumakovitschi (Fig 2). Trichuris schumakovitschi had a significantly higher infection intensity in immature sheep (Permutation test, Z = -2.265, p-value = 0.023), with yearlings having significantly higher infection intensity than young adults (Pairwise permutation test, W = -3.081, p-value = 0.012) and mature adults (Pairwise permutation test, W = -2.851, p-value = 0.013). The opposite trend was observed for M. marshalli, with higher infection intensity in mature adults than in young adults (Pairwise permutation test, W = 2.681, p-value = 0.0219). The infection intensity among the remaining parasite species did not differ among age classes. Comparison of infection intensity between sexes was done only in yearlings as no older males were captured. The infection intensity of T. schumakovitschi in male sheep (n = 8, median = 65, range 11–210) was significantly higher than in females (n = 8, median = 16.5, range = 5–62) (Permutation test, Z = -1.84, p-value = 0.021). There was no difference of infection intensity between sexes for other parasite species.
Fig 2. Infection intensity of gastrointestinal helminth species in adult Dall’s sheep categorized by age class.
Lambs and old adults were excluded from pairwise comparison because of small sample size (n<5). Significant difference among categories (p<0.05) are represented with “*”.
Infection intensity of parasites and body condition
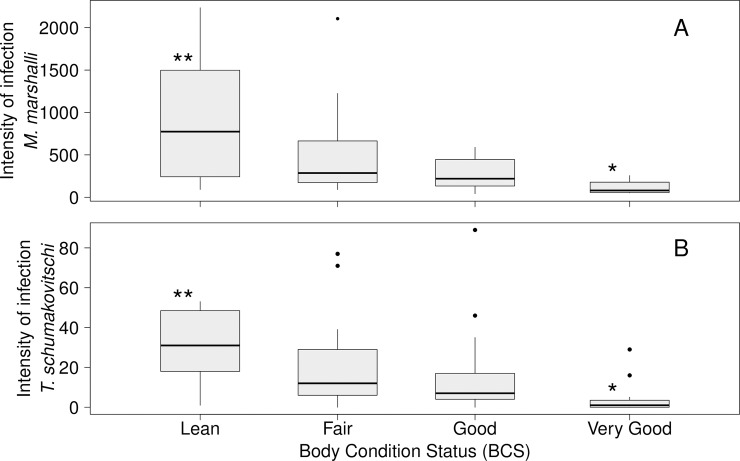
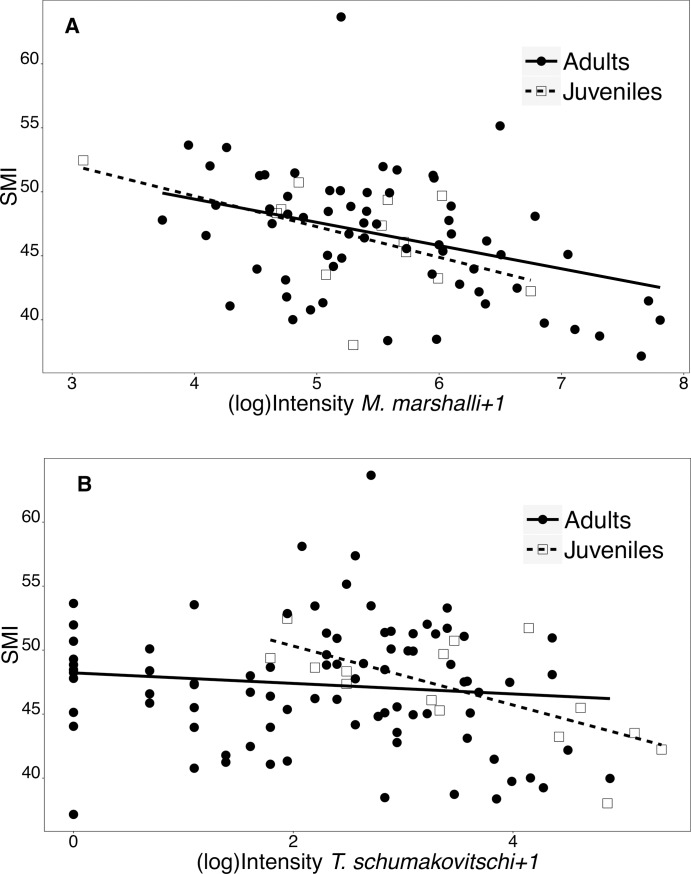
The body condition of adult ewes was negatively associated with the infection intensity of gastrointestinal parasites. Information in BCS was available only for 63 adults and 12 juveniles. The total count of gastrointestinal helminths (log10-transformed) in adult females with both Lean and Fair BCS was significantly higher than in sheep in Very Good BCS (ANOVA, F3,38 = 6.965, p< 0.001). For individual parasites species, only the intensity of M. marshalli and T. schumakovitschi was negatively associated with BCS (M. marshalli, n = 42: Permutation test, Z = -2.966, p-value = 0.0013, Fig 3A; T. schumakovitschi, n = 61: Permutation test, Z = -2.756, p = 0.005, Fig 3B). The infection intensity of M. marshalli (median = 223.5, range = 42–2457) was significantly higher than T. schumakovitschi (median = 14, range = 1–131) (Permutation test, p<0.001). The BCS in juvenile sheep was not associated with either total count of gastrointestinal helminths, M. marshalli or any parasite species. However, the sample size was low and there was an unbalanced distribution among BCS categories. With respect to SMI, adult Dall’s sheep with lower SMI had significantly higher infection intensities of Marshallagia marshalli (Fig 4). Juvenile sheep also had the same pattern of higher infection intensity of M. marshalli with lower SMI, however, the association was barely not significant. Trichuris schumakovitschi also had a negative association with SMI in juvenile sheep (Fig 4). No other single species, nor total intestinal parasites as a whole, were negatively associated with SMI.
Fig 3. Infection intensity of gastrointestinal helminths in adult Dall’s sheep categorized by body condition (BCS).
A) Total infection intensity of M. marshalli (Pairwise comparisons, n = 42, Lean-Very Good, W = -2.674, p = 0.0249). B) Infection intensity of T. schumakovitschi (Pairwise comparisons, n = 61, Lean-Very Good, W = -2.816, p = 0.0291). Significant differences among categories (p<0.05) are represented with “*”.
Fig 4. Relationship between SMI and infection intensity of parasites in Dall’s sheep.
The lines and dashed lines represent the predicted SMI for adults and yearling Dall’s sheep, respectively, estimated by a linear regression. A) Marshallagia marshalli intensity (Linear regression, Adults (n = 66): coefficient = -1.813, p = 0.004, R2 = 0.124; Yearlings (n = 13): coefficient = -2.392, p = 0.062, R2 = 0.28) and B) Trichuris schumakovitschi intensity (Linear regression, Adults (n = 86): coefficient = -0.411, p = 0.311, R2 = 0.012; Yearlings (n = 16): coefficient = -2.302, p = 0.004, R2 = 0.434).
A similar negative association between body condition indicators and the intensity of gastrointestinal parasites was observed when data were analyzed using a multivariate approach. In the case of BCS, the best model included M. marshalli infection intensity/100 (log odds ratio = 0.83, 95% CI = 0.71 0.95, Table 3) and the infection intensity of intestinal parasites/100 (log odds ratio = 0.78, 95% CI = 0.61 0.96, Table 3). Marshallagia marshalli was present in the first six models with the lowest AIC and always had a significant negative effect on BCS regardless of the other variables included in the model (S2 Table). Similarly, the best model describing SMI in Dall’s sheep included the effects of M. marshalli infection intensity and T. schumakovitschi (Table 3). The first nine models with the lowest AIC contained the effect of M. marshalli infection intensity (S2 Table). The effect of pregnancy status was also included in the best model although its effect on SMI was non-significant (Table 3).
Table 3. Final Generalized Linear Models for BCS, SMI and pregnancy status of adult female Dall’s sheep from Mackenzie Mountains.
| Model | Approach | n | Fixed effects | Estimate | SE | P-value | 95% CI |
|---|---|---|---|---|---|---|---|
| BCS Dall’s sheep | Ordinal regression | 42 | M. marshalli intensity/100 | -0.1807 | 0.072 | 0.013 | -0.335–0.047 |
| Intestinal parasites intensity/100 | -0.2190 | 0.169 | 0.049 | -0.484–0.010 | |||
| SMI Dall’s sheep | Gaussian | 66 | M. marshalli intensity/100 | -0.0074 | 0.0028 | 0.014 | -0.013–0.0018 |
|
T. schumakovitschi intensity/100 |
-0.0564 | 0.0612 | 0.360 | -0.176 0.0608 | |||
| Pregnancy status | Binomial | 66 | M. marshalli intensity/100 | -0.1391 | 0.0594 | 0.0191 | -0.273–0.032 |
In the PLS-R analysis, where 39 adult females had complete information for all parasite species, the first component explained a 23.22% (R2) of the original variance in the SMI and the second component explained 8% of the remaining unexplained variance (Table 4). The remaining 12 components included in the analysis had negligible importance in explaining SMI. In component one, M. marshalli, N. archari and T. schumakovitschi, in decreasing order, had the greatest negative effect on SMI and explained 76.98% of the information. Interestingly, either N. andersoni or T. schumakovitschi were significantly associated with SMI in univariate analyzes in the same dataset (Linear Regression; N. andersoni (n = 39), coefficient = -0.030, p = 0.079, R2 = 0.081; T. schumakovitschi (n = 39), coefficient = -0.055, p = 0.133, R2 = 0.059). In component two, M. marshalli and N. spathiger explained 84.91% of the component but only M. marshalli was negatively associated with SMI.
Table 4. Results of the Partial Least Squares Regression (PLS-R) explaining the effects of several gastrointestinal parasite species on body condition in adult female Dall’s sheep (n = 39).
| Component 1 | Component 2 | |||
|---|---|---|---|---|
| Weight | % Variance explained | Weight | % Variance explained | |
| Parasite species | ||||
| Nematodirus andersoni | -0.399 | 15.94 | -0.065 | 0.43 |
| Nematodirus davtiani | -0.103 | 1.06 | 0.267 | 7.12 |
| Nematodirus oiratianus interruptus | -0.154 | 2.38 | 0.251 | 6.30 |
| Nematodirus spathiger | 0.034 | 0.11 | 0.553 | 30.63 |
| Trichuris schumakovitschi | -0.343 | 11.77 | -0.083 | 0.68 |
| Skrjabinema ovis | -0.211 | 4.44 | -0.255 | 6.54 |
| Moniezia sp. | -0.171 | 2.91 | 0.211 | 4.48 |
| Trichostrongylus spp. | -0.145 | 2.12 | 0.290 | 8.41 |
| Marshallagia marshalli | -0.701 | 49.25 | -0.737 | 54.28 |
| Age | -0.161 | 2.58 | -0.170 | 2.89 |
| Parasite diversity | -0.253 | 6.44 | 0.173 | 2.99 |
| Pregnancy | 0.095 | 0.91 | -0.225 | 5.07 |
| R2 | 0.232 | 0.080 | ||
Weights of each variable in the first and second PLS-R components and R2 representing the variance in the response variable accounted for in each component in the PLS-R.
Infection intensity of parasites and pregnancy status
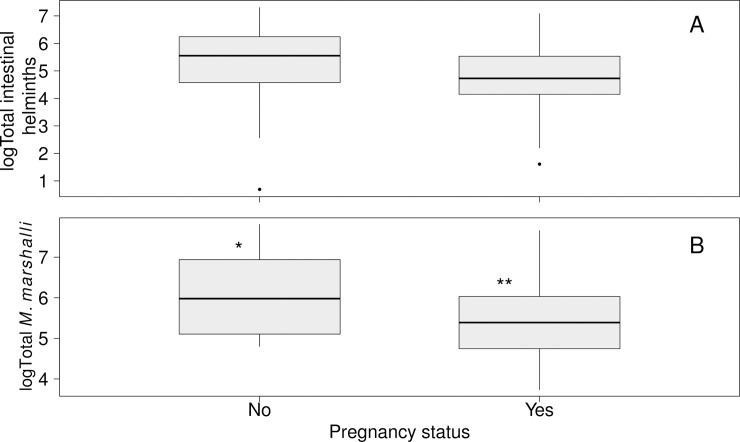
Pregnancy was negatively associated with the infection intensity of gastrointestinal parasites in adult female Dall’s sheep (Fig 5). This negative relationship seemed to be largely determined by M. marshalli; pregnant sheep had significantly lower log10-M. marshalli infection intensity than non-pregnant sheep (Fig 5, Table 5). There were no statistically significant associations of pregnancy with other individual species or intestinal parasites as a whole (Fig 5). The age (in years) of pregnant (Median = 5.8, range = 2.8–11.8) and non-pregnant (Median = 6.8, range = 2.8–12.8) ewes was not significantly different (Permutation test, Z = 0.556, p-value = 0.6181). The model with the better-fit explaining pregnancy in Dall’s sheep included only the effect of M. marshalli infection intensity (Table 4). There was a significant negative effect of M. marshalli infection intensity on Dall’s sheep pregnancy rate (estimated log odds ratio per 100 adult M. marshalli = 0.87, 95% CI = 0.76 0.97).
Fig 5. Infection intensity of gastrointestinal helminths in adult female Dall’s sheep categorized by pregnancy status.
A) Total infection intensity of intestinal helminths (t-test, t = 1.598, df = 25.96, p-value = 0.122). B) Infection intensity of Marshallagia marshalli (: t-test, t = 2.444, df = 24.753, p-value = 0.0220). Significant difference among categories (p<0.05) are represented with different numbers of “*”.
Table 5. Infection intensity and prevalence of gastrointestinal helminths in Dall´s sheep categorized by pregnancy status.
| Non Pregnant | Pregnant | |||||||
|---|---|---|---|---|---|---|---|---|
| n | No. of infected | Prevalence % (95% CI) | Median (Range) |
n | No. of infected | Prevalence % (95% CI) | Median (Range) | |
| Abomasal parasites | ||||||||
| Ostertagia gruehneri | ||||||||
| Marshallagia marshalli | 17 | 17 | 100 (77.1–100) | 395 (122–2457) | 49 | 49 | 100 (90.6–100) | 219 (42–2106)* |
| Small intestine parasites | ||||||||
| Moniezia sp. | 19 | 4 | 26.6 (6.9–46.1) | 1 (1–1) | 67 | 9 | 13.4 (6.6–24.5) | 1 (1–3) |
| Trichostrongylus spp. | 19 | 1 | 5.2 (0.2–28.1) | 1 (1) | 67 | 1 | 1.5 (0.08–9.1) | 3 (3) |
| Nematodirus andersoni | 9 | 5 | 55.6 (22.7–84.7) | 66 (5–189) | 33 | 16 | 48.5 (31.2–66.1) | 23 (2–152) |
| Nematodirus davtiani | 9 | 3 | 33.3 (9.1–69.1) | 18 (11–400) | 33 | 10 | 30.3 (16.2–48.9) | 45 (3–490) |
| Nematodirus oiratianus interruptus | 9 | 8 | 88.9 (50.7–99.4) | 48.5 (4–428) | 33 | 17 | 51.5 (33.9–68.8) | 67 (4–469) |
| Nematodirus spathiger | 9 | 9 | 100 (62.9–100) | 144 (6–485) | 33 | 31 | 93.9 (78.4–98.9) | 55 (2–473) |
| Large intestine parasites | ||||||||
| Trichuris schumakovitschi | 19 | 18 | 94.7 (71.9–99.7) | 15 (1–131) | 67 | 57 | 85.1 (73.8–92.2) | 14 (1–89) |
| Skrjabinema ovis | 19 | 11 | 57.8 (33.9–78.9) | 2 (1–24) | 67 | 44 | 66.7 (52.9–76.6) | 5.5 (1–45) |
| Parasites grouped | ||||||||
| Total Nematodirus spp. | 19 | 19 | 100 (79.1–100) | 220 (1–1427) | 67 | 62 | 92.5 (82.7–97.2) | 58.5 (1–1158) |
| Total Intestinal parasites | 19 | 19 | 100 (79.1–100) | 258 (2–1481) | 69 | 67 | 97.1 (88.9–99.5) | 83 (5–1191) |
| Total Gastrointestinal | 17 | 17 | 100 (79.1–100) | 477 (165–3715) | 49 | 49 | 100 (90.6–100) | 364 (65–2200) |
Significant differences in intensity between pregnancy status are indicated by * (p<0.05, Randomization test).
Discussion
Analyzing the Simmons Collection allowed us to describe the diversity, intensity and prevalence of gastrointestinal helminths in Dall’s sheep from the Mackenzie Mountains in 1971–1972. The parasite fauna identified in this study was similar to that reported and summarized elsewhere in the Northwest Territories and Alaska[28–30,52]. By linking this parasitological data with reproductive and physiological parameters of the host we found strong evidence of a negative association between infection intensity of parasites and both host body condition and pregnancy status. These results were largely determined by the infection intensity of the nematode M. marshalli, suggesting that this species can have detrimental effects on Dall’s sheep fitness. We first discuss the species diversity, then impacts, and conclude with discussing the value of the Simmons collection and of data and sample archiving.
Species diversity and distribution
The diversity of parasite species identified in our study is similar to what has been previously described in Dall’s sheep and other wild ungulates in North America[28,53]. Consistent with other trichostrongylines, M. marshalli is a dimorphic ostertagiine nematode with a direct life cycle that occurs in the abomasum of several wild ungulates in North America such as muskoxen (Ovibos moschatus), bighorn sheep (Ovis canadensis), thinhorn sheep (Ovis dalli), and pronghorn (Antilocapra americana), among others[37]. This species has remarkable ecological tolerance and resilience to persist in extreme environmental conditions. For instance, the eggs remain viable after extended freezing[28] and the infective stage (L3) is also tolerant to freezing temperatures, allowing transmission in the Arctic winter[54,55]. Nematodirus spp. are primarily nematodes infecting the small intestine, are commonly found in Arctic and Subarctic ungulates, and are well adapted to life at high latitudes[28,56]. The four Nematodirus species reported in our study are the same as previously observed in Dall’s sheep from various locations in Alaska[29,39]. It appears that N. andersoni is the correct name for nematodirines previously considered to represent another species, N. archari, according to Durette-Desset and Samuel [40]; until shown otherwise, N. archari is now considered to be limited in distribution to wild sheep from eastern Eurasia where it was originally described. The most prevalent and numerically dominant Nematodirus species seems to vary among Dall’s sheep populations which may be due to the interaction between differences in the natural history among parasite species (e.g. development and hatching strategies, specific seasonal development), the sampling strategies in each sheep population (e.g. season of sampling, parts of the intestine examined), and perhaps geographic locality reflecting complex histories of population fragmentation and isolation[29,57]. Species of Trichuris are commonly found in the large intestine of Dall’s sheep[29] and bighorn sheep[58], but apparently are rare in other sympatric wild ungulates such as caribou and muskoxen[28]. Trichuris schumakovitschi is the only species of Trichuris identified to the species level in Dall’s sheep. The pinworm Skrjabinema ovis is also common in the large intestine and caecum of Dall’s sheep, although at low to moderate prevalence and intensity[30]. This pinworm is also found in bighorn sheep and mountain goats, but rarely observed in caribou, moose and muskoxen[28,29,59–61]. Tapeworms of the genus Moniezia are considered rare in Dall’s sheep and M. benedeni is the only species previously reported[30]. Recent discovery of a complex of cryptic species attributed to Moniezia in the Arctic suggests that this species identification requires confirmation [62]. Ostertagia gruehneri was found in a single individual. This nematode is typically found in high numbers in the abomasum of the sympatric mountain woodland caribou, however, it is highly seasonal, being most abundant in the summer[63]. The Simmons Collection represents only winter parasite diversity and it is probable, based on previous fecal analyses, that O. gruehneri and other related strongyles (e.g. Teladorsagia boreoarcticus), may be more common in Dall’s sheep during the summer, particularly in populations with high sympatry with caribou and muskoxen[28].
Impact of parasites on Dall’s sheep fitness
Our data demonstrated a negative association between the infection intensity of M. marshalli and both body condition and pregnancy status of Dall’s sheep. The association between M. marshalli and host fitness has been examined for caribou and reindeer with contrasting results. In a cross-sectional study, Steele [64] determined that body condition indicators (i.e. protein mass index, kidney fat index, carcass weight) of female barrenground caribou from Kangerlussuaq-Sisimiut, west Greenland, were negatively associated with M. marshalli intensity, however, contrary to our study, there was no association with pregnancy rate. Dall’s sheep, possibly relate on larger body mass and the related higher amount of ingesta to cope with nutritional requirements during pregnancy. On the other hand, in an experiment using a delayed release anthelmintic bolus to control parasite intensity, Carlsson et al. [65] found no association between M. marshalli and body condition indicators in live wild Svalbard reindeer. The differences in the seasonal dynamics of M. marshalli among hosts species and populations, and differences in the natural history of the hosts themselves, might account for the variations in these results. For instance, egg production and infection intensity by M. marshalli in Dall’s sheep occurs year-round, with a slight decrease in the summer (Kutz et al. unpublished data,[29], suggesting a sustained pressure on the host throughout the entire year. In addition, this sustained pressure might increase with age as the intensity of M. marshalli significantly increased with age in Dall’s sheep. Conversely, M. marshalli in Svaldbard reindeer presents a strong seasonal pattern peaking in late spring and decreasing almost to zero during summer months suggesting a shorter seasonal impact[66]. The differences in parasite diversity among host populations might be also a source for divergent results. In Svalbard reindeer the abomasal fauna is largely dominated year-round by O. gruehneri, with a comparatively moderate increase in M. marshalli intensity during winter months but practically absent during summer[5]. Conversely, in the caribou population from Kangerlussuaq-Sisimiut in west Greenland, O. gruehneri is absent and, as occurs in Dall’s sheep, M. marshalli is the more prevalent species and with the higher intensity[64].
Marshallagia marshalli seems to be a species capable of producing a strong cumulative detrimental effect in Dall’s sheep. Marshallagia marshalli is the gastrointestinal helminth with the highest prevalence and infection intensity throughout Dall’s sheep distributional range[28–30]. It is an important cause of parasitic gastroenteritis in wild and domestic sheep[31,67] and can cause marked alterations in the abomasal physiology of the infected host[31,33]. In Dall’ sheep, this detrimental effect may occur through a variety of mechanisms that have been extensively studied for related nematodes in domestic ungulates. These include appetite depression, detrimental changes on host gastrointestinal function, alteration of protein metabolism and/or inducing a nutritionally demanding immune response[68–70]. The magnitude of these effects might be also influenced by the strong seasonality in the Mackenzie Mountains. As winter progresses, the forage availability can be limited only to wind-blown areas as snow cover becomes packed and crusted and sheep cannot dig through it, exacerbating the deficient nutritional uptake of highly parasitized Dall’s sheep[71]. A similar interaction between parasite effect and limited forage periods occurred in the soay sheep/Teladorsagia circumcincta system on St. Kilda, Scotland. The intensity of T. circumcincta, another abomasal ostertagiine, was negatively associated with daily survival in sheep experiencing food shortage during severe winters. However, experimentally infected sheep with ad libitum diet had only minor pathology and 100% survival, supporting that the impact of T. circumcincta can be exacerbated by reduced food availability[11]. According to this hypothesis, the effects of M. marshalli in Dall’s sheep fitness are perhaps more easily detected during periods of nutritional stress, e.g., winter months, which is when our study was performed.
Trichuris schumakovitschi was also negatively associated with body condition, however, this association was not consistent across all the analyses. In domestic ungulates, the intensity and prevalence of Trichuris spp. tends to be higher in yearling and young individuals and clinical signs include chronic gastroenteritis and, in heavy infections, diarrhea, anorexia and weight loss[72]. The intensity and effects of Trichuris spp. are not known in free-ranging arctic ungulates but they also appear to be more important in young age classes. For instance, variable intensity of T. ovis in captive muskoxen have been regularly associated with diarrhea in calves[73]. In our study, the infection intensity of T. schumakovitschi in Dall’s sheep significantly decreased with age, suggesting a similar age distribution than in domestic animals and a higher impact in young sheep.
The negative effect of multiple gastrointestinal species (co-infection) on fitness indicators and the view of hosts as complex “ecosystems of parasites” need also be considered in Dall’s sheep. For instance, although M. marshalli, under a co-infection approach (PLS-R), was still the most important species associated with fitness indicators in adult ewes, the negative association of N. andersoni with body condition was unexpected and not observed in any of the previous univariate analyzes. This outcome could have been the consequence of complex interactions between top down regulations (e.g. immune response of the host) and bottom up regulations (e.g. competition for resources among parasite species) only visible under a co-infection approach. The study of these interactions and the price of neglecting parasite group/species when addressing the cost of parasites to host fitness is gaining increasing attention in the disease ecology community [74,75].
The simmons collection
The research presented herein would not have been possible without the foresight and attention to detail of Dr. Norman Simmons and colleagues over 40 years ago. The Simmons Collection is an unprecedented source of parasitological and ecological information on Dall’s sheep that would be nearly impossible to replicate today. For a variety of biological, ethical and political reasons, the collection of a similar number of Dall’s sheep for scientific purpose would be unlikely to be approved by the public and wildlife management agencies[76]. The Simmons Collection not only represents a parasitological dataset with an intrinsic value to assess ecological questions, it also, and maybe even more importantly, highlights the relevance of proper archiving samples in accredited repositories for the study of wildlife diseases[77,78]. Archival collections are the window to study past geographic and genetic distribution of disease agents and also are a direct opportunity to maximize the impact of federal and private funds over time[14,79–81].
On the other hand, there are also some limitations with respect of The Simmons Collection. First, there is no information on diversity and intensity of parasite larval stages in the sheep. Evidence from domestic[82] and wild[7,28] ungulates suggest that larval stages of ostertagiine nematodes in the abomasal mucosa can cause significant pathology and clinical effects on the host, particularly as a consequence of the mechanical damage when they emerge from the mucosa (e.g. Type II Ostertagiasis). Second, since the parasite specimens were preserved in formalin they are not suitable for molecular studies due to the highly fragmented nature of the DNA, even with the advent of nexgen methodologies. Recent studies suggest it may be possible to extract and sequence DNA from formalin-fixed specimens for use in phylogenetic analyses but the results still are highly variable and largely unreliable depending on specimen age and preservation conditions[83]. Finally, the sampling period in both years was restricted to February. Since the prevalence, intensity and composition of parasite communities is highly influenced by season[28], the information possible to obtain from The Simmons Collection on the life history of the parasites and parasite populations dynamics is limited to interpretation within the context of the season collected.
In conclusion, there is mounting evidence showing that gastrointestinal parasites can have strong influence at individual and population levels for wild ungulates[4–7,10,11,64,84]. In the case of Dall’s sheep, our study shows that M. marshalli, the most prevalent and numerically dominant species, might negatively influence fitness of their hosts by decreasing both body condition and pregnancy rates. Further research is needed to confirm and quantify the magnitude of this effect and to understand the main physiological, behavioural and ecological mechanisms involved. Further the Simmon’s Collection and the large collections of helminths in Dall’s sheep by Nielsen and Neiland (1974) from Alaska in the 1970’s are critical in establishing baselines to identify and document the outcomes of accelerating climate change and ecological perturbation in high latitude systems.
Supporting information
(PDF)
(PDF)
Acknowledgments
We gratefully acknowledge the Canadian Museum of Nature for their excellent job preserving The Simmons Collection for more than thirty years and for the permission to work in their facilities. We thank Brent Wagner for technical support. We thank the Killam Program and NSERC CREATE Host Parasite Interaction Program for funding O. Alejandro Aleuy. Some initial assessments of the Simmons Collections were supported through the Beringian Coevolution Project (BCP), funded by the National Science Foundation (DEB 0196095 and 0415668), an exploration of parasite diversity and history across the northern roof of the world, planned and coordinated by Joseph A. Cook (University of New Mexico) and Eric P. Hoberg (former United States National Parasite Collection).
Data Availability
The underlying data has been uploaded to (https://osf.io/ey7kx/).
Funding Statement
We thank the Killam Program and NSERC CREATE Host Parasite Interaction Program for funding O. Alejandro Aleuy. Some initial assessments of the Simmons Collections were supported through the Beringian Coevolution Project (BCP), funded by the National Science Foundation (DEB 0196095 and 0415668), an exploration of parasite diversity and history across the northern roof of the world, planned and coordinated by Joseph A. Cook (University of New Mexico) and Eric P. Hoberg (former United States National Parasite Collection). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hudson PJ, Rizzoli A, Grenfell BT, Heesterbeek H, Dobson AP. The ecology of wildlife diseases Oxford University Press; Oxford; 2002. [Google Scholar]
- 2.Altizer S, Nunn CL, Thrall PH, Gittleman JL, Antonovics J, Cunningham AA, et al. Social Organization and Parasite Risk in Mammals: Integrating Theory and Empirical Studies. Annu Rev Ecol Evol Syst. 2003;34: 517–547. [Google Scholar]
- 3.Scott ME, Dobson A. The Role of Parasites in Regulating Host Abundance. Parasitol Today. 1989;5: 177–181. [DOI] [PubMed] [Google Scholar]
- 4.Albon SD, Stien A, Irvine RJ. The role of parasites in the dynamics of a reindeer population. Proceedings of the Royal Society of London B. rspb.royalsocietypublishing.org; 2002;269: 1625–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stien A, Irvine RJ, Ropstad E, Halvorsen O, Langvatn R, Albon SD. The impact of gastrointestinal nematodes on wild reindeer: experimental and cross-sectional studies. J Anim Ecol. Blackwell Science Ltd; 2002;71: 937–945. [Google Scholar]
- 6.Irvine RJ, Corbishley H, Pilkington JG, Albon SD. Low-level parasitic worm burdens may reduce body condition in free-ranging red deer (Cervus elaphus). Parasitology. 2006;133: 465–475. doi: 10.1017/S0031182006000606 [DOI] [PubMed] [Google Scholar]
- 7.Hughes J, Albon SD, Irvine RJ, Woodin S. Is there a cost of parasites to caribou? Parasitology. 2009;136: 253–265. doi: 10.1017/S0031182008005246 [DOI] [PubMed] [Google Scholar]
- 8.Craig BH, Tempest LJ, Pilkington JG, Pemberton JM. Metazoan-protozoan parasite co-infections and host body weight in St Kilda Soay sheep. Parasitology. 2008;135: 433–441. doi: 10.1017/S0031182008004137 [DOI] [PubMed] [Google Scholar]
- 9.Festa-Bianchet M. Numbers of lungworm larvae in feces of bighorn sheep: yearly changes, influence of host sex, and effects on host survival. Can J Zool. 1991;69: 547–554. [Google Scholar]
- 10.Craig BH, Jones OR, Pilkington JG, Pemberton JM. Re-establishment of nematode infra-community and host survivorship in wild Soay sheep following anthelmintic treatment. Vet Parasitol. 2009;161: 47–52. doi: 10.1016/j.vetpar.2008.11.027 [DOI] [PubMed] [Google Scholar]
- 11.Gulland FM. The role of nematode parasites in Soay sheep (Ovis aries L.) mortality during a population crash. Parasitology. 1992;105 Pt 3): 493–503. [DOI] [PubMed] [Google Scholar]
- 12.Wesche SD, Chan HM. Adapting to the impacts of climate change on food security among Inuit in the Western Canadian Arctic. Ecohealth. 2010;7: 361–373. doi: 10.1007/s10393-010-0344-8 [DOI] [PubMed] [Google Scholar]
- 13.Settele J, Scholes R, Betts R, Bunn S, Leadley P, Nepstad D, et al. Terrestrial and inland water systems In: Climate Change 2014: Impacts,Adaptation, and Vulnerability. IPCC; 2014. [Google Scholar]
- 14.Kutz SJ, Hoberg EP, Molnár PK, Dobson A, Verocai GG. A walk on the tundra: Host–parasite interactions in an extreme environment. Int J Parasitol Parasites Wildl. 2014;3: 198–208. doi: 10.1016/j.ijppaw.2014.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoberg EP, Kutz SJ, Cook JA, Galaktionov K, Haukisalmi V, Henttonen H, et al. Parasites in terrestrial, freshwater and marine systems In: Arctic Biodiversity Assessment- Status and Trends in Arctic Biodiversity. Meltofte H, editor. Conservation of Arctic Flora and Fauna, Arctic Council; 2013. [Google Scholar]
- 16.Kutz SJ, Jenkins EJ, Veitch AM, Ducrocq J, Polley L, Elkin B, et al. The Arctic as a model for anticipating, preventing, and mitigating climate change impacts on host–parasite interactions. Vet Parasitol. 2009;163: 217–228. doi: 10.1016/j.vetpar.2009.06.008 [DOI] [PubMed] [Google Scholar]
- 17.Festa-Bianchet M. Ovis dalli. The IUCN Red List of Threatened Species. Version 2015.2 [Internet]. 2008 [cited 1 Dec 2017]. Available: http://www.iucnredlist.org/details/39250/0
- 18.Goodrowe KL, Smak B, Presley N, Nlonfort SL. Reproductive, Behavioral, and Endocrine Characteristics of the Dall’s Sheep (Ovis dalli). Zoo Biol. 1996;15: 45–54. [Google Scholar]
- 19.Rachlow JL, Bowyer RT. Interannual Variation in Timing and Synchrony of Parturition in Dall’s Sheep. J Mammal. [American Society of Mammalogists, Oxford University Press]; 1991;72: 487–492. [Google Scholar]
- 20.Lambert Koizumi C, Carey J, Branigam M, Callaghan K. Status of Dall’s Sheep (Ovis dalli dalli) in the Northern Richardson Mountains. Whitehorse, Yukon, Canada: Yukon Fish and Wildlife Branch; 2011. Report No.: TRC-11-01.
- 21.Nadasdy P. Reevaluating the Co-management Success Story. Arctic. 2003;56: 367–380. [Google Scholar]
- 22.Zarnke RL, Dubey JP, Kwok OC, Ver Hoef JM. Serologic survey for Toxoplasma gondii in selected wildlife species from Alaska. J Wildl Dis. 2000;36: 219–224. doi: 10.7589/0090-3558-36.2.219 [DOI] [PubMed] [Google Scholar]
- 23.Clark GW, Colwell DA. Eimeria dalli sp. n. (Protozoa: Eimeriidae) from Dall Sheep Ovis dalli. J Protozool. Blackwell Publishing Ltd; 1974;21: 197–199. [DOI] [PubMed] [Google Scholar]
- 24.Kutz SJ, Veitch AM, Hoberg EP, Elkin BT, Jenkins EJ, Polley L. New host and geographic records for two protostrongylids in Dall’s sheep. J Wildl Dis. 2001;37: 761–774. doi: 10.7589/0090-3558-37.4.761 [DOI] [PubMed] [Google Scholar]
- 25.Hoberg EP, Kutz SJ, Nagy J, Jenkins E, Elkin B, Branigan M, et al. Protostrongylus stilesi (Nematoda: Protostrongylidae): Ecological Isolation and Putative Host-Switching Between Dall’s Sheep and Muskoxen in a Contact Zone. Comp Parasitol. 2002;69: 1–9. [Google Scholar]
- 26.Jenkins EJ, Veitch AM, Kutz SJ, Bollinger TK, Chirino-Trejo JM, Elkin BT, et al. Protostrongylid parasites and pneumonia in captive and wild thinhorn sheep (Ovis dalli). J Wildl Dis. 2007;43: 189–205. doi: 10.7589/0090-3558-43.2.189 [DOI] [PubMed] [Google Scholar]
- 27.Jenkins EJ, Veitch AM, Kutz SJ, Hoberg EP, Polley L. Climate change and the epidemiology of protostrongylid nematodes in northern ecosystems: Parelaphostrongylus odocoilei and Protostrongylus stilesi in Dall’s sheep Ovis d. dalli). Parasitology. 2006;132: 387–401. doi: 10.1017/S0031182005009145 [DOI] [PubMed] [Google Scholar]
- 28.Kutz SJ, Ducrocq J, Verocai GG, Hoar BM, Colwell DD, Beckmen KB, et al. Chapter 2—Parasites in Ungulates of Arctic North America and Greenland: A View of Contemporary Diversity, Ecology, and Impact in a World Under Change In: And D Rollinson, editor. Advances in Parasitology. Academic Press; 2012. pp. 99–252. doi: 10.1016/B978-0-12-398457-9.00002-0 [DOI] [PubMed] [Google Scholar]
- 29.Neilsen C, Neiland K. Sheep Disease Report, Project Progress Report, Federal Aid in Wildlife Restoration. Alaska Department of Fish and Game; 1974. Report No.: Volume XIV.
- 30.Neiland K. Sheep Disease Studies. Alaska Department of Fish and Game; 1972. Report No.: Volume I.
- 31.Moradpour N, Borji H, Razmi G, Maleki M, Kazemi H. Pathophysiology of Marshallagia marshalli in experimentally infected lambs. Parasitology. 2013;140: 1762–1767. doi: 10.1017/S0031182013001042 [DOI] [PubMed] [Google Scholar]
- 32.Moradpour N, Borji H, Razmi G, Maleki M, Kazemi H. The Effect of Marshallagia marshalli on Serum Gastrin Concentrations in Experimentally Infected Lambs. J Parasitol. 2016;102: 436–439. doi: 10.1645/15-860 [DOI] [PubMed] [Google Scholar]
- 33.Moradpour N, Borji H, Razmi G, Kazemi H, Maleki M. Comparison of two methods of Marshallagia marshalli donor sheep production. J Parasit Dis. 2014;38: 289–292. doi: 10.1007/s12639-013-0243-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kutz SJ, Checkley S, Verocai GG, Dumond M, Hoberg EP, Peacock R, et al. Invasion, establishment, and range expansion of two parasitic nematodes in the Canadian Arctic. Glob Chang Biol. 2013;19: 3254–3262. doi: 10.1111/gcb.12315 [DOI] [PubMed] [Google Scholar]
- 35.Simmons NM, Bayer MB, Sinkey LO. Demography of Dall’s Sheep in the Mackenzie Mountains, Northwest Territories. J Wildl Manage. Wiley on behalf of the Wildlife Society; 1984;48: 156–162. [Google Scholar]
- 36.Hoberg EP, Monsen KJ, Kutz S, Blouin MS. Structure, Biodiversity, and Historical Biogeography of Nematode Faunas in Holarctic Ruminants: Morphological and Molecular Diagnoses for Teladorsagia boreoarticus n. sp. (Nemadota: Ostertagiinae), Dimorphic Cryptic Species in Muskoxen (Ovibos moschatus). The Journal of Parasitology. 1999;85: 910–934. [PubMed] [Google Scholar]
- 37.Hoberg EP, Abrams A, Pilitt PA, Jenkins EJ. Discovery and description of a new trichostrongyloid species (Nematoda: Ostertagiinae), abomasal parasites in mountain goat, Oreamnos americanus, from the Western Cordillera of North America. J Parasitol. 2012;98: 817–846. doi: 10.1645/GE-3047.1 [DOI] [PubMed] [Google Scholar]
- 38.Hoberg EP, Lichtenfels JR. Phylogenetic systematic analysis of the Trichostrongylidae (Nematoda), with an initial assessment of coevolution and biogeography. J Parasitol. 1994;80: 976–996. [PubMed] [Google Scholar]
- 39.Rickard LG, Lichtenfels JR. Nematodirus archari (Nematoda: Trichostrongyloidea) from ruminants in North America with a description of the synlophe and the female. Canadian Journal of Zoology. 1989; 67: 1708–1714. [Google Scholar]
- 40.Durette-Desset M-C, Samuel WM. Nematodirinae (Nematoda: Trichostrongyloidea) d’Antilocapra et d'Ovis en Alberta, Canada. Ann Parasitol Hum Comp. EDP Sciences; 1989;64: 469–477. doi: 10.1051/parasite/1989646469 [DOI] [PubMed] [Google Scholar]
- 41.Lichtenfels JR, Pilitt PA. Cuticular Ridge Patterns of Nematodirus (Nematoda: Trichostrong yloidea) Parasitic in Domestic Ruminants of North America, with a Key to Species. Proceedings of the Helminthological Society of Washington. 1983;50: 261–274. [Google Scholar]
- 42.Lichtenfels JR, Hoberg EP. The systematics of nematodes that cause ostertagiasis in domestic and wild ruminants in North America: an update and a key to species. Vet Parasitol. 1993;46: 33–53. [DOI] [PubMed] [Google Scholar]
- 43.Peig J, Green AJ. New perspectives for estimating body condition from mass/length data: the scaled mass index as an alternative method. Oikos. Blackwell Publishing Ltd; 2009;118: 1883–1891. [Google Scholar]
- 44.Peig J, Green AJ. The paradigm of body condition: a critical reappraisal of current methods based on mass and length. Funct Ecol. Blackwell Publishing Ltd; 2010;24: 1323–1332. [Google Scholar]
- 45.Labocha MK, Schutz H, Hayes JP. Which body condition index is best? Oikos. Blackwell Publishing Ltd; 2014;123: 111–119. [Google Scholar]
- 46.Bush AO. An ecological analysis of the helminth parasites of the white ibis in Florida. University of Florida. 1973. [Google Scholar]
- 47.Leong TS, Holmest JC. Communities of metazoan parasites in open water fishes of Cold Lake, Alberta. J Fish Biol. Blackwell Publishing Ltd; 1981;18: 693–713. [Google Scholar]
- 48.Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49: 1373–1379. [DOI] [PubMed] [Google Scholar]
- 49.Ogundimu EO, Altman DG, Collins GS. Adequate sample size for developing prediction models is not simply related to events per variable. J Clin Epidemiol. 2016;76: 175–182. doi: 10.1016/j.jclinepi.2016.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carrascal LM, Galvan I, Gordo O. Partial least squares regression as an alternative to current regression methods used in ecology. Oikos. 2009;118: 681–690. [Google Scholar]
- 51.Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr. 1974;19: 716–723. [Google Scholar]
- 52.Neiland KA. Sheep Disease Studies. Alaska Department of Fish and Game; 1977. Report No.: Volume XVII.
- 53.Hoberg EP, Kocan A, Rickard LG. Gastrointestinal strongyles in wild ruminants In: W Samuel MPAAK, editor. Parasitic Diseases of Wild Mammals. 2nd Iowa State University Press; 2001. pp. 193–227. [Google Scholar]
- 54.Carlsson AM, Justin Irvine R, Wilson K, Piertney SB, Halvorsen O, Coulson SJ, et al. Disease transmission in an extreme environment: nematode parasites infect reindeer during the Arctic winter. Int J Parasitol. 2012;42: 789–795. doi: 10.1016/j.ijpara.2012.05.007 [DOI] [PubMed] [Google Scholar]
- 55.Carlsson AM, Irvine RJ, Wilson K, Coulson SJ. Adaptations to the Arctic: low-temperature development and cold tolerance in the free-living stages of a parasitic nematode from Svalbard. Polar Biol. Springer-Verlag; 2013;36: 997–1005. [Google Scholar]
- 56.Gibson TE. The Development and Survival of the Pre-Parasitic Stages of Nematodirus Spp. on Pasture Herbage. J Comp Pathol Ther. 1958;68: 338–344. [DOI] [PubMed] [Google Scholar]
- 57.van Dijk J, Morgan ER. Hatching behaviour of Nematodirus filicollis in a flock co-infected with Nematodirus battus. Parasitology. 2009;136: 805–811. doi: 10.1017/S003118200900609X [DOI] [PubMed] [Google Scholar]
- 58.Knight RA, Uhazy LS. Redescription of Trichuris = Trichocephalus) schumakovitschi (Savinkova, 1967) from Canadian Rocky Mountain Bighorn Sheep (Ovis canadensis canadensis). J Parasitol. [American Society of Parasitologists, Allen Press]; 1973;59: 136–140. [PubMed] [Google Scholar]
- 59.Blood DA. Parasites from California bighorn sheep in southern British Columbia. Can J Zool. 1963;41: 913–918. [Google Scholar]
- 60.Becklund WW, Senger CM. Parasites of Ovis canadensis canadensis in Montana, with a checklist of the internal and external parasites of the Rocky Mountain bighorn sheep in North America. J Parasitol. 1967;53: 157–165. [PubMed] [Google Scholar]
- 61.Kerr GR, Holmes JC. Parasites of Mountain Goats in West Central Alberta. J Wildl Manage. [Wiley, Wildlife Society]; 1966;30: 786–790. [Google Scholar]
- 62.Haukisalmi V, Laaksonen S, Oksanen A, Beckmen K, Halajian A, Yanagida T, et al. Molecular taxonomy and subgeneric classification of tapeworms of the genus Moniezia Blanchard, 1891 (Cestoda, Anoplocephalidae) in northern cervids (Alces and Rangifer). Parasitol Int. 2018;67: 218–224. doi: 10.1016/j.parint.2017.12.006 [DOI] [PubMed] [Google Scholar]
- 63.Hoar BM, Ruckstuhl K, Kutz S. Development and availability of the free-living stages of Ostertagia gruehneri, an abomasal parasite of barrenground caribou (Rangifer tarandus groenlandicus), on the Canadian tundra. Parasitology. 2012;139: 1093–1100. doi: 10.1017/S003118201200042X [DOI] [PubMed] [Google Scholar]
- 64.Steele JF. The Devil’s in the Diversity: Divergent Parasite Faunas and their Impacts on Body Condition in Two Greenland Caribou Populations. University of Calgary; 2013. [Google Scholar]
- 65.Carlsson AM, Albon SD, Coulson SJ, Ropstad E, Stien A, Wilson K, et al. Little impact of over‐winter parasitism on a free‐ranging ungulate in the high Arctic. Funct Ecol. 2018; 1–11. [Google Scholar]
- 66.Irvine RJ, Stien A, Halvorsen O, Langvatn R, Albon SD. Life-history strategies and population dynamics of abomasal nematodes in Svalbard reindeer (Rangifer tarandus platyrhynchus). Parasitology. 2000;120 Pt 3): 297–311. [DOI] [PubMed] [Google Scholar]
- 67.Eslami A, Meydani M, Maleki S, Zargarzadeh A. Gastrointestinal nematodes of wild sheep (Ovis orientalis) from Iran. J Wildl Dis. 1979;15: 263–265. [DOI] [PubMed] [Google Scholar]
- 68.Fox MT. Pathophysiology of infection with gastrointestinal nematodes in domestic ruminants: recent developments. Vet Parasitol. 1997;72: 285–97; discussion 297–308. [DOI] [PubMed] [Google Scholar]
- 69.Forbes AB, Huckle CA, Gibb MJ, Rook AJ, Nuthall R. Evaluation of the effects of nematode parasitism on grazing behaviour, herbage intake and growth in young grazing cattle. Vet Parasitol. 2000;90: 111–118. [DOI] [PubMed] [Google Scholar]
- 70.Eberhardt AT, Costa SA, Marini MR, Racca A, Baldi CJ, Robles MR, et al. Parasitism and physiological trade-offs in stressed capybaras. PLoS One. 2013;8: e70382 doi: 10.1371/journal.pone.0070382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Simmons NM. Seasonal Ranges of Dall’s Sheep, Mackenzie Mountains, Northwest Territories. Arctic. 1982;35: 512–518. [Google Scholar]
- 72.Jacobs D, Fox M, Gibbons L, Hermosilla C. Principles of Veterinary Parasitology. John Wiley & Sons; 2015. doi: 10.1016/j.vetpar.2014.12.004 [Google Scholar]
- 73.Seider KB, Rowell JE. Canadian Muskoxen i n Centra l Europe—A Zoo Veterinary Review. Rangifer. 1996;16: 79–85. [Google Scholar]
- 74.Serrano E, Millán J. What is the price of neglecting parasite groups when assessing the cost of co-infection? Epidemiol Infect. 2014;142: 1533–1540. doi: 10.1017/S0950268813002100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Oliver-Guimerá A, Martínez-Carrasco C, Tvarijonaviciute A, Ruiz de Ybáñez MR, Martínez-Guijosa J, López-Olvera JR, et al. The physiological cost of male-biased parasitism in a nearly monomorphic mammal. Parasit Vectors. 2017;10: 200 doi: 10.1186/s13071-017-2060-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rodríguez-estrella R, Moreno MCB. Rare, Fragile Species, Small Populations, and the Dilemma of Collections. Biodivers Conserv. Kluwer Academic Publishers; 2006;15: 1621–1625. [Google Scholar]
- 77.Hoberg EP, Polley L, Jenkins EJ, Kutz SJ, Veitch AM, Elkin BT. Integrated approaches and empirical models for investigation of parasitic diseases in northern wildlife. Emerg Infect Dis. 2008;14: 10–17. doi: 10.3201/eid1401.071119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hoberg EP, Galbreath KE, Cook JA, Kutz SJ, Polley L. Northern Host-Parasite Assemblages: History and Biogeography on the Borderlands of Episodic Climate and Environmental Transition. Adv Parasitol. 2012;79: 1–97. doi: 10.1016/B978-0-12-398457-9.00001-9 [DOI] [PubMed] [Google Scholar]
- 79.DiEuliis D, Johnson KR, Morse SS, Schindel DE. Opinion: Specimen collections should have a much bigger role in infectious disease research and response. Proc Natl Acad Sci U S A. 2016;113: 4–7. doi: 10.1073/pnas.1522680112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hoberg EP, Kutz SJ, Galbreath KE. Arctic biodiversity: from discovery to faunal baselines-revealing the history of a dynamic ecosystem. J Parasitol. bgs.ucalgary.ca; 2003; Available: http://www.bgs.ucalgary.ca/files/bgs/Hoberg_kutz_galbreath_cook_2003.pdf [Google Scholar]
- 81.Cook JA, Galbreath KE, Bell KC, Campbell ML, Carrière S, Colella JP, et al. The Beringian Coevolution Project: Holistic collections of mammals and associated parasites reveal novel perspectives on changing environments in the north. Arctic Science. 2017;In press. [Google Scholar]
- 82.Armour J, Duncan M. Arrested larval development in cattle nematodes. Parasitol Today. 1987;3: 171–176. [DOI] [PubMed] [Google Scholar]
- 83.Hykin SM, Bi K, McGuire JA. Fixing Formalin: A Method to Recover Genomic-Scale DNA Sequence Data from Formalin-Fixed Museum Specimens Using High-Throughput Sequencing. PLoS One. 2015;10: e0141579 doi: 10.1371/journal.pone.0141579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Craig BH, Pilkington JG, Pemberton JM. Gastrointestinal nematode species burdens and host mortality in a feral sheep population. Parasitology. 2006;133: 485–496. doi: 10.1017/S0031182006000618 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
Data Availability Statement
The underlying data has been uploaded to (https://osf.io/ey7kx/).